Abstract
A novel mating-type switching-defective mutant showed a highly unstable rearrangement at the mating-type locus (mat1) in fission yeast. The mutation resulted from local amplification of a 134-bp DNA fragment by the mat1-switching phenomenon. We speculate that the rolling-circle-like replication and homologous recombination might be the general mechanisms for local genome region expansion.
The fission yeast (Schizosaccharomyces pombe) cell usually exists in a haploid state, with P (for plus) or M (for minus) cell/mating type. These cell types efficiently interconvert by the mat1-switching phenomenon (reviewed in Egel 2005 and Klar 2007). The mating-type loci are composed of three homologous cassettes, spanning a 30-kb region located in the middle of chromosome 2 (Figure 1A). The mating-type-determining cassette, mat1, is transcriptionally active and contains either P or M allele-specific DNA sequence. The other two cassettes, mat2P or mat3M, exist in an epigenetically silenced state, serving as donors of genetic information required to switch the mat1 locus through recombination. The recombination process is initiated by a site-specific “imprint” placed at the junction of homology box H1 and the mat1 allele-specific sequence (Beach 1983; Beach and Klar 1984; Egel et al. 1984; Klar and Miglio 1986; Nielsen and Egel 1989). The imprint is thought to be a single-strand nick and/or two ribonucleotides incorporated into the specific DNA strand (Klar 1987; Singh and Klar 1993; Kaykov and Arcangioli 2004; Vengrova and Dalgaard 2004). Replication of the imprinted chromosome creates a transient double-strand break (DSB) at the imprint site (Beach 1983; Beach and Klar 1984; Arcangioli 1998; Dalgaard and Klar 2000). The DSB is repaired by the intrachromosomal gene conversion event, replacing the mat1 gene with a copy derived from one of the mat2 or mat3 donor loci, resulting in a cell-type switch.
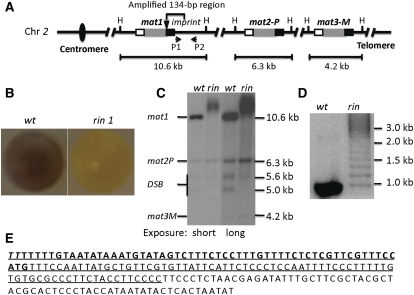
Figure 1 .
Broadly diffused mat1-DNA bands of the rin1 mutant. (A) Mating-type loci. The three mating-type cassettes are indicated. The internal P (1104-bp-long DNA sequence) and M (1128-bp) allele-specific sequences are shown in shaded boxes; the flanking homology boxes H2 (135 bp) and H1 (59 bp) are shown in open and solid boxes, respectively; the solid triangle represents the imprint site; H indicates the HindIII site. The sizes of three mat cassette-containing fragments generated by HindIII digestion of genomic DNA are indicated. A is not drawn to scale. (B) Iodine-vapor-stained colonies of the wild type (wt; strain SP976) and the rin1 mutant (SP2579). (C) Southern blot analysis of the wild type (SP2533) and the rin1 mutant (SP2579) using standard DNA extraction and Southern blot methods (Moreno et al. 1991). The blot was probed with a mat1P-containing, HindIII 10.6-kb fragment. This probe also detected mat2 and mat3 bands due to the shared homology between cassettes defined in A. To depict low-intensity bands, the same blot was exposed to X-ray film for 1 hr (short exposure) or for 5 hr (long exposure). (D) PCR analysis of mat1-distal sequences. The location of primers P1 (ATTCATTCTCCCTCCAATTTTCCC) and P2 (GGAGATAGGAGTGTGATTGAAGGTG) is shown in A. The PCR reactions were performed according to the vendor’s protocol (Clonetaq Titanium Taq, catalog no. 639210). The PCR products were separated on 3% agarose gel and stained with ethidium bromide before taking the picture. (E) The amplified sequence of the rin1 mutant. The sequence shows a 200-bp region, which includes the 59-bp H1 (boldface type) and the H1-distal sequence. The 134-bp sequence amplified in the rin1 mutant is underlined. The italicized TT bases located at the 5′ end of the sequence indicate the imprint site. The genotype of strain is listed in Table S1.
Characterization of a DNA Unstable Mutant
The homothallic cells, called h90 cells, efficiently switch their mating type in ∼40% of cell divisions (Miyata and Miyata 1981; Egel 1984; Egel and Eie 1987; Klar 1990). Consequently, yeast colonies are composed of an equal proportion of P and M cells. Cells of opposite type can mate under nitrogen starvation conditions, and the resulting zygotic diploid cell usually undergoes meiosis and sporulation to produce four haploid spores, called ascospores. The spores synthesize starch, but the vegetative growing cells do not. Because starch reacts with iodine, efficiently switching colonies stain dark, and those of low-switching mutants stain lighter when exposed to iodine vapors (Leupold 1955). By screening with the iodine-staining procedure, we obtained one mutant that showed a very-light-staining phenotype (Figure 1B). To explain several unusual phenotypes exhibited by the mutant (described below), we defined the molecular nature of its switching defect.
Genetic crosses showed that the mutation-causing switching defect is tightly linked to mat1 (47 parental ditypes: 0 nonparental ditypes: 0 tetratype tetrads) by a distance of <1.0 cM. Southern blot analysis showed that the mutant lacks the 10.6-kb mat1-containing HindIII restriction fragment found in wild-type cultures; instead, the mutant produced larger, broadly diffused band(s) ranging from >12 to 16 kb in length (Figure 1C). The 10.6-kb mat1-containing HindIII restriction fragment is broken during the DNA extraction process, due to imprint-generated fragility at the site, into 5.6- and 5.0-kb bands in wild-type cultures (Figure 1C) (Beach 1983; Beach and Klar 1984). In comparison, the mutant’s 5.6-kb band was much weaker than that of the wild-type stock, and the imprint-distal 5.0-kb band was missing. The bands derived from the mat2-P and mat3-M donor loci were not altered.
Interestingly, the mutant spontaneously generated stable derivatives with a dark-staining phenotype when screened with iodine vapors at a frequency of ∼1/1000 cells. These derivatives had reverted to the wild-type constitution, showing the same band pattern as that of the wild-type strain (data not shown). Thus, the mat1 switching that occurs at a reduced rate in the mutant possibly repairs the mutation.
The diffused mat1 band of the mutant suggested a high level of DNA replication instability at the mat1 locus; therefore, the mutation was named rin1 (replication instability 1). To our knowledge, the highly unstable mat1-fragment length phenomenon, detectable by Southern blot analysis, has been observed only in the telomeric regions of the chromosome (Dahlen et al. 2003). The telomeres’ repeat number is not uniform in different cells due to the unusual mode of replication of chromosome ends by the telomerase activity. As the mat1 locus is found in the middle of the chromosome, a telomerase-based explanation of the rin1 mutation is unlikely. We next entertained the idea that some DNA rearrangements in the 5.0-kb imprint-distal region cause both replication instability and the switching defect in the mutant. It is known that only a sequence of 200 bp (Figure 1E) located distal to the mat1 imprint site is required for efficient mat1 switching (Arcangioli and Klar 1991). Because the rin1 mutant exhibited a very low level of imprinting (Figure 1B), and because it is genetically linked to mat1, the mutation likely lies very close to mat1. We analyzed the structure of the mat1 distal region using polymerase chain reaction (PCR) with P1 and P2 primers (Figure 1A). A single PCR product band of the expected size was obtained from the wild-type control strain. Interestingly, the rin1 allele showed multiple bands (Figure 1D). Those PCR products were subcloned into Escherichia coli. Twenty independent, transformed colonies were genotyped to determine the size of their inserts, and five size classes were identified. One sample from each class was subjected to DNA sequencing. Analyses of sequences showed that the shortest one was identical to that of the wild-type mat1 allele, and others carried one to four tandem copies of the 134-bp DNA sequence (Figure 1E). We also isolated and analyzed three rin1-reverted alleles, and their mat1 distal 500-bp sequence was found to be identical to that of the wild-type mat1 allele. These data showed that the rin1 allele is composed of the 134-bp tandem repeats at the mat1 distal region. From the size of the diffused mat1 bands, we estimate that the majority of rin1 cells contain 15–40, 134-bp DNA repeats. We hypothesize that the mutant originated from a spontaneous 134-bp duplication, possibly generated by a DNA replication and/or mat1 switching error.
Analysis of Experimentally Generated rin Mutants
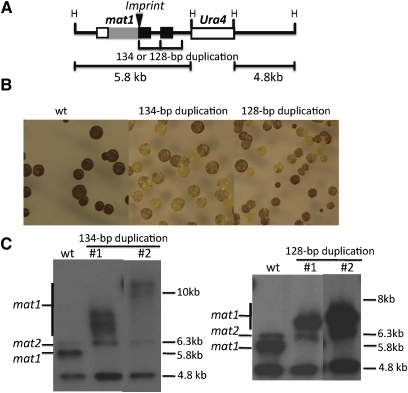
To test our hypothesis for generating the rin1 mutant, we engineered a directly duplicated 134-bp containing DNA fragment at the mat1 locus in wild-type h90 cells by homologous recombination through the DNA-mediated transformation procedure. For transformants selection, we employed the ura4+ marker inserted at a site 250 bp distal to the imprint site in the fragment that we used for transfomation (Figure 2A). The following observations indicated that we had generated a new rin allele. First, we observed that iodine staining produced a highly variegated color phenotype in the transformants (Figure 2B). Second, the transformants’ PCR analyses showed mat1 fragments with multiple bands (Supporting Information, Figure S1), a result similar to that observed with the original rin1 mutant (Figure 1C). Third, we found an association between the lower switching efficiency indicated by iodine staining and a higher repeat copy number (Figure S1). Fourth, Southern blot analyses of cultures derived from two very-light-staining colonies showed diffused bands (Figure 2C); the bands observed were between 8 and 12 kb in length, indicating that between 20 and 50, 134-bp repeats had been generated.
Figure 2 .
Generation of new rin alleles. (A) Schematic structure of genetically engineered rin alleles. A 134- or 128-bp duplicated sequence, together with the 1.8-kb ura4+-containing HindIII fragment, was placed into the mat1 locus using the standard lithium acetate transformation procedure by selecting for the ura4+ marker (Moreno et al. 1991). The symbols are those as defined Figure 1A. (B) Iodine-staining phenotype of new rin mutants. The wild-type CY83 control strain contained only the ura4+ mark insertion, and in addition, the CY96 and CY84 strains contained the 134- and the 128-bp duplication, respectively. (C) Southern blot analyses of HindIII-digested genomic DNA of engineered rin derivatives. Two independent, very-light-iodine-staining colonies derived from strains CY96 or CY84 were analyzed along with the CY83 control. The probe used was as described in Figure 1C.
Similarly, we created a 128-bp duplicated allele that was 6 bp shorter at the 3′ end of the 134-bp allele (Figure 1E). The duplicated allele showed the unstable staining colony phenotype, and its lighter-staining derivatives also produced a mat1 locus with diffused bands (Figure 2, B and C). Our generation of engineered rin alleles demonstrates that DNA repeats located near the imprint sites are unstable and that the original rin1 mutant was likely generated by a spontaneous 134-bp sequence duplication.
rin Mutant Phenotypes Require the mat1-Switching Process
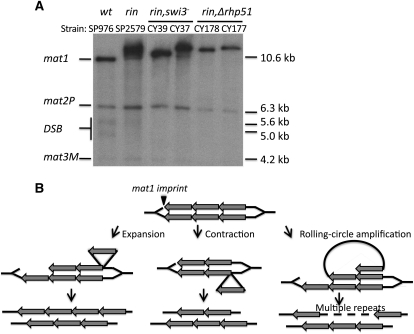
Although eukaryotic genomes contain repeated sequences, including hundreds of copies of the rDNA gene, they are all relatively stable. In contrast, we found that the repeats at the mat1 locus are remarkably unstable. Might the instability result from the mat1 switching process? To test this possibility, we checked the genetic interaction of the rin1 mutation with mutations in the genes required for mat1 switching (Figure 3A). Swi3 is a DNA replication fork pause factor that is required for efficient mat1 imprinting/switching (Egel et al. 1984; Klar and Bonaduce 1993; Dalgaard and Klar 2000). Two separately constructed rin1, swi3− double-mutant CY37 and CY39 strains were analyzed. Southern analysis showed that CY37 contained a more repeats while CY39 contained a smaller number at mat1. Both the rin1, swi3− double mutants showed a more condensed mat1 band than the single rin1 mutant strain SP2579 (Figure 3A), indicating that the mating-type switch and/or the imprint synthesis promotes the instability of rin1 repeats.
Figure 3 .
Genetic interaction of rin1 with swi3 and rhp51. (A) Southern blot analysis of the rin1 swi3− (CY37and CY39) or rin1 Δrhp51 (CY177, CY178) double mutant. Southern analyses of two independently generated, switching-defective rin mutants with indicated duplication are shown. (B) Models for repeat expansion, contraction, and rolling-circle replication by the DSB repair pathway. The two parallel lines represent newly replicated sister chromatids. The DNA replication fork moves from right to left at the mat1 locus. When the leading replicated DNA strand reaches the imprint site, it generates a chromatid ending with a DSB. The broken end invades the repetitive homologous DNA of the sister chromatid and starts the copying process by the DSB repair pathway (Strathern et al. 1982; Szostak et al. 1983). Different choices of pairing partners could lead to repeat expansion or contraction, as shown. Further amplification might result from a rolling-circle type of replication when the DSB repair occurs by using repeats residing on the same chromatid.
The fission yeast Rhp51, the only counterpart of Rad51 in human and budding yeast, is required for mating-type switching and general homologous recombination (HR). The h90, Δrhp51 cells die because their DSB at mat1 cannot be repaired due to an HR defect (Roseaulin et al. 2008). In contrast, we found that the rin1, Δrhp51 double mutant proliferates, suggesting that the rin1 mutant has a reduced level of the imprint/DSB at mat1. This idea was supported by the reduction of mat1 broken bands on Southern blots of the rin1 mutant (Figure 1C and Figure 3A). Moreover, the rin1, Δrhp51 double mutant showed a larger, much sharper mat1 band in comparison to that of the rin1 SP2579 strain (Figure 3A). These results demonstrate that the instability of the rin1 allele is a result of HR.
On the basis of these results, we propose a model to explain the instability of the rin1 and the experimentally generated mutant alleles (Figure 3B). When the replication fork reaches the imprint site, the chromatid with a single broken end forms, and it searches for homologous sequences residing in donor loci to repair itself by the synthesis-dependent strand-annealing pathway in wild-type cells (Klar and Bonaduce 1993; Arcangioli and de Lahondes 2000; Yamada-Inagawa et al. 2007). We hypothesize that the broken chromatid uses repetitive homologous sequences in the intact sister chromatid in the rin1 mutant instead of the normally used donor loci as the template for repair. Depending on which repeat was invaded, the repeat numbers can expand or contract following repair of the DSB (Figure 3B). This way, occasionally wild-type mat1 would be generated through healing the mutation. One study showed that, during the strand-copying process of homologous recombination, the invading strand can dissociate and re-anneal with different possible alignments on the tandem repeated template (Paques et al. 1998). Such frequent invading strand dissociation and re-annealing possibilities explain the high level of instability of rin alleles. In principle, the extrachromosomal 134-bp repeat circles could form by HR and act as intermediates for repeat expansion/contraction. The 134-bp sequence does not contain autonomously replicating sequence, and by Southern blot analysis we did not detect it as an extrachromosomal DNA species in multiple rin-derived alleles. Thus, we think this possibility to be unlikely to explain our results.
A recent report showed that the copy number of a chromosome fragment can be increased from one to two or three by a DNA re-replication mechanism (Green et al. 2010). Similarly, the direct, 134-bp duplication might have been generated by a DNA replication error or from an HR event between the TT residues residing 134 bp apart (Figure 1C). Such spontaneous events are expected to be rare, consistent with our finding of only one such mutant among the >500 switching mutants that we analyzed. Our experiments showed that a directly duplicated DNA fragment could further amplify up to 50 copies located adjacent to a DNA fragile/imprint site. Once a duplication has been generated as proposed above, it is easy to imagine that the mat1 imprint-associated DSB might invade sequence repeats existing nearby in the same chromatid for repair synthesis, analogous to a version of the rolling-circle model of DNA replication (Figure 3B). Such a rolling-circle mechanism for DNA amplification has also been envisioned for transposon-mediated events in maize (Zhang and Peterson 2004; J. Zhang and T. Peterson, personal communication). We speculate that similar mechanisms may contribute to the local DNA amplification during genome evolution and cancer development in humans (Tanaka et al. 2005).
Supplementary Material
Acknowledgments
We thank members of the Amar Klar, Jeff Strathern, and Thomas Peterson laboratories for discussions and advice. The Intramural Research Program of the National Cancer Institute of the National Institutes of Health supported this work.
Footnotes
Communicating editor: J. Sekelsky
Literature Cited
- Arcangioli B., 1998. A site- and strand-specific DNA break confers asymmetric switching potential in fission yeast. EMBO J. 17: 4503–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangioli B., de Lahondes R., 2000. Fission yeast switches mating type by a replication-recombination coupled process. EMBO J. 19: 1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangioli B., Klar A. J., 1991. A novel switch-activating site (SAS1) and its cognate binding factor (SAP1) required for efficient mat1 switching in Schizosaccharomyces pombe. EMBO J. 10: 3025–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach D. H., 1983. Cell type switching by DNA transposition in fission yeast. Nature 305: 682–688. [Google Scholar]
- Beach D. H., Klar A. J., 1984. Rearrangements of the transposable mating-type cassettes of fission yeast. EMBO J. 3: 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlen M., Sunnerhagen P., Wang T. S., 2003. Replication proteins influence the maintenance of telomere length and telomerase protein stability. Mol. Cell. Biol. 23: 3031–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgaard J. Z., Klar A. J., 2000. swi1 and swi3 perform imprinting, pausing, and termination of DNA replication in S. pombe. Cell 102: 745–751. [DOI] [PubMed] [Google Scholar]
- Egel R., 1984. Two tightly linked silent cassettes in the mating-type region of Schizosaccharomyces pombe. Curr. Genet. 8: 199–203. [DOI] [PubMed] [Google Scholar]
- Egel R., 2005. Fission yeast mating-type switching: programmed damage and repair. DNA Repair (Amst.) 4: 525–536. [DOI] [PubMed] [Google Scholar]
- Egel R., Eie B., 1987. Cell lineage asymmetry in Schizosaccharomyces pombe: unilateral transmission of a high-frequency state for mating-type switching in diploid pedigrees. Curr. Genet. 12: 429–433. [Google Scholar]
- Egel R., Beach D. H., Klar A. J., 1984. Genes required for initiation and resolution steps of mating-type switching in fission yeast. Proc. Natl. Acad. Sci. USA 81: 3481–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. M., Finn K. J., Li J. J., 2010. Loss of DNA replication control is a potent inducer of gene amplification. Science 329: 943–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaykov A., Arcangioli B., 2004. A programmed strand-specific and modified nick in S. pombe constitutes a novel type of chromosomal imprint. Curr. Biol. 14: 1924–1928. [DOI] [PubMed] [Google Scholar]
- Klar A. J., 1987. Differentiated parental DNA strands confer developmental asymmetry on daughter cells in fission yeast. Nature 326: 466–470. [DOI] [PubMed] [Google Scholar]
- Klar A. J., 1990. The developmental fate of fission yeast cells is determined by the pattern of inheritance of parental and grandparental DNA strands. EMBO J. 9: 1407–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., 2007. Lessons learned from studies of fission yeast mating-type switching and silencing. Annu. Rev. Genet. 41: 213–236. [DOI] [PubMed] [Google Scholar]
- Klar A. J., Bonaduce M. J., 1993. The mechanism of fission yeast mating-type interconversion: evidence for two types of epigenetically inherited chromosomal imprinted events. Cold Spring Harb. Symp. Quant. Biol. 58: 457–465. [DOI] [PubMed] [Google Scholar]
- Klar A. J., Miglio L. M., 1986. Initiation of meiotic recombination by double-strand DNA breaks in S. pombe. Cell 46: 725–731. [DOI] [PubMed] [Google Scholar]
- Leupold U., 1955. Methods concerning genetics of Schizosaccharomyces pombe. Schweiz. Z. Allg. Pathol. Bakteriol. 18: 1141–1146 (in German). [PubMed] [Google Scholar]
- Miyata H., Miyata M., 1981. Mode of conjugation in homothallic cells of Schizosaccharomyces pombe. J. Gen. Appl. Microbiol. 27: 365–371. [Google Scholar]
- Moreno S., Klar A., Nurse P., 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194: 795–823. [DOI] [PubMed] [Google Scholar]
- Nielsen O., Egel R., 1989. Mapping the double-strand breaks at the mating-type locus in fission yeast by genomic sequencing. EMBO J. 8: 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paques F., Leung W. Y., Haber J. E., 1998. Expansions and contractions in a tandem repeat induced by double-strand break repair. Mol. Cell. Biol. 18: 2045–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseaulin L., Yamada Y., Tsutsui Y., Russell P., Iwasaki H., et al. , 2008. Mus81 is essential for sister chromatid recombination at broken replication forks. EMBO J. 27: 1378–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J., Klar A. J., 1993. DNA polymerase-alpha is essential for mating-type switching in fission yeast. Nature 361: 271–273. [DOI] [PubMed] [Google Scholar]
- Strathern J. N., Klar A. J., Hicks J. B., Abraham J. A., Ivy J. M., et al. , 1982. Homothallic switching of yeast mating type cassettes is initiated by a double-stranded cut in the MAT locus. Cell 31: 183–192. [DOI] [PubMed] [Google Scholar]
- Szostak J. W., Orr-Weaver T. L., Rothstein R. J., Stahl F. W., 1983. The double-strand-break repair model for recombination. Cell 33: 25–35. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Bergstrom D. A., Yao M. C., Tapscott S. J., 2005. Widespread and nonrandom distribution of DNA palindromes in cancer cells provides a structural platform for subsequent gene amplification. Nat. Genet. 37: 320–327. [DOI] [PubMed] [Google Scholar]
- Thon G., Klar A. J., 1993. Directionality of fission yeast mating-type interconversion is controlled by the location of the donor loci. Genetics 134: 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengrova S., Dalgaard J. Z., 2004. RNase-sensitive DNA modification(s) initiates S. pombe mating-type switching. Genes Dev. 18: 794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada-Inagawa T., Klar A. J., Dalgaard J. Z., 2007. Schizosaccharomyces pombe switches mating type by the synthesis-dependent strand-annealing mechanism. Genetics 177: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Peterson T., 2004. Transposition of reversed Ac element ends generates chromosome rearrangements in maize. Genetics 167: 1929–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.