Summary
Background and objectives
Fibroblast growth factor-23 (FGF23) regulates mineral metabolism. Circulatory FGF23 levels are increased and predict outcomes in CKD. However, the relation of FGF23 to albuminuria and disease progression in patients with CKD and one underlying diagnosis is unknown.
Design, setting, participants, & measurements
Prospective, observational study in 180 patients with IgA nephropathy (IgAN), CKD stage 1–4, and median 55-month follow-up (range, 12–177 months). Primary outcomes were (1) time-averaged albuminuria, (2A) progression to CKD stage 5 or ≥50% loss of estimated GFR, (2B) progression to CKD stage 5 or ≥25% loss of estimated GFR within 10 years, and (3) annual loss of estimated GFR.
Results
FGF23 was independently associated with baseline and time-averaged albuminuria (change in 1 g/24 hour albuminuria per increase in log FGF23: β = 0.26; P=0.02). Log FGF23 predicted CKD progression in crude models and after adjustment for mineral metabolites (endpoints 2A and 2B). It remained significant after adjustments for age, sex, serum albumin, calcium, phosphate, parathyroid hormone, 25-hydroxyvitamin D, baseline albuminuria, baseline estimated GFR, mean arterial BP, body mass index, and angiotensin-converting enzyme inhibitors/angiotensin-receptor blocker use in endpoint 2B (hazard ratio, 2.53; P=0.02) but not endpoint 2A (hazard ratio, 2.01; P=0.43). Log FGF23 predicted annual loss of estimated GFR in the same model (change in ml/min per 1.73 m2 per increase in log FGF23, 1.50; P=0.008).
Conclusions
In patients with CKD and IgAN, FGF23 was associated with albuminuria and CKD progression, a finding that suggests its role as a potential biomarker in IgAN.
Introduction
Fibroblast growth factor-23 (FGF23) is a circulating hormone that plays a vital role in the systemic regulation of mineral metabolism by promoting renal phosphate excretion and reducing the synthesis of active 1,25-dihydroxyvitamin D (1,25 [OH] vitamin D) by direct actions on the kidney (1,2). In CKD, renal excretion of phosphate is compromised, leading to a compensatory increase in FGF23 level and development of secondary hyperparathyroidism (3,4). Mild increments in FGF23 are commonly detected in CKD stage 2–3, whereas its circulatory levels are markedly elevated in ESRD (5–9).
High systemic FGF23 levels, both in patients with CKD and those without, are linked to adverse outcomes, including cardiovascular disease (CVD) and death (10–12). It remains to be elucidated whether FGF23 per se is toxic to the cardiovascular system or whether FGF23 is merely a biomarker of multiple biochemical and metabolic derangements that parallel the progression of CKD. Recent studies indicate that the predictive value of FGF23 is higher and more consistently associated with outcomes than are other previously reported risk factors within mineral metabolism, such as hyperphosphatemia and elevated parathyroid hormone (PTH) (10,11). Experimental data favor a direct pathophysiologic role of the FGF23 co-receptor Klotho in renal fibrosis and vascular calcification (13,14), potentially explaining its relation to CKD progression and CVD.
Albuminuria is a major cardiovascular risk factor both in the community (15,16) and in the CKD population (17). Despite the linkage of FGF23 to structural or dynamic cardiovascular changes, such as endothelial dysfunction, atherosclerosis, and left ventricular hypertrophy (7,8,18–21), its relation to albuminuria has not been systematically examined. Further, the predictive value of FGF23 for CKD progression has been evaluated in heterogeneous cohorts but not in patients with a single renal diagnosis.
Materials and Methods
Patient Cohort
Study participants were 180 patients with CKD stage 1–4, a renal diagnosis of IgA nephropathy (IgAN), and a follow-up time of at least 1 year. Median time from diagnosis (i.e., renal biopsy) to inclusion was 23 months (range, 4–93 months). This evaluation is a post hoc analysis based on an ongoing prospective study in patients with a biopsy-confirmed diagnosis of IgAN treated at Karolinska University Hospital or Danderyd University Hospital, Stockholm, Sweden. Exclusion criteria were inconclusive biopsy findings; an overlapping histopathologic diagnosis; or a concomitant clinical diagnosis of diabetes, malignancy, and chronic rheumatologic or inflammatory bowel disease. Twenty-three patients (13%) had a diagnosis of IgAN with initial manifestations of Henoch-Schönlein purpura.
Most patients were receiving antihypertensive medications at time of inclusion: angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin-receptor blockers (ARBs) (73%) or any antihypertensive drug (76%). Three percent of the patients were taking calcitriol supplementation; 2%, phosphate binders; 17%, hydroxymethylglutaryl coenzyme A reductase inhibitors; 8%, fish oil; and 4%, immunosuppressive treatment with prednisolone. At last follow-up, the proportion of patients receiving ACEIs or ARBs had increased to 81%, and the proportion receiving any antihypertensive drug had increased to 83%; 13% were taking vitamin D receptor activators, 29%, statins; and 17%, fish oil. Further, 9% of the patients had been treated with immunosuppressives before baseline evaluation and 7% after. ACEI or ARB therapy was withdrawn in seven patients (4%) during the study. Five patients had CVD at baseline (ischemic heart disease, valvular stenosis, arrhythmia, stroke).
The Ethics Committee of Karolinska University Hospital, Stockholm, Sweden, approved the study protocol, and informed consent was obtained from all patients.
Biochemical Analysis
Blood samples were drawn after an overnight fast at baseline, and routine biochemistries were analyzed immediately. Additional blood samples were spun and serum stored in −70°C until further analysis. For the assessment of albuminuria, patients had provided a 24-hour urine sample (30%) or a morning urine sample for analysis of the urine albumin-to-creatinine ratio (reference < 3.0 mg/mmol). From the latter samples, 24-hour albuminuria was calculated using the Cockcroft-Gault formula in accordance with Fournier and Achard (22). FGF23 was measured using C-terminal ELISA (Immutopics, San Clemente, CA). 25(OH) vitamin D was analyzed with the IDS 25-Hydroxy Vitamin D RIA kit (immunodiagnosticsystems, Boldon, UK) Calcium was corrected for serum albumin using the following equation:
| albumin-corrected \ calcium = serum \ calcium + 0.02 × (40 − serum \ albumin). |
GFR was estimated by four-parameter Modification of Diet in Renal Disease (MDRD) equation (23) and by CKD Epidemiology Collaboration (CKD-EPI) equation (24).
Follow-up Data
Follow-up data were collected from the patient’s records once yearly, and the number of follow-up points ranged from 1 to 15. Some patients had their clinical visits more rarely because of a benign prognosis or low attendance (n=18). Time-averaged albuminuria was calculated as the average of all follow-up values.
Definition of Renal Disease Progression
Disease progression was defined a priori by two different endpoints: (2A) entering ESRD (CKD stage 5) or ≥50% reduction in estimated GFR (eGFR) and (2B) entering ESRD or ≥25% reduction in eGFR by 10 years. Endpoint 2B was predefined to reduce the number of patients misclassified because of a limited follow-up period.
The rate of decline in renal function was expressed as the yearly slope of eGFR, which was obtained by fitting a straight line through the calculated eGFR values, using linear regression and the principal of least squares. All patients with at least three measurements of serum creatinine during follow-up (n=175) were included in this analysis.
Statistical Analyses
Values are expressed as mean ± SD or median (interquartile range) as appropriate unless otherwise indicated. Nominal variables are expressed as percentages. A Spearman rank test was used for all univariate correlations. FGF23 and PTH were natural log–transformed in continuous models because of their skewed distribution. In a multiple regression model, the correlation of log FGF23 to time-averaged albuminuria and to the annual loss of eGFR (negative eGFR slope) was examined after stepwise inclusion of possible confounders. Survival analyses were made with the Kaplan-Meier survival curve and the Cox proportional hazards model, comparing highest tertile versus middle and lowest tertiles of FGF23 as a categorical variable and using log FGF23 as a continuous variable. Because of the limited number of events, we selected the models according to known confounders of mineral metabolism (model 1) or established progression risk factors (model 2). In primary analysis, we adjusted for a maximum of four variables in the different models, choosing only variables that were predictive at a significant level in crude analysis. A P value less than 0.05 was regarded as representing a statistically significant difference. Stata software, version 12 (Stata Corp., College Station, TX), was used for all calculations.
Results
Baseline Analysis
Clinical and biochemical characteristics of the study cohort are presented in Table 1. At baseline, median FGF23 values were 18.5 relative units [RU]/ml (range, 5.4– 138.7 RU/ml) and median albuminuria was 0.3 g/24 hours (range, 0.0–7.8 g/24 hours). FGF23 was significantly correlated with albuminuria (r = 0.36; P<0.001), serum albumin (r = −0.33; P<0.001), creatinine (r = 0.45; P<0.001), eGFR (r = −0.39; P<0.001), calcium (r = 0.19; P=0.01), PTH (r = 0.29; P<0.001), mean arterial BP (MAP) (r =0.16; P=0.04), body mass index (BMI) (r = 0.17; P=0.02), and the number of antihypertensive drugs used (r = 0.21; P=0.004). There was no association between FGF23 and phosphate, 25(OH) vitamin D, age, or systolic or diastolic BP. Patients who were treated with ACEIs or ARBs had higher FGF23 levels (20.0 RU/ml [interquartile range, 14.2–29.8 RU/ml]) than those without treatment (15.9 RU/ml [interquartile range, 11.7–22.2 RU/ml]; P=0.004).
Table 1.
Baseline clinical and biochemical characteristics of the IgAN patient cohort
| Clinical and Biochemical Measures | Total Cohort (n=180) |
|---|---|
| Age (yr) | 41 (19–75) |
| Men/women (%) | 71/29 |
| Systolic BP (mmHg) | 131±15 |
| Diastolic BP (mmHg) | 81±10 |
| Mean arterial BP (mmHg) | 98 (93– 103) |
| Body mass index (kg/m2) | 24.9 (23.0–28.2) |
| eGFR (mL/min per 1.73 m2) | 71 (54–85) |
| CKD stage at baseline (%) | |
| 1 (eGFR ≥90 ml/min per 1.73 m2) | 20 |
| 2 (eGFR 60–89 ml/min per 1.73 m2) | 48 |
| 3 (eGFR 30–59 ml/min per 1.73 m2) | 27 |
| 4 (eGFR 15–29 ml/min per 1.73 m2) | 5 |
| Creatinine (μM) | 96 (81–120) |
| Calcium (mM) | 2.33±0.10 |
| Phosphate (mM) | 1.21±0.24 |
| PTH (ng/L) | 43.0 (28.8–59.8) |
| FGF23 (RU/mL) | 18.5 (12.9–25.7) |
| 1,25-dihydroxyvitamin D (ng/L) | 48.1±18.2 |
| Serum albumin (g/L) | 41 (39–44) |
| Albuminuria (g/24 hr) | 0.30 (0.10–0.97) |
| Antihypertensive drugs (n) | 1.0 (range, 0–6) |
Values are reported as mean ± SD or median (interquartile range) as appropriate unless otherwise stated. Estimated glomerular filtration rate (eGFR) is derived by the Modification of Diet in Renal Disease formula. PTH, parathyroid hormone; FGF23, fibroblast growth factor-23.
FGF23 and Time-Averaged Albuminuria during Follow-Up
Patients had an average of 4.4 albuminuria measurements during follow-up (median time-averaged albuminuria, 0.18 g/24 hours [range, 0–7.4 g/24 hours]). FGF23 correlated significantly with time-averaged albuminuria (r = 0.32; P<0.001), and log FGF23 remained a predictor of time-averaged albuminuria in multivariate models adjusting for age, sex, serum albumin, calcium, phosphate, PTH, 25(OH) vitamin D, baseline albuminuria, baseline eGFR, MAP, BMI, and the use of ACEIs or ARBs (Table 2).
Table 2.
Multivariate regression model predicting time-averaged albuminuria (g/24 hr)
| Variable | Regression Coefficient for Change in Time-Averaged Albuminuria (95% CI) | P Value for Trend |
|---|---|---|
| Per increase in log FGF23 (RU/ml) | 0.26 (0.05–0.47) | 0.02 |
| Per 10 ml/min/1.73 m2 of eGFR | −0.05 (−0.10 to −0.005) | 0.03 |
| Per 0.1 mmol/L of calcium | −0.24 (−0.35 to −0.13) | <0.001 |
| Per 1 g/24 hours of albuminuria | 0.45 (0.34–0.57) | <0.001 |
In a stepwise regression model log, fibroblast growth factor-23 (FGF23), age, sex, serum albumin, baseline albuminuria, baseline estimated GFR (eGFR), calcium, phosphate, parathyroid hormone, 25-hydroxyvitamin D, mean arterial BP, body mass index, and use of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers were included. Log FGF23, eGFR, calcium, and albuminuria were retained as significant (adjusted R2 = 0.44 for the whole model, P<0.001). The regression coefficients are adjusted for the listed variables. CI, confidence interval; RU, relative units.
Renal Survival Analysis Using Endpoint A
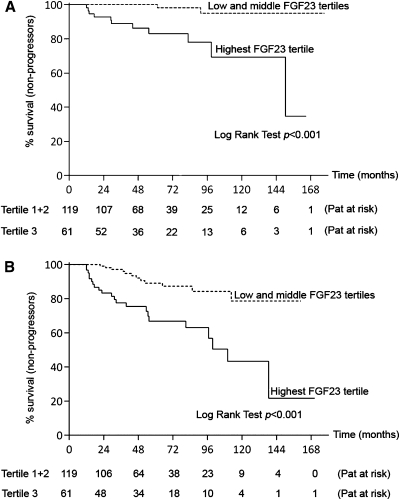
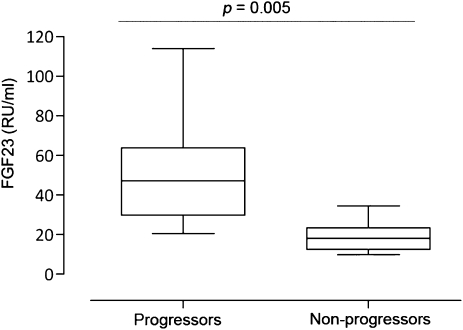
During follow-up (median, 55 months [range, 12–177 months]), 15 patients (8%) were defined as progressors. In Kaplan-Meier analysis, FGF23 levels in the highest tertile (>23 RU/ml) were significantly associated with CKD progression (Figure 1A). In Cox proportional hazards model, log FGF23 was associated with disease progression after adjustment for factors of mineral metabolism (model 1), for baseline eGFR and MAP, or for baseline albuminuria alone, but not after adjustment for a combination of these factors (model 2) (Table 3). However, log FGF23 was an independent predictor of outcome in each model when using the CKD-EPI formula for eGFR estimation, which identified a total of 17 patients (9%) reaching endpoint 2A (Table 3). Log FGF23 did not predict outcome 2A in a secondary model adjusting for age, sex, serum albumin, calcium, phosphate, PTH, 25(OH) vitamin D, baseline albuminuria, baseline eGFR, MAP, BMI, and the use of ACEIs or ARBs (hazard ratio [HR], 2.01 [95% confidence interval (CI), 0.35–11.50], P=0.43 using MDRD; HR, 2.17 [95% CI, 0.52–9.10], P=0.29 using CKD-EPI). The median FGF23 level was significantly higher in the progressor than the nonprogressor group (Figure 2). Inspired by the prognosis model by Berthoux et al. (25), we calculated a risk score that could be used during clinical follow-up, including the presence of hypertension, albuminuria ≥1 g/d, and FGF23 levels >23 RU/mL. Ten of 22 patients with all three risk factors at baseline (45%) reached endpoint 2A (HR compared with 0–2 risk factors, 20.53 [95% CI, 6.43–65.54]; P<0.001).
Figure 1.
Higher fibroblast growth factor-23 (FGF23) levels are associated with increased CKD progression. Kaplan-Meier survival analysis shows that higher FGF23 levels are associated with faster CKD progression, defined as (A) entering CKD stage 5 or ≥50% reduction in eGFR or (B) entering CKD stage 5 or ≥25% reduction in estimated GFR within 10 years. Pat, patients.
Table 3.
Relation of FGF23 to CKD disease progression
| Endpoint | Hazard Ratio (95% CI) | ||||
|---|---|---|---|---|---|
| Crude | Adjusted for Baseline eGFRa and MAP | Adjusted for Baseline Albuminuria | Model 1 | Model 2 | |
| A | |||||
| highest FGF23 tertile versus otherb | 12.53 (2.82–55.6) (P<0.001) | 3.95 (0.83–18.70) (P=0.08) | 7.47 (1.60–35.01) (P=0.01) | 5.49 (1.16–25.94) (P=0.03) | 2.21 (0.42–11.57) (P=0.35) |
| log FGF23 as continuous variableb | 11.07 (4.67–26.21) (P<0.001) | 3.70 (1.31–10.41) (P=0.01) | 7.52 (3.22–17.5) (P<0.001) | 4.07 (1.38–12.07) (P=0.01) | 2.72 (0.92–8.07) (P=0.07) |
| log FGF23, using CKD-EPI eGFR equationc | 8.59 (3.92–18.84) (P<0.001) | 3.84 (1.50–9.82) (P=0.005) | 6.13 (2.78–13.42) (P<0.001) | 3.68 (1.37–9.85) (P=0.01) | 3.05 (1.13–8.20) (P=0.03) |
| B | |||||
| highest FGF23 tertile versus otherb | 3.92 (1.94–7.92) (P<0.001) | 2.28 (1.06–4.90) (P=0.03) | 3.02 (1.41–6.50) (P=0.005) | 2.32 (1.09–4.94) (P=0.03) | 1.99 (0.86–4.60) (P=0.11) |
| log FGF23 as continuous variableb | 4.58 (2.66–7.86) (P<0.001) | 2.92 (1.55–5.53) (P<0.001) | 3.90 (2.22– 6.82) (P<0.001) | 2.74 (1.43–5.24) (P=0.002) | 2.94 (1.51–5.74) (P=0.002) |
| log FGF23, using CKD-EPI eGFR equationc | 4.21 (2.52–6.99) (P<0.001) | 2.91 (1.61–5.27) (P<0.001) | 3.62 (2.14–6.12) (P<0.001) | 2.58 (1.40–4.76) (P=0.002) | 2.88 (1.54–5.36) (P<0.001) |
Relation in serum fibroblast growth factor-23 (FGF23) to CKD progression, defined as entering CKD stage 5 or ≥50% reduction in estimated GFR (eGFR; endpoint A) or progression to CKD stage 5 or ≥25% reduction in eGFR over 10 years (endpoint B) in Cox proportional hazards models. FGF23 in the upper tertile as categorical variable and log-transformed FGF23 as continuous variable were adjusted for factors that had been predictive in crude analysis: model 1, factors of mineral metabolism (albumin, phosphate, log parathyroid hormone); model 2, established risk factors for CKD progression (baseline eGFR, baseline albuminuria, and mean arterial BP [MAP]). CI, confidence interval; CKD-EPI, CKD Epidemiology Collaboration.
By 10 ml/min per 1.73 m2.
Using four-measure Modification of Diet in Renal Disease eGFR equation, with 15 patients (8%) reaching endpoint A and 34 patients (19%) reaching endpoint B,
Using CKD Epidemiology Collaboration eGFR equation, which identified 17 patients (9%) as reaching endpoint A and 39 patients (22%) reaching endpoint B.
Figure 2.
FGF23 levels in patients who progressed to CKD stage 5 or >50% loss of estimated GFR compared with levels in nonprogressors. The lines in the box plots and the error bars are median and 10–90 percentiles.
Renal Survival Analysis Using Endpoint B
During follow-up (median, 52 months [range, 12–171 months]), 34 patients (19%) were defined as progressors. In Kaplan-Meier analysis, FGF23 levels in the highest tertile were associated with CKD progression (Figure 1B). Log FGF23 remained significantly associated with endpoint 2B using the same multivariable models as for endpoint 2A. Using the CKD-EPI equation for eGFR identified 39 patients (22%) reaching endpoint 2B and slightly reinforced the results (Table 3). Of note, FGF23 predicted outcome 2B in a secondary model adjusting for age, sex, serum albumin, calcium, phosphate, PTH, 25(OH) vitamin D, baseline albuminuria, baseline eGFR, MAP, BMI, and the use of ACEIs or ARBs (HR, 2.53 [95% CI, 1.14–5.60], P=0.02 using MDRD; HR, 2.41 [95% CI, 1.15–5.02], P=0.02 using CKD-EPI). The HR for endpoint 2B in the subgroup with the combined risk factors hypertension, albuminuria ≥1 g/d, and FGF23 levels >23 RU/ml was 9.71 (95% CI, 4.86–19.40; P<0.001). Fifteen of those 22 patients (68%) reached endpoint 2B.
CKD Progression Defined as Annual Loss of eGFR
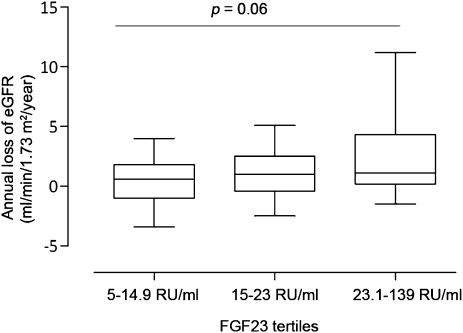
The median annual loss of eGFR was 0.8 ml/min per 1.73 m2 (range, −8.4 to 32.3 ml/min per 1.73 m2). FGF23 correlated significantly to the annual loss of eGFR (r = 0.20; P<0.007). In multiple regression analysis, log FGF23 levels independently predicted the eGFR slope (Table 4). Similarly, the negative slope of eGFR increased over tertiles of FGF23 with borderline significance (P=0.06) (Figure 3).
Table 4.
Multivariate regression model predicting the rate of renal function loss as determined by the negative eGFR slope (ml/min per 1.73 m2 per year)
| Variable | Regression Coefficient for Change in Annual Estimated GFR Loss (95% CI) | P Value for Trend |
|---|---|---|
| Per increase in log FGF23 (RU/ml) | 1.50 (0.40–2.60) | 0.008 |
| Per 0.1 mmol/L of calcium | −1.04 (−1.66 to −0.41) | 0.001 |
| Per 1 g/24 hr of albuminuria | 1.46 (0.85–2.07) | <0.001 |
In a stepwise regression model, log fibroblast growth factor-23 (FGF23), age, sex, serum albumin, baseline albuminuria, baseline estimated GFR, calcium, phosphate, parathyroid hormone, 25-hydroxyvitamin D, mean arterial BP, body mass index, and the use of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers were included. Log FGF23, calcium, and albuminuria were retained as significant (adjusted R2 = 0.22 for the whole model; P<0.001).The regression coefficients are adjusted for the listed variables. RU, relative units.
Figure 3.
Relation between fibroblast growth factor-23 (FGF23) and annual loss of estimated GFR (eGFR). The annual loss of eGFR (negative eGFR slope) is shown over FGF23 tertiles. The P value is the result of nonparametric ANOVA test comparing all tertiles. The lines in the box plots and the error bars are median and 10–90 percentiles.
Secondary Analyses
Exclusion of patients with prevalent CVD at baseline (n=5) or individuals who commenced therapy with renin-angiotensin-aldosterone system (RAAS) inhibitors or vitamin D receptor activators (i.e., drugs that may influence FGF23) after study onset (n=43 [24%]) or adjustments for immunosuppressive treatment received at any time during the study did not modify our results (data not shown). Using multiplicative interaction terms, we found no evidence for interaction between log FGF23, eGFR, or baseline albuminuria in relation to any outcome (P>0.05 for both variables). Finally, there was no relation between FGF23 and interstitial fibrosis in a subset of 16 biopsy samples that were obtained within 3 months from baseline and available for Oxford-MEST histopathologic classification, which describes the presence of mesangial hypercellularity (M 0-1), endocapillary hypercellularity (E 0-1), segmental glomerulosclerosis (S 0-1), and tubular atrophy/interstitial fobrosis (T 0-2) (data not shown).
Discussion
This study analyzed the relation between FGF23, albuminuria, and CKD progression in a Swedish cohort of patients with chronic IgAN. We report on novel associations between FGF23 and albuminuria across the spectrum of CKD stages 1–4. Higher FGF23 levels were similarly associated with faster decline in renal function, suggesting a role for FGF23 as a potential biomarker of CKD progression in patients with IgAN.
The concept of FGF23 as a potential biomarker in CKD has gradually emerged on the basis of clinical studies linking FGF23 to hard endpoints, such as mortality, cardiovascular events, CKD progression (10–12,27–29), and allograft loss among kidney transplant recipients (30). Although it can be viewed as strength that FGF23 predicts outcome in heterogeneous CKD populations, this does not exclude the possibility that such relations are modified or absent in diagnosis-specific CKD populations. To our knowledge, this study for the first time demonstrates an association between FGF23 and CKD progression in nondiabetic patients with CKD who have one single renal diagnosis. Further, to the best of our knowledge, the relation of FGF23 to albuminuria has previously been described only in patients with prevalent CVD (31) and in a small cohort of patients with diabetic nephropathy (32). This study shows that FGF23 correlates not only to baseline albuminuria but also to time-averaged albuminuria during follow-up.
Circulating FGF23 levels are determined by many different factors, including hyperphosphatemia (3,33), dietary phosphate load (34–38), the degree of secondary hyperparathyroidism (39), and vitamin D exposure (40,41); therefore, it is reasonable to assume that FGF23 may reflect the overall risk exposure related to these factors. Indeed, some studies support that FGF23 potentially is a more robust biomarker of outcomes than any other measure of mineral metabolism (11,30). However, we found that FGF23 associations with albuminuria and CKD progression were not influenced by adjustments for changes in mineral metabolism, suggesting that a concurrent disturbance in mineral metabolism is not the major explanatory pathway for FGF23 relation to these outcomes. In contrast, FGF23 association with CKD progression was weakened after adjustment for baseline eGFR, albuminuria, and other potential confounders in multivariate models. These data should, however, be carefully interpreted on the basis of the relatively few events during follow-up, which limits the validity of heavily adjusted models.
The relation of FGF23 to time-averaged albuminuria is a novel finding, although causality remains unclear. FGF23 may directly contribute to glomerular damage and enhanced proteinuria because the kidney is the primary target organ for FGF23. FGF23 signaling, however, is localized to the tubuli, where its co-receptor Klotho is expressed, so this scenario would require Klotho-independent FGF23 signaling. This requirement was evidenced in a recent study by Faul et al., who demonstrated such effects on cardiomyocytes (42). Further studies should examine whether FGF23 modulates glomerular basement membrane characteristics. Alternatively, FGF23 may reflect vascular status that secondarily aggravates a decline in renal function or proteinuria. Unfortunately, no information on vascular status was available in this study, whereas previous studies have shown that higher FGF23 levels were associated with markers of cardiovascular abnormality, such as endothelial dysfunction, atherosclerosis, and left ventricular hypertrophy (7,8,19,20).
Another scenario is that FGF23 is linked to albuminuria through its effects on vitamin D metabolism. Vitamin D receptors are expressed in the glomeruli, and vitamin D treatment has proven to reduce proteinuria alone or in combination with blockers of the RAAS (43). However, this is contradicted by the fact that, unlike FGF23, 25(OH) vitamin D was not associated with baseline or time-averaged albuminuria and did not modulate this relationship in multivariable models. Finally, the relation between FGF23 and albuminuria could also be mediated by RAAS activation because this has shown to reduce the FGF23 co-receptor Klotho, which presumably leads to increased FGF23 expression (44), and in vivo delivery of Klotho ameliorates angiotensin II–induced renal damage (45). Of note, FGF23 levels were significantly increased in patients who received ACEIs or ARBs compared with those who did not, but it remains uncertain whether this can be explained by treatment-by-indication bias.
Some potential limitations should be acknowledged. The moderate-sized cohort and small number of events during follow-up reduced the power in our estimates. The cohort represents a cross-section of prevalent and incident IgAN patients; thus, there is large interindividual variation in the actual number of years with preceding kidney damage before FGF23 was measured. However, this could also be viewed as strength given that it more accurately reflects the clinical situation in which IgAN is diagnosed at various stages after onset of disease. Finally, we cannot exclude the possibility of treatment changes over time as a potential bias, although only a small proportion of the patients were introduced to drugs that are known to influence FGF23 and exclusion of these participants did not alter the results. Further, any potential bias by variations in treatment would most likely conservatively bias our risk estimates.
In conclusion, circulating FGF23 is associated with albuminuria and CKD progression in patients with IgAN. This study extends the role of FGF23 as a potential biomarker for CKD progression in IgAN and poses the question of whether FGF23 modifies albuminuria or fibrosis as part of an “off-target” effect that was recently evidenced in the cardiovascular system (42).
Disclosures
None.
Acknowledgments
This work was supported by the Swedish Foundation for Strategic Research (T.E.L.), Swedish Research Council (K2012-55P-22137-01-06; T.E.L.), Swedish Kidney Foundation (T.E.L.), and Karolinska Institutet grants (T.E.L., S.L., S.H.J.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.ADHR Consortium : Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26: 345–348, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T: Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA 98: 6500–6505, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsson T, Nisbeth U, Ljunggren O, Jüppner H, Jonsson KB: Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 64: 2272–2279, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M: Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Westerberg PA, Linde T, Wikström B, Ljunggren O, Stridsberg M, Larsson TE: Regulation of fibroblast growth factor-23 in chronic kidney disease. Nephrol Dial Transplant 22: 3202–3207, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Marsell R, Grundberg E, Krajisnik T, Mallmin H, Karlsson M, Mellström D, Orwoll E, Ohlsson C, Jonsson KB, Ljunggren O, Larsson TE: Fibroblast growth factor-23 is associated with parathyroid hormone and renal function in a population-based cohort of elderly men. Eur J Endocrinol 158: 125–129, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M: Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119: 2545–2552, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirza MA, Larsson A, Lind L, Larsson TE: Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis 205: 385–390, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Isakova T, Wolf MS: FGF23 or PTH: which comes first in CKD? Kidney Int 78: 947–949, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, Shlipak MG, Whooley MA, Ix JH: The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med 152: 640–648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M: Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 286: 8655–8665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW: Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22: 124–136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damsgaard EM, Frøland A, Jørgensen OD, Mogensen CE: Microalbuminuria as predictor of increased mortality in elderly people. BMJ 300: 297–300, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE, Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group : Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106: 1777–1782, 2002 [DOI] [PubMed] [Google Scholar]
- 17.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey AS, de Jong PE, Gansevoort RT, Levey A, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T, Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79: 1341–1352, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Kanbay M, Nicoleta M, Selcoki Y, Ikizek M, Aydin M, Eryonucu B, Duranay M, Akcay A, Armutcu F, Covic A: Fibroblast growth factor 23 and fetuin A are independent predictors for the coronary artery disease extent in mild chronic kidney disease. Clin J Am Soc Nephrol 5: 1780–1786, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirza MA, Hansen T, Johansson L, Ahlström H, Larsson A, Lind L, Larsson TE: Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol Dial Transplant 24: 3125–3131, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE: Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis 207: 546–551, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Yilmaz MI, Sonmez A, Saglam M, Yaman H, Kilic S, Demirkaya E, Eyileten T, Caglar K, Oguz Y, Vural A, Yenicesu M, Zoccali C: FGF-23 and vascular dysfunction in patients with stage 3 and 4 chronic kidney disease. Kidney Int 78: 679–685, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Fournier A, Achard JM: Mnemotechnical note on the use of Cockcroft creatinine clearance formula for the validation of a 24-h urine collection. Nephrol Dial Transplant 15: 1677–1678, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration : Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berthoux F, Mohey H, Laurent B, Mariat C, Afiani A, Thibaudin L: Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol 22: 752–761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, König P, Kraatz G, Mann JF, Müller GA, Köhler H, Riegler P, MMKD Study Group : Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 18: 2600–2608, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Jean G, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C: High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant 24: 2792–2796, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Larsson TE: The role of FGF-23 in CKD-MBD and cardiovascular disease: Friend or foe? Nephrol Dial Transplant 25: 1376–1381, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Wolf M: Fibroblast growth factor 23 and the future of phosphorus management. Curr Opin Nephrol Hypertens 18: 463–468, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Wolf M, Molnar MZ, Amaral AP, Czira ME, Rudas A, Ujszaszi A, Kiss I, Rosivall L, Kosa J, Lakatos P, Kovesdy CP, Mucsi I: Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol 22: 956–966, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ix JH, Shlipak MG, Wassel CL, Whooley MA: Fibroblast growth factor-23 and early decrements in kidney function: The Heart and Soul Study. Nephrol Dial Transplant 25: 993–997, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Titan SM, Zatz R, Graciolli FG, dos Reis LM, Barros RT, Jorgetti V, Moysés RM: FGF-23 as a predictor of renal outcome in diabetic nephropathy. Clin J Am Soc Nephrol 6: 241–247, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta A, Winer K, Econs MJ, Marx SJ, Collins MT: FGF-23 is elevated by chronic hyperphosphatemia. J Clin Endocrinol Metab 89: 4489–4492, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Antoniucci DM, Yamashita T, Portale AA: Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab 91: 3144–3149, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Burnett SM, Gunawardene SC, Bringhurst FR, Jüppner H, Lee H, Finkelstein JS: Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res 21: 1187–1196, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Ferrari SL, Bonjour JP, Rizzoli R: Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab 90: 1519–1524, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA: Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology 146: 5358–5364, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Vervloet MG, van Ittersum FJ, Büttler RM, Heijboer AC, Blankenstein MA, ter Wee PM: Effects of dietary phosphate and calcium intake on fibroblast growth factor-23. Clin J Am Soc Nephrol 6: 383–389, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T: PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: A bone parathyroid feedback loop. Am J Physiol Renal Physiol 299: F882–F889, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Collins MT, Lindsay JR, Jain A, Kelly MH, Cutler CM, Weinstein LS, Liu J, Fedarko NS, Winer KK: Fibroblast growth factor-23 is regulated by 1alpha,25-dihydroxyvitamin D. J Bone Miner Res 20: 1944–1950, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Nishi H, Nii-Kono T, Nakanishi S, Yamazaki Y, Yamashita T, Fukumoto S, Ikeda K, Fujimori A, Fukagawa M: Intravenous calcitriol therapy increases serum concentrations of fibroblast growth factor-23 in dialysis patients with secondary hyperparathyroidism. Nephron Clin Pract 101: c94–c99, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG,, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D: Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): A randomised controlled trial. Lancet 376: 1543–1551, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Yoon HE, Ghee JY, Piao S, Song JH, Han DH, Kim S, Ohashi N, Kobori H, Kuro-o M, Yang CW: Angiotensin II blockade upregulates the expression of Klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy. Nephrol Dial Transplant 26: 800–813, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, Amaki T, Mori I, Nakamura Y, Sato M, Nangaku M, Hirata Y, Nagai R: In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension 39: 838–843, 2002 [DOI] [PubMed] [Google Scholar]