Abstract
Background:
Since the advent of the Industrial Revolution in the late 19th century, we have all been unfortunately exposed to an increasingly toxic and polluted world. Among the most dangerous of these pollutants is mercury, which is considered to be the most toxic non-radioactive heavy metal. Fermented foods may help cleanse the body of heavy metals. Fermentation breaks down the nutrients in foods by the action of beneficial microorganisms and creates natural chelators that are available to bind toxins and remove them from the body.
Aims:
The current study was designed to determine the impact of feeding a high fiber probiotic fermented mare's milk on the biological effects of mercury toxicity in rat model.
Methods and Materials:
The high fiber fermented mare's milk containing probiotics was prepared and its sensory properties, chemical composition, and antioxidant activity were determined. A rat model of mercury toxicity was used. The effect of feeding the high fiber probiotic fermented mare's milk to rats, along with mercury ingestion, was determined by the analysis of several biochemical markers in serum and histopathological examinations of brain and kidney.
Results:
The high fiber fermented mare's milk containing probiotics was found to be acceptable by all test panels and volunteers. Mercury ingestion was found to cause biochemical and histopathological alterations in rat serum and tissues. The mercury-treated rats showed a decrease in body weight and an increase in kidney weight. Sera of the mercury treated rats showed alterations in biochemical parameters, and histopathological changes in brain and kidney. However, the rats fed high fiber fermented mare`s milk along with mercury ingestion showed improved histopathology of kidney and brain, and there was restoration of the biochemical parameters in serum to almost normal values.
Conclusions:
Feeding high fiber fermented mare`s milk may reduce the toxic effects of mercury.
Keywords: Mare's milk, high-fiber probiotic from fermented mare's milk, mercury contamination
Introduction
Mercury, a toxic divalent heavy metal without any biological function, causes several deleterious effects in adults and developing organisms[1,2]. Mercury exposure primarily affects the central nervous system and renal system[3–6]. The three most commonly encountered forms of mercury are metal vapor (Hg), ionic compounds (Hg2+) and organic mercury, principally methyl mercury (HgCH3)+. Each form has its own effects, routes of absorption and tissue specificity. Organic mercury is the most deadly of the mercury compounds, probably due to its ability to penetrate cells. Within the cell it can destroy various components selectively or in total by releasing lysosomes, damaging DNA and rupturing the cell membrane. Its effects upon the neurological and reproductive system have been extensively documented[7].
The toxicity of high levels of mercury contamination was brought to public attention in the 1950s as a result of mercury dumping in the Minamata Bay in Japan 8 9]. Consumption of seafood from the bay led to widespread neurological damage and teratogenic effects. The general population is primarily exposed to mercury via food, fish being a major source of methyl mercury exposure[10]. Mercury is incorporated into the food chain as methyl mercury, primarily through the action of bacteria and other microbes transforming elemental or inorganic forms. Humans are also exposed to mercury via thimerosal (a preservative added to vaccines and many other pharmaceuticals), and “amalgam” or mercury-based dental fillings[11]. Mercury vapor released from mercury dental fillings is absorbed very rapidly and thoroughly by the body, mainly through inhalation and swallowing[12]. Mercury from amalgams is readily methylated by bacteria in the mouth[13]. Adverse health effects, particularly of a neurological nature, have resulted from low exposure levels, especially to the fetus in pregnant women[14].
Using young rats as a model of mercury toxicity, results from previous studies and from our group have demonstrated that exposure to HgCl2 (25 mg/kg) for only five consecutive days causes cerebral and whole body weight losses, increases in kidney weight, mercury accumulation in tissues, inhibition of porphobilinogen synthase activity[15–17], alterations in renal and hepatic functions and glycemia[4]. Metal intoxication is usually treated with chelating agents, which are not specific and are often more toxic than the metal itself[15,18]. Because chelation therapy for heavy metals intoxication can also be toxic, researchers are looking for natural preventive and therapeutic agents for heavy metal toxicity.
Kumis, also known as koumiss, is an alcoholic beverage made from fermented mare's milk. This beverage originated with the nomads of central Asia. Mare's milk is usually not consumed raw, because it tends to have a strong laxative effect, although this effect is sometimes used medically. Instead, mare's milk is almost always fermented into kumis. Mare's milk contains significantly more lactose than cow's milk. Fermented mare's milk is prepared by lactic acid fermentation[19]. Fermentation destroys lactose in milk, converting it into lactic acid, ethanol, and carbon dioxide. This makes kumis acceptable for lactose intolerant people. Traditional kumis is prepared by mixing fermented milk with fresh raw mares’ milk. Fermentation takes place in 3-8 hours and is carried out by a mixture of indigenous yeasts and lactic acid bacteria[20]. Currently, Kumis is manufactured at an industrial level in some countries using pure cultures of yeasts and lactic acid bacteria[21].
The medical use of kumis has been documented in clinical tests[22]. Kumis is rich in ferments, trace elements, antibiotics, vitamins A, B1, B2, B12, D, E, C, ethyl alcohol, lactic acid and carbonic acid. Kumis drinking has a healing influence on the gastrointestinal tract, metabolism, cardiovascular and nervous systems, and kidneys; it aids the development of immunity; and has been used to treat weight loss and anemia[22,23].
Adding soluble fiber to the diet has also been shown to have positive effects on health. Various studies have shown a reduction in colon cancer risk and enhanced health by consuming inulin and oligo fructose in ratios ranging from 2% to 10%[24,25].
Prebiotics and probiotics have been proposed as human health-enhancing food additives[26]. Probiotics include viable lactic acid bacteria and other bacilli that are able to survive in the intestine. Prebiotics include carbohydrates that are not digested in the upper part of the gastrointestinal tract, but selectively fermented by bacteria in the colon. This selective fermentation affects the composition of the intestinal microflora by stimulating Bifidobacteria and Lactobacilli, both in humans and in animals, where these bacteria have health promoting properties[26].
We have combined these three health-promoting materials to produce a functional food composed of fermented mare's milk plus a high level (6%) of soluble fiber and probiotics. The current study was designed to determine if feeding the high fiber probiotic fermented mare's milk could alleviate the toxic effects of mercury in rat model.
Materials and Methods
Starter cultures of Streptococcus thermophilus, Lactobacillus acidophilus, and Bifidobacterium bifidum were obtained from Hansen's Laboratory, Copenhagen, Denmark. Dandelion root (Taraxacum officinale) was purchased from Haraz Herbal Market, Cairo, Egypt. Fresh mare's milk was obtained from lactating and healthy Arabian mares in the Experimental Animal Unit, College of Agriculture and Veterinary Medicine, Qassim University, Saudi Arabia.
Preparation of probiotic fermented mare's milk
Probiotic fermented mare's milk was prepared using standard methods[27–29]. Briefly, fresh mare's milk (11% total solid) was heated at 85° C for 15 minutes, cooled to 40° C, then inoculated with the 1.0 × 109 probiotic bacteria Streptococcus thermophilus, Lactobacillus acidophilus and Bifidobacterium bifidum and was then incubated for 4-8 hours at 42° C. After coagulation, the curd was tested for pH and stored refrigerated (4-6° C).
Preparation of dandelion root fiber
A hot water extract of soluble dandelion root fiber was prepared according the methods described by Abdel-Salam et al.[30]. Dandelion roots were cut into small pieces and boiled for 10 minutes in distilled water. The mixture was filtered twice: first through cheesecloth (50% cotton/50% polyester), and then through filter paper (Whatman No.2). The solutions obtained were preserved in sterile dark bottles in a cool environment (4° C) until use.
Preparation of high fiber probiotic fermented mare's milk
The high fiber probiotic mare's milk was prepared by mixing the concentrated hot water extract of dandelion root with the fermented mare's milk at 6% total solids.
Sensory evaluation of the product
A sensory evaluation test of the fermented milk products was adopted from the National Aeronautics and Space Administration (N.A.S.A.)[31]. The evaluation was performed by a panel composed of the staff and students in the Department of Food Science and Human Nutrition. The evaluation rated the appearance, color, odor, flavor, and overall impression of the product, and results were expressed as very good (+++), good (++), acceptable (+), or unacceptable (-).
Antioxidant capacity assay: of fresh, fermented and high fiber fermented mare's milk
Antioxidant capacity was measured with a 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay according to the method of Brand-Williams et al.[32] with some modifications. A stock solution was prepared by dissolving 24 mg of DPPH in 100 mL methanol. The working solution was obtained by mixing 10 mL stock solution with 45 mL methanol to obtain an absorbance of 1.1 ± 0.02 units at 515 nm using a spectrophotometer. Milk extracts (750 μL) were allowed to react with 1500 μL of the DPPH solution for 5 minutes in the dark. The absorbance was read at 515 nm. Antioxidant capacity was measured against a Trolox standard. Trolox is a water-soluble derivative of vitamin E It is an antioxidant, like vitamin E, and is used in biological or biochemical applications to reduce oxidative stress or damage. Trolox equivalent antioxidant activity (TEAC) is a measurement of antioxidant strength based on Trolox, measured in units called Trolox Equivalents (TE), Trolox equivalency is used as a benchmark for the antioxidant capacity of such a mixture. Results are expressed in μmol Trolox/100g dry matter. The standard curve was linear between 25 and 800 μmol Trolox.
Animals and experimental feeding groups
Thirty male adult Swiss albino rats weighing 200-250 g ± 40 g were used. The animals were housed in standard cages and were assigned to a specific treatment diet. The experiment was approved by the animal care and use committee of Qassim University.
The rats were divided into three groups of 10 animals each. Each group was fed a defined basal diet plus water ad lib. The composition of the basal diet was as follows: milk protein (12%), sucrose (5%), fat (10%), vitamin mixtures (1%), salt mixtures (4%), fiber (4%) and starch (64 %).
The negative control group was fed on the basal diet only. The positive control group was fed the basal diet and received 25 ppm mercury as mercuric chloride (HgCl2) in drinking water. The third group was fed the basal diet, received 25 ppm mercury as mercuric chloride (HgCl2) in drinking water, and was fed the high fiber fermented mare's milk solution containing probiotics. The weights of the rats were measured at the beginning and end of the experiment.
The feeding experiment continued for 6 weeks. At the end of this period, the rats were anesthetized with diethyl ether and killed by exsanguination. Tissue samples were taken for histological examination. Kidney weights were measured. Blood samples were collected and centrifuged at 3000 X g for 30 minutes. Sera were harvested, labeled and stored frozen (-20° C) pending analysis.
Biochemical Analysis
In sera the following parameters were analyzed: Urea was determined according to the methods of Tietz[33]. Creatinine was determined according to the method of Bonnes and Taussky[34]. Triglycerides were determined according to the methods of Stein and Myers[35]. Glutathione-S-transferase (GST) activity was determined according to Habig et.al.[36]. Lactate dehydrogenase (LDH) activity was determined according to Bergmeyer[37].
Histopathological examination
Samples of brain, cerebellum, and kidneys were taken, and fixed in 10% neutral buffered formalin for 24 hours. Paraffin sections 6 μm thick were prepared and stained with hematoxylin and eosin (H & E)[38] for the examination of tissue and cellular changes by light microscopy.
Statistical analysis
Mean and standard deviation of the data from each experimental group were calculated and according the method described by Miller and Miller[39]. The percent coefficient of variation was also calculated.
Results
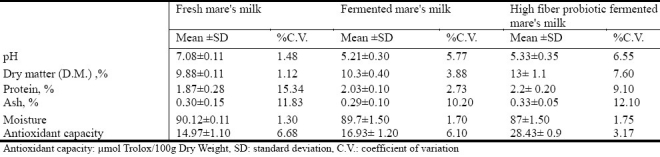
The results of the sensory evaluation of the high fiber fermented mare's milk containing probiotics showed that it's appearance and color were rated “good”, and it's odor, flavor, and overall impression were rated “acceptable” by the test panel. Table 1 shows a comparison of the composition and antioxidant capacity of fresh, fermented, and high fiber fermented mare's milk containing probiotics. Although the composition of all 3 was similar, the antioxidant capacity was highest in the high fiber probiotic fermented mare's milk compared to fresh or fermented mare's milk.
Table 1.
Chemical compositions and antioxidant capacity of fresh, fermented, and high fiber fermented mare's milk
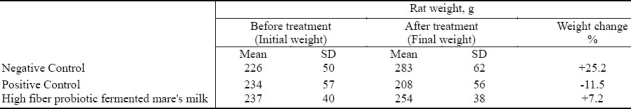
As shown in Table 2, the untreated (negative control) rats gained weight during this experiment, while there was a large decrease in the weight of the rats in the positive control group. The weight of the rats consuming the high fiber probiotic fermented mare's milk treated group increased rather than decreased during the course of this experiment.
Table 2.
Body weight changes following different treatments
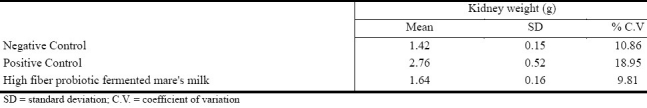
Treatment with mercuric chloride (positive control)resulted in an increase in the rat's kidney weight compared to the untreated negative control rats (Table 3). Less kidney weight increase was observed in rats treated with mercury and high fiber probiotic fermented mare's milk, compared to the positive control animals.
Table 3.
Kidney weight following different treatments
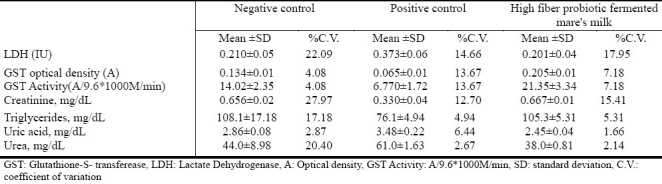
The values of several biochemical markers measured in serum are shown in Table 4. There was a decrease in the activity of glutathione-S- transferase (GST) in the sera of the positive control rats compared to the untreated negative control rats. However, GST activity increased in the rats receiving the high fiber probiotic fermented mare's milk in addition to mercury. The activity of lactate dehydrogenase (LDH) increased in the group treated with mercuric chloride only; however, LDH activity decreased in the group that received mercuric chloride and high fiber probiotic fermented mare's milk. Serum creatinine levels in the positive control group decreased compared to the negative control. However, treatment with high fiber probiotic fermented mare's milk resulted in serum creatinine levels returning to normal values. There was a decrease in the concentration of serum triglycerides in the control positive group receiving mercury only, but the concentration of serum triglycerides was almost normal in the group that received mercury together with high fiber probiotic fermented mare's milk. There was a significant increase in the level of uric acid and urea in serum of the positive control group compared to the negative controls. However, uric acid and urea were lower in the group that ingested high fiber probiotic fermented mare's milk with mercury than in the negative control group.
Table 4.
Biochemical markers of kidney function measured in serum
Histopathological changes in the brain and kidney sections stained with H&E were evaluated by light microscopy. A summary of the evaluations is shown in Table 5. There was generalized and localized edema in the white matter of cerebrum and cerebellum in the positive control rats. The positive controls also showed congested blood vessels in both cerebrum and cerebellum which may occur prior to edema. These sections showed neuronal necrosis in cerebrum, necrosis and chromatolysis of Purkinje cells in the cerebellum, and demyelination in the axons of the neuropil.
Table 5.
Histopathological changes in rat brain and kidney following different treatments
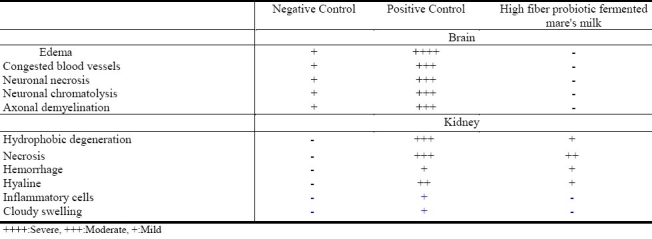
The histopathological changes in kidneys of rats were also evaluated. In the positive control group, the renal epithelium suffered vacuolar and hydropic degeneration in most tubules. Necrotic debris occasionally formed acidophil casts within the tubuli. In some cases, necrosis of the whole tubules was observed. A few tubules showed hyaline droplet degeneration in their epithelium, together with some hyaline casts within tubular lumen. Mild congestion in inter tubular blood vessels was observed. Perivascular and interstitial inflammatory cellular infiltrate with pyknosis of the nucleus was observed, as was severe cloudy swelling of renal tubular epithelium with moderate necrosis and hydropic degeneration but no significant histological alterations were shown in the glomeruli. Administration of high fiber probiotic fermented mare's milk partially protected the kidneys from the histopathological changes produced by mercury. The histopathological changes were severe with mercury alone but they were moderate to mild when mercury was administered together with high fiber probiotic fermented mare's milk.
Rats treated with high fiber probiotic fermented mare's milk showed no histopathological changes in the brain. Mild changes were observed in kidneys as manifested by hydrophobic degeneration, necrosis, and hemorrhage, but no inflammatory cells or cloudy swelling were observed. These results indicate that high fiber probiotic fermented mare's milk protected the rats against the adverse effects produced by mercury in brain and kidney.
Discussion
In recent years the popularity of complementary medicine, such as the use of probiotics and functional foods has increased. Knowledge is accumulating that microbiota modulates gut physiology, immunological functions, and may produce other beneficial effects. This has led scientists to investigate the efficacy of probiotics, prebiotics and synbiotics in the treatment of diseases and toxicities. Increasing the variety of functional foods is a challenge, and the promotion and formulation of functional foods and may enhance human health[40].
It is well documented that HgCl2 exposure promotes Hg accumulation in all tissues[41], with the highest levels in the liver, followed by the kidney and blood. Mercury causes kidney damage, which is reversible after exposure has stopped.
Kidney toxicity was seen in this rat model. The increased blood urea and creatinine are in agreement with the results obtained by Rana et al.[41] in male rats treated with mercury. Elevated blood urea is known to be a function of or related to increased protein catabolism in mammals and/or the conversion of ammonia to urea as a result of increased synthesis of arginase enzyme involved in urea production[42]. In agreement with this, the present results show that body weights decreased in animals treated with mercury indicating body wasting and increased protein catabolism. The increase in plasma urea and creatinine concentrations in the present experiment may be due to kidney dysfunction, as suggested by the increased weight of the kidneys[43].
The decrease in the activity of GST in serum of rats receiving mercury is similar to the findings reported by El-Missiry and Shalaby[44], who found that the heavy metal cadmium decreased the activity of GST in brain and testes of male rats. El-Demerdash[45] also reported that cadmium caused a decrease in GST activity in fish. GST catalyses the reaction of alkylating agents with the thiol (-SH) group of glutathione, thereby neutralizing an electrophilic site and rendering the products more water soluble[46]. The decline in serum GST activity may be due to the effect of mercury on glutathione. Mercury has a high affinity to this molecule where a sulfhydryl, an amino and two carboxylic acid groups, as well as two peptide linkages, represents reactive sites for metals. Reactions of metals with glutathione may lead to either the formation of complexes or the oxidation of glutathione[45].
High fiber probiotics fermented mare's milk was found to have a high antioxidant capacity (Table 1). This high antioxidant capacity may contribute in ameliorating the oxidative stress effects and damage produced by mercury.
Our results were similar to those of Hussain et al[47], who found that the nervous system is affected by mercuric chloride toxicity. In our study, cellular and tissue changes were noticed in the positive control, including generalized and localized edema in the white mater of cerebrum and cerebellum. Edema is a clear sign of compromised blood-brain barrier to mercuric chloride intoxication. The positive control also showed congested blood vessels in both cerebrum and cerebellum which could be prior to edema. The neuronal changes could be due to oxidative stress secondary to mercuric chloride toxicity[47]. In the rats fed the high fiber fermented mare's milk containing probiotic, the histological picture improved; this could be due to antioxidative stress effect of the high fiber fermented mare's milk containing probiotic.
Oxidative stress may contribute to the development of neurodegenerative disorders caused by mercury intoxication. Oxidative stress may induce peroxidation injury in the membrane of lipids and protein as well as DNA fragmentation, which can result in disruption of nerve cell function and integrity. It is well known that brain is more sensitive tissue to oxidative damage because of its high concentration of[43] unsaturated lipids and its high rate of oxidative metabolism[47].
Conclusion
We conclude that high fiber fermented mare's milk containing probiotic had a protective effect against brain and kidney alterations produced by mercury toxicity. The mechanism is not clear and under investigation by authors.
Acknowledgments
The authors would like to acknowledge the editorial assistance of Dr. Belinda Peace.
References
- 1.Emanuelli T, Rocha JB, Pereira ME, et al. Effect of mercuric chloride intoxication and dimercaprol treatment on delta-aminolevulinate dehydratase from brain, liver and kidney of adult mice. Pharmacol Toxicol. 1996;79:136–143. doi: 10.1111/j.1600-0773.1996.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 2.Shigematsu J, Yasuda T, Goto Y, Tanaka K, Tobimatsu S, Kato M. Recovery of brain dysfunction after methylmercury exposure in rats. J Neurol Sci. 2000;182:61–68. doi: 10.1016/s0022-510x(00)00450-0. [DOI] [PubMed] [Google Scholar]
- 3.Rocha JB, Rocha LK, Emanuelli T, Pereira ME. Effect of mercuric chloride and lead acetate treatment during the second stage of rapid post-natal brain growth on the behavioral response to chlorpromazine and on delta-ALA-D activity in weaning rats. Toxicol Lett. 2001;125:143–150. doi: 10.1016/s0378-4274(01)00435-0. [DOI] [PubMed] [Google Scholar]
- 4.Peixoto NC, Pereira ME. Effectiveness of ZnCl2 in protecting against nephrotoxicity induced by HgCl2 in newborn rats. Ecotoxicol Environ Saf. 2007;66:441–446. doi: 10.1016/j.ecoenv.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Magos L, Webb M, Butler WH. The effect of cadmium pretreatment on the nephrotoxic action and kidney uptake of mercury in male and female rats. Br J Exp Pathol. 1974;55:589–594. [PMC free article] [PubMed] [Google Scholar]
- 6.Peixoto NC, Roza T, Morsch VM, Pereira ME. Behavioral alterations induced by HgCl2 depend on the postnatal period of exposure. Int J Dev Neurosci. 2007;25:39–46. doi: 10.1016/j.ijdevneu.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Geier MR, Geier DA. A case-series of adverse events, positive re-challenge of symptoms, and events in identical twins following hepatitis B vaccination: analysis of the Vaccine Adverse Event Reporting System (VAERS) database and literature review. Clin Exp Rheumatol. 2004;22:749–755. [PubMed] [Google Scholar]
- 8.George TS. Minamata: Pollution and the Struggle for Democracy in Postwar Japan. Cambridge, MA USA: Harvard University Press; 2001. [Google Scholar]
- 9.Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- 10.Lindh U, Hudecek R, Danersund A, Eriksson S, Lindvall A. Removal of dental amalgam and other metal alloys supported by antioxidant therapy alleviates symptoms and improves quality of life in patients with amalgam-associated ill health. Neuro Endocrinol Lett. 2002;23:459–482. [PubMed] [Google Scholar]
- 11.Renzoni A, Zino F, Franchi E. Mercury levels along the food chain and risk for exposed populations. Environ Res. 1998;77:68–72. doi: 10.1006/enrs.1998.3832. [DOI] [PubMed] [Google Scholar]
- 12.Drexler H, Schaller KH. The mercury concentration in breast milk resulting from amalgam fillings and dietary habits. Environ Res. 1998;77:124–129. doi: 10.1006/enrs.1997.3813. [DOI] [PubMed] [Google Scholar]
- 13.Leistevuo J, Leistevuo T, Helenius H, et al. Dental amalgam fillings and the amount of organic mercury in human saliva. Caries Res. 2001;35:163–166. doi: 10.1159/000047450. [DOI] [PubMed] [Google Scholar]
- 14.International Programme on Chemical Safety. Inorganic mercury. Environmental health criteria. Geneva: World Health Organization; 1991. World Health Organization; p. 168. [Google Scholar]
- 15.Rocha M, Kruger A, Van Rooijen N, Schirrmacher V, Umansky V. Liver endothelial cells participate in T-cell-dependent host resistance to lymphoma metastasis by production of nitric oxide in vivo. Int J Cancer. 1995;63:405–411. doi: 10.1002/ijc.2910630318. [DOI] [PubMed] [Google Scholar]
- 16.Roza T, Peixoto NC, Welter A, Flores EM, Pereira ME. 2,3-Dimercapto-1-propanol does not alter the porphobilinogen synthase inhibition but decreases the mercury content in liver and kidney of suckling rats exposed to HgCl2. Basic Clin Pharmacol Toxicol. 2005;96:302–308. doi: 10.1111/j.1742-7843.2005.pto960405.x. [DOI] [PubMed] [Google Scholar]
- 17.Peixoto NC, Roza T, Flores EM, Pereira ME. Effects of zinc and cadmium on HgCl2-delta-ALA-D inhibition and Hg levels in tissues of suckling rats. Toxicol Lett. 2003;146:17–25. doi: 10.1016/j.toxlet.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Bapu C, Purohit RC, Sood PP. Fluctuation of trace elements during methylmercury toxication and chelation therapy. Hum Exp Toxicol. 1994;13:815–823. doi: 10.1177/096032719401301201. [DOI] [PubMed] [Google Scholar]
- 19.Ørskov ER. A traveller's view of Outer Mongolia. Outlook on Agriculture. 1995;24:127–129. [Google Scholar]
- 20.Litopoulou-Tzanetaki E, Tzanetakis N. In: Fermented milks, in Encyclopedia of food microbiology. Robinson RK, editor. London, UK: Academic Press; 1999. pp. 774–784. [Google Scholar]
- 21.Tamime AY, Muir DD, Wszolek M. Kefir, koumiss and kishk. Dairy Industries International. 1999;64:32–33. [Google Scholar]
- 22.Thompson WG. Practical dietetics: with special reference to diet in disease. New York, NY USA: D. Appleton and Company; 1905. [Google Scholar]
- 23.Solaroli G, Pagliarini E, Peri C. Compositional and nutritional quality of mare's milk. Italian Journal of Food Science. 1993;5:3–10. [Google Scholar]
- 24.Pool-Zobel BL. Inulin-type fructans and reduction in colon cancer risk: review of experimental and human data. Br J Nutr. 2005;93(Suppl 1):S73–90. doi: 10.1079/bjn20041349. [DOI] [PubMed] [Google Scholar]
- 25.Flamm G, Glinsmann W, Kritchevsky D, Prosky L, Roberfroid M. Inulin and oligofructose as dietary fiber: a review of the evidence. Crit Rev Food Sci Nutr. 2001;41:353–362. doi: 10.1080/20014091091841. [DOI] [PubMed] [Google Scholar]
- 26.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 27.Tamine AY, Robinson RK. Yoghurt: Science and Technology. Cambridge, UK: Woodhead Publishing; 1999. [Google Scholar]
- 28.Tamime AY. Fermented milks: a historical food with modern applications--a review. Eur J Clin Nutr. 2002;56(Suppl 4):S2–S15. doi: 10.1038/sj.ejcn.1601657. [DOI] [PubMed] [Google Scholar]
- 29.Al-Wabel N, Mousa HM, Omer OH, Abdel-Salam AM. Biological evaluation of synbiotic fermented milk containing, honey, garlic, gensin, cod liver oil and chicory inulin against lead acetatecontamination in rat. J Food Agric Environ. 2007:169–172. [Google Scholar]
- 30.Abdel-Salam AM, Ammar AS, Gala WK. Evaluation and properties of formulated low calories functional yoghurt cake. J Food Agric Environ. 2009;7:218–221. [Google Scholar]
- 31.N.A.S.A., Space Food and Nutrition An Educator's Guide, in Johnson Space Center Laboratory NB Editor. 1999. http://spacelink.nasa.gov/products: Houston, TX USA .
- 32.Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Lebensmittel Wissenschaft Und Technologie. 1995;28:25–30. [Google Scholar]
- 33.Tietz NW, editor. Fundamentals of Clinical Chemistry. Philadelphia, PA USA: W. B. Saunders Co; 1970. [Google Scholar]
- 34.Bonnes RW, Taussky HH. On the calorimetric determination of creatinine by the Jaffe reaction. J Biol Chem. 1945;158:1585–1591. [Google Scholar]
- 35.Stein EA, Myers GL. National Cholesterol Education Program recommendations for triglyceride measurement: executive summary.The National Cholesterol Education Program Working Group on Lipoprotein Measurement. Clin Chem. 1995;41:1421–1426. [PubMed] [Google Scholar]
- 36.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases.The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 37.Bergmeyer HU, Bernt E, Bergmeyer HU, editors. Lactate dehydrogenase. Methods of Enzymatic Analysis. Vol. 2. Verlag Chemie: New York, NY; 1974. [Google Scholar]
- 38.Humason GL. Tissue Techniques. San Francisco, CA USA: W. N. Freeman and Company; 1979. [Google Scholar]
- 39.Miller JC, Miller JN. New York, NY USA: Ellis Horweed; 1992. Statistics for Analytical Chemistry. [Google Scholar]
- 40.Abdel-Salam AM. Functional Foods: Hopefulness to Good Health. American Journal of Food Technology. 2010;5:86–99. [Google Scholar]
- 41.Rana SV, Rekha S, Seema V. Protective effects of few antioxidants on liver function in rats treated with cadmium and mercury. Indian Journal of Experimental Biology. 1996;34:177–179. [PubMed] [Google Scholar]
- 42.Harper HA, Rodwell VW, Mayes PA. Review of Physiological Chemistry. Los Altos, CA USA: Lange Medical Publications; 1979. [Google Scholar]
- 43.Ali BH, Al-Wabel N, Mahmoud O, Mousa HM, Hashad M. Curcumin has a palliative action on gentamicin-induced nephrotoxicity in rats. Fundam Clin Pharmacol. 2005;19:473–477. doi: 10.1111/j.1472-8206.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- 44.El-Missiry MA, Shalaby F. Role of beta-carotene in ameliorating the cadmium-induced oxidative stress in rat brain and testis. J Biochem Mol Toxicol. 2000;14:238–243. doi: 10.1002/1099-0461(2000)14:5<238::AID-JBT2>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 45.El-Demerdash FM, Yousef MI, Elagamy EI. Influence of paraquat, glyphosate, and cadmium on the activity of some serum enzymes and protein electrophoretic behavior (in vitro) J Environ Sci Health B. 2001;36:29–42. doi: 10.1081/pfc-100000914. [DOI] [PubMed] [Google Scholar]
- 46.Regoli F, Principato G. Glutathione, glutathione-dependent and antioxidant enzymes in mussel, Mytilus galloprovincialis, exposed to metals under field and laboratory conditions: implications for the use of biochemical biomarker. Aquatic Toxicology. 1995;31:143–164. [Google Scholar]
- 47.Hussain S, Rodgers DA, Duhart HM, Ali SF. Mercuric chloride-induced reactive oxygen species and its effect on antioxidant enzymes in different regions of rat brain. J Environ Sci Health B. 1997;32:395–409. doi: 10.1080/03601239709373094. [DOI] [PubMed] [Google Scholar]


