Abstract
OBJECTIVES
We evaluated highly active antiretroviral therapy (HAART) utilization in youth infected with HIV through risk behaviors (BIY) who met treatment criteria for HAART. We assessed the impact of receiving care at an adult or pediatric HIV clinical site on initiation and discontinuation of the first HAART regimen in BIY.
METHODS
This was a retrospective analysis of treatment-naive BIY, aged 12–24, who enrolled in the HIV Research Network (HIVRN) between 2002 and 2008 and who met criteria for HAART. The outcomes were time from meeting criteria to initiation of HAART and time to discontinuation of the first HAART regimen. Analyses were conducted using Cox proportional hazards regression.
RESULTS
Of 287 treatment-eligible youth, 198 (69%) received HAART and 58/198 (29.3%) subsequently discontinued HAART. In multivariable analyses, there was no significant difference in the time between meeting treatment criteria and initiating HAART for BIY followed at adult or pediatric HIV clinical sites. However, BIY followed at adult sites discontinued HAART sooner than BIY followed at pediatric HIV clinical sites (AHR 3.19 [1.26–8.06]).
CONCLUSIONS
Two thirds of treatment-eligible BIY in the HIVRN cohort initiated HAART; however, one third who initiated HAART discontinued HAART during the study period. Identifying factors associated with earlier HAART initiation and HAART sustainability can inform interventions to enhance HAART utilization among treatment-eligible youth. The finding of earlier HAART discontinuation for youth at adult care sites deserves further study.
Keywords: adolescents, youth, highly active antiretroviral therapy (HAART), disparities, utilization, HIV Research Network, clinical site
Background
An estimated 50% of incident human immunodeficiency virus (HIV) infections in the United States are acquired during the second decade of life 1;2. Although behaviorally-infected youth (BIY) often present early in infection with fewer HIV sequelae than adults, many meet criteria for antiretroviral treatment1. Most medical providers of HIV-infected youth strive to balance the main goal of prescribing a potent antiretroviral regimen with providing support for and reducing barriers to adherence3. However, successful HIV treatment in youth is challenging due to poor clinical attendance, intolerance of side effects, limited parental involvement, and depression. In addition, especially among BIY, cognitive and developmental immaturity often create a sense of invincibility and underestimation of personal risk, which may adversely affect adherence to medication 2;4. Several studies have noted lower adherence (29–50%) in youth who started HAART 5–7 when compared to the 80–90% reported in adult cohorts on similar regimens8;9. We previously examined HAART initiation in BIY as compared to adult patients ≥25 years old and found significantly lower rates of HAART initiation among BIY. Lower CD4 and better HIV clinic attendance were associated with increased likelihood of HAART initiation 10;11. Given those findings, we hypothesized that HIV clinic site, specifically, pediatric or adult site, may impact HAART utilization among BIY.
The ages of BIY span from 12 to 24 years, with the large majority between the ages of 18 and 22 at diagnosis and entry into care. Many pediatric HIV clinical centers see patients through age 24, and some adult clinical centers may see patients as young as age 16. Therefore, BIY can be referred to either pediatric or adult HIV clinical sites, where they may be cared for by providers with varied training and experience with this population (e.g., pediatric, adolescent, or internal medicine-trained providers). There may be specific clinic and provider factors that may differ at pediatric-centered vs. adult-centered facilities, including but not limited to comfort with the patient population, and youth-friendly services and facilities. Differences in care and outcomes between adult and pediatric providers have been documented in other clinical conditions 12,13. Clinic-related features may influence the likelihood that BIY patients initiate or continue a HAART regimen.
Discontinuation of HAART may be related to poor outcome expectancy and self-efficacy, difficulty with pills, and poor tolerability of side effects 14,15. Given differences in pill burden, side effects, and tolerability between protease inhibitor (PI) and non-nucleoside reverse transcriptase inhibitor (NNRTI) based regimens, sustainability of HAART may vary by regimen type among BIY.
We conducted analyses of BIY meeting treatment criteria in a large national cohort to assess the impact of HIV clinical site on initiation and continuation of HAART. We examined whether site of care (adult vs. pediatric) and type of regimen affects HAART initiation and sustainability for BIY.
METHODS
Study Setting and Participants
This was a retrospective cohort study of BIY between the ages of 12 and 24 who were enrolled in the HIV Research Network (HIVRN). HIVRN is a consortium of 18 high-volume U.S. clinic sites that provide primary and subspecialty care to HIV-infected children, youth, and adult patients. The fourteen sites, three pediatric and 11 adult, that contributed patients to this analysis were geographically distributed: Northeast (3), Southern (5), West (4), Midwest (2)16. Sites annually abstracted specified data elements from patients’ medical records; abstracted data were assembled into a uniform database, as previously described16. Adolescents and young adults were followed at pediatric or adult sites, as there were no adolescent-only sites that exclusively cared for BIY included in the network. The study was approved by the Johns Hopkins Institutional Review Board (IRB) as well as the IRB at each participating institution.
Patients were included if they were between 12 and 24 years old, had at least one CD4 value, HIV-1 RNA assessment, and one outpatient visit within any given calendar year between the years 2002 and 2008, and were treatment-naïve when first meeting treatment criteria. Patients classified as BIY had acquired HIV through sexual exposure (either heterosexual or men who have sex with men [MSM]), injection drug use [IDU], or a combination of risk factors [i.e. MSM and IDU]). Patients were excluded if they acquired infection perinatally, through blood transfusion, or if the mode of acquisition was unknown (to avoid inclusion of perinatally-infected youth who were diagnosed late), as these patients have very different clinical trajectories and are more likely to have initiated HAART at very young ages.
Data Collection and Measures
Demographic and clinical data were collected from the clinical database. Race/ethnic group was based on data recorded in the medical record and classified as White, Black, Hispanic, and other. The number of outpatient clinic/office visits was determined for each calendar year and categorized as <4 or ≥ 4 HIV provider visits per year based on the Department of Health and Human Services’ (DHHS) recommendation for quarterly clinical follow-up17. Outpatient clinic visits excluded emergency department visits and were limited to visits to an HIV healthcare provider, not including administrative, laboratory testing, nurse-only, psychiatry, or visits in which an HIV healthcare provider was not seen. Clinical site was classified as adult or pediatric. Mental health and substance abuse data were incomplete and therefore not evaluated.
HAART was defined as concomitant use of ≥ 3 antiretroviral drugs from at least two classes, including acceptable triple NRTI regimens18. In the retrospectively abstracted data, HAART initiation was specifically defined as HAART being prescribed at any time after meeting treatment criteria. DHHS’ guidelines were used to categorize patients’ need for HAART during a calendar year. Patients were classified as meeting DHHS criteria based on having two CD4 measurements <350 cells/mm3 subsequent to enrollment. This CD4 level was the recommended threshold in the guidelines between 2002 and 200817. Meeting treatment criteria was considered permanent; specifically, once patients met treatment criteria, they were considered to always be in need of treatment.
Analysis of time to HAART initiation and factors associated with HAART initiation among BIY as compared to adults ≥25 years has been previously conducted by our group11. Therefore, we focused this analysis specifically on the impact of clinical site on HAART initiation. In addition, BIY youth aged 12–17 were not included in prior analyses. Time to initiation was calculated from the date patients first met CD4 criterion for treatment and the prescription date of the first HAART regimen. For the assessment of HAART initiation, patients were censored at their last clinical visit. The duration of the first HAART regimen was calculated from the start and stop dates of the first HAART regimen and any subsequent regimens. Discontinuation was defined as being off of HAART for at least 60 days. For examining discontinuation of HAART, patients were censored at their last known HIV clinical visit. For patients who discontinued HAART regimen, we assessed the year after discontinuation of the regimen to see if patients were still in care and if HAART was subsequently re-initiated.
Statistical analysis
The impact of clinical site on time to HAART initiation and discontinuation was evaluated. We used Chi-squared tests and Wilcoxon rank sum tests to assess differences in demographic and clinical variables between pediatric and adult sites. The primary outcomes of interest were time to HAART initiation and, once initiated, time to discontinuation of the first HAART regimen. Cox proportional hazards regression analysis was used to assess covariates associated with earlier initiation and discontinuation of the first HAART regimen. The final models included gender, race, CD4 cell count category when meeting treatment criteria (<200 vs. 200–350 cells/mm3), number of outpatient visits made in the year after treatment eligibility (<4 vs. ≥4), the calendar year the patient met treatment criteria, insurance during the year the patient met treatment criteria, and adult or pediatric HIV provider clinical site. HIV acquisition risk was not included in the analysis as nearly all youth in this cohort were infected sexually. We examined the impact of regimen type, categorized as protease inhibitor (PI)- based vs. non-nucleoside reverse transcriptase inhibitor (NNRTI)-based vs. triple NRTI-based HAART, on duration of the first HAART regimen. We assessed potential interactions between key variables and none were found to be significant. Kaplan-Meier estimates were used to graphically examine the time-to-event analyses; and the log rank test was used to compare the survival curves. The proportionality assumption was assessed graphically. Data were analyzed using STATA 10.0 (Stata Corp., College Station, TX).
RESULTS
287 treatment-eligible HAART-naïve BIY were identified. The majority of youth (78.4%) were followed at adult clinical sites. At the time of initial treatment eligibility, the median age was 21 years [IQR: 20–23]); 23 (8.0%) were under 18 years old (Table 1). Approximately 72% were male, 67.6% black, and 58.2% had an HIV risk factor of MSM. Youth followed at pediatric sites tended to be younger, to have attended ≥4 HIV provider visits in the calendar year after meeting treatment criteria, and to have had a greater likelihood of being covered by public insurance. Of patients meeting DHHS treatment criteria, 198 (69.0%) were treated with HAART during the study period, including 40/62 (64.5%) of those at pediatric and 158/225 (70.2%) (p=.39) of those followed at adult HIV clinical sites. There were a total of 2,840 patient-years of follow-up. The median time from meeting treatment criteria to HAART initiation was 198 days (IQR: 35–988 days).
Table 1.
Characteristics of treatment-naïve youth ages 12–24 enrolled in the HIVRN cohort meeting treatment criteria in or after 2002 through 2008
| Pediatric site N=62 (21.6%) |
Adult site N=225 (78.4%) |
Total N=287(100%) |
P-value | |
|---|---|---|---|---|
| Age, years (median (inter-quartile range)) | 20 (17–21) (Range 14–24) | 22 (20–23) (Range 15–24) | 21 (20–23) | .001 |
| Male | 38 (61.3) | 169 (75.1) | 207 (72.1) | .081 |
| Race | .015 | |||
| Black | 52 (83.9) | 142 (63.1) | 194 (67.6) | |
| Hispanic | 3 (4.8) | 47 (20.9) | 50 (17.4) | |
| White | 7 (11.3) | 29 (12.9) | 36 (12.5) | |
| Other/unknown | 0 (0) | 7 (3.1) | 7 (2.4) | |
| HIV acquisition risk1,2 | .058 | |||
| MSM | 27 (43.6) | 140 (62.2) | 167 (58.2) | |
| Hetero | 34 (54.8) | 78 (34.7) | 112 (39.0) | |
| IDU3 | 1 (1.6) | 3 (1.3) | 4 (1.4) | |
| Hetero + IDU | 0 (0.0) | 3 (1.3) | 3 (1.1) | |
| MSM + IDU | 0 (0.0) | 1 (0.4) | 1 (0.3) | |
| CD4+ T cells when first met criteria (cells/mm3) | .711 | |||
| <200 | 18 (29.0) | 60 (26.7) | 78 (27.2) | |
| 200–350 | 44 (71.0) | 165 (73.3) | 209 (72.8) | |
| ≥4 HIV provider visits within a year of meeting treatment criteria | 49 (79.0) | 129 (57.3) | 178 (62.0) | .002 |
| Insurance⊥ | .002 | |||
| Uninsured/Ryan White/Other | 27 (43.5) | 139 (61.8) | 166 (57.8) | |
| Private | 2 (3.2) | 20 (8.9) | 22 (7.7) | |
| Public | 33 (53.2) | 63 (28.0) | 96 (33.4) | |
| Unknown | 1 (0.0) | 3 (1.3) | 3 (1.1) | |
| Year first met treatment criteria | .041 | |||
| 2002 | 4 (6.4) | 9 (4.0) | 13 (4.5) | |
| 2003 | 7 (11.3) | 20 (8.9) | 27 (9.4) | |
| 2004 | 7 (11.3) | 39 (17.3) | 46 (16.0) | |
| 2005 | 9 (14.5) | 41 (18.2) | 50 (17.4) | |
| 2006 | 19 (30.6) | 30 (13.3) | 49 (17.1) | |
| 2007 | 7 (11.3) | 41 (18.2) | 48 (16.7) | |
| 2008 | 9 (14.5) | 45 (20.0) | 54 (18.8) | |
| HAART Ever Started | 40 (64.5) | 158 (70.2) | 198 (69.0) | .390 |
Excluded if HIV acquired vertically, via blood transfusion, or if unknown mode of acquisition;
Categories mutually exclusive.
Insurance coverage included private insurance, public (Medicaid, Medicare, Dual insurance with Medicaid/Medicare), and Uninsured/Ryan White/Other (e.g., Veteran’s administration, county health program), and Unknown insurance coverage.
In multivariate analyses (Table 2), CD4 count <200 vs. 200–350 cells/mm3 (Adjusted hazard ratio [AHR] 2.00 [95% CI 1.39–2.87]) and ≥4 outpatient HIV provider visits in the year after meeting treatment criteria (AHR 2.20 [95% CI 1.48–3.26]) were associated with a greater hazard of being initiated on HAART. Receiving care at an adult clinical site was not significantly associated with HAART initiation (Adjusted hazard ratio [AHR] 1.23 [95% CI 0.80–1.87).
Table 2.
Patient characteristics associated with HAART initiation in treatment-naïve youth (12–24 years old) meeting treatment criteria
| Univariate (HR (95% CI))* | Multivariate (AHR (95% CI))* | |
|---|---|---|
| Female | 0.86 (0.60–1.24) | 1.05 (0.71–1.54) |
| Black | 0.86 (0.60–1.24) | 0.80 (0.52–1.23) |
| Hispanic | 0.87 (0.55–1.35) | 0.69 (0.41–1.18) |
| White | 1.0 (Ref) | 1.0 (Ref) |
| HIV acquisition risk* | — | |
| IDU | 0.58 (0.23–1.50) | |
| CD4+ T cells when first met criteria | ||
| <200 cells/mm3 | 2.57 (1.81–3.64) | 2.00 (1.39–2.87) |
| 200–350 cells/mm3 | 1.0 (Ref) | 1.0 (Ref) |
| Attending ≥4 outpatient HIV provider visits in the 365 days after meeting treatment criteria | 1.67 (1.14–2.43) | 2.20 (1.48–3.26) |
| < 4 visits | 1.0 (Ref) | 1.0 (Ref) |
| Insurance⊥ | ||
| Uninsured/RW/Other | 1.0 (Ref) | 1.0 (Ref) |
| Private | 0.94 (0.47–1.88) | 1.01 (0.52–1.98) |
| Public | 0.80 (0.54–1.18) | 0.82 (0.56–1.18) |
| Clinical site characteristic | ||
| Pediatric | 1.0 (Ref) | 1.0 (Ref) |
| Adult | 1.15 (0.78–1.70) | 1.23 (0.80–1.87) |
| Year first meeting treatment criteria for HAART | ||
| 2002 | 1.0 (Ref) | 1.0 (Ref) |
| 2003 | 0.44 (0.15–1.26) | 0.49 (0.17–1.36) |
| 2004 | 0.66 (0.25–1.69) | 0.48 (0.19–1.19) |
| 2005 | 0.68 (0.26–1.76) | 0.47 (0.19–1.16) |
| 2006 | 0.63 (0.24–1.66) | 0.50 (0.20–1.25) |
| 2007 | 0.44 (0.16–1.21) | 0.32 (0.12–0.81) |
| 2008 | 0.75 (0.28–1.97) | 0.86 (0.33–2.21) |
Cox proportional hazards regression multivariate model sex, race/ethnicity,, clinic utilization, insurance in the year treatment criteria met, CD4 category, year met criteria for HAART; and adult or pediatric HIV clinical site. Output is hazard ratio (HR) and adjusted hazard ratio (AHR). Bolded values indicates significant findings.
Insurance coverage included private insurance, public (Medicaid, medicare, Dual insurance with Medicaid/medicare), and Other/Unknown insurance (e.g., Veteran’s administration, county health program).
Discontinuation of HAART
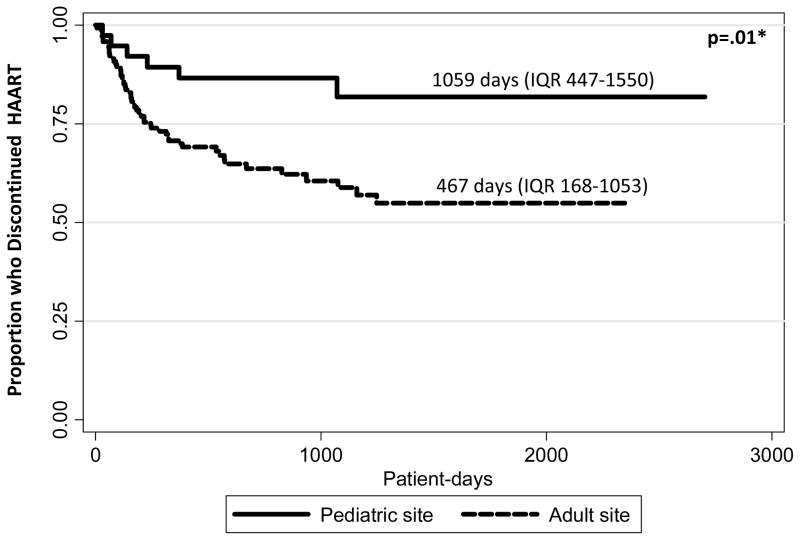
Fifty-eight BIY (29.3%) discontinued their first HAART regimen; 6/40 (15.0%) vs. 52/158 (32.9%) of those followed at pediatric vs. adult clinical sites, p=.026. The duration of the first regimen was longer for those followed at pediatric sites (1059 days [IQR 447–1550]) than for those followed at adult sites (467 days [IQR 168–1053]); p=.01. Forty-one of 58 (70.7%) had virologic data available at the time of discontinuation; 22/41 (53.7%) had log 10 HIV-1 RNA measurements > 400 copies/mL. Examination of virologic non-suppression and HAART discontinuation by site type revealed that 3/3 (100%) of those at pediatric sites and 19/38 (50%) at adult sites were not suppressed at the time of HAART discontinuation, p=.09. In Cox regression analyses, the only factor that was independently associated with earlier discontinuation of the first HAART regimen was receiving care at an adult HIV clinical site (AHR 3.19 [95% CI 1.26–8.06]) (Table 3) (Figure 1).
Table 3.
Hazard of discontinuing the first HAART regimen for treatment-naïve youth (12–24 years old) criteria in the HIV Research Network
| Univariate (HR (95% CI))* | Multivariate (AHR 95% CI))* | |
|---|---|---|
| Female | 0.85 (0.44–1.61) | 0.85 (0.35–1.74) |
| Race | ||
| Black | 0.99 (0.54–1.79) (0.84–2.01) | 0.69 (0.35–1.34) |
| Hispanic | 0.44 (0.19–1.01) | 0.39 (0.15–0.99) |
| White | 1.0 (Ref) | 1.0 (Ref) |
| CD4+ T cells when first met criteria | ||
| <200 cells/mm3 | 1.0 (Ref) | 1.0 (Ref) |
| 200–350 | 0.95 (0.54–1.65) | 1.04 (0.57–1.87) |
| Attending ≥4 outpatient HIV provider visits in the year after meeting treatment criteria | 0.64 (0.36–1.16) | 0.68 (0.36–1.25) |
| <4 visits | 1.0 (Ref) | 1.0 (Ref) |
| Clinical site characteristic | ||
| Pediatric | 1.0 (Ref) | 1.0 (Ref) |
| Adult | 2.93 (1.26–6.85) | 3.19 (1.26–8.06) |
| Insurance⊥ | ||
| Uninsured/R-W/Other | 1.0 (Ref) | 1.0 (Ref) |
| Private | 1.12 (0.32–3.90) | 0.62(0.18–2.11) |
| Public | 0.64 (0.31–1.31) | 0.56 (0.27–1.13) |
Cox proportional hazards regression multivariate model including sex, race/ethnicity, clinic utilization, insurance in the year treatment criteria met, CD4 category, year met criteria for HAART; and adult or pediatric HIV clinical site. Output is hazard ratio (HR) and adjusted hazard ratio (AHR). Bolded values indicate significant findings.
Insurance coverage included private insurance, public (Medicaid, Medicare, Dual insurance with Medicaid/Medicare), and Other/Unknown insurance (e.g., Veteran’s administration, county health program).
Fig 1. Kaplan-Meier estimate of time to discontinuing HAART for behaviorally HIV-Infected youth who have initiated HAART.
Kaplan-Meier univariate estimate of time to HAART discontinuation. *Log-rank test.
For the 58 BIY who discontinued HAART, six followed at pediatric and 52 at adult clinical sites; 3(50.0%) and 31 (59.6%) were in care the subsequent year, with no difference between pediatric and adult clinical sites (p=.65). All three (100%) vs. 24/31 (77.4%) of those who remained in care at pediatric vs. adult clinical sites, respectively, were on HAART during the subsequent year; p=.36.
Regimen details
The medications comprising the HAART regimen were available on 176/198 (88.9%) of patients who started HAART. Ninety-one (51.7%) started NNRTI-based, 85 (48.3%) started PI-based. By Kaplan-Meier univariate estimations, PI-based regimens were used longer (median 581 [IQR 203–1200] days) than NNRTI-based regimens (median 447 [IQR 245–1059] days, p=.02). However, in the Cox proportional hazards regression analysis of HAART discontinuation, no significant differences in likelihood of discontinuation by HAART regimen were observed for PI-based (AHR 1.26 [95% CI 0.70–2.27]) compared to NNRTI-based regimens. (Results not shown) Adult site was again, the only variable significantly associated with HAART discontinuation (AHR 3.64 [95% CI 1.35–0.83]).
DISCUSSION
In this longitudinal study examining HAART initiation and maintenance in a cohort of BIY, we found that 69% of those eligible for treatment were prescribed HAART over the study period. This study indicates that BIY in need of HAART are starting therapy at lower rates than reported for adult cohorts19;20, such as the HAART utilization rate of 91% in HIVRN adult patients ≥18 years of age (median of 41 years) meeting treatment criteria reported by Gebo et al 19. The lower rates seen in BIY youth are similar to those seen in marginalized populations such as IDUs 19;20, persons with mental health diagnoses 21, and commercial sex workers. 22 The factors associated with HAART initiation in BIY are similar to those reported in adult cohorts, including lower CD4 count and adherence to quarterly clinic visits23. Of note, factors associated with HAART in adult cohorts, such as race, risk group, and insurance had little variation in the BIY sample, which may lead to nonsignificant results.
While studies in BIY have focused on barriers to adherence in this population 15;24;25, few studies have examined disparities in HAART within the BIY population. The comparison of the BIY HAART initiation rates with those reported for adults and not those reported for perinatally-infected youth in this study is deliberate, as the longstanding relationships with providers and recommendations for early and continued HAART in young children make perinatally-infected youth quite different from BIY. The lower likelihood of HAART initiation for BIY underscores the importance of designing novel mechanisms to engage and maintain BIY youth into care. Studies that delineate specific factors associated with HAART initiation are critical for informing appropriate interventions to improve management in this population.
Importantly, our study found that BIY receiving care at adult clinical sites were equally as likely to start HAART as those being seen at pediatric sites. However, receiving care at an adult clinical site was independently associated with a greater hazard of discontinuing one’s first HAART regimen, the regimen most likely to achieve durable virologic suppression and immune recovery 26;27. Furthermore, there was no evidence that this difference was driven by level of immunosuppression or attending regular clinic visits. Greater HAART discontinuation in adult HIV clinical sites in this study may reflect differences in clinic structure, available programs to support HAART adherence and sustainability, or an increased emphasis on social services and case management. These findings could also reflect differences in provider and staff approach to adolescent and young adult patients, as well as BIY interactions with clinical staff. However, we were unable to evaluate the impact of these factors in the current study.
Differences in treatment practices between pediatric and adult providers have been observed in other chronic illnesses, such as end stage renal disease 12;13. Pediatric clinical sites are more likely to have providers and support staff who are familiar with and/or have received specific training about adolescents and young adult development and behavior, lower patient to provider ratios, youth-friendly facilities and programs, such as technologies (e.g., texting) targeted at youth to encourage attendance to appointments and adherence to HAART 28–31. Although the reason for the observed difference was not specifically studied, the finding that HAART utilization in youth may differ by being cared for at a pediatric facility is intriguing and may have implications for best practice guidelines for this vulnerable population. It is possible that caring for youth in a clinic that addresses the unique developmental and psychosocial needs of this population may be the ideal treatment setting, particularly as BIY engage into care and initiate HAART; however, more study is needed. Furthermore, the difference observed lends further support for the value of systematic transition from pediatric to adult-centered care to minimize loss to follow up and ensure optimal HAART outcomes32;33. For patients maintained on HAART, we did not examine subsequent virologic suppression, CD4 outcomes, or resistance. Though this was beyond the scope of these analyses, future studies should address this critical outcome to truly understand best practice and optimize care for this population.
Lower CD4 counts have previously been associated with a greater likelihood of starting HAART19. As therapies have become more simplified with less recognized toxicities, recommendations for initiation of HAART have shifted to higher CD4 thresholds. It is therefore expected that increased numbers of BIY will qualify for therapy according to the new HAART guidelines;, however, if there is not an accompanying increase in the likelihood of initiating HAART, it is possible that the proportion of patients who need HAART and receive it may actually decrease34,35.
Attendance at four or more outpatient clinic visits in the year after meeting treatment criteria was used as a marker of adequate outpatient utilization, based on the DHHS recommendations 3, similar to prior adult HIVRN studies36. While in a prior analysis there was an association of attendance to outpatient visits and starting HAART, there was no relationship between attendance and HAART discontinuation in this analysis23. Clinic attendance may be a marker for unmeasured variables including patient engagement into care; greater access to providers, clinic support personnel, and services; and better monitoring of immune status. However, it is intriguing that clinic attendance was not independently associated with HAART discontinuation. Likewise, regimen type was not associated with HAART discontinuation.
It should be noted that while our study design has allowed us to identify associations, we were unable to determine causal relationships. Also, although the sites in the sample encompass a broad geographic distribution, the sample is small and not nationally representative and may not generalize to all HIV clinical sites; however, the use of multiple sites does afford greater generalizability than a single-site study would provide. Moreover, the sites in the HIVRN were all highly experienced in the management of HIV infection; results may differ at sites with smaller caseloads of HIV-infected patients. Additionally, in identifying patients who were not receiving HAART, we were unable to assess whether they refused it or whether other complex medical decision-making by them and their providers resulted in their not being on HAART; these data are not available in the database. We do not have data on why HAART was discontinued. We did not have access to socioeconomic and psychosocial variables such as income, educational level, family awareness/support of HIV diagnosis, mental health and substance abuse treatment, or unstable housing which have previously been shown to be associated with HAART utilization 20. We did not assess outcomes (e.g., decreases in HIV-1 RNA) as we were underpowered and it was beyond the scope of the current analysis; future analyses will need to examine the long-term outcomes of HAART in youth, including virologic suppression, CD4 outcomes, and development of resistance.
It is highly probable that increased numbers of youth in need of treatment will be identified as the CDC guidelines for universal opt-out testing are implemented and with newer data supporting initiating treatment at higher CD4 levels34;37. The association of pediatric clinical site with HAART sustainability should prompt further study into the clinical and psychosocial characteristics as well as the types of targeted interventions that may enhance effective HAART utilization for this vulnerable population.
Acknowledgments
Sponsorship: Supported by the Agency for Healthcare Research and Quality (290-01-0012) and the National Institute on Aging, NIH (RO1 AG026250). Dr. Gebo also received support from the Johns Hopkins University Richard Ross Clinician Scientist Award.
Dr. Agwu is supported by the National Institutes of Allergy and Infectious Diseases (1K23AI084549-01A1) and the Johns Hopkins Ross Clinician Scientist Award. Dr. Korthuis is supported by the National Institute on Drug Abuse (K23DA019809).
Abbreviations
- HAART
highly active antiretroviral therapy
- HIV
human immunodeficiency virus
- HIVRN
HIV Research Network
- BIY
youth infected through risk behaviors
- DHHS
Department of Health and Human Services
- MSM
men who have sex with men
- IDU
injection drug use
Footnotes
Disclaimer: The views expressed in this paper are those of the authors. No official endorsement by DHHS, the National Institutes of Health, or the Agency for Healthcare Research and Quality is intended or should be inferred.
Implications and Contributions: To our knowledge, this is the first study to specifically examine the impact of clinic type (adult or pediatric-focused) on HAART utilization and sustainablity in this population. The findings may have implications for best practices in the care of HIV-infected youth.
Participating Sites
Alameda County Medical Center, Oakland, California (Howard Edelstein, M.D.)
Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania (Richard Rutstein, M.D.)
Community Health Network, Rochester, New York (Roberto Corales, D.O.)
Drexel University, Philadelphia, Pennsylvania (Jeffrey Jacobson, M.D., Sara Allen, C.R.N.P.)
Johns Hopkins University, Baltimore, Maryland (Kelly Gebo, M.D., Richard Moore, M.D., Allison Agwu M.D.)
Montefiore Medical Group, Bronx, New York (Robert Beil, M.D., Carolyn Chu, M.D.)
Montefiore Medical Center, Bronx, New York (Lawrence Hanau, M.D.)
Oregon Health and Science University, Portland, Oregon (P. Todd Korthuis, M.D.)
Parkland Health and Hospital System, Dallas, Texas (Laura Armas-Kolostroubis, M.D.)
St. Jude’s Children’s Hospital and University of Tennessee, Memphis, Tennessee (Aditya Gaur, M.D.)
St. Luke’s Roosevelt Hospital Center, New York, New York (Victoria Sharp, M.D.)
Tampa General Health Care, Tampa, Florida (Charurut Somboonwit, M.D.)
University of California, San Diego, La Jolla, California (Stephen Spector, M.D.)
University of California, San Diego, California (W. Christopher Mathews, M.D.)
Wayne State University, Detroit, Michigan (Jonathan Cohn, M.D.)
Sponsoring Agencies
Agency for Healthcare Research and Quality, Rockville, Maryland (Fred Hellinger, Ph.D., John Fleishman, Ph.D., Irene Fraser, Ph.D.)
Health Resources and Services Administration, Rockville, Maryland (Robert Mills, Ph.D.)
Data Coordinating Center
Johns Hopkins University (Richard Moore, M.D., Jeanne Keruly, C.R.N.P., Kelly Gebo, M.D., Cindy Voss, M.A., Bonnie Cameron, M.S.)
Conflict of interest: Dr. Gebo has received research funding from Tibotec.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Catallozzi M, Futterman DC. HIV in Adolescents. Curr Infect Dis Rep. 2005;7:401–5. doi: 10.1007/s11908-005-0015-z. [DOI] [PubMed] [Google Scholar]
- 2.Futterman DC. HIV in adolescents and young adults: half of all new infections in the United States. Top HIV Med. 2005;13:101–5. [PubMed] [Google Scholar]
- 3.Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 1-29-2008. Ref Type: Internet Communication. [Google Scholar]
- 4.Blum RW, McNeely C, Nonnemaker J. Vulnerability, risk, and protection. J Adolesc Health. 2002;31:28–39. doi: 10.1016/s1054-139x(02)00411-1. [DOI] [PubMed] [Google Scholar]
- 5.Murphy DA, Belzer M, Durako SJ, Sarr M, Wilson CM, Muenz LR. Longitudinal antiretroviral adherence among adolescents infected with human immunodeficiency virus. Arch Pediatr Adolesc Med. 2005;159:764–70. doi: 10.1001/archpedi.159.8.764. [DOI] [PubMed] [Google Scholar]
- 6.Murphy DA, Wilson CM, Durako SJ, Muenz LR, Belzer M. Antiretroviral medication adherence among the REACH HIV-infected adolescent cohort in the USA. AIDS Care. 2001;13:27–40. doi: 10.1080/09540120020018161. [DOI] [PubMed] [Google Scholar]
- 7.Flynn PM, Rudy BJ, Douglas SD, Lathey J, Spector SA, Martinez J, Silio M, Belzer M, Friedman L, D’Angelo L, McNamara J, Hodge J, Hughes MD, Lindsey JC. Virologic and immunologic outcomes after 24 weeks in HIV type 1-infected adolescents receiving highly active antiretroviral therapy. J Infect Dis. 2004;190:271–79. doi: 10.1086/421521. [DOI] [PubMed] [Google Scholar]
- 8.Pulido F, Arribas JR, Miro JM, Costa MA, Gonzalez J, Rubio R, Pena JM, Torralba M, Lonca M, Lorenzo A, Cepeda C, Vazquez JJ, Gatell JM. Clinical, virologic, and immunologic response to efavirenz-or protease inhibitor-based highly active antiretroviral therapy in a cohort of antiretroviral-naive patients with advanced HIV infection (EfaVIP 2 study) J Acquir Immune Defic Syndr. 2004;35:343–50. doi: 10.1097/00126334-200404010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Matthews GV, Sabin CA, Mandalia S, Lampe F, Phillips AN, Nelson MR, Bower M, Johnson MA, Gazzard BG. Virological suppression at 6 months is related to choice of initial regimen in antiretroviral-naive patients: a cohort study. AIDS. 2002;16:53–61. doi: 10.1097/00002030-200201040-00008. [DOI] [PubMed] [Google Scholar]
- 10.Agwu AL, Ellen J, Rutstein R, Gaur AH, Siberry GK, Spector SA, Warford R, Gebo KA. Significantly Lower Rates of HAART Utilization in HIV-1 Infected Youth vs. Adults Meeting Treatment Criteria in a Large Multisite US Cohort. Infectious Diseases Society of America; 2009. Ref Type: Conference Proceeding. [Google Scholar]
- 11.Agwu AL, Fleishman JA, Korthuis PT, Siberry GK, Ellen JM, Gaur AH, Rutstein R, Gebo KA and for the HIV Research Network. Disparities in Antiretroviral Treatment: A Comparison of Behaviorally-HIV-Infected Youth and Adults in the HIV Research Network. Journal of the Acquired Immune Deficiency Syndrome. 2011 doi: 10.1097/QAI.0b013e31822327df. Ref Type: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furth SL, Hwang W, Yang C, Neu AM, Fivush BA, Powe NR. Relation between pediatric experience and treatment recommendations for children and adolescents with kidney failure. JAMA. 2001;285:1027–33. doi: 10.1001/jama.285.8.1027. [DOI] [PubMed] [Google Scholar]
- 13.Furth SL, Powe NR, Hwang W, Neu AM, Fivush BA. Does greater pediatric experience influence treatment choices in chronic disease management? Dialysis modality choice for children with end-stage renal disease. Arch Pediatr Adolesc Med. 1997;151:545–50. doi: 10.1001/archpedi.1997.02170430011002. [DOI] [PubMed] [Google Scholar]
- 14.Rudy BJ, Murphy DA, Harris DR, Muenz L, Ellen J. Patient-related risks for nonadherence to antiretroviral therapy among HIV-infected youth in the United States: a study of prevalence and interactions. AIDS Patient Care STDS. 2009;23:185–94. doi: 10.1089/apc.2008.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy DA, Sarr M, Durako SJ, Moscicki AB, Wilson CM, Muenz LR. Barriers to HAART adherence among human immunodeficiency virus-infected adolescents. Arch Pediatr Adolesc Med. 2003;157:249–55. doi: 10.1001/archpedi.157.3.249. [DOI] [PubMed] [Google Scholar]
- 16.Gebo KA, Moore RD, Fleishman JA. The HIV Research Network: a unique opportunity for real time clinical utilization analysis in HIV. Hopkins HIV Rep. 2003;15:5–6. [PubMed] [Google Scholar]
- 17.Department of Health and Human Services. Archived Department of Health and Human Services (DHHS) Guidelines. Internet. 2008 Nov 3; 12-15-2008. Ref Type: Electronic Citation. [Google Scholar]
- 18.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in HIV-1 Infected Adults and Adolescents. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. 12-1-2009. 12-23-2009. Ref Type: Electronic Citation
- 19.Gebo KA, Fleishman JA, Conviser R, Reilly ED, Korthuis PT, Moore RD, Hellinger J, Keiser P, Rubin HR, Crane L, Hellinger FJ, Mathews WC. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38:96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham WE, Markson LE, Andersen RM, Crystal SH, Fleishman JA, Golin C, Gifford A, Liu HH, Nakazono TT, Morton S, Bozzette SA, Shapiro MF, Wenger NS. Prevalence and predictors of highly active antiretroviral therapy use in patients with HIV infection in the united states. HCSUS Consortium. HIV Cost and Services Utilization. J Acquir Immune Defic Syndr. 2000;25:115–23. doi: 10.1097/00042560-200010010-00005. [DOI] [PubMed] [Google Scholar]
- 21.Himelhoch S, Chander G, Fleishman JA, Hellinger J, Gaist P, Gebo KA. Access to HAART and utilization of inpatient medical hospital services among HIV-infected patients with co-occurring serious mental illness and injection drug use. Gen Hosp Psychiatry. 2007;29:518–25. doi: 10.1016/j.genhosppsych.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shannon K, Bright V, Duddy J, Tyndall MW. Access and utilization of HIV treatment and services among women sex workers in Vancouver’s Downtown Eastside. J Urban Health. 2005;82:488–97. doi: 10.1093/jurban/jti076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agwu A, Rutstein R, Gaur A, Spector S, Warford R, Gebo K and HIV Research Network. Starting Late and Stopping Early: Disparities in HAART Utilization for Behaviorlally HIV-Infected Youth. 18th Conference on Retroviruses and Opportunistic Infections; 2011. Feb 27, Ref Type: Conference Proceeding. [Google Scholar]
- 24.Murphy DA, Sarr M, Durako SJ, Moscicki AB, Wilson CM, Muenz LR. Barriers to HAART adherence among human immunodeficiency virus-infected adolescents. Arch Pediatr Adolesc Med. 2003;157:249–55. doi: 10.1001/archpedi.157.3.249. [DOI] [PubMed] [Google Scholar]
- 25.Murphy DA, Lu MC, Martin D, Hoffman D, Marelich WD. Results of a pilot intervention trial to improve antiretroviral adherence among HIV-positive patients. J Assoc Nurses AIDS Care. 2002;13:57–69. doi: 10.1177/1055329002238026. [DOI] [PubMed] [Google Scholar]
- 26.Palella FJ, Chmiel JS, Moorman AC, Holmberg SD and the HIV Outpatient Study Investigators. Durability and predictors of success of highly active antiretroviral therapy for ambulatory HIV-infected patients. AIDS. 2002;16:1617–26. doi: 10.1097/00002030-200208160-00007. [DOI] [PubMed] [Google Scholar]
- 27.Ledergerber B, Egger M, Opravil M, Telenti A, Hirschel B, Battegay M, Vernazza P, Sudre P, Flepp M, Furrer H, Francioli P, Weber R. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Swiss HIV Cohort Study. Lancet. 1999;353:863–68. doi: 10.1016/s0140-6736(99)01122-8. [DOI] [PubMed] [Google Scholar]
- 28.Johnson RL, Botwinick G, Sell RL, Martinez J, Siciliano C, Friedman LB, Dodds S, Shaw K, Walker LE, Sotheran JL, Bell D. The utilization of treatment and case management services by HIV-infected youth. J Adolesc Health. 2003;33:31–38. doi: 10.1016/s1054-139x(03)00158-7. [DOI] [PubMed] [Google Scholar]
- 29.Dodds S, Blakley T, Lizzotte JM, Friedman LB, Shaw K, Martinez J, Siciliano C, Walker LE, Sotheran JL, Sell RL, Botwinick G, Johnson RL, Bell D. Retention, adherence, and compliance: special needs of HIV-infected adolescent girls and young women. J Adolesc Health. 2003;33:39–45. doi: 10.1016/s1054-139x(03)00157-5. [DOI] [PubMed] [Google Scholar]
- 30.Johnson RL, Martinez J, Botwinick G, Bell D, Sell RL, Friedman LB, Dodds S, Shaw K, Siciliano C, Walker LE, Sotheran JL. Introduction: what youth need--adapting HIV care models to meet the lifestyles and special needs of adolescents and young adults. J Adolesc Health. 2003;33:4–9. doi: 10.1016/s1054-139x(03)00161-7. [DOI] [PubMed] [Google Scholar]
- 31.Preston KE, Walhart TA, O’Sullivan AL. Prompting Health Behavior via Text Messaging in Adolescents and Young Adults. American Journal of Lifestyle Medicine. 2011 [Google Scholar]
- 32.Wiener LS, Kohrt B, Battlers HB, Pao M. The HIV Experience: Youth Identified Barriers for Transitioning from Pediatric to Adult Care. Journal of Pediatric Psychology. 2011;36:141–54. doi: 10.1093/jpepsy/jsp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miles K, Edwards S, Clapson M. Transition from paediatric to adult services: experiences of HIV-positive adolescents. AIDS Care. 2004;16:305–14. doi: 10.1080/09540120410001665312. [DOI] [PubMed] [Google Scholar]
- 34.Hammer SM, Eron JJ, Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, Fischl MA, Gatell JM, Hirsch MS, Jacobsen DM, Montaner JS, Richman DD, Yeni PG, Volberding PA. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 35.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Dec 1, 2009. pp. 1–161. 2009. 5-10-2010. Ref Type: Report. [Google Scholar]
- 36.Fleishman JA, Gebo KA, Reilly ED, Conviser R, Christopher MW, Todd KP, Hellinger J, Rutstein R, Keiser P, Rubin H, Moore RD. Hospital and outpatient health services utilization among HIV-infected adults in care 2000–2002. Med Care. 2005;43:III40–III52. doi: 10.1097/01.mlr.0000175621.65005.c6. [DOI] [PubMed] [Google Scholar]
- 37.Bransom B, Handsfield H, Lampe M, Janssen R, Taylor A, Lyss S, Clark J. Revised Recommendations for HIV Testing of Adults, Adolescents, and Pregnant Women in Health-Care Settings. MMWR. 2008;55:1–17. [PubMed] [Google Scholar]