Abstract
Background
Vascular occlusion after tissue transfer is a devastating complication that can lead to complete flap loss. Spatial frequency domain imaging is a new, noncontact, noninvasive, wide-field imaging technology capable of quantifying oxygenated and deoxygenated hemoglobin levels, total hemoglobin, and tissue saturation.
Methods
Pedicled fasciocutaneous flaps on Wistar rats (400 to 500 g) were created and underwent continuous imaging using spatial frequency domain imaging before and after selective vascular occlusion. Three flap groups (control, selective arterial occlusion, and selective venous occlusion) and a fourth group composed of native skin between the flaps were measured.
Results
There were no statistically significant differences between the control flap group and the experimental flap groups before selective vascular occlusion: oxyhemoglobin (p = 0.2017), deoxyhemoglobin (p = 0.3145), total hemoglobin (p = 0.2718), and tissue saturation,(p = 0.0777). In the selective arterial occlusion flap group, percentage change in total hemoglobin was statistically different from that of the control flap group (p = 0.0218). The remaining parameters were not statistically different from those of the control flap: percentage change in oxyhemoglobin (p = 0.0888), percentage change in deoxyhemoglobin (p = 0.5198), and percentage change in tissue saturation (p = 0.4220). The selective venous occlusion flap group demonstrated changes statistically different compared with the control flap group: percentage change in oxyhemoglobin (p = 0.0029) and deoxyhemoglobin, total hemoglobin, and tissue saturation (p < 0.0001).
Conclusions
Spatial frequency domain imaging provides two-dimensional, spatially resolved maps of tissue oxyhemoglobin, deoxyhemoglobin, total hemoglobin, and tissue saturation. Results presented here indicate that this can be used to quantify and detect physiologic changes that occur after arterial and venous occlusion in a rodent tissue transfer flap model. This portable, noncontact, noninvasive device may have a high clinical applicability in monitoring postoperative patients.
The process of reconstructive surgery using tissue transfer is a reliable method; however, it is not without complications, some of which can be devastating for the patient. These complications include vascular occlusion resulting in partial or complete flap loss. Overall thrombosis rates between 3 and 16 percent have been reported after reconstructive surgery using free tissue transfer.1–6 Venous thrombosis occurs more frequently than arterial thrombosis, with rates reported between 2 and 8 percent,1–3,5 whereas the rates of arterial thrombosis alone are reported to range from 0.7 to 2 percent.1,3,5,6 It has been well established in the literature that early detection followed by early reexploration of vascular compromised flaps results in improved salvage rates.1,3,5,7,8 Compared with arterial ischemia alone, the pathophysiology involved in venous thrombosis is associated with a greater degree of irreversible damage to the microvascular system of the flap9–11 and thus results in lower salvage rates.1,5 Early detection and surgical reexploration after venous thrombosis is imperative if a flap is to be salvaged. Both arterial and venous thromboses involve injury to the microvascular system of the flap, the development of microthrombosis, and tissue edema.9–11 The development of tissue edema and microthrombosis within the capillary beds results in continued impairment of oxygen diffusion capacity within the flaps after reestablishment of blood flow.9,10 Hjortdal et al.9,10 demonstrated that oxygen transport from the vascular system to the tissues remains diminished after reestablishment of blood flow. This phenomenon is more severe after venous thrombosis because of a greater degree of congestion within the flap after venous thrombosis compared with isolated arterial thrombosis. Arterial thrombosis is a rare event but may be difficult to detect based on clinical examination alone, as the flap does not become congested in appearance, but rather becomes pale.5 The difficulty in detecting arterial thrombosis is the rationale for the use of adjuncts to clinical examination, such as audible bedside Doppler checks of the pedicle, pin prick tests, and laser Doppler flowmeter monitoring.12–14
In this pilot study, we investigated the capabilities of a new optical imaging technology to detect vascular occlusion in a pedicled flap model. This device is based on the principles of spatial frequency domain imaging.15–19 The prototype device, called the Tissue OxImager, is being developed by Modulated Imaging, Inc. (Irvine, Calif.) and marketed under the name Introspective Medical (www.introspectivemedical.com). The prototype device used in this experiment was a portable device on a cart measuring 2 × 2 × 3 feet. A new, second-generation, handheld device has since been constructed.
The ideal tissue flap monitoring device should be capable of rapid data collection, be noninvasive and noncontact, and provide quantitative information, which will assist the clinician in evaluating the flap's viability. The Tissue OxImager is a noncontact spectral imaging device that uses near-infrared light and is capable of rapid, wide-field measurements from which two-dimensional maps of the optical properties of a tissue can be generated.15–19 This prototype spatial frequency domain imaging device is able to measure the absolute amount of deoxygenated hemoglobin and oxygenated hemoglobin in millimoles per liter and from these data calculate the total level of hemoglobin in the tissue and tissue saturation without the use of injectable contrast dyes. The device is different from other tissue spectroscopy devices in that it is an imaging device able to generate two-dimensional images of the tissue in a rapid fashion without scanning or movement of an imaging probe. The technique of using spatial frequency domain imaging to obtain wide-field quantitative maps of oxyhemoglobin and deoxyhemoglobin concentrations is, at this time, unique. There are other devices that are commercially available, such as the T.Ox (ViOptix, Fremont, Calif.) and the T-Stat (Spectros Corp., Portola Valley, Calif.), that are also capable of reporting local oxyhemoglobin and deoxyhemoglobin concentrations. However, those devices are fiber probe based, which requires contact with the tissue, and each probe interrogates only a small region of the tissue with which it is placed in contact.
Other groups have demonstrated that near-infrared tissue spectroscopy can detect and differentiate between arterial and venous occlusion, but none of the technologies used were imaging methods.20–23 In the study described here, we hypothesize that the spatial frequency domain imaging device will detect and differentiate a viable flap from those compromised by arterial or venous occlusion. In addition, we have examined the performance of the device to quantitatively deduce changes in oxyhemoglobin, deoxyhemoglobin, and total hemoglobin levels and tissue saturation.
MATERIALS AND METHODS
Bilateral fasciocutaneous pedicled flaps based on the inferior epigastric vessels were created in 11 Wistar rats (400 to 500 g). All flaps were created by a single surgeon, with the left-sided flap raised and set first, followed by the same process on the animal's right side. The flaps on each animal were randomized to one of three groups: control flap (n = 7 flaps), selective arterial ligation (n = 8 flaps), and selective venous ligation (n = 7 flaps). In addition to the three flap groups, an additional fourth group of native skin between the flaps on each animal was imaged simultaneously. The native skin group consisted of a region of skin between the bilateral groin flaps that was not surgically manipulated, and consisted of tissue near the flaps that remained outside of the surgical field. Figure 1 illustrates the orientation of the control and experimental flaps, the region of the native skin group, and the location of the caudal incisions used to selectively ligate the vessels in the experimental flaps. For each flap, 6–0 silk sutures were placed around the femoral artery and vein just proximal and distal to the inferior epigastric vessels. These sutures were used to selectively ligate the vessels during the imaging process by means of an incision caudal to the flap. The sutures on the control flaps were placed around the vessels but not ligated. The animals were imaged using the spatial frequency domain imaging device continuously for a period of 10 minutes after setting the second flap (from time −10 minutes to 0 minutes). This was performed before selective ligation of the experimental flaps at 0 minutes. This was followed by continuous measurements for an additional 55 minutes (from time 0 to 55 minutes) after ligation of the experimental flaps (selective arterial or selective venous occlusion flaps). The bilateral flaps and the native skin in the region between the flaps were imaged simultaneously during the experiment. Induction and maintenance of anesthesia was achieved using intraperitoneal injections of ketamine (75 to 100 mg/kg) and xylazine (5 to 10 mg/kg) in accordance with the University of California, Irvine Institutional Animal Care Use Committee–approved protocol (no. 2006–2693).
Fig. 1.
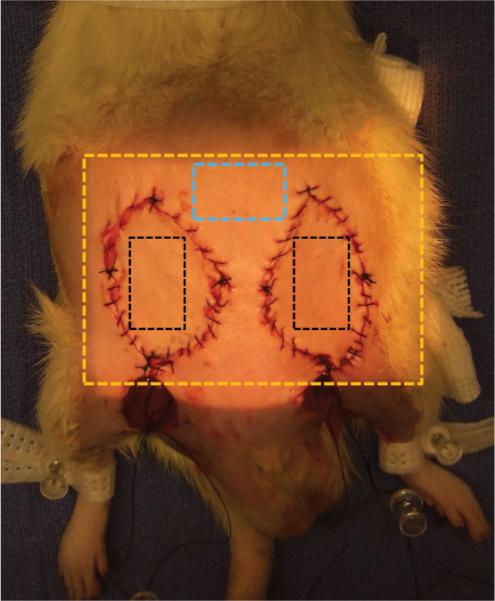
Diagram of typical orientation of the flaps on the animal and the region imaged by the prototype spatial frequency domain imaging device (Tissue OxImager). The illumined region out-lined by the golden box is the region imaged by the device. The blue box is the area outside of the surgical field used as the native skin group. The black boxes represent the area on either the control flap or experimental flaps (selective arterial or selective venous occlusion flaps). Note the incisions caudal to the flaps used to perform selective ligation of the vessels.
Imaging
The flaps were imaged using the Tissue Ox-Imager, a prototype device that uses spatial frequency domain imaging principles and that has been described previously.17,18 In brief, the device uses a sequential pattern of light that appears to the user like parallel lines (actually a sine wave pattern) of various spacing, which is used to illuminate the tissue region of interest. The light that is reflected/remitted from the tissue is then detected by a charge-coupled device camera. In this study, we used light at 670, 730, 790, and 850 nm because these wavelengths correspond with spectral features inherent in oxyhemoglobin and deoxyhemoglobin. A mathematical algorithm that describes light transport through a scattering and absorbing medium such as tissue is used to model the “blurring” of these spatial patterns. This algorithm allows us to quantify the scattering and absorption properties of the tissue of interest. Once we know the absorption properties at each wavelength of interest, we can apply Beer's law to determine the concentrations of oxyhemoglobin and deoxyhemoglobin from which total hemoglobin and tissue saturation are subsequently calculated.19 The spatial frequency domain imaging device provides quantified values for each of the aforementioned chromophores in a two-dimensional format as illustrated in Figure 2.15,16,19
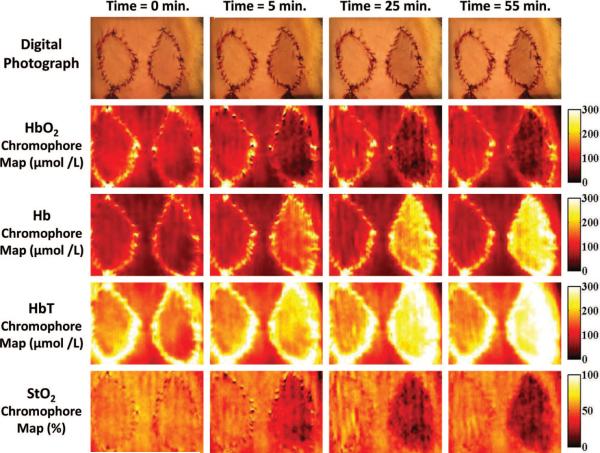
Fig. 2.
Sample of digital photograph and the corresponding chromophore maps as generated by the Tissue OxImager for all four chromophores parameters (oxyhemoglobin, deoxyhemoglobin, total hemoglobin, and tissue saturation). The flap on the left of each image represents a control flap, whereas the flap on the right is an experimental flap that underwent selective venous occlusion. The images at time 0 were obtained just before selective venous occlusion to the flap on the right side. Note the similar appearance between the control flap and the experimental flap at this time. The photographs and chromophore maps at time 5, 25, and 55 minutes illustrate the changes over time after selective venous occlusion of the flap on the right of each image. Note how the Tissue OxImager detects changes in oxyhemoglobin, deoxyhemoglobin, total hemoglobin, and tissue saturation by 5 minutes after selective venous occlusion, whereas the clinical appearance of the flap as illustrated by digital photography is only subtly different in appearance even after 55 minutes.
Figure 2 illustrates typical results obtained in the control flap and selective venous occlusion flap groups. Spatial maps of oxyhemoglobin and deoxyhemoglobin concentrations, and calculated total hemoglobin and tissue saturation, are depicted in addition to a corresponding image acquired using standard digital photography as a point of reference. Note that in Figure 2 the control flap on the left of each image and the experimental flap on the right are initially similar in appearance at time 0. After selective venous occlusion of the flap on the right, the spatial frequency domain imaging device detects a progressive decline in the amount of oxyhemoglobin and tissue saturation and a corresponding increase in deoxyhemoglobin and total hemoglobin after selective venous occlusion. This is an expected result, as the venous outflow to the flap is occluded and the flap becomes engorged with deoxygenated blood.
For the purpose of the statistical analysis described below, the quantitative chromophore maps generated by the spatial frequency domain imaging device were used to determine the average value of each chromophore within the flap. This was performed by selecting an area within each flap and calculating the average quantity of a given chromophore within the region of tissue selected. During the continuous imaging portion of the experiment, each flap was imaged several times per minute, and the mean value of all the repetitions for a given 1-minute interval over a given region on the flap was used to compare the difference between the flaps. Thus, the recorded value for each minute represents the average chromophore concentration in the selected portion of the tissue on the flap and the averaged values over several repetitions performed in a given 1-minute interval. The chromophore values were then analyzed to determine whether the device was able to detect changes in the chromophore values in pedicle flaps undergoing selective vascular occlusion.
Statistical Analysis
A one-way analysis of variance with Dunnett's posttest analysis was performed (GraphPad Prism version 5.00; GraphPad Software, Inc., San Diego, Calif.) to compare the control flap group to native skin outside the operative field, and to the two experimental flap groups (selective arterial occlusion and venous occlusion). A Dunnett's posttest analysis allows for a direct comparison of the baseline chromophore values (oxyhemoglobin, deoxyhemoglobin, total hemoglobin, and tissue saturation) between the control flap group and the three other groups (selective arterial occlusion flap, selective venous occlusion flap, and native skin groups). A one-way analysis of variance with Dunnett's posttest analysis was also used to compare the difference among the three flap types in terms of operative times and time from inset of the flaps to first baseline measurement.
The effects of selective occlusion were compared in terms of percentage change from baseline. The baseline values for each animal were defined as the average chromophore values measured during the 10-minute period after setting the flaps but before selective vascular occlusion (i.e., the average of values obtained for each flap between–10 and 0 minutes). This was performed to correct for any intraanimal variability in the values at baseline between animals. The percentage change from baseline for each chromophore parameter (oxyhemoglobin, deoxyhemoglobin, total hemoglobin, and tissue saturation) was compared among the different flap groups. By definition, percentage change in oxyhemoglobin, deoxyhemoglobin, total hemoglobin, and tissue saturation at 0 minutes were equal to 0. Curves for each chromophore based on percentage change from baseline versus time were generated for the experimental flap groups and were compared with the values measured in the control flap using a repeated measures two-way analysis of variance test. A Bonferroni posttest analysis was used to compare the differences at given time points along the curves. Four curves representing the four different chromophore parameters were generated for each flap group and for the native skin group. Use of Bonferroni posttest analysis allows for comparison of the data in a paired fashion. The pairing of the data among all four groups was performed based on the length of time elapsed from selective arterial or venous occlusion in experimental flaps (i.e., the time elapsed from 0 minutes).
RESULTS
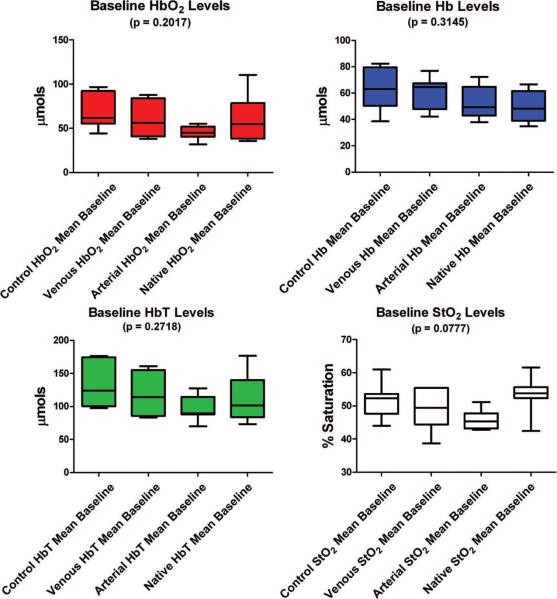
The operative times required to elevate and set each flap group (control flaps, selective arterial occlusion flaps, and selective venous occlusion flaps) did not vary statistically among the three flap groups (p = 0.4006). Figure 3 and Table 1 illustrate that there was no statistically significant difference in baseline values for each of the chromophores among all four groups (control flap group, selective venous occlusion flap group, selective arterial occlusion flap group, and native skin group) based on a one-way analysis of variance analysis for each of the following measured parameters: oxyhemoglobin (p = 0.2017), deoxyhemoglobin (p = 0.3145), total hemoglobin (p = 0.2718), and tissue saturation(p = 0.0777).
Fig. 3.
Comparison of measured chromophore values among all four groups (control flaps, selective venous occlusion flaps, selective arterial occlusion flaps, and native skin) during the baseline measurement period from −10 minutes to 0 minutes. The mean values are depicted as the center line and the error bars represent the range. The confidence interval of 2.5 to 97.5 percent is depicted by the colored boxes.
Table 1.
Measured Baseline Values for Each Individual Chromophore for the Control Flap, Selective Arterial Occlusion Flap, Selective Venous Occlusion Flap, and Native Skin Groups*
| Group | Hbo2 (μM/liter) | Hb (μM/liter) | HbT (μM/liter) | Sto2 (%) |
|---|---|---|---|---|
| Control flap | 67.93 ± 7.302 | 62.62 ± 6.077 | 130.5 ± 12.63 | 51.44 ± 2.041 |
| Selective arterial occlusion flap | 45.00 ± 2.989 | 52.47 ± 4.615 | 97.54 ± 7.325 | 46.06 ± 1.108 |
| Selective venous occlusion flap | 58.85 ± 7.813 | 59.16 ± 4.746 | 118.0 ± 11.59 | 48.77 ± 2.326 |
| Native skin | 61.00 ± 9.940 | 50.70 ± 4.449 | 112.0 ± 13.98 | 53.29 ± 2.160 |
| p | 0.2017 | 0.3145 | 0.2018 | 0.0777 |
HbO2, oxyhemoglobin; Hb, deoxyhemoglobin; HbT, total hemoglobin; StO2, tissue saturation.
Baseline values represent the average value for each chromophore measured during the 10-minute period (−10 minutes to 0 minutes), just before selective vascular occlusion in the experimental flap groups (selective arterial and selective venous occlusion flap groups). Statistical analysis was performed using a one-way analysis of variance with a Dunnett's posttest analysis allowing for a direct comparison of the baseline chromophore values between the control flap group and the three other groups (selective arterial occlusion flap, selective venous occlusion flap, and native skin groups).
Selective Arterial Occlusion Results
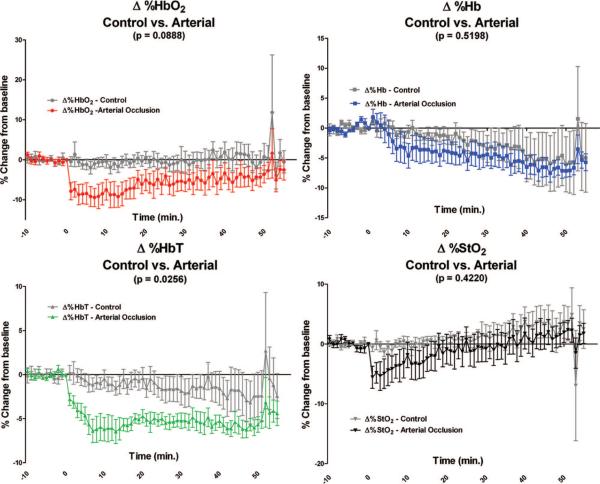
When comparing the curves of the control flap group to the selective arterial occlusion flap group in Figure 4, only the percentage change in total hemoglobin was statistically different (p = 0.0256), with the selective arterial occlusion flap group decreasing over time. However, there was no statistical difference in the percentage change in total hemoglobin between the control flap group and the selective arterial occlusion flap group at any given time point when a Bonferroni posttest analysis was applied. The percentage change in oxygenated hemoglobin in the selective arterial occlusion flap group decreased compared with the control flap group but did not become statistically significant over the entire postligation time course (p = 0.0888). There were no statistically significant differences between the control flap group and the selective arterial occlusion group in terms of percentage change in deoxygenated hemoglobin (p = 0.5198). The percentage change in tissue saturation was also not statistically different during the postligation measurement period (p = 0.4220).
Fig. 4.
Graphic illustration of the percentage changes from baseline for each parameter measured in the control flap group compared with the selective arterial occlusion flap group (note the scale differences on the y axis). Baseline measurements are from −10 minutes to 0 minutes. Selective arterial occlusion occurred at time 0 minutes. The p values reflect the differences over the entire time course between the curve representing the control flap group (gray) and the selective arterial occlusion flap group (color). Only percentage change in total hemoglobin was found to be statistically different between the control flap group and the selective arterial occlusion flap group over the entire postocclusion time course (time 0 to 55 minutes). A Bonferroni posttest analysis did not reveal statistically significant differences at any given individual time point in any of the four chromophore parameters (percentage change in oxyhemoglobin, deoxyhemoglobin, total hemoglobin, and tissue saturation).
Selective Venous Occlusion Results
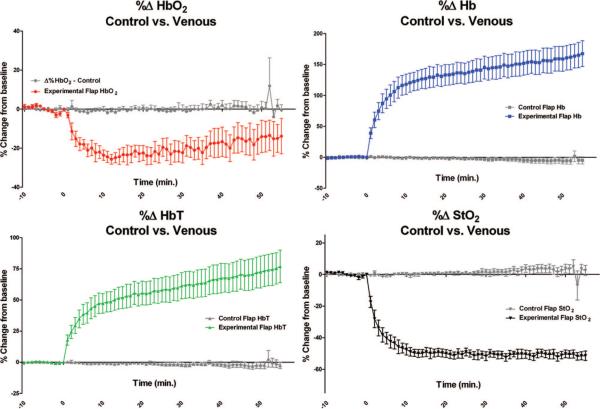
After selective venous ligation, there were statistically significant changes in all four measured parameters over time between the control flap group and the selective venous occlusion flap group, as can be seen in Figure 5. The percentage change in oxyhemoglobin was statistically different between the control flap group and the selective venous occlusion flap group (p = 0.0029). Using a Bonferroni posttest analysis, the difference between the control flap group and the selective venous occlusion flap group first occurred at 8 minutes after selective venous ligation. The percentage change in deoxyhemoglobin, total hemoglobin, and tissue saturation had a statistically significant difference between the control flap group and the selective venous occlusion flap group (p < 0.0001) following selective venous occlusion. Using a Bonferroni posttest analysis, the percentage change in deoxyhemoglobin was first statistically different within 2 minutes after selective venous ligation, whereas percentage change in total hemoglobin was different at 4 minutes after ligation and percentage change in tissue saturation was different within the first minute after venous ligation.
Fig. 5.
Graphic illustration of the percentage changes from baseline for each parameter measured in the control flap group compared with the selective venous occlusion flap group (note the scale differences on the y axis). Baseline measurements are from −10 minutes to 0 minutes. Selective venous occlusion occurred at 0 minutes. The p values reflect the differences over the entire time course between the curve representing the control flap group (gray) and the selective venous occlusion flap group (color). A Bonferroni posttest analysis found a statistically significant difference (p < 0.05) in percentage change in oxyhemoglobin at 8 minutes, percentage change in deoxy hemoglobin at 2 minutes, percentage change in total hemoglobin at 4 minutes, and percentage change in tissue saturation at 1 minute following selective venous ligation.
Native Skin Results
As expected, there were no statistically significant differences between the control flap group and the nearby native skin group, which did not undergo surgical manipulation. When comparing the percentage change in the control flap group with that of the native skin group, there was no difference in percentage change in oxyhemoglobin (p = 0.6074), deoxyhemoglobin (p = 0.1775), total hemoglobin (p = 0.1770), or tissue saturation (p = 0.3355). Using a Bonferroni posttest analysis, there were no differences at any time point along curves between the native skin group and the control flap group.
DISCUSSION
The use of free tissue transfer has become standard practice for complex reconstructions after trauma or oncologic surgical resections, but the technique is not without complications. Vascular occlusion can occur to the vessels supplying the flap, resulting in partial or complete flap loss.1–3,6,24,25 This can be a devastating complication for the patient. Several large studies have found that isolated venous thrombosis is far more frequent than isolated arterial thrombosis.1,3,5,6 A majority of both venous and arterial occlusion occurs early in the postoperative period, within the first 48 to 72 hours after surgery.3,6,13
The published literature suggests that early detection of a vascular compromised flap followed by early exploration improves salvage rates.5 Because most arterial and venous occlusions occur within the first 48 to 72 hours after surgery, several different monitoring devices have been investigated as adjuncts to serial clinical examinations in the early postoperative period. These adjunctive flap monitoring tools have been used as a means of allowing for early detection of a compromised flap and thereby expediting surgical reexploration in an attempt to improve salvage rates of flaps compromised by vascular occlusion.
Control Flap, Native Skin, and Preselective Vascular Occlusion Data
There was no statistically significant difference among all four groups in terms of oxyhemoglobin (p = 0.2017), deoxyhemoglobin (p = 0.3145), total hemoglobin (p = 0.2718), and tissue saturation (p = 0.0777) before selective vascular occlusion. The apparent trend toward a difference with regard to tissue saturation between the native skin group and the flap groups is likely attributable to multiple factors. We believe this likely reflects the effects of raising and setting the flaps on the tissue within the flap compared with the native skin in the two groin flaps, which has not undergone surgical manipulation. If the native skin group is excluded from the analysis, and the baseline tissue saturation values are compared in the control flap group, the selective arterial occlusion flap group, and the selective venous occlusion flap group, there is no statistically significant difference among the groups (p = 0.2316).
It has been demonstrated by prior authors that the process of elevating a flap affects the physiology of the microcirculation within the flap.26 Given that the native skin group had the highest measured tissue saturation within the tissue imaged, we believed that the process of elevating and setting the flap affects the microcirculation of the flap, resulting in a greater degree of deoxyhemoglobin within the flap's tissues. One possible explanation for this phenomenon is that in the process of elevating the flap there is disruption of the secondary venous drainage system of the tissues, resulting in some degree of venous congestion.27,28 Another possible explanation for a higher tissue saturation within the native skin group compared with the tissues within the three flap groups at baseline is that surgical manipulation results in injury to the flaps, thereby resulting in an increased metabolic demand, which in turn increases the oxygen extraction from oxyhemoglobin within the flaps, resulting in an overall lower measured tissue saturation, which appears to be detected by the spatial frequency domain imaging device.
As expected, the control flap group demonstrated stable values for all four parameters measured (oxyhemoglobin, deoxyhemoglobin, total hemoglobin, and tissue saturation) during the entire time course after raising and setting the flap (from −10 minutes to 55 minutes). Both the selective arterial occlusion flap group and the selective venous occlusion flap group demonstrated similar stable values in the preselective vascular occlusion imaging period (from −10 minutes to 0 minutes).
Selective Arterial Occlusion
In the flaps undergoing selective arterial occlusion, we hypothesized that the spatial frequency domain imaging device would detect specific patterns of change that have been described by other groups using near-infrared spectroscopy.20 After selective arterial occlusion, the amount of measured oxyhemoglobin should decrease nearly instantaneously as oxygenated blood flow into the flap stops. The amount of deoxyhemoglobin should initially increase as the tissue within the flap continues to extract oxygen from the oxyhemoglobin, thereby converting oxyhemoglobin to deoxyhemoglobin. This would be followed by a decrease in deoxyhemoglobin as the remaining blood within the flap drains out of the flap because of gravity by means of the patent venous system to more dependent tissues. The expected pattern of change for total hemoglobin would be a progressive decline in the total amount of hemoglobin in the flap as blood drains from the flap out of the patent venous system because of gravity. Tissue oxygen saturation would be predicted to decrease progressively as the amount of oxyhemoglobin remaining in the flap diminishes over time after selective arterial occlusion.20
In our study, we found that when comparing the control flap group to the selective arterial occlusion flap group, only percentage change in total hemoglobin was statistically different (p = 0.0256). The measured change in percentage change in total hemoglobin levels was as expected and similar to the changes described by prior authors,20 with the total amount of hemoglobin in the flap decreasing over time as blood within the flap drains out of the flap by means of the patent venous system because of gravity. This is consistent with prior authors' description of the expected changes seen after selective arterial occlusion in a tissue transfer flap.20
The measured percentage change in oxyhemoglobin appeared to initially be similar to the expected pattern change, with a decrease in the measured levels of oxyhemoglobin. However, this did not become statistically significant over the time course of the experiment (p = 0.0888). The measured levels of percentage change in oxyhemoglobin in the selective arterial occlusion flap group appeared to initially have a rapid decline in oxyhemoglobin levels as was expected. However, after approximately 10 minutes after selective arterial occlusion, the levels of oxyhemoglobin appeared to increase slowly. One possible explanation is that the Tissue OxImager is measuring the levels of oxyhemoglobin in the abdominal wall musculature deep to the fasciocutaneous flap, as the average sampling depth of the device is approximately 3 to 5 mm, which is deeper than the thickness of the fasciocutaneous flaps in this model.17,18,29 As the blood drains from the flap following selective arterial occlusion, the maximal sampling depth of the spatial frequency domain imaging becomes greater. This would be analogous to light penetrating into the ocean from the surface. If the clarity of the ocean is great, light is able to pass to greater depths; yet if the water is murky, the amount of light reaching the sea floor will be considerably less. Thus, as the blood drains from the flap after selective arterial occlusion, the clarity of the flap becomes greater and the signal detected becomes proportionally more from the tissues deep to the flap than from the flap itself. Another possible explanation for the slow increase in percentage change in oxyhemoglobin several minutes after selective arterial occlusion is that this represents the effects of diffusion of oxygen from the surface of the flap because of plasmatic diffusion and imbibition from the tissues deep to the flap, in a similar fashion as occurs with full-thickness skin grafts.
After selective arterial occlusion, the measured level of percentage change in deoxyhemoglobin within the selective arterial occlusion flap group was not significantly different from the control flap group levels (p = 0.5198). Other groups have demonstrated an increase in measured deoxyhemoglobin after selective arterial ligation.20 The control flap group and the selective arterial occlusion flap group appear to have a decline in the measured deoxyhemoglobin levels, as shown in Figure 4. It is possible that the declining deoxyhemoglobin in the control flap group represents the recovery of the flap and the deeper surrounding tissues near the flap after the trauma caused by surgical elevation and setting of the flap. The decline in the selective arterial occlusion flap group represents the drainage of blood from the flap caused by gravity and the fact that the differences between the two curves are not statistically significant but are attributable to different mechanisms. It is also possible that the decline in percentage change in deoxyhemoglobin in both the control flap group and the selective arterial occlusion flap group is more indicative of the characteristics of the tissues deep to the fasciocutaneous flap rather than the flap itself.
Tissue saturation, which represents the ratio between oxyhemoglobin and total hemoglobin, was not statistically different between the control flap group and the selective arterial occlusion flap group (p = 0.4220), as can be seen in Figure 4. The selective arterial occlusion flap group appeared to initially have a modest decrease in tissue saturation following occlusion of the arterial inflow, as would be expected based on previously published data using tissue spectroscopy to detect selective arterial ischemia.10,11,20,21,30–32 However, after the initial decline, the measured tissue saturation appeared to improve gradually, becoming near baseline approximately 20 minutes after selective arterial occlusion and greater than baseline after 40 minutes of selective arterial occlusion. The selective arterial occlusion flap group consistently had a lower measured tissue saturation compared with the control flap group but was never statistically different at a given time point after selective arterial occlusion. The initial decrease in measured percentage change in tissue saturation suggests an initial desaturation of hemoglobin caused by lack of arterial inflow. However, the following increase in the percentage change in tissue saturation implies that the ratio of oxyhemoglobin to total hemoglobin measured eventually increases over the time course of the experiment. Again, a likely explanation for this observed phenomenon is that the spatial frequency domain imaging device is measuring the tissues deep to the fasciocutaneous flaps and the tissues within the flap, and that as the amount of chromophores present in the flap decreases, the interrogation depth increased as the tissues become less “opaque” to the specific wavelengths of light used by the device. Thus, as the total amount of blood within the flap decreased, the spatial frequency domain imaging device's interrogation depth increases, and the “background signal” from the deeper tissues beneath the flap becomes more prominent as the signal generated by the fasciocutaneous flap diminishes. This dynamic phenomenon may also have contributed to the unexpected results found in the selective arterial occlusion flap group. Given the limitations of the rat pedicle fasciocutaneous flap model used attributable to the thickness of the flaps, we intend to carry out a similar set of experiments using a model that more closely mimics the properties and thickness associated with human tissues. A model using a swine-based pedicle flap model would provide a flap with a thickness greater than the maximal interrogation depth of the spatial frequency domain imaging device and would remove this confounding variable. A swine model would also provide a tissue type more similar to human skin compared with a rat-based model.33,34
Selective Venous Occlusion
Using the spatial frequency domain imaging device, we observed a rapid decrease from baseline in the measured values of oxyhemoglobin immediately following selective venous occlusion. This decrease from baseline became significantly different between the control flap group and the selective venous occlusion group after 8 minutes. We expected to observe an initial increase in the measured amount of oxyhemoglobin within the flap, as arterial inflow continued for some time until the pressure within the venous system became elevated enough to equal the arterial inflow pressure and thereby result in cessation of blood flow in the arterial system to the flap.20
The expected physiologic changes within the tissue with regard to deoxyhemoglobin, total hemoglobin, and tissue saturation were observed. As expected, following obstruction of venous outflow from a flap, the amount of deoxyhemoglobin and total hemoglobin measured within the flap by the spatial frequency domain imaging device increased dramatically over time as the flaps became engorged with deoxygenated blood. This is similar to the data reported by other groups that have used near-infrared spectroscopy to assess venous congested flaps.20,23,35
CONCLUSIONS
Near-infrared spectroscopy and imaging is a set of evolving diagnostic modalities that has many potential applications in medicine.22 Several authors have published results using various devices based on near-infrared spectroscopic principles as an adjunct to clinical examination after tissue transfer.26,30,32,35–38 Generally, the majority of devices described in the literature are able to detect oxyhemoglobin, deoxyhemoglobin, total hemoglobin, and tissue saturation from only a small localized region and require movement of a probe over multiple locations on a flap to determine the chromophore concentrations throughout the flap. The spatial frequency domain imaging device is capable of rapid, wide-field spectral imaging in a noncontact manner, and is able to generate chromophore maps, over surface areas as large as 100 cm2, simultaneously generating maps of oxyhemoglobin, deoxyhemoglobin, total hemoglobin, and tissue saturation.15–19 In the study carried out here, the clinical model was “fair skinned” (unpigmented), which reduces the complexity of the optical problem of separating oxyhemoglobin and deoxyhemoglobin concentration from, for example, melanin. In the next phase of research, a more advanced light propagation algorithm will correct the optical signals for skin of arbitrary pigmentation. Another limitation of this particular study is that each imaging cycle required 15.3 seconds to obtain and requires tissue to be relatively motionless within the focal zone of the detection camera. However, neither of these issues is a fundamental limitation of the technique. The next generation spatial frequency domain imaging device that is being tested is able to acquire an imaging cycle in less than 1 second and will continue to improve. The ability to acquire data quickly reduces artifacts related to subject motion.
The spatial frequency domain imaging device has potential as an objective monitor of flaps after pedicled or free tissue transfer following reconstructive surgery. Further studies using a large-animal model that more closely simulates the properties and thicknesses encountered in human skin are needed to determine whether the device can detect and differentiate between a healthy flap and a flap compromised by arterial and/or venous occlusion. Ultimately, we hope to assess the capabilities of the spatial frequency domain imaging device to improve patient management after reconstructive surgery.
ACKNOWLEDGMENTS
Funding for this study was provided by internal funds from the Department of Surgery and the Aesthetic and Plastic Surgery Institute at the University of California, Irvine Medical Center (Orange, Calif.). Additional support was provided by the National Institutes of Health National Center for Research Resources Biomedical Technology Research Center (Laser Microbeam and Medical Program: 5P-41RR01192), the Military Photomedicine Program (Air Force Office of Scientific Research grant FA9550-08-1-0384), and the Beckman Foundation.
Funding for the establishment of Modulated Imaging, Inc., and Introspective Medical, Inc., was provided by a National Institutes of Health Small Business Technology Transfer phase II grant.
Footnotes
Presented in part at the 20th Annual Scientific Meeting of the Southern California Chapter of the American College of Surgeons, in Santa Barbara, California, January 17, 2009; SPIE Photonics West 2009, in San Jose, California, January 25, 2009; and the 95th Annual American College of Surgeons Clinical Congress, in Chicago, Illinois, October 14, 2009.
Disclosures: Dr. Cuccia and Dr. Durkin have a financial interest in Modulated Imaging, Inc., and Introspective Medical, Inc., which developed the Tissue OxImager and provided the device used in this study. The other authors have no financial interests or commercial associations that might pose or create a conflict of interest with the information presented in this article.
REFERENCES
- 1.Bui DT, Cordeiro PG, Hu QY, Disa JJ, Pusic A, Mehrara BJ. Free flap reexploration: Indications, treatment, and outcomes in 1193 free flaps. Plast Reconstr Surg. 2007;119:2092–2100. doi: 10.1097/01.prs.0000260598.24376.e1. [DOI] [PubMed] [Google Scholar]
- 2.Khouri RK, Cooley BC, Kunselman AR, et al. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. 1998;102:711–721. doi: 10.1097/00006534-199809030-00015. [DOI] [PubMed] [Google Scholar]
- 3.Kroll SS, Schusterman MA, Reece GP, et al. Timing of pedicle thrombosis and flap loss after free-tissue transfer. Plast Reconstr Surg. 1996;98:1230–1233. doi: 10.1097/00006534-199612000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Panchapakesan V, Addison P, Beausang E, Lipa JE, Gilbert RW, Neligan PC. Role of thrombolysis in free-flap salvage. J Reconstr Microsurg. 2003;19:523–530. doi: 10.1055/s-2004-815638. [DOI] [PubMed] [Google Scholar]
- 5.Brown JS, Devine JC, Magennis P, Sillifant P, Rogers SN, Vaughan ED. Factors that influence the outcome of salvage in free tissue transfer. Br J Oral Maxillofac Surg. 2003;41:16–20. doi: 10.1016/s0266-4356(02)00260-7. [DOI] [PubMed] [Google Scholar]
- 6.Nakatsuka T, Harii K, Asato H, et al. Analytic review of 2372 free flap transfers for head and neck reconstruction following cancer resection. J Reconstr Microsurg. 2003;19:363–368. doi: 10.1055/s-2003-42630. discussion 369. [DOI] [PubMed] [Google Scholar]
- 7.Hidalgo DA, Jones CS. The role of emergent exploration in free-tissue transfer: A review of 150 consecutive cases. Plast Reconstr Surg. 1990;86:492–498. discussion 499–501. [PubMed] [Google Scholar]
- 8.Goodstein WA, Buncke HJ., Jr. Patterns of vascular anastomoses vs. success of free groin flap transfers. Plast Reconstr Surg. 1979;64:37–40. doi: 10.1097/00006534-197907000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Hjortdal VE, Hansen ES, Hauge E. Myocutaneous flap ischemia: Flow dynamics following venous and arterial obstruction. Plast Reconstr Surg. 1992;89:1083–1091. [PubMed] [Google Scholar]
- 10.Hjortdal VE, Hauge E, Hansen ES. Differential effects of venous stasis and arterial insufficiency on tissue oxygenation in myocutaneous island flaps: An experimental study in pigs. Plast Reconstr Surg. 1992;89:521–529. doi: 10.1097/00006534-199203000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Kerrigan CL, Wizman P, Hjortdal VE, Sampalis J. Global flap ischemia: A comparison of arterial versus venous etiology. Plast Reconstr Surg. 1994;93:1485–1495. discussion 1496–1497. [PubMed] [Google Scholar]
- 12.Heller L, Levin LS, Klitzman B. Laser Doppler flowmeter monitoring of free-tissue transfers: Blood flow in normal and complicated cases. Plast Reconstr Surg. 2001;107:1739–1745. doi: 10.1097/00006534-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Jones NF. Intraoperative and postoperative monitoring of microsurgical free tissue transfers. Clin Plast Surg. 1992;19:783–797. [PubMed] [Google Scholar]
- 14.Yuen JC, Feng Z. Monitoring free flaps using the laser Doppler flowmeter: Five-year experience. Plast Reconstr Surg. 2000;105:55–61. doi: 10.1097/00006534-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Ayers FR, Cuccia DJ, Kelly KM, Durkin AJ. Wide-field spatial mapping of in vivo tattoo skin optical properties using modulated imaging. Lasers Surg Med. 2009;41:442–453. doi: 10.1002/lsm.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuccia DJ, Bevilacqua F, Durkin AJ, Ayers FR, Tromberg BJ. Quantitation and mapping of tissue optical properties using modulated imaging. J Biomed Opt. 2009;14:024012. doi: 10.1117/1.3088140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuccia DJ, Bevilacqua F, Durkin AJ, Tromberg BJ. Modulated imaging: Quantitative analysis and tomography of turbid media in the spatial frequency domain. Opt Lett. 2005;30:1354–1356. doi: 10.1364/ol.30.001354. [DOI] [PubMed] [Google Scholar]
- 18.Cuccia DJ, Levenson R, Durkin A, et al. A new method for quantitative, depth-resolved imaging of tissue fluorescence using spatially modulated illumination. Paper presented at the Third Annual Meeting of the Society of Molecular Imaging; September 9–12, 2004; St. Louis, Mo: [Google Scholar]
- 19.Gioux S, Mazhar A, Cuccia DJ, Durkin AJ, Tromberg BJ, Frangioni JV. Three-dimensional surface profile intensity correction for spatially modulated imaging. J Biomed Opt. 2009;14:034045. doi: 10.1117/1.3156840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irwin MS, Thorniley MS, Doré CJ, Green CJ. Near infra-red spectroscopy: A non-invasive monitor of perfusion and oxygenation within the microcirculation of limbs and flaps. Br J Plast Surg. 1995;48:14–22. doi: 10.1016/0007-1226(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 21.Thorniley MS, Sinclair JS, Barnett NJ, Shurey CB, Green CJ. The use of near-infrared spectroscopy for assessing flap viability during reconstructive surgery. Br J Plast Surg. 1998;51:218–226. doi: 10.1054/bjps.1997.0145. [DOI] [PubMed] [Google Scholar]
- 22.Cohn SM. Near-infrared spectroscopy: Potential clinical benefits in surgery. J Am Coll Surg. 2007;205:322–332. doi: 10.1016/j.jamcollsurg.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Hampson NB, Piantadosi CA. Near infrared monitoring of human skeletal muscle oxygenation during forearm ischemia. J Appl Physiol. 1988;64:2449–2457. doi: 10.1152/jappl.1988.64.6.2449. [DOI] [PubMed] [Google Scholar]
- 24.Kroll SS. Fat necrosis in free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg. 2000;106:576–583. doi: 10.1097/00006534-200009030-00008. [DOI] [PubMed] [Google Scholar]
- 25.Blondeel PN, Arnstein M, Verstraete K, et al. Venous congestion and blood flow in free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg. 2000;106:1295–1299. doi: 10.1097/00006534-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Figus A, Mosahebi A, Ramakrishnan V. Microcirculation in DIEP flaps: A study of the haemodynamics using laser Doppler flowmetry and lightguide reflectance spectrophotometry. J Plast Reconstr Aesthet Surg. 2006;59:604–612. doi: 10.1016/j.bjps.2005.09.047. discussion 613. [DOI] [PubMed] [Google Scholar]
- 27.Tsao CK, Chen HC, Chuang CC, Chen HT, Mardini S, Coskunfirat K. Adequate venous drainage: The most critical factor for a successful free jejunal transfer. Ann Plast Surg. 2004;53:229–234. doi: 10.1097/01.sap.0000116286.61316.28. [DOI] [PubMed] [Google Scholar]
- 28.Yazar S, Chen HC, Mardini S. Augmentation of venous drainage by a venous anastomosis for pedicled flaps. J Reconstr Microsurg. 2008;24:369–376. doi: 10.1055/s-2008-1080531. [DOI] [PubMed] [Google Scholar]
- 29.Cerussi AE, Berger AJ, Bevilacqua F, et al. Sources of absorption and scattering contrast for near-infrared optical mammography. Acad Radiol. 2001;8:211–218. doi: 10.1016/S1076-6332(03)80529-9. [DOI] [PubMed] [Google Scholar]
- 30.Keller A. Noninvasive tissue oximetry for flap monitoring: An initial study. J Reconstr Microsurg. 2007;23:189–197. doi: 10.1055/s-2007-974655. [DOI] [PubMed] [Google Scholar]
- 31.Payette JR, Kohlenberg E, Leonardi L, et al. Assessment of skin flaps using optically based methods for measuring blood flow and oxygenation. Plast Reconstr Surg. 2005;115:539–546. doi: 10.1097/01.prs.0000148415.54546.ca. [DOI] [PubMed] [Google Scholar]
- 32.Repez A, Oroszy D, Arnez ZM. Continuous postoperative monitoring of cutaneous free flaps using near infrared spectroscopy. J Plast Reconstr Aesthet Surg. 2008;61:71–77. doi: 10.1016/j.bjps.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 33.DeLuca L, Beckenstein M, Guyuron B. Yucatan pig: An optimal hairless model for a true random cutaneous flap. Aesthetic Plast Surg. 1997;21:205–206. doi: 10.1007/s002669900111. [DOI] [PubMed] [Google Scholar]
- 34.Kerrigan CL, Zelt RG, Thomson JG, Diano E. The pig as an experimental animal in plastic surgery research for the study of skin flaps, myocutaneous flaps and fasciocutaneous flaps. Lab Anim Sci. 1986;36:408–412. [PubMed] [Google Scholar]
- 35.Holzle F, Loeffelbein DJ, Nolte D, Wolff KD. Free flap monitoring using simultaneous non-invasive laser Doppler flowmetry and tissue spectrophotometry. J Craniomaxillofac Surg. 2006;34:25–33. doi: 10.1016/j.jcms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Cai ZG, Zhang J, Zhang JG, et al. Evaluation of near infrared spectroscopy in monitoring postoperative regional tissue oxygen saturation for fibular flaps. J Plast Reconstr Aesthet Surg. 2008;61:289–296. doi: 10.1016/j.bjps.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 37.Scheufler O, Andresen R. Tissue oxygenation and perfusion in inferior pedicle reduction mammaplasty by near-infrared reflection spectroscopy and color-coded duplex sonography. Plast Reconstr Surg. 2003;111:1131–1146. doi: 10.1097/01.PRS.0000046615.36917.3E. [DOI] [PubMed] [Google Scholar]
- 38.Scheufler O, Exner K, Andresen R. Investigation of TRAM flap oxygenation and perfusion by near-infrared reflection spectroscopy and color-coded duplex sonography. Plast Reconstr Surg. 2004;113:141–152. doi: 10.1097/01.PRS.0000095940.96294.A5. discussion 153–155. [DOI] [PubMed] [Google Scholar]