Abstract
Acute liver failure (ALF) is a condition of acute hepatic emergency where rapid deterioration of hepatocyte function leads to hepatic encephalopathy, coagulopathy, cerebral edema (CE), infection and multi-organ dysfunction syndrome resulting in a high mortality rate. Urgent liver transplantation is the standard of care for most of these patients in Western countries. However, in India, access to liver transplantation is severely limited and, hence, the management is largely based on intensive medical care. With earlier recognition of disease, better understanding of pathophysiology and improved intensive care, ALF patients have shown a significant improvement in spontaneous survival. An evidence base for practice for supportive care is still lacking; however, intensive organ support as well as control of infection and CE are likely to be key to the successful outcome in this acute and potentially reversible condition without any sequel. A structured approach to decision making about intensive care is important in each case. Unlike in Western countries where acetamenophen is the most common cause of ALF, the role of a specific agent, such as N-acetylcysteine, is limited in India. Ammonia-lowering therapy is still in an evolving phase. The current review highlights the important medical management issues in patients with ALF in general as well as the management of major complications associated with ALF. We performed a MEDLINE search using combinations of the key words such as acute liver failure, intensive treatment of acute liver failure and fulminant hepatic failure. We reviewed the relevant publications with regard to intensive care of patients with ALF.
Keywords: Acute liver failure, intensive, treatment
Introduction
Acute liver failure (ALF) is a condition with rapid deterioration of hepatocyte function resulting in hepatic encephalopathy (HE) and/or coagulopathy in patients with previously normal liver.[1] This is a devastating syndrome, which results in death or the need for liver transplantation in over 50% of the cases.[2] Urgent liver transplantation has become standard of care for most ALF patients in Western countries where ALF survival rates have shown progressive and substantial improvement, with 1-year survival exceeding 80%.[2,3] However, in India, access to liver transplantation and other extracorporeal liver-assist devices is severely limited and, hence, the management is largely supportive. Fortunately, the spontaneous survival of ALF patients has increased over the last 20 years because of earlier disease recognition, better understanding of pathophysiology of various insults and improved intensive care management.[3,4] Indeed, ALF is an acute event and a potentially reversible condition, where survivors recover completely without any sequel. Therefore, if the individual can be supported properly throughout the acute event, recovery will follow the rapid regeneration of hepatocytes. A structured approach to decision making about intensive critical care is important for achieving a good outcome in ALF. The current review highlights the important issues in the management of patients with ALF at centers where a liver transplantation facility is not available.
We performed a MEDLINE search using combinations of key words such as acute liver failure, intensive treatment and fulminant hepatic failure. Between 1975 and 2011, the search yielded over 700 citations. In an attempt to provide a review of comprehensive medical management of patients with ALF, we selected 52 relevant publications with some important cross-references from the list of publications.
Early evaluation
Each patient should be managed in an intensive care unit. Early evaluation should include obtaining a detailed history and assessment of patient's mental status, liver size and presence or absence of ascites and stigmata of chronic liver disease. Initial laboratory investigations must be extensive in order to evaluate the etiology, disease severity and complications. It should include complete blood count, liver function tests, INR, glucose, creatinine, urea, sodium, potassium, chloride, bicarbonate, calcium, magnesium, phosphate, arterial blood gas, lactate, arterial ammonia, chest X-ray, cultures of blood and urine, bedside ultrasonography, viral markers, autoimmune markers, serum ceruloplasmin and pregnancy test (female). Subsequently, daily microbiological surveillance and frequent monitoring of neurological status, hemodynamic parameters, serum electrolytes, urea, creatinine, blood sugar, blood gas and INR should be done.
General measures in management
In general, ALF patients should be kept in a quiet environment with limited stimulus. We should avoid sudden change in position, head rotation, head flexion and heavy chest physiotherapy. Even prophylactic intravenous lidocaine has been suggested before an endotracheal suctioning.[5] With the aim of avoiding compromise of jugular venous drainage and to improve cerebrospinal fluid drainage, the head should be in a neutral position with the head end of the bed elevated to 30 degrees.[6,7] During elevation of the head of the bed, the mean arterial pressure (MAP) should be maintained to ensure a good cerebral perfusion.[8] Fever exacerbates intracranial hypertension;[9] therefore, it should be promptly controlled by cooling. It is better to avoid nonsteroidal antiinflammatory drugs and acetaminophen in ALF patients. Shivering, which may also increase intracranial pressure (ICP), should be treated with increased sedation. Furthermore, any drugs with hepatotoxic potential, such as antituberculosis drugs, should be discontinued.
Specific treatments based on the etiology of ALF
In Western countries, acetamenophen is the most common cause of ALF where a specific agent, N-acetylcysteine (NAC), has a proven benefit.[10,11] Doses for intravenous NAC administration vary according to the protocol; one suggested schedule includes 150 mg/kg IV in 250 mL of 5% dextrose in water (D5W) over 1 h, followed by 50 mg/kg in 500 mL of D5W over 4 h, 125 mg/kg in 1000 mL of D5W over 19 h and 150 mg/kg in 1000 mL of D5W over 24 h for an additional 48 h (72 h total). In nonacetaminophen ALF, benefit of NAC is small and is limited only to patients with early HE (grades I-II).[12] Therefore, an early attempt to identify an etiology may not be important in India, where ALF is mostly due to hepatotrophic viruses, the most common being hepatitis E virus.[13] Except for hepatitis B-induced ALF, where lamivudine has been suggested to cause improvement in survival compared with historical control subjects,[14] specific therapy for other viruses is largely unavailable. A small retrospective series of patients with autoimmune hepatitis presenting as ALF found no improvement in outcomes with the administration of corticosteroids.[15] For women with acute fatty liver of pregnancy or the hemolysis-elevated liver enzymes-low platelet syndrome, a prompt delivery of the fetus is required to reverse ALF.[16] However, data is insufficient to recommend any specific therapies for pregnant women with ALF due to other etiologies.
Decision on endotracheal intubation and mechanical ventilation
The usual indications for endotracheal intubation in ALF patients include respiratory failure, advanced HE (grade III or more) and severe agitation. Intubation should be done under appropriate sedation in order to prevent transient rise in ICP. There are insufficient data to recommend a standard ventilation strategy for ALF patients. In patients with acute lung injury, tidal volume and plateau pressure should be limited to 6 mL/kg predicted body weight and <30 cm H2O, respectively.[17] Because the decrements in tidal volume increase Pco2, which thereby increases ICP, the respiratory rate must be increased to maintain a stable Pco2. Also, the lowest level of positive end-expiratory pressure (PEEP) that achieves adequate oxygenation should be applied in such patients because high levels of PEEP may also increase ICP.[18] Acute hyperventilation is recommended only as emergency rescue therapy of patients with evidence of brain herniation, because hypocapnia-induced cerebral vasoconstriction decreases ICP.[19,20] However, prophylactic hyperventilation is not recommended because such vasoconstriction can also reduce cerebral oxygen utilization,[19] and had no effect on the development of cerebral edema (CE) in one study.[21]
Blood sugar and nutritional management
ALF patients are prone to hypoglycemia; therefore, monitoring for blood glucose should be performed at frequent intervals (e.g., every 1-2 h) and intravenous glucose infusion (1.5–2.0 g/kg/day) should be used in patients who develop hypoglycemia. Marked hyperglycemia should be avoided, as this may impair control of ICP.[22] ALF patients are catabolic and, hence, adequate nutritional supplementation is needed.[23,24] No study exists to guide nutritional therapy in ALF patients.[25,26] Enteral nutrition should be preferred whenever possible. The feeds should be of dense caloric value to avoid hypoosmolality, which may exacerbate CE. Lipid emulsions appear to be safe in ALFpatients.[26] Caloric goals for patients who have ALF should be approximately 25–30 kcal/kg/day. Protein intake of approximately 1 g/kg/day does not appear to worsen hyperammonemia. Excess glutamine should be avoided given the role of glutamine in the production of ammonia.
Management of fluids, electrolytes and renal failure
Volume resuscitation is an important aspect of initial management in ALF patients. It is important to establish whether the ALF patient is euvolemic. At presentation, most ALF patients have varying degrees of intravascular volume depletion, which may lead to renal insufficiency. Therefore, an intravenous fluid challenge (crystalloid and colloid; 1–1.5 L) should be given to exclude prerenal azotemia. A large volume of glucose-containing solutions should be avoided. The volume resuscitation is best done judiciously to prevent problems associated with excess fluid administration, such as increased brain edema, herniation and death. It should be noted that measurement of central venous pressure only poorly reflects intravascular volume. Electrolyte imbalance of all types can accompany ALF, especially when complicated by renal dysfunction, and may be particularly deleterious. Hyponatremia should be strictly avoided, because it may exacerbate CE.[27] Other electrolyte concentrations (magnesium, phosphate, bicarbonate) should be kept within the normal range.
There are insufficient data to recommend when to start or discontinue renal replacement therapy (RRT) in patients with ALF. In patients with worsening renal function or acidosis, RRT should be started to avoid volume overload and to control acid-base-electrolyte balance. Patients with ALF poorly tolerate intermittent hemodialysis because of hemodynamic instability, fluid shifts and risk of increase in ICP.[28] Therefore, we should prefer continuous RRT[29] even in hemodynamically stable patients.[30]
Decision on vasopressor therapy
There are considerable alterations in systemic hemodynamic parameters in ALF patients. Early hemodynamic changes include increased portal pressure, splanchnic sequestration of blood and decreased central venous return.[31] This hemodynamic picture in ALF closely mimics septic shock, and is marked by a state of high cardiac output with decreased systemic vascular resistance.[32] The assessment and correction of volume status is important in the management of hypotension in ALF patients. Vasopressors are recommended for systemic hypotension to maintain a MAP >60 mmHg to ensure an acceptable cerebral perfusion. Norepinephrine is preferred over dopamine because it provides a more consistent and predictable increase in cerebral perfusion than dopamine.[33] Epinephrine should not be used because of its propensity to decrease mesenteric blood flow, which may compromise hepatic blood flow in patients with ALF.[34] Vasopressin is also avoided because it causes cerebral vasodilation.[35] A trial of hydrocortisone may be considered in patients with persistent hypotension despite a volume challenge and norepinephrine. More recently, however, in another small study, terlipressin appeared to increase cerebral perfusion in ALF patients.[36]
Antibiotic prophylaxis and treatment
Infection, which may be subtle in clinical presentation, remains one of the major causes of mortality in ALF patients.[37] Prophylactic antibiotics have not been shown to improve outcome in such patients, although these studies may not be adequately powered to definitively preclude the benefit.[38] Antibiotics should strongly be recommended in the presence of positive surveillance cultures, worsening encephalopathy, hemodynamic instability or systemic inflammatory response syndrome.[37,39,40] Careful daily surveillance for infection is needed in all patients, and the threshold for treatment should be low. The common sites of infection are the lung, urinary tract and blood.[37–40] The most frequently identified organisms in Indian series of ALF patients are gram negative bacilli, followed by gram positive bacterial and fungal infections.[41] The selection of empirical antibiotics must take into account the need to cover a broad spectrum of microorganisms. In general, third-generation cephalosporin drugs are recommended. When line infection is suspected, vancomycin should be started pending results of cultures. An antifungal agent should be added any patient without prompt improvement in signs of infection after institution of antibiotics.
Management of CE
The management of CE remains a difficult task to date. CE is one of the major causes of morbidity and mortality in ALF patients.[42] When invasive monitoring is available, the ICP should be maintained below 25 mmHg. A sustained ICP >40 mmHg or cerebral perfusion pressure (CPP) <40 mmHg for over 2 h is associated with brainstem herniation.[43] Antiedema therapies should be considered when there is a clinical evidence of CE or when ICP exceeds 25 mmHg. A vasopressor may be required to elevate MAP to ensure CPP between >50 and 60 mmHg.[44] Although invasive ICP monitoring remains the only objective gold standard for measuring and monitoring ICP, the placement of an ICP monitor is still controversial because of insufficient data to recommend it, risk of bleeding complications and no survival advantage shown in a nonrandomized study.[45–47] Despite lack of consensus, many centers place ICP monitors in patients with advanced encephalopathy, wherein the ICP monitor also provides prognostic information regarding neurologic recovery after liver transplantation.
Administration of intravenous mannitol is the recommended first-line therapy for CE. Mannitol should be administered as repeated 0.25–1.0 g/kg intravenous boluses, provided serial serum osmolality remains <320 mOsm/L.[48,49] The lower doses of mannitol (0.25–0.5 g/kg) may be as effective as the higher doses (1.0 g/kg). However, mannitol is not effective in patients with a very high ICP (>60 mmHg),[50] and it cannot be used once patients develop significant renal injury. The standard therapy of CE-refractory mannitol is not known. Hypertonic saline boluses have been found to have similar or even superior efficacy to mannitol.[51,52] Many preparations and doses have been used to treat CE, including 23.4% saline (30 mL) and 7.5% saline (2.0 mL/kg) boluses repeated every 2–3 h. Hypertonic saline can also be used as a prophylactic measure with a goal of achieving sodium of 145–155 mEq/L.[53]
Although not universally accepted, induced moderate hypothermia (32–33°C) may decrease ICP in ALF patients.[54] However, hypothermia is also associated with increased risk of arrhythmia, infection, bleeding, electrolyte imbalance, hyperglycemia and alteration in drug metabolism.[55] Pentobarbital (3–5 mg/kg intravenous loading bolus followed by 1–3 mg/kg/h intravenous infusion) may also be tried in refractory cases.[56] However, it has the potential to cause severe adverse effects, including hypotension, hypothermia, immunosuppression, hypokalemia and prolonged coma. Further, its benefit is unclear if the patient is already in stage 4 encephalopathy. Indomethacin (25 mg intravenously over 1 min) has also been shown to decrease ICP and, thus, may be considered as salvage therapy.[57] CE in ALF has not been shown to improve with steroid[58] ; therefore, steroids are not recommended. Hyperammonemia in ALF contributes to the pathogenesis of CE.[59] Currently, there are insufficient data to recommend the use of lactulose or nonabsorbable antibiotics as ammonia-lowering therapy in patients with ALF. Furthermore, lactulose may increase gaseous distention of the bowel, risk of aspiration and may, rarely, precipitate megacolon.[60] A recent large study on human ALF patients revealed that L-ornithine L-aspartate is ineffective in lowering arterial ammonia or improving survival.[61] The failure is likely because of regeneration of ammonia from glutamine and, therefore, combining phenylacetate with L-ornithine may overcome this problem because glutamine would be trapped as phenylacetylglutamine, which is excreted. The efficacy of L-ornithine and phenylacetate has been confirmed in pig ALF.[62]
Avoid pain and agitation
Pain and agitation may increase intracranial hypertension in patients with ALF.[63,64] Therefore, adequate analgesia and cautious sedation is required, especially in patients with advanced hepatic encephalopathy and before any invasive procedure. For sedation, propofol should be preferred over benzodiazepines because of shorter recovery time, which may allow early and more reliable neurologic examination.[65] In addition, propofol decreases cerebral blood flow and lowers ICP.[66] However, both these sedatives can increase γ-aminobutyric acid-ergic neurotransmission and, therefore, may exacerbate hepatic encephalopathy.[67,68] For analgesia, agents with a shorter half-life, such as fentanyl, are preferred over morphine and meperidine.
Seizure prophylaxis
Nonconvulsive seizure activity has been documented in a high proportion of patients with ALF with advanced HE.[69] However, there are insufficient data to recommend prophylactic anticonvulsants in all patients with ALF because two studies using prophylactic phenytoin have come up with conflicting results.[69,70] It should be noted that propofol or benzodiazepine used for sedation also provide potent antiseizure prophylaxis.
Management of coagulopathy
ALF patients are severely coagulopathic; however, spontaneous and clinically significant bleeding is uncommon.[71] Prophylactic fresh frozen plasma (FFP) does not reduce the risk of significant bleeding; rather, it may increase the risks of volume overload and may obscure the trend of prothrombin time as a prognostic marker.[72] Therefore, the routine correction of coagulopathy and thrombocytopenia is not required. However, such corrections are attempted in patients with clinically significant bleeding or before placement of invasive devices. The exact goal of treatment with FFP and FFP is not clear. A rough target would be to correct the INR to approximately 1.5 and platelet count to approximately 50,000/mm3. Vitamin K deficiency may contribute to the coagulopathy of ALF in a minority of the patients. Therefore, the administration of Vitamin K is recommended empirically. The cryoprecipitate is recommended in patients who have significant hypofibrinogenemia (<100 mg/dL). Antifibrinolytic agents such as aminocaproic acid should be considered in patients with diffuse mucosal and puncture wound oozing.[73] Recombinant factor VIIa (rFVIIa) administration may be considered in circumstances where FFP has failed to correct INR. Because the use of rFVIIa may increase the risk of thrombotic complications, it should not be given to patients with a history of myocardial infarction, stroke or unstable angina within 2 weeks, deep venous thrombosis, pregnancy or Budd-Chiari syndrome. In subjects with persistent coagulopathy despite FFP who have contraindications to rFVIIa, plasma exchange is effective and should be considered.[74] The incidence of upper gastrointestinal bleeding in ALF patients has been shown to be decreased by gastric acid suppression.[75] Therefore, intravenous histamine-2 blockers or proton pump inhibitors (intravenous or oral) are recommended.
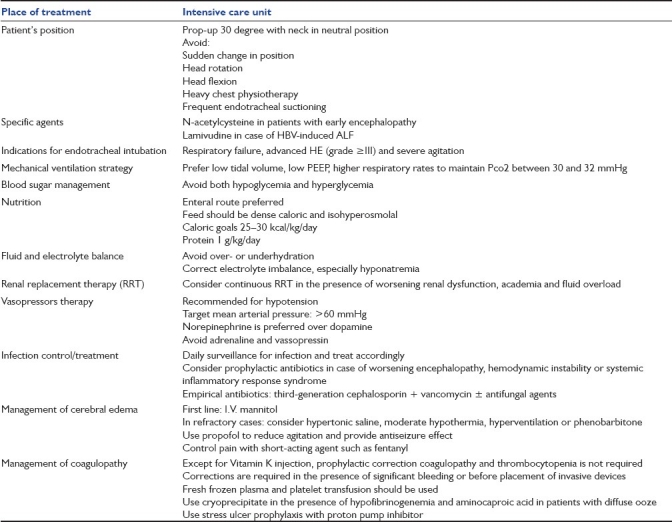
In conclusion, ALF should not be considered as a universally fatal condition without liver transplantation. With intensive medical management, the rate of spontaneous survival can be improved considerably. A summary of medical management strategies is mentioned in Table 1. This is particularly pertinent to areas where access to liver transplantation is grossly limited and where hepatitis E virus is the major cause of ALF, which has the best survival rates among ALF due to other etiologies.[13]
Table 1.
Summary of medical management strategy in patients with acute liver failure
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342:273–5. doi: 10.1016/0140-6736(93)91818-7. [DOI] [PubMed] [Google Scholar]
- 2.Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 3.Wigg AJ, Gunson BK, Mutimer DJ. Outcomes following liver transplantation for seronegative acute liver failure: experience during a 12-year period with more than 100 patients. Liver Transpl. 2005;11:27–34. doi: 10.1002/lt.20289. [DOI] [PubMed] [Google Scholar]
- 4.Hughes RD, Wendon J, Gimson AE. Acute liver failure. Gut. 1991;Suppl:S86–91. doi: 10.1136/gut.32.suppl.s86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yano M, Nishiyama H, Yokota H, Kato K, Yamamoto Y, Otsuka T. Effect of lidocaine on ICP response to endotracheal suctioning. Anesthesiology. 1986;64:651–3. doi: 10.1097/00000542-198605000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Marrero J, Martinez FJ, Hyzy R. Advances in critical care hepatology. Am J Respir Crit Care Med. 2003;168:1421–6. doi: 10.1164/rccm.200303-361UP. [DOI] [PubMed] [Google Scholar]
- 7.Ng I, Lim J, Wong HB. Effects of head posture on cerebral hemodynamics: Its influences on intracranial pressure, cerebral perfusion pressure, and cerebral oxygenation. Neurosurgery. 2004;54:593–7. doi: 10.1227/01.neu.0000108639.16783.39. discussion 598. [DOI] [PubMed] [Google Scholar]
- 8.Rosner MJ, Coley IB. Cerebral perfusion pressure, intracranial pressure, and head elevation. J Neurosurg. 1986;65:636–41. doi: 10.3171/jns.1986.65.5.0636. [DOI] [PubMed] [Google Scholar]
- 9.Muñoz SJ, Moritz MJ, Bell R, Northrup B, Martin P, Radomski J. Factors associated with severe intracranial hypertension in candidates for emergency liver transplantation. Transplantation. 1993;55:1071–4. doi: 10.1097/00007890-199305000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose.Analysis of the national multicenter study (1976 to 1985) N Engl J Med. 1988;319:1557–62. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 11.Harrison PM, Keays R, Bray GP, Alexander GJ, Williams R. Improved outcome of paracetamol-induced fulminant hepatic failure by late administration of acetylcysteine. Lancet. 1990;335:1572–3. doi: 10.1016/0140-6736(90)91388-q. [DOI] [PubMed] [Google Scholar]
- 12.Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856–64. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar R, Shalimar, Bhatia V, Khanal S, Sreenivas V, Gupta SD, et al. Antituberculosis therapy-induced acute liver failure: magnitude, profile, prognosis, and predictors of outcome. Hepatology. 2010;51:1665–74. doi: 10.1002/hep.23534. [DOI] [PubMed] [Google Scholar]
- 14.Tillmann HL, Hadem J, Leifeld L, Zachou K, Canbay A, Eisenbach C, et al. Safety and efficacy of lamivudine in patients with severe acute or fulminant hepatitis B, a multicenter experience. J Viral Hepat. 2006;13:256–63. doi: 10.1111/j.1365-2893.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 15.Ichai P, Duclos-Vallée JC, Guettier C, Hamida SB, Antonini T, Delvart V, et al. Usefulness ofcorticosteroids for the treatment of severe and fulminant forms of autoimmune hepatitis. Liver Transpl. 2007;13:996–1003. doi: 10.1002/lt.21036. [DOI] [PubMed] [Google Scholar]
- 16.Mabie WC. Acute fatty liver of pregnancy. Crit Care Clin. 1991;7:799–808. [PubMed] [Google Scholar]
- 17.The Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 18.Bonnet F, Richard C, Glaser P, Lafay M, Guesde R. Changes in hepatic flow induced by continuous positive pressure ventilation in critically ill patients. Crit Care Med. 1982;10:703–5. doi: 10.1097/00003246-198211000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Wendon JA, Harrison PM, Keays R, Williams R. Cerebral blood flow and metabolism in fulminant liver failure. Hepatology. 1994;19:1407–13. [PubMed] [Google Scholar]
- 20.Strauss GI, Møller K, Holm S, Sperling B, Knudsen GM, Larsen FS. Transcranial doppler sonography and internal jugular bulb saturation during hyperventilation in patients with fulminant hepatic failure. Liver Transpl. 2001;7:352–8. doi: 10.1053/jlts.2001.23075. [DOI] [PubMed] [Google Scholar]
- 21.Ede RJ, Gimson AE, Bihari D, Williams R. Controlled hyperventilation in the prevention of cerebral oedema in fulminant hepatic failure. J Hepatol. 1986;2:43–51. doi: 10.1016/s0168-8278(86)80007-1. [DOI] [PubMed] [Google Scholar]
- 22.Kodakat S, Gopal P, Wendon J. Hyperglycemia is associated with intracranial hypertension in patients with acute liver failure. Liver Transpl. 2001;7:C21. [Google Scholar]
- 23.Walsh TS, Wigmore SJ, Hopton P, Richardson R, Lee A. Energy expenditure in acetaminophen-induced fulminant hepatic failure. Crit Care Med. 2000;28:649–54. doi: 10.1097/00003246-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 24.O’Keefe SJ, El-Zayadi AR, Carraher TE, Davis M, Williams R. Malnutrition and immuno-incompetence in patients with liver disease. Lancet. 1980;2:615–7. doi: 10.1016/s0140-6736(80)90284-6. [DOI] [PubMed] [Google Scholar]
- 25.Munoz SJ. Nutritional therapies in liver disease. Semin Liver Dis. 1991;11:278–91. doi: 10.1055/s-2008-1040446. [DOI] [PubMed] [Google Scholar]
- 26.Schütz T, Bechstein WO, Neuhaus P, Lochs H, Plauth M. Clinical practice of nutrition in acute liver failure-a European survey. Clin Nutr. 2004;23:975–82. doi: 10.1016/j.clnu.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Rabinstein AA. Treatment of brain edema in acute liver failure. Curr Treat Options Neurol. 2010;12:129–41. doi: 10.1007/s11940-010-0062-0. [DOI] [PubMed] [Google Scholar]
- 28.Davenport A, Will EJ, Davison AM. Early changes in intracranial pressure during haemofiltration treatment in patients with grade 4 hepatic encephalopathy and acute oliguric renal failure. Nephrol Dial Transplant. 1990;5:192–8. doi: 10.1093/ndt/5.3.192. [DOI] [PubMed] [Google Scholar]
- 29.Mehta RL. Continuous renal replacement therapy in the critically ill patient. Kidney Int. 2005;67:781–95. doi: 10.1111/j.1523-1755.2005.67140.x. [DOI] [PubMed] [Google Scholar]
- 30.Davenport A, Will EJ, Davidson AM. Improved cardiovascular stability during continuous modes of renal replacement therapy in critically ill patients with acute hepatic and renal failure. Crit Care Med. 1993;21:328–38. doi: 10.1097/00003246-199303000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Pinsky MR, Matuschak GM, Bernardi L, Klain M. Hemodynamic effects of cardiac cyclespecific increases in intrathoracic pressure. J Appl Physiol. 1986;60:604–12. doi: 10.1152/jappl.1986.60.2.604. [DOI] [PubMed] [Google Scholar]
- 32.Guimond JG, Pinsky MR, Matuschak GM. Effect of synchronous increase in intrathoracic pressure on cardiac performance during acute endotoxemia. J Appl Physiol. 1990;69:1502–8. doi: 10.1152/jappl.1990.69.4.1502. [DOI] [PubMed] [Google Scholar]
- 33.Steiner LA, Johnston AJ, Czosnyka M, Chatfield DA, Salvador R, Coles JP, et al. Direct comparison of cerebrovascular effects of norepinephrine and dopamine in head-injured patients. Crit Care Med. 2004;32:1049–54. doi: 10.1097/01.ccm.0000120054.32845.a6. [DOI] [PubMed] [Google Scholar]
- 34.De Backer D, Creteur J, Silva E, Vincent JL. Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: which is best? Crit Care Med. 2003;31:1659–67. doi: 10.1097/01.CCM.0000063045.77339.B6. [DOI] [PubMed] [Google Scholar]
- 35.Shawcross DL, Davies NA, Mookerjee RP, Hayes PC, Williams R, Lee A, et al. Worsening of cerebral hyperemia by the administration of terlipressin in acute liver failure with severe encephalopathy. Hepatology. 2004;39:471–5. doi: 10.1002/hep.20044. [DOI] [PubMed] [Google Scholar]
- 36.Eefsen M, Dethloff T, Frederiksen HJ, Hauerberg J, Hansen BA, Larsen FS. Comparison of terlipressin and noradrenalin on cerebral perfusion, intracranial pressure, and cerebral extracellular concentrations of lactate and pyruvate in patients with acute liver failure in need of inotropic support. J Hepatol. 2007;47:381–6. doi: 10.1016/j.jhep.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 37.Rolando N, Philpott-Howard J, Williams R. Bacterial and fungal infection in acute liver failure. Semin Liver Dis. 1996;16:389–402. doi: 10.1055/s-2007-1007252. [DOI] [PubMed] [Google Scholar]
- 38.Rolando N, Gimson A, Wade J, Philpott-Howard J, Casewell M, Williams R. Prospective controlled trial of selective parenteral and enteral antimicrobial regimen in fulminant liver failure. Hepatology. 1993;17:196–201. [PubMed] [Google Scholar]
- 39.Vaquero J, Polson J, Chung C, Helenowski I, Schiodt FV, Reisch J, et al. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology. 2003;125:755–64. doi: 10.1016/s0016-5085(03)01051-5. [DOI] [PubMed] [Google Scholar]
- 40.Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734–9. doi: 10.1053/jhep.2000.17687. [DOI] [PubMed] [Google Scholar]
- 41.Acharya SK, Panda SK, Saxena A, Gupta SD. Acute hepatic failure in India: a perspective from the East. J Gastroenterol Hepatol. 2000;15:473–9. doi: 10.1046/j.1440-1746.2000.02073.x. [DOI] [PubMed] [Google Scholar]
- 42.Ware AJ, D’Agostino AN, Combes B. Cerebral edema: A major complication of massive hepatic necrosis. Gastroenterology. 1971;61:877–84. [PubMed] [Google Scholar]
- 43.Lidofsky SD, Bass NM, Prager MC, Washington DE, Read AE, Wright TL, et al. Intracranial pressure monitoring and liver transplantation for fulminant hepatic failure. Hepatology. 1992;16:1–7. doi: 10.1002/hep.1840160102. [DOI] [PubMed] [Google Scholar]
- 44.Daas M, Plevak DJ, Wijdicks EF, Rakela J, Wiesner RH, Piepgras DG, et al. Acute liver failure: results of a 5-year clinical protocol. Liver Transpl Surg. 1995;1:210–9. doi: 10.1002/lt.500010403. [DOI] [PubMed] [Google Scholar]
- 45.Keays RT, Alexander GJ, Williams R. The safety and value of extradural intracranial pressure monitors in fulminant hepatic failure. J Hepatol. 1993;18:205–9. doi: 10.1016/s0168-8278(05)80247-8. [DOI] [PubMed] [Google Scholar]
- 46.Vaquero J, Fontana RJ, Larson AM, Bass NM, Davern TJ, Shakil AO, et al. Complications and use of intracranial pressure monitoring in patients with acute liver failure and severe encephalopathy. Liver Transpl. 2005;11:1581–9. doi: 10.1002/lt.20625. [DOI] [PubMed] [Google Scholar]
- 47.Blei AT, Olafsson S, Webster S, Levy R. Complications of intracranial pressure monitoring in fulminant hepatic failure. Lancet. 1993;341:157–8. doi: 10.1016/0140-6736(93)90016-a. [DOI] [PubMed] [Google Scholar]
- 48.Canalese J, Gimson AE, Davis C, Mellon PJ, Davis M, Williams R. Controlled trial of dexamethasone and mannitol for the cerebral oedema of fulminant hepatic failure. Gut. 1982;23:625–9. doi: 10.1136/gut.23.7.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marshall LF, SMith RW, Rauscher LA, Shapiro HM. Mannitol dose requirements in brain-injured patients. J Neurosurg. 1978;48:169–72. doi: 10.3171/jns.1978.48.2.0169. [DOI] [PubMed] [Google Scholar]
- 50.Hanid MA, Davies M, Mellon PJ, Silk DB, Strunin L, McCabe JJ, et al. Clinical monitoring of intracranial pressure in fulminant hepatic failure. Gut. 1980;21:866–9. doi: 10.1136/gut.21.10.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Battison C, Andrews PJ, Graham C, Petty T. Randomized, controlled trial on the effect of a 20% mannitol solution and a 7.5% saline/6% dextran solution on increased intracranial pressure after brain injury. Crit Care Med. 2005;33:196–202. doi: 10.1097/01.ccm.0000150269.65485.a6. discussion 257-8. [DOI] [PubMed] [Google Scholar]
- 52.Ware ML, Nemani VM, Meeker M, Lee C, Morabito DJ, Manley GT. Effects of 23.4% sodium chloride solution in reducing intracranial pressure in patients with traumatic brain injury: A preliminary study. Neurosurgery. 2005;57:727–36. discussion 727-36. [PubMed] [Google Scholar]
- 53.Murphy N, Auzinger G, Bernel W, Wendon J. The effect of hypertonic sodium chloride on intracranial pressure in patients with acute liver failure. Hepatology. 2004;39:464–70. doi: 10.1002/hep.20056. [DOI] [PubMed] [Google Scholar]
- 54.Jalan R, Olde Damink SW, Deutz NE, Hayes PC, Lee A. Moderate hypothermia in patients with acute liver failure and uncontrolled intracranial hypertension. Gastroenterology. 2004;127:1338–46. doi: 10.1053/j.gastro.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Polderman KH. Application of therapeutic hypothermia in the intensive care unit: opportunities and pitfalls of a promising treatment modality: part 2. Practical aspects and side effects. Intensive Care Med. 2004;30:757–69. doi: 10.1007/s00134-003-2151-y. [DOI] [PubMed] [Google Scholar]
- 56.Forbes A, Alexander GJ, O’Grady JG, Keays R, Gullan R, Dawling S, et al. Thiopental infusion in the treatment of intracranial hypertension complicating fulminant hepatic failure. Hepatology. 1989;10:306–10. doi: 10.1002/hep.1840100309. [DOI] [PubMed] [Google Scholar]
- 57.Tofteng F, Larsen FS. The effect of indomethacin on intracranial pressure, cerebral perfusion, and extracellular lactate and glutamate concentrations in patients with fulminant hepatic failure. J Cereb Blood Flow Metab. 2004;24:798–804. doi: 10.1097/01.WCB.0000125648.03213.1D. [DOI] [PubMed] [Google Scholar]
- 58.Report from the European Association for the Study of the Liver (EASL): Randomised trial of steroid therapy in acute liver failure. Gut. 1979;20:620–3. doi: 10.1136/gut.20.7.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blei AT. Pathophysiology of brain edema in fulminant hepatic failure, revisited. Metab Brain Dis. 2001;16:85–94. doi: 10.1023/a:1011670713730. [DOI] [PubMed] [Google Scholar]
- 60.Wright RA. Lactulose-induced megacolon. Gastrointest Endosc. 1988;34:489–90. doi: 10.1016/s0016-5107(88)71452-2. [DOI] [PubMed] [Google Scholar]
- 61.Acharya SK, Bhatia V, Sreenivas V, Khanal S, Panda SK. Efficacy of L-ornithine L aspartate in acute liver failure: a double-blind, randomized, placebo-controlled study. Gastroenterology. 2009;136:2159–68. doi: 10.1053/j.gastro.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 62.Ytrebø LM, Kristiansen RG, Maehre H, Fuskevåg OM, Kalstad T, Revhaug A, et al. L-ornithine phenylacetate attenuates increased arterial and extracellular brain ammonia and prevents intracranial hypertension in pigs with acute liver failure. Hepatology. 2009;50:165–74. doi: 10.1002/hep.22917. [DOI] [PubMed] [Google Scholar]
- 63.Muñoz SJ, Moritz MJ, Bell R, Northrup B, Martin P, Radomski J. Factors associated with severe intracranial hypertension in candidates for emergency liver transplantation. Transplantation. 1993;55:1071–4. doi: 10.1097/00007890-199305000-00025. [DOI] [PubMed] [Google Scholar]
- 64.Citerio G, Cormio M. Sedation in neurointensive care: advances in understanding and practice. Curr Opin Crit Care. 2003;9:120–6. doi: 10.1097/00075198-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–41. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 66.Wijdicks EF, Nyberg SL. Propofol to control intracranial pressure in fulminant hepatic failure. Transplant Proc. 2002;34:1220–2. doi: 10.1016/s0041-1345(02)02804-x. [DOI] [PubMed] [Google Scholar]
- 67.Basile AS, Hughes RD, Harrison PM, Murata Y, Pannell L, Jones EA, et al. Elevated brain concentrations of 1,4-benzodiazepines in fulminant hepatic failure. N Engl J Med. 1991;325:473–8. doi: 10.1056/NEJM199108153250705. [DOI] [PubMed] [Google Scholar]
- 68.Marik PE. Propofol: therapeutic indications and side-effects. Curr Pharm Des. 2004;10:3639–49. doi: 10.2174/1381612043382846. [DOI] [PubMed] [Google Scholar]
- 69.Ellis AJ, Wendon JA, Williams R. Subclinical seizure activity and prophylactic phenytoin infusion in acute liver failure: A controlled clinical trial. Hepatology. 2000;32:536–41. doi: 10.1053/jhep.2000.9775. [DOI] [PubMed] [Google Scholar]
- 70.Bhatia V, Batra Y, Acharya SK. Prophylactic phenytoin does not improve cerebral edema or survival in acute liver failure-a controlled clinical trial. J Hepatol. 2004;41:89–96. doi: 10.1016/j.jhep.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 71.Pereira SP, Langley PG, Williams R. The management of abnormalities of hemostasis in acute liver failure. Semin Liver Dis. 1996;16:403–14. doi: 10.1055/s-2007-1007253. [DOI] [PubMed] [Google Scholar]
- 72.Gazzard BG, Henderson JM, Williams R. Early changes in coagulation following a paracetamol overdose and a controlled trial of fresh frozen plasma therapy. Gut. 1975;16:617–20. doi: 10.1136/gut.16.8.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shami VM, Caldwell SH, Hespenheide EE, Arseneau KO, Bickston SJ, Macik BG. Recombinant activated factor VII for coagulopathy in fulminant hepatic failure compared with conventional therapy. Liver Transpl. 2003;9:138–43. doi: 10.1053/jlts.2003.50017. [DOI] [PubMed] [Google Scholar]
- 74.Munoz SJ, Ballas SK, Moritz MJ, Martinez J, Friedman LS, Jarrell BE, et al. Perioperative management of fulminant and subfulminant hepatic failure with therapeutic plasmapheresis. Transplant Proc. 1989;21:3535–6. [PubMed] [Google Scholar]
- 75.MacDougall BR, Williams R. H2-receptor antagonist in the prevention of acute upper gastrointestinal hemorrhage in fulminant hepatic failure: A controlled trial. Gastroenterology. 1978;74:464–5. [PubMed] [Google Scholar]


