Summary
Background and objectives
Sudden death is common in hemodialysis patients, but whether modifiable practices affect the risk of sudden death remains unclear.
Design, setting, participants, & measurements
This study analyzed 37,765 participants in 12 countries in the Dialysis Outcomes and Practice Patterns Study to explore the association of the following practices with sudden death (due to cardiac arrhythmia, cardiac arrest, and/or hyperkalemia): treatment time [TT] <210 minutes, Kt/V <1.2, ultrafiltration volume >5.7% of postdialysis weight, low dialysate potassium [KD <3]), and prescription of Q wave/T wave interval–prolonging drugs. Cox regression was used to estimate effects on mortality, adjusting for potential confounders. An instrumental variable approach was used to further control for unmeasured patient-level confounding.
Results
There were 9046 deaths, 26% of which were sudden (crude mortality rate, 15.3/100 patient-years; median follow-up, 1.59 years). Associations with sudden death included hazard ratios of 1.13 for short TT, 1.15 for large ultrafiltration volume, and 1.10 for low Kt/V. Compared with KD ≥3 mEq/L, the sudden death rate was higher for KD ≤1.5 and KD=2–2.5 mEq/L. The instrumental variable approach yielded generally consistent findings. The sudden death rate was elevated for patients taking amiodarone, but not other Q wave/T wave interval–prolonging drugs.
Conclusions
This study identified modifiable dialysis practices associated with higher risk of sudden death, including short TT, large ultrafiltration volume, and low KD. Because KD <3 mEq/L is common and easy to change, KD tailoring may prevent some sudden deaths. This hypothesis merits testing in clinical trials.
Introduction
Cardiovascular disease accounts for approximately 50% of the highly premature mortality observed in hemodialysis patients. Close to 50% of cardiovascular deaths in hemodialysis patients are sudden; sudden death thus accounts for 20%–25% of all deaths in hemodialysis patients (1,2). Although sudden death is also common among patients on peritoneal dialysis (1,2), there are reasons to speculate that practices related to the hemodialysis treatment itself may play a specific role. These include the well established risk of arrhythmias associated with electrolyte imbalances, especially potassium shifts (3), as well as the increased risk of sudden death in the 12-hour period that begins with the start of the hemodialysis session or at the end of the long hemodialysis-free interval (4).
Several patient characteristics—such as heart failure, left ventricular hypertrophy, and coronary artery disease—have been associated with a high risk of sudden death in hemodialysis patients (1,5). However, whether readily modifiable practices are associated with the risk of sudden death among hemodialysis patients has received relatively little attention (6). We postulated that selected characteristics of the hemodialysis regimen and/or drug treatment may also affect the risk of sudden death. Specifically, we hypothesized that the rapid changes in extracellular fluid and electrolyte concentrations that occur during shorter dialysis sessions, using low dialysate potassium (KD) and at a higher ultrafiltration rate, may trigger arrhythmias and other cardiac events that may lead to higher incidence of sudden death. To explore these hypotheses, we analyzed the detailed data from the Dialysis Outcomes and Practice Patterns Study (DOPPS), taking advantage of the substantial variability of practices across the 12 participating countries (7).
Materials and Methods
Study Design and Data Sources
DOPPS is a prospective, observational study of hemodialysis practices and outcomes in 12 countries. Details on the study design have been described previously (7). We analyzed data from participants in the first three phases (1, 1996–2001; 2, 2002–2004; 3, 2005–2008). Baseline patient characteristics including demographics, detailed comorbidities, laboratory values, and dialysis treatment characteristics are obtained at the time of study entry by medical record abstraction. Clinical variables and medical events, including mortality and cause of death, are updated every 4 months. The study sample comprised 37,765 patients on thrice-weekly hemodialysis from 930 dialysis facilities in 12 countries. Participants were followed until the earliest incidence of death, transplant, change of treatment modality, withdrawal from dialysis, transfer to another facility, or study end. Time at risk was defined as the period from study enrollment until the end of patient follow-up.
Exposure Variables
This study considered the following four modifiable dialysis practices: short duration of dialysis sessions (prescribed treatment time <210 minutes), low dialysis dose (single pool Kt/V <1.2), large ultrafiltration volume (>5.7% of the postdialysis weight), and KD <3 mEq/L (categorized as ≤1.5 and 2–2.5 mEq/L). Prescription of β blockers and Q wave/T wave interval (QTI)–prolonging drugs (amiodarone, selected antidepressants, antipsychotics, and others) (8,9) was also considered.
Outcomes
The primary outcomes of interest were sudden death and all-cause mortality. Sudden death was defined as primary cause of death reported as death due to cardiac arrhythmia, cardiac arrest, and/or hyperkalemia. Other deaths were classified as “other cardiovascular death” if due to cardiovascular disease (myocardial infarction, pericarditis, atherosclerotic heart disease, cardiomyopathy, valvular heart disease, pulmonary edema due to exogenous fluid, congestive heart failure, and cerebrovascular accident) and “noncardiovascular death” (all deaths not classified as “sudden death” or “other cardiovascular death”). Cause of death was imputed for patients in whom the cause was not reported or available (see Statistical Analyses).
Statistical Analyses
Standard descriptive statistics were used to describe characteristics of study participants by the four modifiable dialysis treatment practices. Cox regression models were used to assess the associations of the four dialysis practices and QTI-prolonging medications with all-cause mortality, sudden death, other cardiovascular death, and noncardiovascular death. Models were adjusted for age, sex, black race, body mass index, years with ESRD, 14 comorbidity classes (coronary artery disease, other cardiac disease, cerebrovascular disease, congestive heart failure, diabetes, gastrointestinal bleeding, hypertension, peripheral vascular disease, lung disease, neurologic disorder, cancer [excluding skin], psychiatric disease, recurrent cellulitis, and HIV infection), catheter use, serum albumin, phosphorus, calcium, parathyroid hormone, hemoglobin, serum creatinine, ferritin, white blood cell count, and single pool Kt/V (except for models with single pool Kt/V and treatment time as exposure variables). To speed computation and allow for different baseline hazard functions, models were stratified by DOPPS phase and country. Models were also adjusted for indicators of facility achievement of clinical guidelines (facility proportion of patients with hemoglobin <11g/dl, albumin <3.2 g/dl, phosphorus >5.5 mg/dl, Kt/V <1.2, and using a catheter as vascular access). Robust variance estimates (sandwich estimator) were used to account for facility-level clustering.
To partially reduce the effect of unmeasured patient-level confounders, we also applied an instrumental variable approach, using the dialysis facilities as the instruments (10–13). For each of the four dialysis practices, we conducted separate instrumental variable analyses, applying a two-stage model method, which is an extension of two-stage least squares. In the first stage, the patient-level predicted treatment was determined using a linear regression model, including all patient covariates and indicators of facility achievement of clinical guidelines, as well as facility indicators as the instruments. In the second stage, Cox regression was used to estimate the hazard of mortality associated with the patient-level predicted treatment with the same covariate adjustments as in the first stage (14–21). Given the low F statistic observed for two of the practices (Kt/V and ultrafiltration volume), we also used limited information maximum likelihood regression, which is considered more robust when instruments are weak (11,22), that is, when the first stage partial F statistic is <10 (23). Limited information maximum likelihood regression uses linear models for both the first and second stage. We used 1-year mortality as the second stage outcome because the censoring rate is low, and modeled it as a dichotomous variable using linear regression as an approximation to logistic regression. IVEware was used to perform multiple imputations of missing data, including cause of death (24). Estimates and their variances from the multiple imputation results were combined according to the Rubin method (25). Sensitivity analyses limited to nonmissing data were performed. The assumption of proportionality of hazards in the Cox regression model was confirmed by an interaction term between each covariate and log-transformed follow-up time. Analyses were performed using SAS software (version 9.2; SAS Institute, Cary, NC). The authors followed the Strengthening the Reporting of Observational studies in Epidemiology guidelines for reporting observational studies (26).
Results
Patient Characteristics
The overall study sample is described in Table 1. As expected, differences were observed between patients with low and adequate Kt/V, as well as between patients treated versus not treated with each of the other three dialysis practices.
Table 1.
Patient characteristics by treatment practice
| Mean (SD) or % | Treatment Time <210 min | Kt/V <1.2 | Ultrafiltration Volume >5.7% | KD <3 mEq/L | ||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | No | Yes | |
| Patients (n) | 28,878 | 8536 | 18,824 | 7273 | 26,880 | 3970 | 6606 | 29,629 |
| Age (yr) | 61.0 (14.6) | 64.6 (15.3)a | 62.0 (14.8) | 61.2 (14.5)b | 62.5 (14.6) | 57.2 (14.9)a | 64.4 (14.5) | 61.3 (14.8)a |
| Male | 60 | 49a | 53 | 72a | 58 | 55a | 54 | 59a |
| Black | 13 | 15a | 14 | 13a | 13 | 13a | 13 | 14a |
| Vintage | 4.2 (5.5) | 2.0 (3.8)a | 4.9 (5.7) | 2.6 (4.3)a | 3.7 (5.2) | 6.3 (6.2)a | 1.8 (3.5) | 4.1 (5.5)a |
| Body mass index | 24.9 (5.8) | 24.1 (5.2)a | 24.2 (5.6) | 25.4 (5.9)a | 25.0 (5.7) | 21.5 (4.0)a | 25.8 (5.9) | 24.5 (5.6)c |
| Catheter | 23 | 35a | 18 | 28a | 23 | 11a | 41 | 22a |
| Comorbid conditions | ||||||||
| coronary artery disease | 45 | 46 | 46 | 42 | 46 | 40 | 54 | 43a |
| other cardiovascular | 35 | 32c | 36 | 31a | 34 | 35a | 39 | 33a |
| cerebrovascular disease | 16 | 18a | 17 | 16 | 17 | 14a | 20 | 16a |
| congestive heart failure | 35 | 37 | 35 | 33 | 34 | 33a | 42 | 34a |
| diabetes | 38 | 40 | 35 | 44a | 38 | 35b | 46 | 37a |
| Laboratory results | ||||||||
| hemoglobin (g/dl) | 10.9 (1.7) | 10.5 (1.7)a | 11.1 (1.6) | 10.5 (1.8)a | 10.9 (1.7) | 10.6 (1.6) | 10.8 (1.7) | 10.8 (1.7)a |
| albumin (g/dl) | 3.7 (0.5) | 3.6 (0.6)a | 3.7 (0.5) | 3.7 (0.6)a | 3.7 (0.5) | 3.8 (0.5)a | 3.5 (0.6) | 3.7 (0.5)a |
| potassium (mEq/L) | 5.0 (0.8) | 4.8 (0.8)a | 5.0 (0.8) | 4.9 (0.8)a | 4.9 (0.8) | 5.2 (0.8)a | 4.7 (0.8) | 5.0 (0.8)a |
| phosphorous (mg/dl) | 5.6 (1.8) | 5.6 (1.9)a | 5.5 (1.7) | 5.7 (1.9)a | 5.5 (1.8) | 5.9 (1.9)a | 5.4 (1.9) | 5.6 (1.8)a |
| parathyroid hormone (pg/ml) | 177 (275) | 187 (282) | 172 (272) | 184 (269)b | 178 (271) | 175 (297)a | 190 (269) | 178 (280) |
| creatinine (mg/dl) | 9.2 (3.2) | 8.0 (3.1)a | 9.2 (3.0) | 9.2 (3.5)a | 8.9 (3.1) | 10.1 (3.2)a | 7.3 (3.0) | 9.2 (3.2)a |
| ferritin (ng/ml) | 285 (431) | 247 (403)c | 314 (456) | 203 (332)a | 286 (429) | 222 (439)b | 278 (410) | 273 (426)a |
Serum parathyroid hormone and ferritin values are summarized as median (interquartile range). Significance values obtained from logistic and mixed linear regression models adjusted for the Dialysis Outcomes and Practice Patterns Study phase and accounted for facility clustering effects. KD, dialysate potassium.
P<0.001 for “yes” versus “no” column.
0.05≤P<0.10 for “yes” versus “no” column.
0.001≤P<0.05 for “yes” versus “no” column.
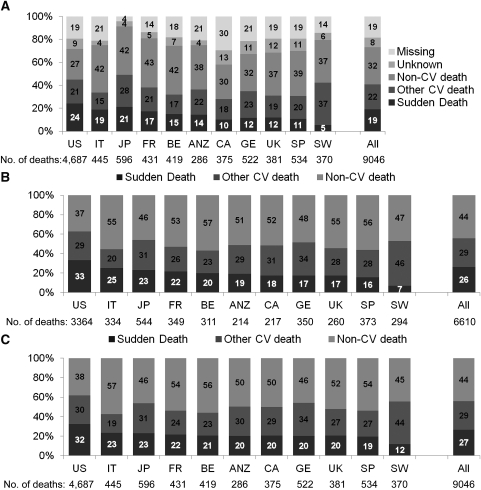
Among the 37,765 DOPPS participants included in these analyses, 9046 died (crude mortality rate, 15.3/100 patient-years; median follow-up, 1.59 years; range, 0.003–5.43). The distribution of causes of death in each DOPPS country is shown in Figure 1. Because the number of deaths without a reported cause was relatively high in some countries (Figure 1A), causes of death are also shown excluding missing causes (Figure 1B) and using imputed causes from one set of the multiple imputation procedure (Figure 1C). When excluding missing/unknown causes, sudden death ranged from 33.4% of deaths in the United States to 6.8% in Sweden. Approximately 6.7% of sudden deaths are reported as due to hyperkalemia.
Figure 1.
Cause of death by country. (A) Observed cause of death by country (n=9046 deaths), as reported. (B) Cause of death by country (n=6610 deaths), with 2436 deaths excluded due to missing/unknown causes. (C) Cause of death by country (n=9046), with imputed causes of death for 2436 with missing/unknown causes. Data from the following phases of the of the Dialysis Outcomes and Practice Patterns Study: phase 1, n=15,589 patients from 308 facilities in seven countries, including France, Germany, Italy, Japan, Spain, the United Kingdom, and the United States; phase 2, n=11,675 patients from 322 facilities in 12 countries, including the above-mentioned countries as well as Australia, New Zealand, Belgium, Canada, and Sweden; and phase 3, n=10,501 patients from 300 facilities in the same 12 countries as in phase 2. US, United States; IT, Italy; JP, Japan; FR, France; BE, Belgium; ANZ, Australia and New Zealand; CA, Canada; GE, Germany; UK, United Kingdom; SP, Spain; SW, Sweden; CV, cardiovascular.
Variation in Dialysis Practices
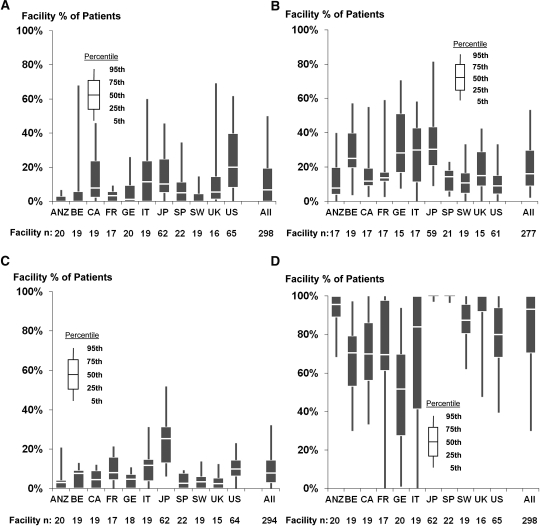
Wide variation in the four dialysis practices was observed both across facilities within each DOPPS country and across countries (Figure 2).
Figure 2.
Facility percentage of patients with short treatment time, low Kt/V, large ultrafiltration volume and low dialysate potassium, by country. Data from phase 3 of the Dialysis Outcomes and Practice Patterns Study, which included 298 facilities with ≥10 patients with each treatment of interest. ANZ, Australia and New Zealand; BE, Belgium; CA, Canada; FR, France; GE, Germany; IT, Italy; JP, Japan; SP, Spain; SW, Sweden; US, United States; UK, United Kingdom; UF, ultrafiltration.
Association of Dialysis Practices with Mortality
Higher risk for sudden death and all-cause mortality was seen for patients with treatment time <210 minutes (hazard ratio [HR], 1.13; 95% confidence interval [95% CI], 1.00–1.27; P=0.04; and HR, 1.06; 95% CI, 1.00–1.13; P=0.04) and ultrafiltration volume >5.7% (HR, 1.15; 95% CI, 1.00–1.32; P=0.04; and HR, 1.09; 95% CI, 1.01–1.19; P=0.03). For Kt/V <1.2, the HR for sudden death was 1.06 (95% CI, 1.00–1.12; P=0.07) and the HR for all-cause mortality was 1.10 (95% CI, 0.97–1.24; P=0.15). For the instrumental variable analyses, the magnitude of the estimates was generally greater than for the standard patient-level analyses, although as expected the estimates were less precise (Table 2).
Table 2.
Association of dialysis treatment practices (treatment time, single pool Kt/V, ultrafiltration volume) with mortality
| Patient Level | Instrumental Variable Approach | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Treatment time <210 min (versus ≥210 min) | ||||||
| all-cause mortality | 1.06 | (1.00–1.13) | 0.04 | 1.09 | (0.94–1.27) | 0.26 |
| sudden death | 1.13 | (1.00–1.27) | 0.04 | 1.38 | (1.04–1.83) | 0.03 |
| other cardiovascular death | 1.00 | (0.89–1.12) | 0.96 | 0.77 | (0.58–1.03) | 0.08 |
| noncardiovascular death | 1.06 | (0.97–1.17) | 0.20 | 1.16 | (0.93–1.44) | 0.18 |
| Kt/V <1.2 (versus ≥1.2) | ||||||
| all-cause mortality | 1.06 | (1.00–1.12) | 0.07 | 1.39 | (1.07–1.81) | 0.01 |
| sudden death | 1.10 | (0.97–1.24) | 0.15 | 1.71 | (1.02–2.87) | 0.04 |
| other cardiovascular death | 0.96 | (0.85–1.08) | 0.48 | 0.95 | (0.60–1.50) | 0.83 |
| noncardiovascular death | 1.10 | (1.00–1.21) | 0.04 | 1.59 | (1.11–2.29) | 0.01 |
| Ultrafiltration volume >5.7% of postweight (versus ≤5.7%) | ||||||
| all-cause mortality | 1.09 | (1.01–1.19) | 0.03 | 1.32 | (0.84–2.08) | 0.23 |
| sudden death | 1.15 | (1.00–1.32) | 0.04 | 1.56 | (0.60–4.03) | 0.35 |
| other cardiovascular death | 1.17 | (1.01–1.36) | 0.04 | 2.36 | (1.02–5.46) | 0.05 |
| noncardiovascular death | 1.00 | (0.88–1.14) | 0.98 | 0.78 | (0.43–1.43) | 0.42 |
N=37,741. HRs and CIs adjusted for variables listed in Table 1 and in the Materials and Methods, stratified by phase and country, and accounted for facility clustering. IV, instrumental variable; HR, hazard ratio; 95% CI, 95% confidence interval.
KD≤1.5 and KD=2–2.5 were associated with increased risk for sudden death (HR, 1.39 for KD ≤1.5; 95% CI, 1.12–1.74; P=0.004; and HR, 1.17 for KD=2–2.5; 95% CI, 1.01–1.37; P=0.04) and all-cause mortality (HR, 1.13 for KD ≤1.5; 95% CI, 1.03–1.25; P=0.01; and HR, 1.08 for KD=2–2.5; 95% CI, 1.01–1.16; P=0.03) compared with KD ≥3 (Table 3). Similar associations of sudden death with low KD were observed when models were further adjusted for serum bicarbonate or dialysate bicarbonate (not shown). For KD ≤1.5, the magnitude of the associations (especially for sudden death) was greater for patients with serum potassium <5 versus ≥5 mEq/L. For KD=2–2.5, the associations were generally comparable for patients with serum potassium <5 versus ≥5 mEq/L.
Table 3.
Association of KDa with mortality
| Patient Level | Instrumental Variable Approach | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KD ≤1.5 (versus ≥3) | KD=2–2.5 (versus ≥3) | KD ≤1.5 (versus ≥3) | KD=2–2.5 (versus ≥3) | |||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| All patients (N=37,741) | ||||||||||||
| all-cause mortality | 1.13 | (1.03–1.25) | 0.01 | 1.08 | (1.01–1.16) | 0.03 | 1.09 | (0.88–1.35) | 0.43 | 1.23 | (1.04–1.45) | 0.01 |
| sudden death | 1.39 | (1.12–1.74) | 0.004 | 1.17 | (1.01–1.37) | 0.04 | 1.67 | (0.99–2.81) | 0.05 | 1.61 | (1.12–2.30) | 0.01 |
| other cardiovascular death | 1.14 | (0.95–1.36) | 0.16 | 1.04 | (0.91–1.19) | 0.54 | 1.11 | (0.75–1.64) | 0.62 | 1.23 | (0.90–1.68) | 0.19 |
| noncardiovascular death | 0.99 | (0.84–1.17) | 0.93 | 1.05 | (0.94–1.16) | 0.38 | 0.84 | (0.61–1.16) | 0.29 | 1.03 | (0.83–1.29) | 0.76 |
| Among patients with serum K ≥5 (n=17,327) | ||||||||||||
| all-cause mortality | 1.09 | (0.95–1.26) | 0.23 | 1.08 | (0.97–1.20) | 0.17 | 1.13 | (0.87–1.47) | 0.37 | 1.23 | (0.99–1.52) | 0.06 |
| sudden death | 1.21 | (0.91–1.61) | 0.18 | 1.11 | (0.90–1.38) | 0.33 | 1.27 | (0.73–2.22) | 0.40 | 1.30 | (0.81–2.08) | 0.28 |
| other cardiovascular death | 1.16 | (0.88–1.52) | 0.29 | 1.00 | (0.82–1.21) | 0.97 | 1.18 | (0.74–1.88) | 0.49 | 1.17 | (0.79–1.72) | 0.44 |
| noncardiovascular death | 0.97 | (0.77–1.22) | 0.81 | 1.10 | (0.93–1.31) | 0.27 | 1.02 | (0.70–1.47) | 0.93 | 1.21 | (0.87–1.69) | 0.25 |
| Among patients with serum K <5 (n=20,414) | ||||||||||||
| all-cause mortality | 1.15 | (1.00–1.33) | 0.04 | 1.06 | (0.98–1.15) | 0.15 | 1.04 | (0.80–1.36) | 0.76 | 1.23 | (1.03–1.46) | 0.02 |
| sudden death | 1.53 | (1.10–2.13) | 0.01 | 1.18 | (0.98–1.42) | 0.08 | 2.01 | (0.96–4.24) | 0.06 | 1.86 | (1.31–2.63) | <0.001 |
| other cardiovascular death | 1.05 | (0.85–1.31) | 0.64 | 1.05 | (0.88–1.24) | 0.58 | 0.94 | (0.56–1.56) | 0.80 | 1.23 | (0.86–1.76) | 0.26 |
| noncardiovascular death | 1.03 | (0.77–1.38) | 0.83 | 1.00 | (0.88–1.15) | 0.95 | 0.77 | (0.51–1.15) | 0.20 | 0.95 | (0.75–1.22) | 0.71 |
HRs and confidence intervals CIs adjusted for variables listed in Table 1 and in the Materials and Methods, stratified by phase and country and accounted for facility clustering. IV, instrumental variable; HR, hazard ratio; 95% CI, 95% confidence interval. KD, dialysate potassium.
KD ≤1.5, n=5493; KD=2–2.5, n=25,552; KD≥3, n=6696.
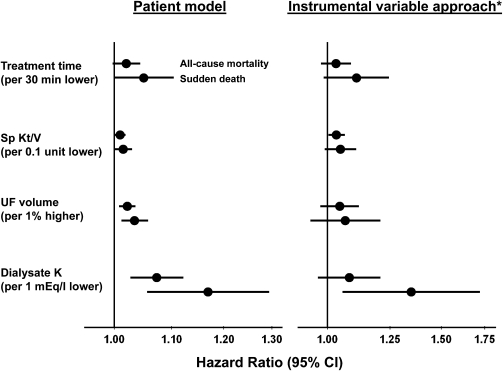
Higher risk of all-cause mortality and sudden death was also found when the four dialysis practices were modeled as continuous variables (Figure 3).
Figure 3.
Association of treatment practices with sudden death. Adjusted for variables listed in Table 1 and in the Materials and Methods, stratified by phase and country and accounted for facility clustering. *Used predicted treatment from first stage. IV, instrumental variable; UF, ultrafiltration; 95% CI, 95% confidence interval.
Medications
Higher risk of sudden death was evident for amiodarone prescription (HR, 1.44; 95% CI, 1.16–1.81; P=0.001), but not for prescription of other QTI-prolonging drugs (HR, 1.10; 95% CI, 0.94–1.28; P=0.22). β blockers were associated with a lower risk of sudden death (HR, 0.88; 95% CI, 0.78–0.99; P=0.03).
Sensitivity Analyses
Our findings were confirmed in a series of sensitivity analyses. Similar associations between dialysis practices and mortality were found in the following: in models restricted to prevalent patients (time on dialysis >6 months) and to “healthier” patients (nondiabetic participants aged <65 years); when observed causes of death were used in place of imputed causes of death; when all four practices were included in the same Cox model, indicating that the association of each practice with sudden death was independent of the other three; and when deaths attributed to hyperkalemia were excluded (data not shown).
Discussion
Among 6610 patients with a recorded cause of death in our study, 1734 (26%) deaths were sudden, similar to previous reports on sudden death in US hemodialysis patients (1,2). However, our DOPPS results demonstrate for the first time substantial differences between countries in the proportion of deaths that are reported as sudden: 33.4% in the United States, 17.5% in Canada, 17.8% in Europe, 19.2% in Australia and New Zealand, and 23.2% in Japan. More importantly, our analysis identified several modifiable practices associated with higher sudden death risk. These include short treatment time, excessive ultrafiltration volume, and low Kt/V (most evident in instrumental variable analysis). A low KD level was also associated with higher sudden death risk, which was most evident in patients with a relatively low (<5) predialysis potassium serum level.
The finding that low KD may trigger some sudden death in hemodialysis patients was previously suggested by the case series of Karnik et al. (27) and Bleyer et al. (4). These authors showed that among patients on hemodialysis who eventually suffered sudden death, the proportion of patients on K0 or K1 dialysate was much higher than in controls. In addition, it has long been known that the relative hypokalemia at the end of hemodialysis sessions and/or the electrolyte shifts during hemodialysis may trigger various arrhythmias, including ventricular ones (3). More recently, Genovesi et al. demonstrated in a small-sized crossover study in 16 hemodialysis patients that K2 dialysate was associated, compared with K3 dialysate, with a longer duration of QTIs corrected for heart rate (28), a risk factor for sudden death in the general population. A recent study of a series of cardiac arrests at outpatient dialysis centers further showed that cardiac arrests were mostly related to ventricular fibrillation, especially those occurring during or shortly after hemodialysis (29). More recently, Kovesdy et al. (30) studied the association between predialysis serum potassium and survival in a cohort of US hemodialysis patients. The best survival was observed in patients with predialysis potassium ranging from 4.6 to 5.3, but these authors were unable to study the effect of either predialysis potassium or KD on sudden death. Similarly, Al-Ghamdi et al. (31) did not detect, after adjustment for malnutrition and inflammation, any association between KD and overall risk of death in 1267 hemodialysis patients from North Alberta. Finally, Pun et al. (6) recently reported that KD <2 was a risk factor for sudden cardiac arrest within hemodialysis clinics in a case-control study. Our results extend those of Pun et al. to the international cohort of DOPPS and demonstrate major differences in sudden death risk and typical KD level between DOPPS countries (Figures 1B and 2D). Most importantly, our findings indicate that the higher mortality risk occurs with KD <3, rather than only <2. The implications of this finding cannot be overemphasized because the median KD level in this study was well <3 in all countries. Finally, we analyzed all sudden death, rather than only sudden cardiac arrest within hemodialysis units; our results are relevant for the potential prevention of many more sudden deaths than those of Pun et al. (6).
In contrast to the suggestion of the recent editorial (32) discussing strategies toward preventing sudden death in hemodialysis patients, our results do not necessarily imply completely banishing low KD (≤2) from hemodialysis units. Both patient-level and instrumental variable analyses, however, indicate that the prescription of KD ≥3 may reduce the risk of sudden death, especially in patients with predialysis potassium level <5. At the same time, the optimal strategy for patients with significant hyperkalemia, especially >6 and recurrent hyperkalemia, remains unclear (in our data, too few patients had both serum potassium >6 and high KD ≥3 for us to study this treatment approach). The challenge in such patients is to avoid both life-threatening predialysis hyperkalemia and postdialysis relative hypokalemia (or at least very rapid decrease of serum potassium level, and the related risk of lethal arrhythmias). Resins such as kayexalate may be used, but may have their own risks (33). Alternative strategies, such as more frequent hemodialysis sessions, may be required in such cases. These possibilities merit additional study.
The mechanisms underlying the risk of sudden death associated with excessively low Kt/V, short treatment time, and large ultrafiltration volume are potentially multiple: they include rapid electrolyte and/or volume shifts, potentially contributing to arrhythmias but also to repeated myocardial stunning and derived injury (34), as well as the persistent accumulation of uremic toxins. Our results on the risk of sudden death associated with short treatment time and low Kt/V are in line with recent DOPPS results on the risk of overall mortality associated with the same characteristics (35). As previously reported in other cohorts (27), the risk of sudden death among DOPPS participants is higher after the long dialysis interval, that is, on Monday for a Monday, Wednesday, and Friday hemodialysis schedule or Tuesday for a Tuesday, Thursday, and Saturday hemodialysis schedule (36). Volume and/or electrolyte abnormalities may contribute to this association, in keeping with the findings presented herein. Interestingly, the authors of the recent Frequent Hemodialysis Network trial reported a significant reduction of left ventricular mass in hemodialysis patients randomized to more frequent (six times per week) hemodialysis versus those remaining on the conventional thrice-weekly schedule (37), a finding again supporting the deleterious effect of large, relatively rapid volume shifts on the heart of hemodialysis patients.
Most previous studies on the risk of sudden death focused on patient characteristics associated with sudden death. These include, not surprisingly, heart failure (4), coronary artery disease, left ventricular hypertrophy (38), inflammation malnutrition complex (2,39), and poor glycemic control (40). We also observed higher risk of sudden death for cardiovascular comorbidities (including congestive heart failure, coronary artery disease, and other cardiovascular and cerebrovascular diseases) and inflammatory markers including ferritin and white blood cell count (data not shown). Except for glycemic control, all of these characteristics are difficult to readily modify in the dialysis unit, in contrast to the dialysis practices predicting sudden death on which our analysis is focused.
This study is the first, to our knowledge, to assess in hemodialysis patients the association between sudden death and the prescription of drugs prolonging the QTI on the electrocardiogram. A potential deleterious effect had been repeatedly proposed by experts in the field (1,41,42) and appeared very plausible in view of the well known polypharmacy of the current hemodialysis population, including many drugs either prolonging the QTI or reducing the metabolism of the former category of drugs. Despite the attractiveness of the hypothesis, we were unable to demonstrate for most of these drugs that their use contributes to the risk of sudden death in hemodialysis patients. This may be due to their low use overall, despite the large size of the database. On the other hand, β blockers were associated with a lower risk of sudden death, in line with their capacity to counteract the increased sympathetic activity common in hemodialysis patients (1,42). This finding is also consistent with previously reported associations of β blockers with lower all-cause and cardiovascular mortality (43). The association of a higher risk of sudden death with amiodarone likely reflects a prescription by indication bias.
This study has multiple strengths, including the large sample size, nationally representative patient samples, prospective data collection, and extensive adjustment for patient characteristics, comorbidities, and laboratory results. In addition, the detailed DOPPS medication database made it possible for the first time to study the effect of drugs that prolong the QTI on the risk of sudden death. Furthermore, all sensitivity analyses show consistent associations between the modifiable practices and sudden death. These associations, obtained using baseline exposures, were consistent over the duration of study follow-up. Finally, the use of facility-level instrumental variable analyses, with results that were qualitatively consistent with the standard patient-level analyses, tends to lessen the risk of patient-level confounding by indication. Here, we utilize facilities as natural “instruments,” mimicking a natural experiment in which patients are “pseudo-randomly” assigned. The idea is that patients at different facilities tend to receive different levels of treatment for reasons not associated with patients’ outcome or patient-level unmeasured confounders, after accounting for patients’ observed characteristics. Interestingly, although the directions of the estimates obtained from the instrumental variable analyses and the patient-level analyses were consistent, in several scenarios the instrumental variable estimates were appreciably larger (e.g., for low KD) (Table 3 and Figure 3). Although these differences may be because the IV approach reduced bias due to unmeasured confounders, we also cannot exclude the possibility of bias from violation of instrumental variable assumptions, such as the possibility of facility-level confounding.
Several limitations must be acknowledged. As is true of any observational study, we are not able to claim causality. We also note that the number of patients who had received an implantable cardiac defibrillator was too small to study the effect of their use on the risk of sudden death (44). In addition, as in any large clinical database, some errors may be present in coding (e.g., cause of death) but these should have favored the null hypothesis, whereas we observed in both patient-level and facility-level analysis consistent associations between some modifiable practices and sudden death, as coded. It should also be mentioned that we did not have information on the time interval between the end of the last hemodialysis session and sudden death. Finally, we did not have electrocardiographic data regarding the presence of left ventricular hypertrophy (38) or prolonged QTI (28), nor potentially relevant biomarker data. Adiponectin levels (39), vitamin D levels (45), and antiheparin antibodies (39) have been implicated as potential contributors to sudden death and/or cardiovascular mortality in hemodialysis patients with diabetes in post hoc analyses of the Die Deutsche Diabetes Dialyse trial.
We have identified several readily modifiable facility practices associated with an independent risk of sudden death, both in standard patient-level analyses and in instrumental variable analyses based on facility variation in practice. Specifically, short treatment time, low dialysis dose, high ultrafiltration volume, and low KD are associated with sudden death as well as overall mortality. Because low KD (<3) is commonly used and it is the easiest practice to modify, our study suggests that more careful adaptation of KD level may help minimize the effect of sudden death observed in hemodialysis patients. Clinical trials are needed to test this possibility, and may be feasible for practices such as KD in which substantial variation in care exists and clinical uncertainty remains significant.
Disclosures
J.T., D.S.F., F.T., Y.L., F.P., and B.M.R. are employed by Arbor Research Collaborative for Health. D.M. has received speaker fees from Amgen, Ortho Biotech, and Genzyme; serves on the advisory boards of Amgen, Ortho Biotech, Genzyme, Shire, Baxter, Novartis, and Takeda; and has research grants from Ortho Biotech (principal investigator, multicenter studies) and Amgen (site investigator).
Acknowledgments
Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health, helped edit this paper.
The DOPPS is administered by Arbor Research Collaborative for Health and is supported by scientific research grants from Amgen, Kyowa Hakko Kirin, Genzyme, Abbott, and Baxter, without restrictions on publications.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Herzog CA, Mangrum JM, Passman R: Sudden cardiac death and dialysis patients. Semin Dial 21: 300–307, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Parekh RS, Plantinga LC, Kao WHL, Meoni LA, Jaar BG, Fink NE, Powe NR, Coresh J, Klag MJ: The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int 74: 1335–1342, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Morrison G, Michelson EL, Brown S, Morganroth J: Mechanism and prevention of cardiac arrhythmias in chronic hemodialysis patients. Kidney Int 17: 811–819, 1980 [DOI] [PubMed] [Google Scholar]
- 4.Bleyer AJ, Hartman J, Brannon PC, Reeves-Daniel A, Satko SG, Russell G: Characteristics of sudden death in hemodialysis patients. Kidney Int 69: 2268–2273, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Bleyer AJ, Russell GB, Satko SG: Sudden and cardiac death rates in hemodialysis patients. Kidney Int 55: 1553–1559, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Pun PH, Lehrich RW, Honeycutt EF, Herzog CA, Middleton JP: Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int 79: 218–227, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Young EW, Goodkin DA, Mapes DL, Port FK, Keen ML, Chen K, Maroni BL, Wolfe RA, Held PJ: The Dialysis Outcomes and Practice Patterns Study (DOPPS): An international hemodialysis study. Kidney Int 57[Suppl 74]: S74–S81, 2000 [Google Scholar]
- 8.Liu BA, Juurlink DN: Drugs and the QT interval - caveat doctor. N Engl J Med 351: 1053–1056, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Arizona CERT: QT drug lists. Available at: http://www.azcert.org Accessed January 2011
- 10.Angrist JD, Imbens GW, Rubin DB: Identification of causal effects using instrumental variables. J Am Stat Assoc 91: 444–455, 1996 [Google Scholar]
- 11.Wooldridge J: Introductory Econometrics: A Modern Approach, 4th Ed., Mason, OH, Cengage South-Western, 2002 [Google Scholar]
- 12.Newhouse JP, McClellan M: Econometrics in outcomes research: The use of instrumental variables. Annu Rev Public Health 19: 17–34, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Greenland S: An introduction to instrumental variables for epidemiologists. Int J Epidemiol 29: 722–729, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Angrist JD, Pischke JS: Mostly Harmless Econometrics: An Empiricist's Companion, Princeton, NJ, Princeton University Press, 2008 [Google Scholar]
- 15.Bradbury BD, Fissell RB, Albert JM, Anthony MS, Critchlow CW, Pisoni RL, Port FK, Gillespie BW: Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2: 89–99, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Pisoni RL, Arrington CJ, Albert JM, Ethier J, Kimata N, Krishnan M, Rayner HC, Saito A, Sands JJ, Saran R, Gillespie B, Wolfe RA, Port FK: Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: An instrumental variable analysis. Am J Kidney Dis 53: 475–491, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ: Analysis of observational studies in the presence of treatment selection bias: Effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA 297: 278–285, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneeweiss S, Seeger JD, Landon J, Walker AM: Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med 358: 771–783, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Ramirez SP, Albert JM, Blayney MJ, Tentori F, Goodkin DA, Wolfe RA, Young EW, Bailie GR, Pisoni RL, Port FK: Rosiglitazone is associated with mortality in chronic hemodialysis patients. J Am Soc Nephrol 20: 1094–1101, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tentori F, Albert JM, Young EW, Blayney MJ, Robinson BM, Pisoni RL, Akiba T, Greenwood RN, Kimata N, Levin NW, Piera LM, Saran R, Wolfe RA, Port FK: The survival advantage for haemodialysis patients taking vitamin D is questioned: Findings from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 24: 963–972, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Pisoni RL, Bragg-Gresham JL, Fuller DS, Morgenstern H, Canaud B, Locatelli F, Li Y, Gillespie B, Wolfe RA, Port FK, Robinson BM: Facility-level interpatient hemoglobin variability in hemodialysis centers participating in the Dialysis Outcomes and Practice Patterns Study (DOPPS): Associations with mortality, patient characteristics, and facility practices. Am J Kidney Dis 57: 266–275, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Stock JH, Wright JH, Yogo M: A survey of weak instruments and weak identification in generalized method of moments. J Bus Econ Stat 20: 518-529, 2002 [Google Scholar]
- 23.Staiger D, Stock JH: Instrumental variables regression with weak instruments. Econometrica 65: 557–586, 1997 [Google Scholar]
- 24.Raghunathan TE, Lepkowski JM, van Hoewyk J, Solenberger P: A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol 27: 85–95, 2001 [Google Scholar]
- 25.Rubin DB: Multiple Imputation for Nonresponse in Surveys, New York, John Wiley & Sons, 1987 [Google Scholar]
- 26.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 370: 1453–1457, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Karnik JA, Young BS, Lew NL, Herget M, Dubinsky C, Lazarus JM, Chertow GM: Cardiac arrest and sudden death in dialysis units. Kidney Int 60: 350–357, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Genovesi S, Dossi C, Viganò MR, Galbiati E, Prolo F, Stella A, Stramba-Badiale M: Electrolyte concentration during haemodialysis and QT interval prolongation in uraemic patients. Europace 10: 771–777, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Davis TR, Young BA, Eisenberg MS, Rea TD, Copass MK, Cobb LA: Outcome of cardiac arrests attended by emergency medical services staff at community outpatient dialysis centers. Kidney Int 73: 933–939, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Kovesdy CP, Regidor DL, Mehrotra R, Jing J, McAllister CJ, Greenland S, Kopple JD, Kalantar-Zadeh K: Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol 2: 999–1007, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Al-Ghamdi G, Hemmelgarn B, Klarenbach S, Manns B, Wiebe N, Tonelli M, Alberta Kidney Disease Network : Dialysate potassium and risk of death in chronic hemodialysis patients. J Nephrol 23: 33–40, 2010 [PubMed] [Google Scholar]
- 32.Young BA: Prevention of sudden cardiac arrest in dialysis patients: Can we do more to improve outcomes? Kidney Int 79: 147–149, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Sterns RH, Rojas M, Bernstein P, Chennupati S: Ion-exchange resins for the treatment of hyperkalemia: Are they safe and effective? J Am Soc Nephrol 21: 733–735, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin J Am Soc Nephrol 4: 914–920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saran R, Bragg-Gresham JL, Levin NW, Twardowski ZJ, Wizemann V, Saito A, Kimata N, Gillespie BW, Combe C, Bommer J, Akiba T, Mapes DL, Young EW, Port FK: Longer treatment time and slower ultrafiltration in hemodialysis: Associations with reduced mortality in the DOPPS. Kidney Int 69: 1222–1228, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Schaubel DE, Kalbfleish JD, Bragg-Gresham JL, Robinson B, Pisoni RL, Canaud B, Jadoul M, Akiba T, Saito A, Saran R: Relationship between dialysis schedule and day-of-week association with mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS) [abstract]. J Am Soc Nephrol 19: 699A, 2008 [Google Scholar]
- 37.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS, FHN Trial Group : In-center hemodialysis six times per week versus three times per week. N Engl J Med 363: 2287–2300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krane V, Heinrich F, Meesmann M, Olschewski M, Lilienthal J, Angermann C, Störk S, Bauersachs J, Wanner C, Frantz S, German Diabetes and Dialysis Study Investigators : Electrocardiography and outcome in patients with diabetes mellitus on maintenance hemodialysis. Clin J Am Soc Nephrol 4: 394–400, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drechsler C, Krane V, Winkler K, Dekker FW, Wanner C: Changes in adiponectin and the risk of sudden death, stroke, myocardial infarction, and mortality in hemodialysis patients. Kidney Int 76: 567–575, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Drechsler C, Krane V, Ritz E, März W, Wanner C: Glycemic control and cardiovascular events in diabetic hemodialysis patients. Circulation 120: 2421–2428, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Gussak I, Gussak HM: Sudden cardiac death in nephrology: Focus on acquired long QT syndrome. Nephrol Dial Transplant 22: 12–14, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Ritz E, Wanner C: The challenge of sudden death in dialysis patients. Clin J Am Soc Nephrol 3: 920–929, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Lopes AA, Bragg-Gresham JL, Ramirez SPB, Andreucci VE, Akiba T, Saito A, Jacobson SH, Robinson BM, Port FK, Mason NA, Young EW: Prescription of antihypertensive agents to haemodialysis patients: Time trends and associations with patient characteristics, country and survival in the DOPPS. Nephrol Dial Transplant 24: 2809–2816, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Herzog CA, Li S, Weinhandl ED, Strief JW, Collins AJ, Gilbertson DT: Survival of dialysis patients after cardiac arrest and the impact of implantable cardioverter defibrillators. Kidney Int 68: 818–825, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Drechsler C, Pilz S, Obermayer-Pietsch B, Verduijn M, Tomaschitz A, Krane V, Espe K, Dekker F, Brandenburg V, März W, Ritz E, Wanner C: Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J 31: 2253–2261, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]