Abstract
Background:
A pregnant woman is usually young and in good health until she suffers from some acute injury. Her prognosis will hopefully be better if she receives timely intensive care.
Materials and Methods:
The aims of this study were to study the indications of medical intensive care unit (MICU) transfers for critically ill pregnant and postpartum females, biochemical and hematological profile, organ failure, ICU interventions, outcome of mother/fetus, APACHE II score and its correlation with mortality.
Study Design and Setting:
It is a prospective observational study, carried out in the MICU of a tertiary care teaching hospital over a period of 18 months. One hundred and twenty-two pregnant and postpartum females (up to 42 days after delivery) were studied.
Results and Conclusion:
Maternal age >30 years was associated with high mortality (68.2%). Majority of the females were admitted in the third trimester (50 patients) and postpartum period (41 patients), and mortality was highest in the postpartum period (39%). Increasing parity and gravida was associated with significantly high mortality (59.5%). Acute viral hepatitis E (45 patients) was most common indication for MICU transfer, followed by malaria and pregnancy-induced hypertension. The mortality rate was 30.3%. The most common cause of death was acute viral hepatitis E (24 patients), with hepatic failure (53 patients) being the most common organ failure. Majority of the females (88 patients) were ANC registered. Low Glasgow coma score and high APACHE II score on admission were associated with significantly high mortality (85.2%). Prompt treatment with oseltamivir in H1N1 infection was associated with good maternal and fetal outcomes.
Keywords: Critical illness, hepatitis, infection, pregnancy-induced hypertension
Introduction
Critical care is a bonafide part of obstetric practice. A critically ill obstetric patient is one who, because of normal or abnormal pregnancy, delivery, and puerperium, or because of the effects of systemic disease, develops complications threatening her life for which she needs intensive monitoring, therapy or life support system. Care of the critically ill pregnant women presents a unique challenge as the assessment, monitoring and the treatment must take into account both maternal and fetal wellbeing. Because the pregnant woman is usually young and in good health until she suffers from some acute injury, her prognosis will hopefully be better if she receives timely intensive care, than that of most other patients admitted to a critical care unit. This is strengthened by a study of 58 obstetric patients showing an observed mortality rate significantly lower than the expected mortality rate calculated by APACHE II score.[1]
“Maternal deaths” are defined by the World Health Organization as the death of a woman while pregnant or within 42 days of termination of pregnancy, irrespective of the duration and the site of the pregnancy, from any cause related to or aggravated by the pregnancy or its management but not from accidental or incidental causes.[2]
Direct obstetric deaths
Those resulting from obstetric complications of the pregnant state (pregnancy, labor and puerperium), from interventions, omissions, incorrect treatment or from a chain of events resulting from any of the above.[2]
Indirect obstetric deaths
Those resulting from previous existing disease or disease that developed during pregnancy and was not due to direct obstetric causes, but which were aggravated by the physiologic effects of pregnancy.[2]
It is estimated that every minute one woman somewhere in the world dies from a complication related to pregnancy or childbirth. This is almost 600,000 women a year, worldwide. Ninety-nine percent of these deaths occur in developing countries. In India, one woman dies every 5 min from a pregnancy-related cause.[3] It is estimated that 15% of the deaths of women in reproductive age in India are maternal deaths. In the developed nations, the maternal mortality rate (MMR) is 9 per 100,000 live births as compared with 450 in developing nations.[4] India has an MMR of 450 deaths per 100,000 live births.[4] A woman's lifetime risk of dying from pregnancy-related complication or during child birth is 1 in 48 in developing countries, whereas it is 1 in 1800 in developed countries.[5]
The NFHS-3 (National Family Health Survey of India, conducted in 2005–2006) reported main reasons behind high maternal mortality in India being:
Deliveries not attended by trained personnel: NFHS-3 reports that only one-third (34%) of the deliveries in India take place in health care facilities, and two-fifth (42%) of the deliveries are unattended by a trained medical professional.
Women not seeking antenatal care: More than one out of every three women (34%) in India did not receive an antenatal check-up for births in the 3 years preceding the survey. Only 7% received antenatal check-up in the third trimester.
Postnatal care is grossly deficient.
It is found that 72% of maternal deaths can be prevented through effective antenatal care. However, as most obstetric emergencies are not predictable and 15% of all pregnant women develop life-threatening complications, antenatal care will not prevent all the maternal deaths. Thus, it is the combination of antenatal care and intensive obstetric care that is essential for reducing maternal mortality.
Basket et al. in 1998 studied the obstetric patients requiring ICU admission over a period of 14 years. The study concluded that the main indications for maternal transfer to intensive care unit are hypertensive disorders, hemorrhage and sepsis along with the medical disorders.[6] One study conducted at Parkland Hospital showed that 1.7% of almost 22,000 women delivered required admission to ICU, and the most common conditions compelling transfer are hypertensive disorders (40%), obstetrical hemorrhage (15%) and pulmonary insufficiency (9%).[7]
In view of this, the present study makes an attempt to study these disorders threatening the lives of both the mother and the child.
Materials and Methods
It is a prospective, observational, cohort study carried out in the Medical Intensive Care Unit (MICU) of a tertiary care teaching hospital in Mumbai, and our aims were to study the indications of MICU transfers for critically ill pregnant and postpartum females, their biochemical and hematological profile, organ failure, ICU interventions, outcome of mother/fetus, Glasgow coma scale (GCS) and APACHE II score on admission and its correlation with mortality, and excluded those with positive HIV status.
They were assessed, investigated and treated as per the existing practices without disturbing their routine protocol. The institute's ethics committee approval was taken for this study. After valid written informed consent, the following data was recorded: name, age, sex, address, pathological condition for admission in ICU, diagnosis, general and clinical examination and vital parameters monitored 6 hourly. Investigations, namely complete blood count, liver function tests, renal function tests and serum electrolytes (i.e., sodium, potassium, calcium, phosphates, magnesium, random blood sugar, arterial blood gas, erythrocyte sedimentation rate, blood culture [±]) were performed. Other factors like APACHE II score on admission, hypotension on admission (±), need for ventilatory support (±), ionotropic support (±), cardiac failure ±), hepatic failure (±), renal failure (±) and duration of MICU stay till either the patient was discharged from MICU or expired were also noted.
All the patients (pts) were given routine MICU care appropriate for the disease condition. Appropriate antibiotics were given according to the prevalent sensitivity patterns and changed as per the culture sensitivity pattern. Interventions like central venous line insertion in hypotension, cardiac failure etc., endotracheal intubation, ventilatory support in respiratory distress or failure were performed. Supportive therapy was given to these patients as follows: nutrition was maintained with IV fluids and Ryle's tube feeding in drowsy or unconscious patients and oral feeding in conscious patients, severe anemia was treated with packed cell transfusion and DIC with fresh frozen plasma (FFP) and blood ± platelets, renal failure with hemodialysis or conservative management, hypoglycemia with dextrose infusions, metabolic acidosis with sodium bicarbonate administration or dialysis. All the treatment was at the discretion of the ICU physicians. The treatment strategy was individualized for each patient and was the sole prerogative of the treating physician.
Study design and setting
It is a prospective observational study. This study was carried out in the MICU of a tertiary care teaching hospital. One hundred and twenty-two consecutive pregnant and postpartum females (up to 42 days after delivery) transferred to the ICU were studied. This study was carried out for a period of 18 months (Mar 2009–Aug 2010).
Statistical analysis
Outcome of each patient was classified as either discharged or expired. Data thus obtained was tabulated and statistically analyzed using the Pearson Chi-square test, continuity correction and Fisher's exact test with the help of SPSS version 18.
Results
In this prospective study, 122 consecutive pregnant and postpartum females (up to 42 days after delivery) transferred to the ICU were studied. Results are noted in Tables 1, 2 and 3 and [Figure 1].
Table 1.
General characteristics of the study population based on parameters
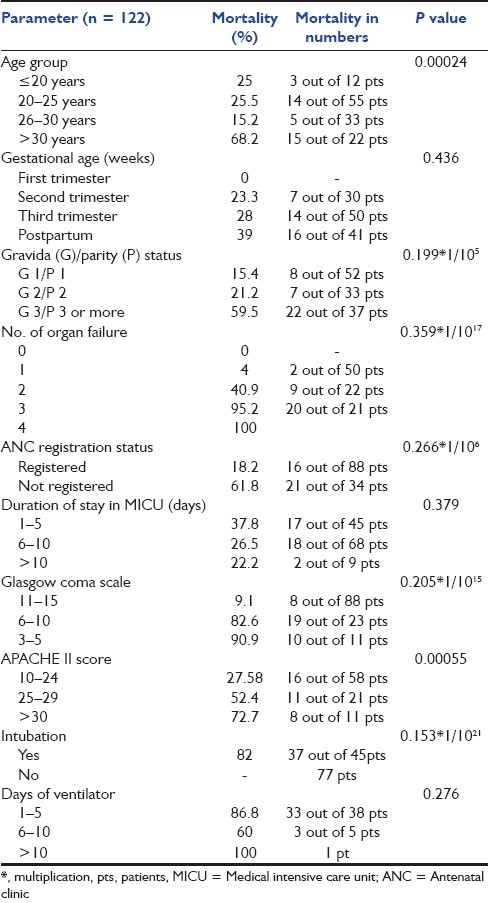
Table 2.
Cause of death in the study population
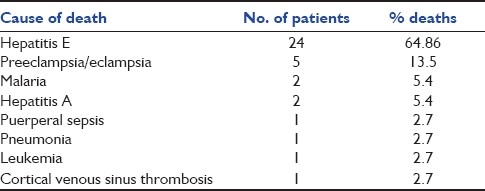
Table 3.
Biochemistry of the study population
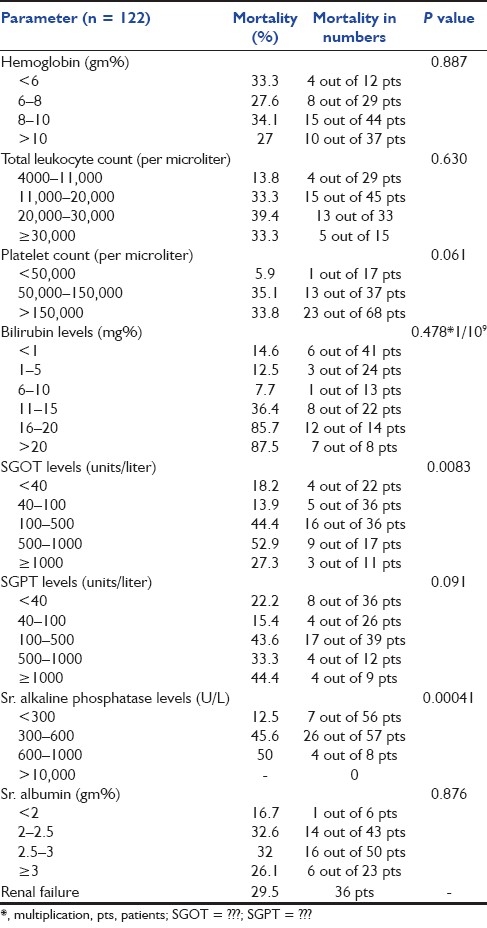
Figure 1.
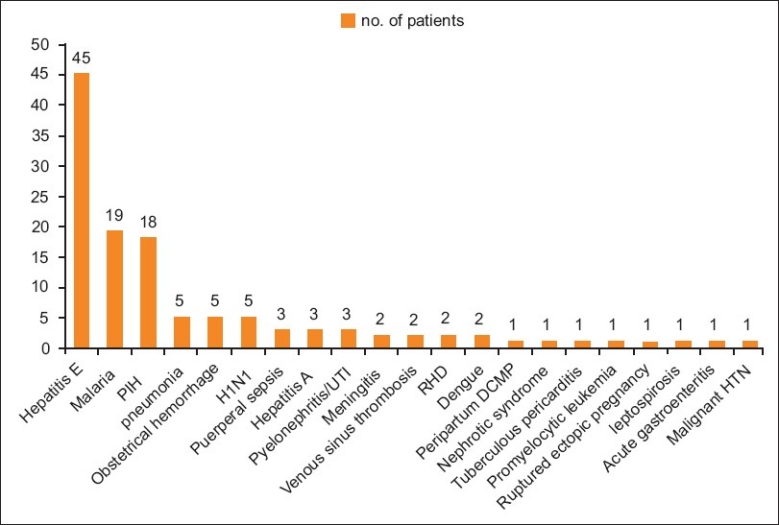
Indications for medical intensive care unit care in pregnant and postpartum females
Discussion
The total number of obstetric admissions in the critical care unit is a vital indicator to incidence of life-threatening complications in the pregnant mother. The data from various studies shows variable results of MICU admission statistics. Obstetric admission rate to MICU was 0.9% and 3.4% in a study done by Mabie et al., and Lewinsohn et al., respectively, whereas our study showed an admission rate of 4.0%.[1,8]
Age is one of the important prognostic factors. In our study, 100 patients were between the ages of 21 and 30 years (82.7%), and 22 (17.3%) patients were more than 30 years of age. The mean distribution of age in studies done by Patkar et al. was 56% for the age group of 21–30 years and 14.7% for those above 30 years, and that done by Rochat et al. was 72% for the age group of 21–30 years.[9,10] These studies thus show that majority of the obstetric patients requiring critical care are in the age group of 21–s30 years, which is comparable to our study. Rochat et al. further showed the maternal mortality of almost 50% in the age group of 21–30 years, and 40.14% in those above 30 years.[10] The present study showed a maternal mortality of 22% in the age group of 21–30 years, whereas studies done by Bhattacharya et al. and Patel et al. showed the mortality ranging from 1.7 to 11.9% in the above age group.[11,12] The mortality rate was significantly high (68%, 15 pts out of 22) in females more than 30 years of age. Thus, the above results indicate that youth (age >20 years) confers protection in critical illness in pregnancy and the adverse outcome of critically ill obstetric patients with advancing age.
In the current study, maximum number of admissions (42.6%) were either primipara or primigravida, but this group showed the least morality of 15.4%, whereas females with three or more pregnancies showed a steep rise in mortality to as high as 59.5%. Mantel et al. and Bhattacharya et al. showed highest maternal mortality in women with three or more parity. Thus, the results in these studies are consistent with our observations of highest mortality in multipara.[11,13] Hence, as the number of pregnancy increases, more are the chances of mortality.
In the present study, out of a total of 122 pts, 81 pts (66.39%) were antepartum and 41 pts (33.6%) were postpartum; mortality rate in antepartum females was 25.9% and in postpartum females mortality was 39%, which was higher as compared with antepartum females. Karnad et al. also reported the mortality rate of 21.6% in both ante- and postpartum groups.[14] In our study, maximum number of obstetric admissions occurred during the antepartum period, with maximum admissions in the 3rd trimester (41%), followed by the postpartum period (33.6%). Only 25% admissions were in the 1st or 2nd trimester. These aspects of gestational age highlight the maximum occurrence of complications in the third trimester and postpartum period and thus the importance of close supervision of patients during these periods. Distribution of gestational age (antepartum, postpartum) in various studies done by Karnad et al. (46.6%, 53.3%), Basket et al. (18%. 82%) and El-solh et al. (62%, 38%) was comparable to our study.[14–16]
In our study, about 22.9% of the patients were admitted with direct causes, the most common being pregnancy-induced hypertension (PIH) (14.75%), obstetrical hemorrhage (4%) and puerperal sepsis (2.45%). Majority (77.1%) were admitted with indirect causes, the most common being acute viral hepatitis E (36.8%), malaria (15.57%) and pneumonia (4%), followed by meningitis, rheumatic heart disease and cortical venous sinus thrombosis. Baskett et al., Mahutte et al., Bekele et al., El-solh et al. and Karnad et al. have shown that PIH, obstetrical hemorrhages and medical disorders of pregnancy were the most common indications requiring maternal transfer to MICU.[14–18] In another large study at the Parkland Hospital ICU, 40% admissions were due to hypertensive disorders, 15% due to obstetric hemorrhage and 40% due to medical disorders aggravated in pregnancy.[19] However, findings in our study are not comparable to above studies. This is because majority of the patients in the present study were suffering from acute viral hepatitis E (37.5%) followed by malaria (16%). Another reason was the high incidence of hepatitis E and malaria in the general population during the period (monsoon) our study took place. Maternal mortality rate in the current study was 30.3%. Direct maternal mortality was 16%. Indirect maternal mortality was 84%. The most common direct cause of mortality was PIH. Among the common indirect causes were hepatitis E, malaria and hepatitis A. Maternal mortality rate seen in the present study is slightly higher than the mortality reported in another study by Bekele et al.[15] However, Lewinsohn et al., Tang et al., Lapinsky et al. and Sriram et al. reported mortality rates of critically ill obstetric patients admitted to the MICU in the range from 0 to 36% thus coinciding with mortality found in the present study.[1,20–22] Our hospital being a tertiary referral center, majority of the patients are referred in advanced stages of disease, which might explain the higher mortality seen in our patients. Direct and indirect causes of maternal mortality in studies done by Bhattacharya et al. was 83.6% and 16.4%, by Patel et al. was 63.7% and 36.3% and by Sharma et al. was 56.3% and 43.7%, respectively; the present study showed 16% as a cause of direct mortality and 84% as a cause of indirect mortality in the critical care unit.[11,12,15] Unlike the above studies, the mortality due to indirect causes was significantly higher than the direct pregnancy-related causes because of majority of deaths occurring due to fulminant hepatitis E (64.86%).
Among the direct causes of mortality, PIH was the most common, accounting for 13.5% of all deaths. Rochat et al. reported mortality as 14.8% in MICU due to preeclampsia/eclampsia, whereas Sibai et al. reported mortality as 10% and Bhagwanjee et al. reported it as 10.8%. Mortality due to PIH in our study was comparable to studies by Rochat, Sibai and Bhagwanjee.[10,23,24] Among indirect causes, fulminant hepatitis E was the most common cause of death, accounting for 64.86% of all deaths followed by malaria (5.4%). No studies done in the past have shown such a high mortality attributed to hepatitis E in critically ill obstetric females.
High mortality attributed to hepatitis E in the present study could be because of the following reasons:
High incidence of hepatitis E cases in the general population during the period (monsoon) the present study took place.
Fulminant course of the disease in pregnant females as compared with the general population.
Large number of fulminant hepatitis E cases referred to our center from the infectious unit of our hospital.
Late referral of such cases to our institute.
Rochat et al., Bhattacharya et al., Patel et al. and Baskett et al. reported a mortality of 27–30% with sepsis and 18–20% with the obstetric hemorrhages.[10–12,15] In the present study, the common causes of sepsis were lower respiratory tract infections, puerperal sepsis and illegal abortion. We found 27% mortality in critically ill obstetric patients due to sepsis, which coincided with the above studies. There was no mortality due to obstetric hemorrhage as against high mortality reported by Rochat et al., Bhattacharya and Bewley et al.[10,15,25]
Global epidemic of H1N1 swine flu during the year 2009–2010 aroused a heightened interest of this infection in the obstetric population due complicated course of this illness in pregnant and postpartum females. Pregnancy increases the risk of complications and hospitalization in H1N1-infected females. The study done by Louie et al. recommended that H1N1 influenza can cause severe illness and death in pregnant and postpartum women, and that regardless of the results of rapid antigen testing, prompt evaluation and antiviral treatment of influenza-like illness should be considered in such women.[26] Miller et al. in their study of 18 pregnant patients with H1N1 infection showed that admitted pregnant patients with H1N1 are at risk for obstetrical complications including fetal distress, premature delivery, emergency cesarean delivery and fetal death.[27] Hence, early antiviral treatment may improve maternal outcomes.
While Oseltamivir and Zanamivir are “Pregnancy Category C” medications, meaning no clinical studies have been conducted to assess the safety of these medications for pregnant women, available data from the Centre for Disease Control, USA, suggest that pregnant women with suspected or confirmed influenza should receive prompt antiviral therapy, and pregnancy should not be considered a contraindication to treatment with Oseltamivir or Zanamivir.[28] Oseltamivir is preferred for treatment of pregnant women because of its systemic activity. Anecdotal reports suggest postpartum women, similar to pregnant women, might be at increased risk for severe complications and death from H1N1 influenza.[28] The transition to normal immune, cardiac and respiratory function occurs quickly, but not immediately after delivery. Therefore, the increased risk associated with pregnancy should be considered to extend for 2 weeks postpartum regardless of the outcome of the pregnancy (including live birth, premature birth, termination of pregnancy, miscarriage, fetal death). Prompt empiric antiviral treatment is indicated for suspected or confirmed H1N1 influenza in women who are up to 2 weeks postpartum regardless of how the pregnancy ended.[28] In the current study, there were five cases of H1N1 infection. Of these, four were pregnant and one female was postpartum. Three females had lower respiratory tract involvement, of which two had severe pneumonia and one required ventilatory support. All females received prompt treatment with oseltamivir 75 mg bid for 5 days from day 1 of admission. All females survived. Pregnant females who received oseltamivir had full-term normal delivery later and babies born who were followed-up for a period of 6 months after birth did not show any significant abnormalities. Thus, in our study, we found that H1N1 infection in pregnancy and in the postpartum period is associated with severe illness, and that prompt empirical treatment in suspected or proven cases of H1N1 infection can significantly improve outcome in the obstetric population.
The outcome of the patient in MICU is also correlated with the number of organ involvement at the time of admission. We found that 41% of the patients had single organ involvement and 40.16% suffered multiorgan involvement. Among the latter, 12% of the patients had four or more organs involved. Karnad et al. demonstrated in their study that 30% of the patients had single organ failure and 33% had multiorgan failure.[14] Bekele et al. demonstrated that failure of one or more organs was present in 65% of the total critically ill obstetric patients.[18] Thus, we had a large number of patients who were admitted with multiorgan failure, which might explain the slightly higher mortality seen in the present study. The most common organ failure seen in our patients was hepatic failure (43.4%), as against the respiratory failure and neurologic failure seen in studies done by Patel et al. and Munnur et al.[12,29] We found that in patients of hepatic failure, high levels of bilirubin, SGOT and alkaline phosphatase were associated with significantly high mortality. As many as 44 patients (36%) had bilirubin levels of more than 10 mg/dL. Twenty-two patients had bilirubin levels of more than 15, and mortality in this subgroup was very high (86%), with only three patients surviving. Thus, bilirubin levels of more than 15 were associated with significantly high mortality in our study. Cardiovascular failure (31.9%) was the second most common organ system failure, followed by renal failure (29.5%). PIH and sepsis were the most common causes of renal failure in the present study (22.2% each), which matches with the study done by Mandal et al., in which sepsis was the most common cause of renal failure followed by PPH and PIH[30] [Table 4]. A total of 14 out of 36 patients of renal failure received dialysis. Among the 14 patients who received dialysis, 10 survived while four died.
Table 4.
Causes of renal failure in the study population
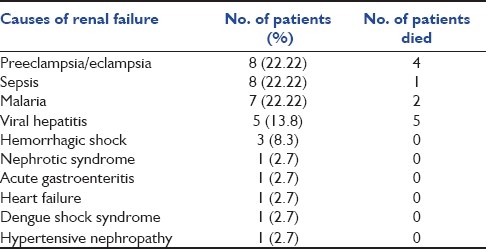
Neurological failure was another organ failure associated with very high mortality (92.8%). Glasgow coma score (GCS) of all patients at the time of admission was documented. It was seen that patients with GCS of ≤ to 10 at the time of admission had significantly high mortality (85.3%) as compared with patients with GCS of more than 10 (9.1%). Most of the patients in our study who had hepatic failure due to hepatitis E were in hepatic encephalopathy at the time of admission, with poor GCS accounting for very high mortality in this subgroup of patients. It was obvious that as the number of failing organs increased, the mortality also increased accordingly. The mortality was 4% in patients with single organ failure as against 100% in four or more organ failures.
Majority of the patients in the current study (63.1%) stayed for more than 5 days in the MICU. Most of them (55.7%) stayed for 6-10 days. Only 7.4% stayed for more than 10 days, while only one patient had a stay of less than 24 h. In the study by Basket et al. in 1998, the average duration of critical care required in majority of the patients (72%) was around 8–10 days, which is comparable to our study.[15] It was seen in our study that mortality rate was high in the first few days of admission (37.8%), whereas patients referred or transferred very late from other hospitals in moribund condition had poor prognosis. This highlights the fact that more number of patients can be made to survive if patients are referred earlier. Similarly, the patients should be provided with initial primary resuscitation at the earliest before transfer.
We tried to study the association of hemoglobin (Hb) levels at the time of admission with mortality. Although majority of the women (70%) were anemic at the time of admission, with 33.6% severely anemic, there was no significant increase in mortality in anemic females as compared with females who had Hb >10 gm/dL. This could be attributed to the fact that majority of the females died due to hepatic failure, due to hepatitis E, in which case Hb levels could not have been contributory in causing death and also to the fact that all anemic females received prompt and adequate transfusions whenever necessary. As noted in the results, a total of 31 patients (25.4%) required blood transfusion, of which seven required four or more transfusions. Patients were transfused with either whole blood or packed red cells depending on the need. In view of the high mortality associated with anaemia, as studied by Munnur et al. and Karnad et al., prevention and prompt treatment would help in cutting short the stay and mortality of patients in the critical care unit.[14,29] In addition to blood, about 66 patients (54.1%) required transfusion of FFP. Of this, 37 patients required more than four FFP. Also, nearly 14% of the patients required platelet transfusions. Approximately 70% of the total patients required either blood and/or component therapy in our study. Basket et al. in 1998 documented that 53% of the patients received blood transfusions, 26% received packed cells, 28% required at least eight units of FFP, 20% required 14 units of platelets and 8% were given cryoprecipitate.[15] Thus, immediate and ample availability of blood and blood components in a critical care unit help in reducing the mortality of obstetrical emergencies.
In the present study, we found that majority of the patients (72%) were ANC (antenatal clinic) registered, and this conferred significant protection in critical illness in pregnancy. Unregistered females had significantly high mortality (61.8%) as compared with registered females (18.6%). This fact was ascertained by a study done by Karnad et al., in which lack of prenatal care and delay in ICU referral were proved to adversely affect the outcome in critically ill pregnant females.[14]
The need for mechanical ventilation and various invasive procedures was also evaluated in this study; 44 patients (36%) of the total number of patients required support of artificial mechanical ventilation. Acute respiratory distress syndrome (ARDS), cardiogenic pulmonary edema, neurological involvement and circulatory shock were among the major causes of respiratory failure requiring artificial ventilatory support. Bekele et al. showed that 45% of the critically ill obstetric patients required mechanical ventilation; in their study, the most common organ failure was respiratory failure, and 15% of the total patients developed ARDS.[18] Tang et al. and Lapinsky et al. in their studies showed that mechanical ventilation was required in 12-55% of the obstetric patients admitted to an ICU.[20,21]
Critically ill obstetric patients were also evaluated for their need of invasive procedures. The commonly performed procedures like central venous lines, intubations, tracheostomies and hemodialysis were accounted for in the present study. These procedures done for different diagnostic and/or therapeutic purposes were infact suggestive of the severity of illness. More the procedures performed, more it suggested the severity of the illness. It is seen that almost every patient required one or more procedure during the MICU stay. The most common procedure performed was insertion of central venous lines in almost 82.8% of the patients. It was done especially in cases of circulatory shock, acute renal failure and pulmonary edema for fluid management purposes. Intubations were performed in 45 patients (36.9%) mostly for ventilatory support and also for prophylactic purposes to secure the airway. Only one tracheostomy was performed in a patient who was on ventilator for 20 days.
One of the aims of the present study was to calculate the APACHE II score of all obstetric patients on admission and to determine whether it can used as a tool to predict mortality in critically ill obstetrics patients. Described by Knaus, the APACHE II prognostic system is one of the most widely used general outcome models.[31] In the present study, we found that as the APACHE II score increases, the mortality also increases significantly. Furthermore, we found that observed mortality in the present study was comparable to the predicted mortality, ascertaining the fact that the APACHE II scoring system is a good predictor of mortality in critically ill obstetric patients. However, findings in the current study were not comparable to some studies done by Lewinsohn et al., Baskett et al. and Karnad et al., who showed that the APACHE II score predicts well over the observed mortality in obstetric patients.[1,14,15] The overall predicted deaths in the present study were 36.66, while the observed deaths were 37, giving a standardized mortality ratio (predicted deaths/observed deaths) of 0.99 as against a ratio of 0.78 in the study by Karnad et al.[14] Thus, from our study, we conclude that the APACHE II score is a good predictor of mortality in critically ill obstetric patients.
We also studied the fetal outcomes in these patients, as noted in [Figure 2]. Of 122 patients, 65 (53.27%) females had full-term normal delivery.
Figure 2.
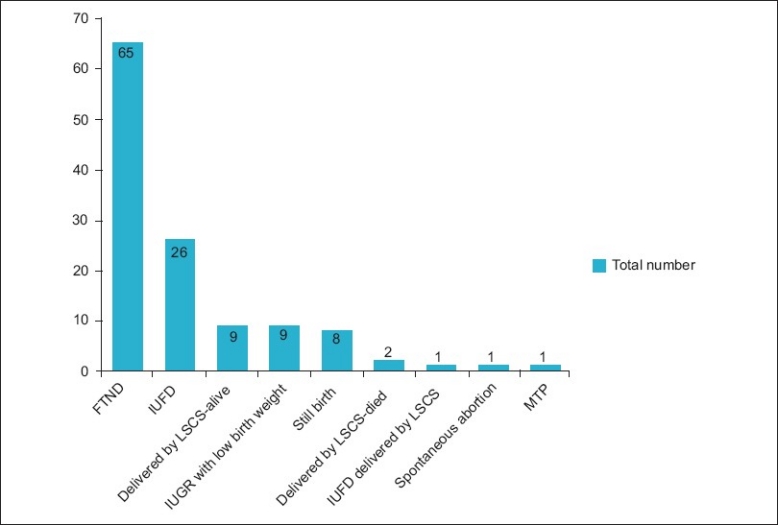
Fetal outcome in the study population
Conclusion
From the experience of the current study, we put forth the following:
Maternal age more than 30 years is associated with significantly high mortality in critically the ill obstetric population; thus, youth (age >20 years) confers an advantage for critically ill obstetric patients. Majority of the females were admitted in the third trimester and postpartum period, and mortality was highest in the postpartum period and third trimester, highlighting the importance of close supervision of critically ill obstetric females during this period. Increasing parity was associated with significantly high mortality, especially in females with gravida or parity status of 3 or more. The most common medical complications requiring critical care were infective causes like viral hepatitis, malaria, pneumonia, H1N1 and sepsis. Majority of the deaths were due to indirect causes, the most common cause of death being acute viral hepatitis E, highlighting fulminant course of this infection in pregnancy with high mortality, which can be prevented by drinking clean and boiled water. PIH was second most common cause of death and the most common direct cause of maternal mortality. Hepatic failure was the most common organ system failure seen in our study. ANC unregistered females had significantly high mortality as compared with registered females. Thus, lack of prenatal care and delay in intensive care referral adversely affect the outcome in critically ill pregnant females. Low GCS and high APACHE II on admission were associated with significantly mortality, and APACHE II score predicts mortality well in critically ill obstetric patients. H1N1 infection in pregnancy is associated with a high rate of complications, and prompt treatment with oseltamivir is associated with good maternal and fetal outcomes. Thus, medical disorders should be treated in the antenatal period itself by the appropriate specialties. Early recognition of an obstetric patient going downhill before one or multiple systems start failing is important, as is the importance of good intensive care once this does occur.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Lewinsohn G, Herman A, Leonov Y, Klinowski E. Critically ill obstetrical patients: outcome and predictability. Crit Care Med. 1994;22:1412–4. doi: 10.1097/00003246-199409000-00010. [DOI] [PubMed] [Google Scholar]
- 2.10. Vol. 2. Geneva: World Health Organization; 1992. [Last accessed on 2011 June 22]. International Statistical Classification of Diseases and Related Health Problems; pp. 98–9. Available from: http://www.who.int/classifications/icd/ICD-10_2nd_ed_volume2.pdf . [Google Scholar]
- 3.Information kit, world health day 1998, World health organization. [Last accessed on 2011 June 22]. Available from: http://www.who.ch/whday/1998/index.html .
- 4.Maternal mortality in 2005: estimates developed by WHO, UNICEF, UNFPA, and the World Bank. [Last accessed on 2011 June 22]. Available from: http://www.who.int/whosis/mme_2005.pdf .
- 5.“Maternal Mortality: A Global Review”. Women's International Network News. 1998;34(3):15–18. Available from: http://www.deathreference.com/Me-Nu/Mortality-Childbirth.html . [Google Scholar]
- 6.Baskett TF, Sternadel J. Maternal intensive care and near miss mortality in obstetrics. Br J Obstet Gynecol. 1998;105:981–4. doi: 10.1111/j.1471-0528.1998.tb10261.x. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham FG. Obstetrics complications. In: Leveno KJ, Steven BL, Hauth JC, Rouse DJ, editors. Williams Obstetrics. 23rd ed. New-York: McGraw Hill; 2011. pp. 706–841. [Google Scholar]
- 8.Mabie WC, Sibai BM. Treatment in an obstetric intensive care unit. Am J Obstet Gynecol. 1990;162:1–4. doi: 10.1016/0002-9378(90)90808-k. [DOI] [PubMed] [Google Scholar]
- 9.Dalal N, Patkar V, Karnik N. Critical care in obstetrics. Bombay Hosp J. 1999;19:127–49. [Google Scholar]
- 10.Rochat RW, Koonin LM, Atrash HK, Jewett JF. Maternal mortality in the United States: report from the Maternal Mortality Collaborative. Obstet Gynecol. 1988;72:91–7. [PubMed] [Google Scholar]
- 11.Bhattacharya S. A study on maternal mortality in Silchar medical college and hospital. J Obstet Gynecol India. 2001;51:67–70. [Google Scholar]
- 12.Patel DA, Gangopadhay S, Vaishnav SB. Maternal mortality at Karamsad-The only rural medical college in Gujarat. J Obstet Gynecol India. 2001;51:63–6. [Google Scholar]
- 13.Mantel GD, Buchmann E, Rees H, Pattinson RC. Severe acute maternal morbidity: a pilot study of definition for a near- miss. Br J Obstet Gynecol. 1998;105:985–90. doi: 10.1111/j.1471-0528.1998.tb10262.x. [DOI] [PubMed] [Google Scholar]
- 14.Karnad DR, Lapsia V, Krishnan A, Salvi VS. Prognostic factors in obstetric patients admitted to an Indian intensive care unit. Crit Care Med. 2004;32:1294–9. doi: 10.1097/01.ccm.0000128549.72276.00. [DOI] [PubMed] [Google Scholar]
- 15.Baskett TC, Sternadel J. Maternal intensive care and near-miss mortality in obstetrics. Br J Obstet Gynecol. 1998;105:981–4. doi: 10.1111/j.1471-0528.1998.tb10261.x. [DOI] [PubMed] [Google Scholar]
- 16.el-Solh AA, Grant BJ. A comparison of severity of illness scoring systems for critically ill obstetric patients. Chest. 1996;110:1299–304. doi: 10.1378/chest.110.5.1299. [DOI] [PubMed] [Google Scholar]
- 17.Mahutte NG, Murphy-Kaulbeck L, Le Q, Solomon J, Benjamin A, Boyd ME. Obstetric admissions to the intensive care unit. Obstet Gynecol. 1999;94:263–6. doi: 10.1016/s0029-7844(99)00274-4. [DOI] [PubMed] [Google Scholar]
- 18.Afessa B, Green B, Delke I, Koch K. Systemic inflammatory response syndrome, organ failure, and outcome in critically ill obstetric patients treated in an ICU. Chest. 2001;120:1271–7. doi: 10.1378/chest.120.4.1271. [DOI] [PubMed] [Google Scholar]
- 19.Pritchard JA, Cunningham FG, Pritchard SA. The parkland memorial hospital protocol for treatment of Eclampsia.Evaluation of 245 cases. Am J Obstet Gynecol. 1984;148:951–63. doi: 10.1016/0002-9378(84)90538-6. [DOI] [PubMed] [Google Scholar]
- 20.Tang LC, Kwok AC, Wang AY, Lee YY, Sun KO, So AP, et al. Critical care in obstetrical patients.An eight year review. Chin Med J (Engl) 1997;110:936–41. [PubMed] [Google Scholar]
- 21.Lapinsky SE, Kruczynski K, Seaward GR, Farine D, Grossman RF. Critical care management of the obstetric patients. Can J Anaesth. 1997;44:325–9. doi: 10.1007/BF03015374. [DOI] [PubMed] [Google Scholar]
- 22.Sriram S, Robertson MS. Critically ill obstetric patients in Australia: a retrospective audit of 8 years’ experience in a tertiary intensive care unit. Crit Care Resusc. 2008;10:124. [PubMed] [Google Scholar]
- 23.Sibai BM. Eclampsia.VI. Maternal-perinatal outcome in 254 consecutive cases. Am J Obstet Gynecol. 1990;163:1049–54. doi: 10.1016/0002-9378(90)91123-t. discussion 1054-5. [DOI] [PubMed] [Google Scholar]
- 24.Bhagwanjee S, Paruk F, Moodley J, Muckart DJ. Intensive care unit morbidity and mortality from eclampsia: an evaluation of acute physiology and chronic health evaluation II score and the Glasgow coma scale score. Crit Care Med. 2000;28:120–4. doi: 10.1097/00003246-200001000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Bewley S, Creighton S. ‘Near- miss’ obstetric enquiry. J Obstet Gynecol. 1997;17:26–9. doi: 10.1080/01443619750114031. [DOI] [PubMed] [Google Scholar]
- 26.Louie JK, Acosta M, Jamieson DJ, Honein MA California Pandemic (H1N1) Working Group. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362:27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 27.Miller AC, Safi F, Hussain S, Subramanian RA, Elamin EM, Sinert R, et al. Novel Influenza A (H1N1) Virus among Gravid Admissions. Arch Intern Med. 2010;170:868–73. doi: 10.1001/archinternmed.2010.126. [DOI] [PubMed] [Google Scholar]
- 28.Updated Interim Recommendations for the Use of Antiviral Medications in the Treatment and Prevention of Influenza for the 2009-2010 Season. [Last accessed on 2009];Centres for Disease Control and Prevention. Available from: http://www.cdc.gov/h1n1flu/recommendations.htm . [Google Scholar]
- 29.Munnur U, Karnad DR, Bandi VD, Lapsia V, Suresh MS, Ramshesh P, et al. Critically ill obstetric patients in an American and an Indian public hospital: comparison of case-mix, organ dysfunction, intensive care requirements, and outcomes. Intensive Care Med. 2005;31:1087–94. doi: 10.1007/s00134-005-2710-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandal D, Munjappa B, Sharma PP, Roy CA, Biswas SC. Dialysis requiring pregnancy related acute kidney injury- A single center experience. Kuwait Med J. 2010;42:124–8. [Google Scholar]
- 31.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]


