Abstract
Vaccines are the most powerful public health tools mankind has created, but malaria parasites are bigger, more complicated, and wilier than the viruses and bacteria that have been conquered or controlled with vaccines. Despite decades of research toward a vaccine for malaria, this goal has remained elusive. Nevertheless, recent advances justify optimism that a licensed malaria vaccine is within reach. A subunit recombinant protein vaccine that affords in the neighborhood of 50% protective efficacy against clinical malaria is in the late stages of clinical evaluation in Africa. Incremental improvements on this successful vaccine are possible and worth pursuing, but the best hope for a highly efficacious malaria vaccine that would improve prospects for malaria eradication may lie with the use of attenuated whole parasites and powerful immune-boosting adjuvants.
Keywords: Plasmodium falciparum, malaria eradication, malaria prevention, malaria immunity, subunit vaccines, whole-organism vaccines
INTRODUCTION
Malaria is a potentially fatal parasitic disease transmitted to humans and other vertebrates by mosquitoes. The malaria parasite is thought to have killed more humans throughout history than any other single cause. Today, along with AIDS and tuberculosis, malaria remains one of the “big three” infectious diseases, every year exacting a heavy toll on human life and health in parts of Central and South America, in large regions of Asia, and throughout most of sub-Saharan Africa, where up to 90% of malaria deaths occur (1). Starting in the late 1990s, new tools, including long-lasting insecticide-impregnated nets and highly efficacious combination drug therapies, led to dramatic reductions in the malaria burden in some areas and complete elimination of malaria in others. These success stories have stimulated a renewed sense of optimism about prospects for global malaria eradication (2).
If it is to succeed, this nascent drive toward country-by-country elimination and possible eventual worldwide eradication of malaria will require powerful new tools, importantly including vaccines. A successful malaria vaccine must produce protective immune responses that surpass those acquired through natural exposure to malaria (3). Successful global or regional campaigns to eradicate smallpox, polio, and measles have all relied on vaccines. Campaigns against yellow fever, hookworm, and yaws—and malaria—have relied primarily on nonvaccine measures such as vector control or drug treatment, and have all failed (4). Although the odds of successful global malaria eradication would be long even with an ideal malaria vaccine, they are virtually nil without one. In the meantime, even a modestly effective vaccine could substantially reduce the continuing heavy burden of malaria-attributable disease and death.
VACCINES AND THE MALARIA LIFE CYCLE
Four species of Plasmodium cause malaria disease primarily in humans: P. falciparum, P. vivax, P. ovale, and P. malariae. A fifth, P. knowlesi, infects mainly nonhuman primates but was recently found also to infect and sicken humans, and hundreds of other malaria species infect other mammals, reptiles and birds. Because it is responsible for most severe malaria disease and deaths, P. falciparum has been the target of most vaccine development efforts and is the main focus of this review.
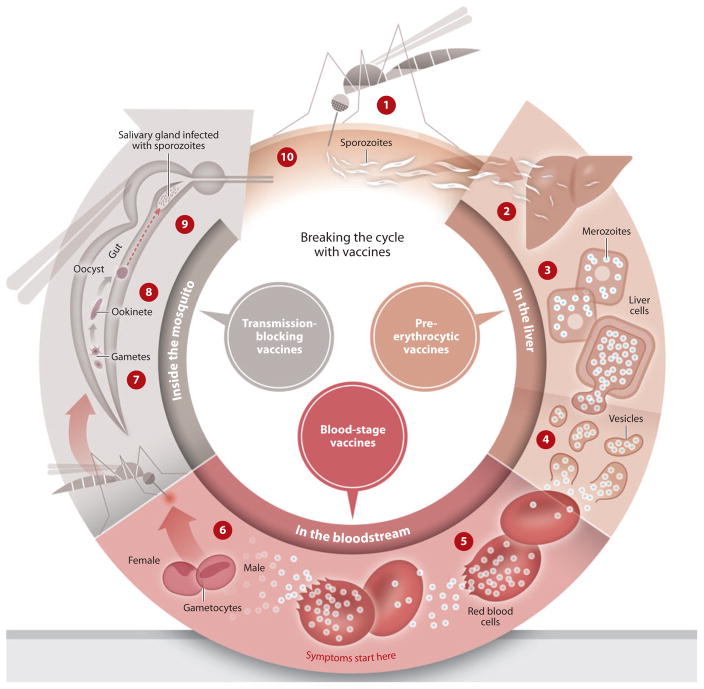
The malaria life cycle begins when the female Anopheles mosquito injects worm-like sporozoites from her salivary gland into the skin as she takes a blood meal (Figure 1). After invading the liver, each sporozoite multiplies over several days into tens of thousands of tiny merozoites packed into a single hepatocyte. These pre-erythrocytic stages cause no clinical signs or symptoms. A highly efficacious pre-erythrocytic vaccine would thus completely block infection, preventing parasites from reaching the blood and causing disease, and also preventing transmission.
Figure 1.
Life cycle of malaria and stages targeted by vaccines. ➀ An infected female Anopheles mosquito bites a person, injecting Plasmodium parasites, in the form of sporozoites, into the bloodstream. ➁ The sporozoites pass quickly into the human liver. ➂ The sporozoites multiply asexually in the liver cells over the next 7–10 days, causing no symptoms. ➃ The parasites, in the form of merozoites, burst from the liver cells. ➄ In the bloodstream, the merozoites invade red blood cells (erythrocytes) and multiply again until the cells burst. Then they invade more erythrocytes. This cycle is repeated, causing fever each time parasites break free and invade blood cells. ➅ Some of the infected blood cells leave the cycle of asexual multiplication. Instead of replicating, the merozoites in these cells develop into sexual forms of the parasite, called gametocytes, that circulate in the bloodstream. ➆ When a mosquito bites an infected human, it ingests the gametocytes, which develop further into mature sex cells called gametes. ➇ The gametes develop into actively moving ookinetes that burrow into the mosquito’s midgut wall and form oocysts. ➈ Inside the oocyst, thousands of active sporozoites develop. The oocyst eventually bursts, releasing sporozoites that travel to the mosquito salivary glands. ➉ The cycle of human infection begins again when the mosquito bites another person. Figure adapted from PATH Malaria Vaccine Initiative.
Rupturing hepatocytes release showers of merozoites into the circulation, initiating the “blood stage” of malaria infection, which is responsible for disease. Merozoites quickly invade erythrocytes and undergo asexual multiplication, dividing, growing, bursting from the erythrocyte, and reinvading in a periodic pattern with each cycle lasting two days (three days in the case of P. malariae), until interrupted by host immunity, drug treatment, or death. Malaria vaccines that target the blood stage are thought of as antidisease vaccines, and are expected to prevent or reduce clinical illness without preventing infection.
Some blood-stage parasites develop into male and female gametocytes. These sexual forms of Plasmodium are taken up by the mosquito during a blood meal, then mate to form a brief diploid stage and develop through further haploid stages before migrating from the gut to the salivary glands (Figure 1). Each mating pair of gametocytes yields up to 1,000 infectious sporozoites, which are injected into the host to complete the transmission cycle. Vaccines targeting the sexual stages would prevent neither infection nor disease in the vaccinated individual and are thought of as transmission-blocking vaccines. Highly efficacious pre-erythrocytic or blood-stage vaccines that prevent sexual reproduction would also block transmission, so the term “transmission-blocking” does not refer exclusively to vaccines against sexual or mosquito stages of the parasite (5).
EPIDEMIOLOGY, DISEASE, AND IMMUNITY
Clinical falciparum malaria originates with changes in the infected erythrocytes. P. falciparum parasites effectively hijack the host cell and its machinery, expressing on the erythrocyte surface highly variant P. falciparum erythrocyte membrane proteins (PfEMP1s), which are encoded by a large, diverse family of up to 60 var genes in each parasite genome (6). PfEMP1s are expressed on the red cell surface in cytoadherent clumps known as knobs. Infected red cells sequester in the microcirculatory compartments of organs, most notably in the brain and placenta, leading to disease. The slow acquisition of immune protection against uncomplicated malaria over years of repeated exposure to malaria is thought to represent the accumulation of protective immune responses to a repertoire of diverse antigens, probably including both PfEMP1s and the surface proteins that are the targets of most vaccine candidates.
The patterns and intensity of malaria transmission are the primary determinants of malaria epidemiology, which in turn drives the prevalence of malaria infection and the incidence of different forms of malaria disease. Depending largely on the degree of host immunity, the manifestations of malaria infection can range from completely asymptomatic parasitemia to mild disease to acute, catastrophic, life-threatening illness. Very young infants are thought to be protected from malaria disease by maternal antibodies and persistent hemoglobin F. Protective immunity against malaria disease is acquired through repeated exposure and is therefore related to transmission intensity.
Where malaria transmission is moderate or high, the risk period for death from malaria is highest in infants and young children who are in the process of developing acquired immunity. Semi-immune adults, although they remain susceptible to asymptomatic parasitemia, are protected against clinical malaria disease, rarely becoming ill even when persistently infected. This protective immunity is lost after a few years in the absence of exposure. Acquired immunity is also diminished in pregnancy, in that women pregnant with their first child are susceptible to severe P. falciparum disease from placental malaria because they lack immunity to placenta-specific cytoadherence proteins. In subsequent pregnancies, as placental immunity develops, there is a reduced risk of adverse effects of malaria on the mother and fetus (7).
Based on these epidemiological patterns, the primary populations targeted for malaria vaccines are infants and young children in areas of moderate and high transmission, who bear the greatest burden of disease and death; and women of child-bearing age in these same areas. Malaria-naïve travelers and military troops would also benefit from a malaria vaccine. As more countries move toward malaria elimination and global eradication is considered (2), the general population of malaria-endemic areas may be vaccinated to drive down transmission (3, 5).
Both humoral and cellular factors contribute to acquired immune protection against malaria. Broadly speaking, cellular immune responses are thought to be more important in controlling the pre-erythrocytic stages of malaria infection, and antibodies are thought to block erythrocyte invasion to suppress blood-stage infection. For these reasons, cellular immune responses are typically emphasized more in the development of pre-erythrocytic vaccines and antibody responses in the development of blood-stage vaccines. However, the basis of protective immunity against malaria is poorly understood, and no specific immune response has been established as an essential correlate of clinical protection, complicating malaria vaccine development.
OBSTACLES TO MALARIA VACCINES
In addition to the lack of an immune correlate of protection that would permit down-selection of vaccine candidates before expensive efficacy trials, several other factors have impeded malaria vaccine development. Chief among these impediments are the size and plasticity of the P. falciparum genome, which has about 23 million bases of DNA organized into 14 chromosomes and about 5,000 genes (8). This is orders of magnitude larger than the genomes of most of the viruses and bacteria for which vaccines have been successfully developed. Through mechanisms that are poorly understood, many of these genes are differentially expressed during the various stages of the highly complex life cycle in vertebrate hosts and mosquitoes (Figure 1). Adding further complexity, mutation during mitotic reproduction in the haploid liver and blood stages and genetic recombination during the diploid sexual reproductive stages in the mosquito result in extensive genetic diversity that is driven by selection pressure from the immune system, as well as by drugs and, when they are deployed, potentially by vaccines (9). All of this complexity and diversity greatly complicates the choice of candidate antigens for vaccine development and raises doubt that monovalent single-antigen vaccines will provide broad cross-protection.
At least 18 different forms of one leading blood-stage antigen (10) and >200 variants of another (11) have been documented in a single African village. If vaccines targeting these antigens generate immune responses that are in-sufficiently cross-protective, vaccines based on just one or two genetic variants are unlikely to be broadly efficacious (9). To date, the choices of which variants of target antigens to include in malaria vaccines have not been made in consideration of the frequencies of these variants in natural populations. Careful molecular epidemiological studies are beginning to pinpoint which of the many polymorphisms in some of these antigens are the most important determinants of strain-specific natural immunity (10, 11), and this approach may help inform the design of polyvalent or chimeric vaccines that protect against diverse parasite strains (9).
Immunization with stage-specific vaccines typically protects against only that life-cycle stage, hence the notion of vaccines that specifically prevent infection, disease, or transmission by targeting the different stages. A highly efficacious pre-erythrocytic vaccine would prevent not only infection but also disease and transmission (3); however, even a single surviving sporozoite could theoretically result in infection, disease, and transmission. This is because of the parasite’s ability to multiply rapidly—one sporozoite gives rise to tens of thousands of merozoites, and each merozoite multiplies roughly tenfold in 48 h, quickly resulting in billions of parasites circulating in the body. The fully sterile protection that would be required to completely prevent infection is a dauntingly high bar for a vaccine to clear. However, the ability of a partially efficacious pre-erythrocytic vaccine to reduce the risk of clinical malaria illness (12) supports the idea that there is some benefit from slowing the rate of parasite reproduction short of complete prevention of blood-stage infection. A so-called “leaky vaccine,” perhaps better thought of as an injectable bed-net, could be a valuable tool for malaria control.
Most successful vaccines prevent infection or illness with pathogens that naturally result in strong and long-lasting immune protection after a single exposure. As described above, naturally acquired protective immunity to malaria is hard-won and short-lived. An effective malaria vaccine will need to produce stronger and faster immune responses than those that develop under even the most intense, continuous natural exposure to malaria. A vaccine intended to prevent infection will need to surpass natural immunity, which gradually protects against clinical illness but does not completely prevent infection.
Finally, because the host immune response contributes to malaria pathogenesis, a vaccine could theoretically increase the risk of harmful inflammatory responses to subsequent infection, especially a vaccine directed against the blood stages responsible for pathology. Vigilance for untoward inflammatory responses to malaria vaccines or to postvaccination malaria infection is an important aspect of clinical malaria vaccine development, especially for blood-stage vaccines.
TYPES OF MALARIA VACCINES
Pre-Erythrocytic Vaccines
The vaccine furthest in clinical development, RTS,S/AS01, targets the pre-erythrocytic circumsporozoite protein (CSP) of P. falciparum. CSP contains a central repeat region that elicits antibody responses (13), flanked on each side by nonrepetitive regions containing T cell epitopes (14). Antibodies directed against the central repeat region cause the protein coat to slough off and block invasion of hepatocytes, suggesting that vaccine-induced antibodies might prevent infection (15). RTS,S is composed of the central repeat region (R) and T cell epitopes (T) of CSP using the hepatitis B surface antigen (S) as a carrier matrix. It is coexpressed in Saccharomyces cerevisiae with additional S, hence “RTS,S.” The clinical development of RTS,S has included progressive improvements in adjuvant systems, resulting in improved efficacy both in challenge trials (16, 17) and in clinical trials in malaria-exposed adults (18) and children (12, 19). The current formulation includes the liposomal-based Adjuvant System AS01, which contains the immunostimulants monophosphoryl lipid A and QS21, a saponin derivative extracted from the bark of the South American soap bark tree Quillaja saponaria. The development of RTS,S supports the notion that strong adjuvants are necessary for an efficacious subunit vaccine for malaria.
RTS,S was the first malaria vaccine to demonstrate meaningful levels of clinical protection in field trials. Trials in children and infants who are naturally exposed to malaria have demonstrated efficacy in the range of 30%–56% against clinical disease and up to 66% against infection, and a good record of safety and tolerability (12, 19). A phase II trial in 1,465 Mozambican children showed 26% efficacy against all malaria episodes over nearly four years, 32% against a first or only episode of clinical malaria, and 38% in preventing severe clinical episodes (20). After 45 months, the prevalence of parasitemia was significantly lower in vaccinees than in the control group (12% versus 19%). The magnitude of the protective effect after nearly four years was thus modest, but importantly, there was no evidence of a postimmunization “rebound” effect—a theoretical concern that the vaccine might interfere with the natural acquisition of protective immunity.
Based on these demonstrations of a level of efficacy that is well below levels of protection expected for vaccines against other common pathogens but rare good news for malaria vaccines, RTS,S/AS01 is currently being evaluated in a large phase III trial of 16,000 children and infants in seven African countries. Early results of the phase III trial suggest similar efficacy to that seen in phase II trials. The cost-effectiveness of licensing and deploying a malaria vaccine with efficacy in the range of 25%–50% is debated, but where the malaria burden remains high, as in much of sub-Saharan Africa, such a vaccine is likely to be sought and used. Strategies being investigated to improve the efficacy of RTS,S/AS01 include adding antigens tested with the same adjuvant system to create a multi-stage, multi-antigen RTS,S-based vaccine (21) and priming with an adenovirus expressing CSP before boosting with RTS,S/AS01 (22).
Several other pre-erythrocytic vaccine candidates have progressed through preclinical and early clinical development (23), including recombinant subunit-protein vaccines as well as DNA vaccines and viral-vectored vaccines (19). Even though some of these have generated seemingly good humoral and especially cellular immune responses when formulated with strong adjuvants (24), protective efficacy has not been achieved in clinical testing in humans. Prime–boost approaches using DNA vaccines or viral vectors have also resulted in improved immunogenicity and sometimes measurable delays in time to infection in experimental sporozoite challenge trials (25). Although prime–boost vaccine strategies have failed to demonstrate meaningful protection in published clinical efficacy trials, recent unpublished reports are more promising, with ~25% sterile protection provided by DNA prime and viral-vector boost using both CSP and the blood-stage antigen AMA1 (T. Richie, personal communication). Efforts to improve these approaches to get more consistent cellular immune responses and higher levels of protection may be hampered by variability in host responses to vaccination. Other novel approaches, such as a self-assembling polypeptide nanoparticle CSP vaccine, are showing promise in preclinical testing (26). Mining of genomic and proteomic data has led to the identification of new pre-erythrocytic vaccine candidate proteins, some of which have shown promising results in animal models (27).
Blood-Stage Vaccines
Most blood-stage malaria vaccine candidates are based on antigens that coat the surface of the invasive merozoites and/or are involved in the process of erythrocyte invasion. The hope is that immunization with these antigens will generate antibodies that block invasion and curtail parasite replication in the blood, reducing the risk or severity of clinical illness. The merozoite surface protein 1, or MSP1, was the first and best characterized of many proteins on the merozoite surface that are being targeted for vaccine development. Naturally acquired antibodies to MSP119 inhibit erythrocyte invasion and are associated with protection from clinical malaria in field studies (28), supporting its potential as a vaccine candidate. An MSP1-based vaccine on the same adjuvant platform as RTS,S produced antibodies in Malian adults that recognized MSP1 from diverse strains of P. falciparum (29), but it had no protective efficacy against clinical malaria in Kenyan children (30). Comparison of the degree of homology with the vaccine strain of MSP1 sequences in the infections experienced by children in the vaccine and control groups will clarify the extent to which the genetic diversity of MSP1 accounted for this lack of efficacy.
The apical membrane antigen 1, or AMA1, resides in the apical complex of the merozoite (31). AMA1 is thought to play a role in erythrocyte invasion (32), and it may play a similar role in hepatocyte invasion (33). Naturally acquired antibodies to AMA1 inhibit erythrocyte invasion in vitro (34) and are associated with protection in field studies (35). Studies in animal models show strain specificity in the inhibitory activity of anti-AMA1 antibodies (34), and these results have been corroborated by subsequent allelic exchange experiments (36, 37).
Sequencing of the gene encoding AMA1 in samples from a single Malian village identified >200 unique AMA1 variants in ~500 P. falciparum infections (11), raising the daunting prospect that a 200-valent AMA1 vaccine might be required to achieve broad protective efficacy. However, molecular epidemiological analyses showed that a group of just eight polymorphic amino acids lying adjacent to the presumed erythrocyte-binding site on AMA1 were responsible for strain-specific naturally acquired immunity, suggesting that a vaccine comprising as few as 10 “serotypes” of AMA1 might be sufficient to protect against 80% of unique variants.
Two AMA1 vaccines have reached the stage of efficacy trials in humans. A bivalent vaccine with two different forms of AMA1 adjuvanted with aluminum hydroxide failed to provide any protection against parasitemia or clinical malaria (38), and molecular analyses of pre-and postimmunization infections turned up no evidence of strain-specific efficacy or selection of nonvaccine variants (39). This disappointing result, following on the heels of the failure of MSP1-based and other blood-stage vaccines to protect against clinical malaria, contributed to a growing inclination to abandon subunit blood-stage vaccines altogether. However, a monovalent AMA1 vaccine formulated with the same adjuvant system as that used with RTS,S was more highly immunogenic (40) in a similar population of African children. Although this vaccine did not prevent infection after experimental sporozoite challenge (41) and showed marginal overall efficacy against clinical malaria, it demonstrated strong strain-specific efficacy, reducing the risk of clinical malaria caused by parasites with AMA1 homologous to the vaccine strain by >60% (42). This encouraging result, along with a post hoc analysis of data from a phase Ib trial of a merozoite surface protein 3 blood-stage vaccine showing efficacy against clinical malaria (42a), reanimated the field of blood-stage malaria vaccines by suggesting that it may be possible to develop a more broadly efficacious multivalent or chimeric next-generation AMA1 vaccine. Efforts are now under way to do this (43, 44).
P. falciparum proteins that are expressed on the surface of infected erythrocytes, mediate cytoadherence and immune evasion, and contribute to pathogenesis would seem to be attractive candidates for antidisease vaccines. These PfEMP1 proteins are encoded by a large family of diverse var genes (45) with up to 60 variants in each parasite genome. Designing a vaccine that would be broadly protective against such an extraordinarily polymorphic target is likely to be very difficult. One possible approach to overcome this difficulty may be the identification of conserved epitopes that are nevertheless immunogenic (46). Because a single PfEMP1 that is somewhat less polymorphic, VAR2CSA, mediates cytoadherence in placental malaria, prospects for a PfEMP1-based vaccine for placental malaria may be better, and research toward this goal is under way (47).
Multi-stage, multi-antigen vaccines that include blood-stage components are discussed in a subsequent section.
Transmission-Blocking Vaccines
Vaccines that are specifically intended to block transmission target molecules that are unique to gametocytes or to subsequent mosquito stages. Antibodies directed against such targets are capable of blocking the development of mosquito stages, thus interrupting transmission (48). In a rare example of a vaccine designed to target multiple species, a vaccine based on mosquito-stage proteins in both P. falciparum and P. vivax was shown to produce dose-dependent antibody-mediated transmission-blocking activity (49). However, the vaccine, which was formulated with the powerful adjuvant Montanide ISA 51, was unacceptably reactogenic. With the recent renewed call for global malaria eradication (2), transmission-blocking vaccines will be increasingly emphasized. In one novel and promising approach, vaccines that target mosquito molecules are being contemplated in hopes of avoiding selection pressure within the host that favors “vaccine-resistant” parasites (50).
Although not traditionally thought of as transmission-blocking vaccines, highly efficacious pre-erythrocytic vaccines that provide sterile immunity would also interrupt transmission. In the context of malaria elimination, a highly efficacious pre-erythrocytic vaccine would thus be the product-development target for “vaccines that interrupt transmission” (5).
Multi-Stage, Multi-Antigen Vaccines
Compared to the viral and bacterial human pathogens for which effective vaccines exist, malaria parasites are big and complex and elicit equally complex and multifaceted immune responses. In retrospect, it may not be surprising that so many candidate vaccines that target a single variant of a single antigen have failed to demonstrate clinical efficacy, especially against heterologous natural challenge. Several attempts have been made to improve on the efficacy of single-antigen vaccines by developing multi-stage, multi-antigen vaccines. One of the earliest was SPf66, a synthetic vaccine consisting of peptides derived from the blood-stage antigen MSP1 linked by the central repeat of the pre-erythrocytic antigen CSP. Although reports of efficacy in initial trials in South America generated great excitement (51), subsequent studies in Africa and Asia showed no significant protective efficacy (52, 53). In another approach that yielded disappointing results, vaccinia virus was used as a vector to express seven P. falciparum genes, but both immunogenicity and efficacy were limited (54). Attempts to develop DNA vaccines with P. falciparum genes (55), using up to 15 genes, were likewise unsuccessful.
Based on the modest efficacy of RTS,S against clinical malaria and evidence that a blood-stage vaccine using a similar adjuvant system can produce strain-specific efficacy, it is reasonable to attempt to construct a multistage, multi-antigen recombinant protein that improves on the efficacy of RTS,S (21).
Whole-Organism Vaccines
Even though live attenuated vaccines were the earliest and remain some of the best vaccines against other pathogens, and even though birds and monkeys had been protected by live attenuated malaria parasites in the earliest vaccine studies (56), the protection seen with irradiated sporozoites in the early 1970s (57) was interpreted not as a direct path to a malaria vaccine but as proof that a vaccine was possible, and as justification for the ensuing decades of research aimed at identifying the “right” vaccine antigen or heterologous expression system. The limited success of these Sisyphean research efforts led to reevaluation of the dogma that it would be impossible to manufacture an attenuated sporozoite vaccine in mosquitoes (58). A radiation-attenuated, metabolically active, nonreplicating sporozoite vaccine has been manufactured in and purified from aseptically raised mosquitoes and was recently evaluated for safety and efficacy in an experimental sporozoite challenge trial in humans (59). The goal was to administer by needle essentially the same immunogen—albeit aseptic, purified, and cryopreserved—that had previously demonstrated 90% protective efficacy in the form of sterilizing immunity when delivered by the bites of at least 1,000 irradiated infected mosquitoes. When administered by intradermal or subcutaneous routes, the sporozoite vaccine did not have significant protective efficacy (60). However, the vaccine sporozoites are highly immunogenic in monkeys when administered intravenously but not subcutaneously (60). The likeliest explanation for this result is therefore thought to be that sporozoites delivered into the skin by needle injection in a comparatively large volume of fluid were unable to reach the liver with efficiency approaching that of sporozoites injected either by a mosquito probing for small blood vessels or intravenously.
The potential for this approach to yield a highly efficacious pre-erythrocytic whole-organism vaccine remains high. Efforts are under way to improve delivery methods to more closely approximate the probing mosquito’s efficient delivery of attenuated sporozoites into the circulation, starting with the intravenous route that is clearly superior in animal models. Strong adjuvants have been key to developing partially efficacious subunit malaria vaccines, and whole-parasite vaccine experiments in the 1940s likewise showed that adjuvants could boost protection; this will likely be tried with the sporozoite vaccine. Genetic attenuation of sporozoites as an alternative to radiation is also being explored (61), although insufficient attenuation and consequent breakthrough infection in an early trial have somewhat dampened enthusiasm for this approach. In a similar “back to the future” paradigm shift, attenuated whole-parasite blood-stage vaccines are now also back on the table (62) more than 60 years after this approach was shown to work in monkeys.
One side benefit to advances in the development of whole-organism vaccines is the availability of aseptically produced Good Manufacturing Practices–grade frozen sporozoites that can be used for experimental infections in challenge trials. In initial tests at the University of Maryland’s Center for Vaccine Development, intradermal injections of such sporozoites have been successfully used to infect humans (63), and injection of precise numbers of thawed sporozoites offers an attractive alternative to challenge by the bite of infectious mosquitoes, which can infect reliably but deliver unknown and variable numbers of sporozoites. With this enabling tool, experimental challenge trials for vaccines as well as drugs may become routine not just at a few specialized centers with established insectaries, but at any malaria clinical trial facility, including those in malaria-endemic countries.
FUTURE DIRECTIONS
Malaria is transmitted in more than 100 countries. Even as about 30 of these are actively trying to eliminate malaria, it is generally agreed that global eradication is not possible without global economic development to the levels that permitted malaria elimination in the United States, Europe, and the former Soviet Union, and/or new tools such as a highly efficacious malaria vaccine that interrupts transmission (5). A powerfully adjuvanted pre-erythrocytic single-antigen recombinant protein vaccine, RTS,S/AS01, significantly reduces the clinical burden of malaria in African children (12, 19), but it does not prevent infection and, somewhat surprisingly, its effect on transmission (if any) has not been reported. The results of a field trial of a similarly adjuvanted blood-stage vaccine (42), as well as a combination of RTS,S with a viral vector in a prime–boost regimen (22), provide a strong rationale for pursuing improvements on RTS,S and similar vaccines, but the dire public health need for a highly efficacious malaria vaccine calls for more than incremental improvements. Whole-parasite vaccines may be the radically different new (and yet very old) approach that is needed. Challenges remain in the production and delivery of such a vaccine, but none are insurmountable.
Many past and recent studies point to the importance of strong adjuvants for subunit vaccines as well as for whole-organism vaccines, and wide access to immunogenic and safe adjuvants will be important for accelerating malaria vaccine development. Researchers are focusing now on the elusive goal of achieving a high degree of efficacy, but the ideal vaccine would be not only safe and efficacious but also thermostable and protective for a long period of time after a single immunization. These characteristics will not be easy to achieve but might be possible through pharmaceutical technologies such as controlled-release formulations.
All predictions of when a malaria vaccine will be available have been overly optimistic, but barring unforeseen setbacks, a moderately efficacious vaccine that substantially reduces the malaria burden should be licensed within just a few years. This magnificent accomplishment will mark not the end of the road for malaria vaccine development but a critical milestone on the path toward the malaria vaccine that the world needs.
Acknowledgments
C.V.P. is supported by the National Institute of Allergy and Infectious Diseases of the U.S. National Institutes of Health, by the Doris Duke Charitable Foundation, and by the Howard Hughes Medical Institute. M.A.T. is supported by cooperative agreement U01AI065683 from the National Institute of Allergy and Infectious Diseases and by grant D43TW001589 from the Fogarty International Center of the U.S. National Institutes of Health. The authors thank Kirsten Lyke, Matthew Laurens, Drissa Coulibaly, and Mark Travassos for critical reading of the manuscript. The authors acknowledge the U.S. Agency for International Development, the Walter Reed Army Institute of Research, GlaxoSmithKline Biologicals, Sanaria Inc., the U.S. Military Malaria Vaccine Program–Naval Medical Research Center, and the Malaria Research and Training Center of the University of Bamako, Mali, for collaboration on malaria vaccine trials.
Glossary
- Pre-erythrocytic
stages of the malaria parasite that are injected by a mosquito and develop in the liver before emerging into the blood where they can cause symptoms. Vaccines targeting pre-erythrocytic stages are intended to prevent infection altogether and, if highly effective, would also prevent disease and block transmission
- PfEMP1
P. falciparum erythrocyte membrane protein 1, a highly variable parasite protein expressed on the surface of host erythrocytes that mediates cytoadherence, pathogenesis, and immune evasion
- AS01
Adjuvant System 01, a proprietary adjuvant system from GlaxoSmithKline Biologicals
- CSP
circumsporozoite protein of P. falciparum, a pre-erythrocytic vaccine target
- Challenge trial
small, experimental phase II/III clinical trial in which healthy volunteers receive a malaria vaccine and are exposed to the bites of malaria-infected mosquitoes or injected with malaria parasites under carefully controlled conditions
- Subunit vaccine
a vaccine based on a small portion of the organism, usually a peptide or protein
- Immunogenicity
the ability of a vaccine to produce specific immune responses (usually antibodies) that recognize the vaccine antigen
- Whole-organism vaccine
a vaccine based on an attenuated or killed whole parasite
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Mahamadou A. Thera, Email: mthera@icermali.org.
Christopher V. Plowe, Email: cplowe@medicine.umaryland.edu.
LITERATURE CITED
- 1.World Health Organization, Roll Back Malaria Partnership. [Accessed Aug 17, 2011];The global malaria action plan for a malaria free world. 2008 Available at http://www.rbm.who.int/gmap/
- 2.Tanner M, de Savigny D. Malaria eradication back on the table. Bull World Health Organ. 2008;86:82. doi: 10.2471/BLT.07.050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plowe CV, Alonso P, Hoffman SL. The potential role of vaccines in the elimination of falciparum malaria and the eventual eradication of malaria. J Infect Dis. 2009;200:1646–49. doi: 10.1086/646613. [DOI] [PubMed] [Google Scholar]
- 4.Henderson DA. Lessons from the eradication campaigns. Vaccine. 1999;17(Suppl 3):S53–S55. doi: 10.1016/s0264-410x(99)00293-5. [DOI] [PubMed] [Google Scholar]
- 5.malERA Consultative Group on Vaccines. A research agenda for malaria eradication: vaccines. PLoS Med. 2011;8:e1000398. doi: 10.1371/journal.pmed.1000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su X, Heatwole VM, Wertheimer SP, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 7.Duffy PE. Plasmodium in the placenta: parasites, parity, protection, prevention and possibly preeclampsia. Parasitology. 2007;134:1877–81. doi: 10.1017/S0031182007000170. [DOI] [PubMed] [Google Scholar]
- 8.Gardner MJ, Hall N, Fung E, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takala SL, Plowe CV. Genetic diversity and malaria vaccine design, testing and efficacy: preventing and overcoming ‘vaccine resistant malaria’. Parasite Immunol. 2009;31:560–73. doi: 10.1111/j.1365-3024.2009.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takala SL, Coulibaly D, Thera MA, et al. Dynamics of polymorphism in a malaria vaccine antigen at a vaccine-testing site in Mali. PLoS Med. 2007;4:e93. doi: 10.1371/journal.pmed.0040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takala SL, Coulibaly D, Thera MA, et al. Extreme polymorphism in a vaccine antigen and risk of clinical malaria: implications for vaccine development. Sci Transl Med. 2009;1:2ra5. doi: 10.1126/scitranslmed.3000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alonso PL, Sacarlal J, Aponte JJ, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–20. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 13.Ballou WR, Rothbard J, Wirtz RA, et al. Immunogenicity of synthetic peptides from circumsporozoite protein of Plasmodium falciparum. Science. 1985;228:996–99. doi: 10.1126/science.2988126. [DOI] [PubMed] [Google Scholar]
- 14.Dame JB, Williams JL, McCutchan TF, et al. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984;225:593–99. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- 15.Hollingdale MR, Nardin EH, Tharavanij S, et al. Inhibition of entry of Plasmodium falciparum and P. vivax sporozoites into cultured cells; an in vitro assay of protective antibodies. J Immunol. 1984;132:909–13. [PubMed] [Google Scholar]
- 16.Gordon DM, McGovern TW, Krzych U, et al. Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit vaccine. J Infect Dis. 1995;171:1576–85. doi: 10.1093/infdis/171.6.1576. [DOI] [PubMed] [Google Scholar]
- 17.Stoute JA, Slaoui M, Heppner DG, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 18.Bojang KA, Milligan PJ, Pinder M, et al. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet. 2001;358:1927–34. doi: 10.1016/S0140-6736(01)06957-4. [DOI] [PubMed] [Google Scholar]
- 19.Bejon P, Lusingu J, Olotu A, et al. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359:2521–32. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacarlal J, Aide P, Aponte JJ, et al. Long-term safety and efficacy of the RTS,S/AS02A malaria vaccine in Mozambican children. J Infect Dis. 2009;200:329–36. doi: 10.1086/600119. [DOI] [PubMed] [Google Scholar]
- 21.Heppner DG, Jr, Kester KE, Ockenhouse CF, et al. Towards an RTS,S-based, multi-stage, multi-antigen vaccine against falciparum malaria: progress at the Walter Reed Army Institute of Research. Vaccine. 2005;23:2243–50. doi: 10.1016/j.vaccine.2005.01.142. [DOI] [PubMed] [Google Scholar]
- 22.Stewart VA, McGrath SM, Dubois PM, et al. Priming with an adenovirus 35-circumsporozoite protein (CS) vaccine followed by RTS,S/AS01B boosting significantly improves immunogenicity to Plasmodium falciparum CS compared to that with either malaria vaccine alone. Infect Immun. 2007;75:2283–90. doi: 10.1128/IAI.01879-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. [Accessed Aug 17, 2011];Initiative for vaccine research: malaria vaccines. 2010 Available at http://www.who.int/vaccine_research/links/Rainbow/en/index.html.
- 24.Cummings JF, Spring MD, Schwenk RJ, et al. Recombinant liver stage antigen-1 (LSA-1) formulated with AS01 or AS02 is safe, elicits high titer antibody and induces IFN-gamma/IL-2 CD4+ T cells but does not protect against experimental Plasmodium falciparum infection. Vaccine. 2010;28:5135–44. doi: 10.1016/j.vaccine.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 25.Hill AV, Reyes-Sandoval A, O’Hara G, et al. Prime-boost vectored malaria vaccines: progress and prospects. Hum Vaccin. 2010;6:78–83. doi: 10.4161/hv.6.1.10116. [DOI] [PubMed] [Google Scholar]
- 26.Kaba SA, Brando C, Guo Q, et al. A nonadjuvanted polypeptide nanoparticle vaccine confers long-lasting protection against rodent malaria. J Immunol. 2009;183:7268–77. doi: 10.4049/jimmunol.0901957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergmann-Leitner ES, Mease RM, De La Vega P, et al. Immunization with pre-erythrocytic antigen CelTOS from Plasmodium falciparum elicits cross-species protection against heterologous challenge with Plasmodium berghei. PLoS ONE. 2010;5:e12294. doi: 10.1371/journal.pone.0012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riley EM, Allen SJ, Wheeler JG, et al. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 1992;14:321–37. doi: 10.1111/j.1365-3024.1992.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 29.Thera MA, Doumbo OK, Coulibaly D, et al. Safety and allele-specific immunogenicity of a malaria vaccine in Malian adults: results of a phase I randomized trial. PLoS Clin Trials. 2006;1:e34. doi: 10.1371/journal.pctr.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogutu BR, Apollo OJ, McKinney D, et al. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PLoS ONE. 2009;4:e4708. doi: 10.1371/journal.pone.0004708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson MG, Marshall VM, Smythe JA, et al. Integral membrane protein located in the apical complex of Plasmodium falciparum. Mol Cell Biol. 1989;9:3151–54. doi: 10.1128/mcb.9.7.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell GH, Thomas AW, Margos G, et al. Apical membrane antigen 1, a major malaria vaccine candidate, mediates the close attachment of invasive merozoites to host red blood cells. Infect Immun. 2004;72:154–58. doi: 10.1128/IAI.72.1.154-158.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Florens L, Washburn MP, Raine JD, et al. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419:520–26. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- 34.Hodder AN, Crewther PE, Anders RF. Specificity of the protective antibody response to apical membrane antigen 1. Infect Immun. 2001;69:3286–94. doi: 10.1128/IAI.69.5.3286-3294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polley SD, Mwangi T, Kocken CH, et al. Human antibodies to recombinant protein constructs of Plasmodium falciparum apical membrane antigen 1 (AMA1) and their associations with protection from malaria. Vaccine. 2004;23:718–28. doi: 10.1016/j.vaccine.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 36.Dutta S, Lee SY, Batchelor AH, et al. Structural basis of antigenic escape of a malaria vaccine candidate. Proc Natl Acad Sci USA. 2007;104:12488–93. doi: 10.1073/pnas.0701464104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Healer J, Murphy V, Hodder AN, et al. Allelic polymorphisms in apical membrane antigen-1 are responsible for evasion of antibody-mediated inhibition in Plasmodium falciparum. Mol Microbiol. 2004;52:159–68. doi: 10.1111/j.1365-2958.2003.03974.x. [DOI] [PubMed] [Google Scholar]
- 38.Sagara I, Dicko A, Ellis RD, et al. A randomized controlled phase 2 trial of the blood stage AMA1-C1/Alhydrogel malaria vaccine in children in Mali. Vaccine. 2009;27:3090–98. doi: 10.1016/j.vaccine.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouattara A, Mu J, Takala-Harrison S, et al. Lack of allele-specific efficacy of a bivalent AMA1 malaria vaccine. Malar J. 2010;9:175. doi: 10.1186/1475-2875-9-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thera MA, Doumbo OK, Coulibaly D, et al. Safety and immunogenicity of an AMA1 malaria vaccine in Malian children: results of a phase 1 randomized controlled trial. PLoS ONE. 2010;5:e9041. doi: 10.1371/journal.pone.0009041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spring MD, Cummings JF, Ockenhouse CF, et al. Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A. PLoS ONE. 2009;4:e5254. doi: 10.1371/journal.pone.0005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thera MA, Doumbo OK, Coulibaly D, et al. A field trial to assess a blood-stage malaria vaccine. N Engl J Med. 2011;365:1004–13. doi: 10.1056/NEJMoa1008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42a.Sirima SB, Cousens S, Druilhe P. Protection against malaria by MSP3 candidate vaccine. N Engl J Med. 2011;365:1062–64. doi: 10.1056/NEJMc1100670. [DOI] [PubMed] [Google Scholar]
- 43.Remarque EJ, Faber BW, Kocken CH, et al. A diversity-covering approach to immunization with Plasmodium falciparum apical membrane antigen 1 induces broader allelic recognition and growth inhibition responses in rabbits. Infect Immun. 2008;76:2660–70. doi: 10.1128/IAI.00170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dutta S, Dlugosz LS, Clayton JW, et al. Alanine mutagenesis of the primary antigenic escape residue cluster, c1, of apical membrane antigen 1. Infect Immun. 2010;78:661–71. doi: 10.1128/IAI.00866-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith JD, Chitnis CE, Craig AG, et al. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–10. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein MM, Gittis AG, Su HP, et al. The cysteine-rich interdomain region from the highly variable Plasmodium falciparum erythrocyte membrane protein-1 exhibits a conserved structure. PLoS Pathog. 2008;4:e1000147. doi: 10.1371/journal.ppat.1000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Avril M, Cartwright MM, Hathaway MJ, et al. Immunization with VAR2CSA-DBL5 recombinant protein elicits broadly cross-reactive antibodies to placental Plasmodium falciparum-infected erythrocytes. Infect Immun. 2010;78:2248–56. doi: 10.1128/IAI.00410-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barr PJ, Green KM, Gibson HL, et al. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med. 1991;174:1203–8. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Y, Ellis RD, Shaffer D, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS ONE. 2008;3:e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dinglasan RR, Valenzuela JG, Azad AF. Sugar epitopes as potential universal disease transmission blocking targets. Insect Biochem Mol Biol. 2005;35:1–10. doi: 10.1016/j.ibmb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Valero MV, Amador LR, Galindo C, et al. Vaccination with SPf66, a chemically synthesised vaccine, against Plasmodium falciparum malaria in Colombia. Lancet. 1993;341:705–10. doi: 10.1016/0140-6736(93)90483-w. [DOI] [PubMed] [Google Scholar]
- 52.D’Alessandro U, Leach A, Drakeley CJ, et al. Efficacy trial of malaria vaccine SPf66 in Gambian infants. Lancet. 1995;346:462–67. doi: 10.1016/s0140-6736(95)91321-1. [DOI] [PubMed] [Google Scholar]
- 53.Alonso PL, Smith T, Schellenberg JR, et al. Randomised trial of efficacy of SPf66 vaccine against Plasmodium falciparum malaria in children in southern Tanzania. Lancet. 1994;344:1175–81. doi: 10.1016/s0140-6736(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 54.Ockenhouse CF, Sun PF, Lanar DE, et al. Phase I/IIa safety, immunogenicity, and efficacy trial of NYVAC-Pf7, a pox-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. J Infect Dis. 1998;177:1664–73. doi: 10.1086/515331. [DOI] [PubMed] [Google Scholar]
- 55.Kumar S, Epstein JE, Richie TL, et al. A multilateral effort to develop DNA vaccines against falciparum malaria. Trends Parasitol. 2002;18:129–35. doi: 10.1016/s1471-4922(01)02207-3. [DOI] [PubMed] [Google Scholar]
- 56.Freund J, Thomson KJ, Sommer HE, et al. Immunization of rhesus monkeys against malarial infection (P. knowlesi) with killed parasites and adjuvants. Science. 1945;102:202–4. doi: 10.1126/science.102.2643.202. [DOI] [PubMed] [Google Scholar]
- 57.Clyde DF, Most H, McCarthy VC, et al. Immunization of man against sporozite-induced falci-parum malaria. Am J Med Sci. 1973;266:169–77. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- 58.Luke TC, Hoffman SL. Rationale and plans for developing a non-replicating, metabolically active, radiation-attenuated Plasmodium falciparum sporozoite vaccine. J Exp Biol. 2003;206:3803–8. doi: 10.1242/jeb.00644. [DOI] [PubMed] [Google Scholar]
- 59.Hoffman SL, Billingsley PF, James E, et al. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum Vaccin. 2010;6:97–106. doi: 10.4161/hv.6.1.10396. [DOI] [PubMed] [Google Scholar]
- 60.Epstein JE, Tweari K, Lyke KE, et al. Live attenuated malaria vacine designed to protect through hepatic D8+ T cell immunity. Science. 2011;334:475–80. doi: 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]
- 61.VanBuskirk KM, O’Neill MT, De La Vega P, et al. Preerythrocytic, live-attenuated Plasmodium falciparum vaccine candidates by design. Proc Natl Acad Sci USA. 2009;106:13004–9. doi: 10.1073/pnas.0906387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCarthy JS, Good MF. Whole parasite blood stage malaria vaccines: a convergence of evidence. Hum Vaccin. 2010;6:114–23. doi: 10.4161/hv.6.1.10394. [DOI] [PubMed] [Google Scholar]
- 63.Lyke KE, Laurens M, Adams M, et al. Plasmodium falciparum malaria challenge by the bite of aseptic Anopheles stephensi mosquitoes: results of a randomized infectivity trial. PLoS ONE. 2010;5:e13490. doi: 10.1371/journal.pone.0013490. [DOI] [PMC free article] [PubMed] [Google Scholar]