Summary
Background and objectives
The principle defect in dense deposit disease and C3 glomerulonephritis is hyperactivity of the alternative complement pathway. Eculizumab, a monoclonal antibody that binds to C5 to prevent formation of the membrane attack complex, may prove beneficial.
Design, setting, participants, & measurements
In this open-label, proof of concept efficacy and safety study, six subjects with dense deposit disease or C3 glomerulonephritis were treated with eculizumab every other week for 1 year. All had proteinuria >1 g/d and/or AKI at enrollment. Subjects underwent biopsy before enrollment and repeat biopsy at the 1-year mark.
Results
The subjects included three patients with dense deposit disease (including one patient with recurrent dense deposit disease in allograft) and three patients with C3 glomerulonephritis (including two patients with recurrent C3 glomerulonephritis in allograft). Genetic and complement function testing revealed a mutation in CFH and MCP in one subject each, C3 nephritic factor in three subjects, and elevated levels of serum membrane attack complex in three subjects. After 12 months, two subjects showed significantly reduced serum creatinine, one subject achieved marked reduction in proteinuria, and one subject had stable laboratory parameters but histopathologic improvements. Elevated serum membrane attack complex levels normalized on therapy and paralleled improvements in creatinine and proteinuria.
Conclusions
Clinical and histopathologic data suggest a response to eculizumab in some but not all subjects with dense deposit disease and C3 glomerulonephritis. Elevation of serum membrane attack complex before treatment may predict response. Additional research is needed to define the subgroup of dense deposit disease/C3 glomerulonephritis patients in whom eculizumab therapy can be considered.
Introduction
Activation of the classic complement pathway by immune complexes of antigen and antibody is inferred when both Ig and complement are detected on immunofluorescence staining of a renal biopsy. This pathology is exemplified by secondary forms of membranoproliferative glomerulonephritis (MPGN) caused by lupus or hepatitis C infection as well as most cases of idiopathic type I MPGN, cryoglobulinemic GN, and acute postinfectious GN. In rare instances, however, a proliferative GN is marked by the presence of complement, usually C3, without Ig. This immunofluorescence pattern implicates activation of the alternative complement pathway and an antibody-independent means of triggering complement deposition within tissues. These lesions have recently been termed C3 glomerulopathies (1) and are best represented by dense deposit disease (DDD) and C3GN (2).
DDD (previously called MPGN type II) is defined by the appearance of intramembranous electron dense material in the glomerular basement membrane. C3GN (previously called idiopathic MPGN with isolated C3 deposits) is marked by subendothelial and/or mesangial electron dense deposits. Both of these disorders display isolated glomerular C3 deposition without concomitant staining for Ig, signaling hyperactivity of the alternative complement pathway. This unrestrained activity can be caused by mutations in complement proteins (e.g., C3, factor B, factor H, and factor I) or acquired autoantibodies that either stabilize the activating complex of the alternative pathway (e.g., C3 nephritic factors) or target the inhibitory complement factors (e.g., factor H autoantibodies) (3,4).
Nonspecific immunomodulatory therapies, such as corticosteroids, cyclophosphamide, and calcineurin inhibitors, have been used in small numbers of patients with varied results in both DDD and C3GN (5,6). Because the principle defect underlying the C3 glomerulopathies is excessive activation of the alternative complement pathway in the fluid phase, with deposition of complement debris including breakdown products of C3b and components of the terminal complement cascade (TCC) in the glomerular basement membrane, therapies that prevent propagation of the TCC may prove beneficial. Eculizumab is a humanized monoclonal antibody that binds with high affinity to C5 and prevents the generation of membrane attack complex. It is Food and Drug Administration-approved for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS), and we hypothesized that it may provide effective, targeted therapy for patients with C3 glomerulopathies.
Materials and Methods
Subjects
This open-label, nonblinded, proof of concept efficacy and safety study of eculizumab (Soliris; Alexion Pharmaceuticals, Cheshire, CT) enrolled six adult subjects with C3 glomerulopathies. To be eligible for study participation, subjects needed to have a biopsy within 6 months of enrollment read as either DDD or C3GN. The other chief inclusion criteria were 24-hour urine protein >1000 mg/d, urine protein to creatinine ratio >1.0, or acute renal failure (defined as ≥50% increase in serum creatinine from baseline). Subjects of childbearing age agreed to use birth control, and all subjects were vaccinated against meningococcal disease or had documentation of previous vaccination. Exclusion criteria were age <18 years, use of rituximab or another monoclonal antibody within 6 months of screening, inability to discontinue other immunomodulatory therapies (e.g., cyclosporine, high-dose steroids, or mycophenolate mofetil) unless indicated for prophylaxis against transplant rejection (e.g., stable doses of mycophenolate mofetil and/or calcineurin inhibitor), inability to taper down to <10 mg/d prednisone (or prednisone equivalent) if on chronic steroid therapy, other renal diseases (e.g., diabetic nephropathy or renal vascular disease) that would interfere with interpretation of the study, and a baseline estimated GFR<30 ml/min per 1.73 m2. All subjects signed an informed consent before enrollment. The study was approved by the Institutional Review Board of Columbia University Medical Center and registered on clinicaltrials.gov (NCT01221181).
Treatment Regimen and Evaluations
Subjects were treated with eculizumab at 900 mg intravenously one time per week for 4 weeks and then 1200 mg intravenously on week 5 and every other week afterward for a total medication period of 53 weeks. This dosing regimen was based on previous experience using eculizumab for aHUS. All subjects underwent pharmacokinetic testing in the third month of treatment to ensure that dosing regimens were sufficient; no dosing adjustments were needed. No changes were made to subjects’ renin-angiotensin-aldosterone system–blocking medications unless clinically indicated (e.g., hyperkalemia) during the treatment period (this change occurred only for subject C3GN1, who was taken off dual angiotensin converting enzyme inhibitor–angiotensin receptor blocker therapy for hyperkalemia after week 24 and continued on angiotensin converting enzyme inhibitor alone). Subjects on chronic immunomodulatory therapies were tapered off during the first one-half of the treatment phase provided that the therapies were not used for rejection prophylaxis.
Laboratory measurements performed every 4 weeks during the study period included basic metabolic panel, complete blood count, hepatic function panel, lipid profile, spot urine protein to creatinine ratio, and serum complements (C3, C4, and CH50). Blood and urine samples were collected immediately before eculizumab infusions. Baseline screening for mutations in several complement genes (e.g., CFH, CFI, CFHR5, and MCP) as well as autoantibodies associated with dysregulation of the alternative pathway (e.g., C3 nephritic factors and factor H autoantibodies) was performed at the University of Iowa as described previously (7,8); in addition, serum and plasma were sent every 4 weeks during the treatment period to the University of Iowa for functional testing of the alternative complement pathway as described previously (7–9). Fundoscopic examination for presence of drusen was performed during the first month of treatment, with repeat examinations after 1 year of therapy in subjects with detectable drusen. Autofluorescent imaging was performed on subjects with drusen using the Spectralis system (Heidelberg Engineering, Carlsbad, CA).
Outcomes
The primary endpoint for subjects enrolled for proteinuria indications was change in proteinuria over treatment period, and the primary endpoint for subjects enrolled for AKI was change in serum creatinine over treatment period. Secondary endpoints included changes in renal histopathology on repeat biopsies performed after 1 year of therapy, which are described in detail in another work (10). In addition, we examined trends in serum creatinine and estimated GFR before and during therapy, changes in serum albumin, changes in fundoscopic examination and autofluorescence, and changes in assays of alternative complement pathway activity.
Statistical Analyses
Given the small number of subjects and the design of this study, no formal statistical analysis was performed. Instead, the data are presented descriptively.
Results
The study included three subjects with DDD, including one subject with recurrent DDD in the allograft, and three subjects with C3GN, including two subjects with recurrent C3GN in the allograft (Table 1). All subjects were white and male; the age range was 20–42 years, with four subjects aged 20–25 years. All but one subject had previously been treated with immunosuppression, including steroids, before initiation of eculizumab therapy. All subjects had impaired renal function at baseline, and median serum creatinine was 1.75 mg/dl (range=1.2–2.0 mg/dl). Four subjects had nephrotic range proteinuria and/or significant hypoalbuminemia at initiation of therapy.
Table 1.
Baseline characteristics of study participants
| ID | Native/ Transplant | Age (years) | Race | Sex | Months from Biopsy Diagnosis | Previous Immunosuppression | Baseline SCr | Baseline UPCR | Baseline Salb |
|---|---|---|---|---|---|---|---|---|---|
| DDD1 | Native | 22 | W | M | 25 | None | 2.0 | 0.7 | 4.6 |
| DDD2 | Native | 32 | W | M | 332 | Steroids | 1.9 | 3.5 | 3.4 |
| DDD3 | Transplant | 42 | W | M | 150 (native), 0.5 (graft) | Steroids (native) tacrolimus, MPA (graft) | 1.2 | 4.5 | 3.8 |
| C3GN1 | Native | 25 | W | M | 162 | Steroids, MMF | 1.6 | 2.6 | 2.9 |
| C3GN2 | Transplant | 22 | W | M | 138 (native), 8 (graft) | Steroids (native) steroids, tacrolimus, MMF (graft) | 1.8 | 4.4 | 3.5 |
| C3GN3 | Transplant | 20 | W | M | 114 (native), 2 (graft) | Tacrolimus, MMF, rituximab (native) steroids, tacrolimus, MMF (graft) | 1.7 | 0.1 | 4.4 |
ID, identification; Scr, serum creatinine (mg/dl); UPCR, urine protein to creatinine ratio (g/g); Salb, serum albumin (g/dl); DDD, dense deposit disease; W, white; M, male; MPA, mycophenolic acid; C3GN, C3 glomerulonephritis; MMF, mycophenolate mofetil.
Genetic testing for mutations in complement genes revealed a pathologic variant of CFH in one subject with DDD and MCP in one subject with C3GN (Table 2). No subjects had mutations in CFI, CFB, or CFHR5. Autoantibodies against factor H or factor B were not detected in any subject, although three subjects (one with DDD and two with C3GN) had C3 nephritic factors. Levels of soluble membrane attack complex (sMAC; C5b-9), an indicator of activation of the terminal complement cascade, were elevated in three subjects (DDD1, C3GN2, and C3GN3). All subjects underwent fundoscopic examination at the initiation of the study, and three (DDD2, DDD3, and C3GN2) showed prominent symmetric macular drusen (Figure 1). Pretreatment biopsies to confirm diagnosis and stage disease, along with post-treatment biopsies to gauge histopathologic response to therapy, are described in detail in another work (10).
Table 2.
Pretreatment genetic and complement testing results
| ID | CFH Mutation | CFI Mutation | CFB Mutation | CFHR5 Mutation | MCP Mutation | C3 Nephritic Factor | Factor H Autoantibodies | sMAC (C5b-9; nl<0.30 mg/L) |
|---|---|---|---|---|---|---|---|---|
| DDD1 | c.2,867C>T; p.Thr956Met | No | No | No | No | Negative | Negative | 1.08 |
| DDD2 | No | No | No | No | No | C3CSAP+1 IFE+1 | Negative | 0.21 |
| DDD3 | No | No | No | No | No | Negative | Negative | —a |
| C3GN1 | No | No | No | No | No | Negative | Negative | 0.07 |
| C3GN2 | No | No | No | No | No | C3CSAP+3 IFE− | Negative | 0.71 |
| C3GN3 | No | No | No | No | c.475+1G>A | C3CSAP+1 IFE− | Negative | 0.32 |
ID, identification; sMAC, soluble membrane attack complex; DDD, dense deposit disease; C3CSAP, C3 convertase stabilizing assay with properdin; C3GN, C3 glomerulonephritis; IFE, immunofixation electrophoresis.
Pretreatment sample for sMAC testing was inadequate in this subject; sMAC level at week 4 was 0.06 mg/L.
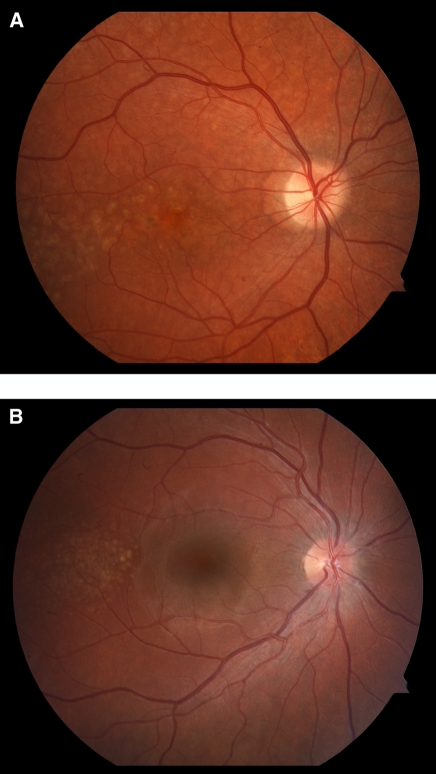
Figure 1.
Drusen formation in C3 glomerulopathies. Drusen are characteristically seen in age-related macular degeneration but have also been reported in individuals with dense deposit disease (DDD), suggesting a common underlying etiology for deposits in the glomerular basement membrane and retina. A shows pretreatment drusen deposition in subject DDD3. B shows pretreatment drusen deposition in subject C3GN2; to our knowledge, this case is the first reported case of drusen in C3GN, although a prior case report of drusen in membranoproliferative glomerulonephritis type I may, in retrospect, have been a case of C3GN (18). Neither subject showed qualitative change in drusen deposition over the 1-year course of therapy despite clinical and/or histopathologic improvements in renal disease.
Subject DDD1 was diagnosed approximately 2 years before initiating therapy and had a creatinine trend of 1.8–2.1 mg/dl over this time period. He showed complete inhibition of the terminal complement cascade (CH50=0) immediately after beginning therapy, a change that was accompanied by a decline in serum creatinine from 1.8 to 1.5 mg/dl within 4 weeks (Table 3). This improvement in renal function persisted throughout the duration of therapy, with final creatinine in the 1.3–1.4 mg/dl range, and it was accompanied by normalization of elevated sMAC levels (1.08 mg/L pretherapy and <0.30 mg/L on therapy). Proteinuria levels remained consistently low throughout the course of therapy. Repeat biopsy after 1 year of treatment showed decreased activity with no evidence of endocapillary proliferation; 4 weeks after cessation of eculizumab, creatinine rose to 1.5 mg/dl, and 8 weeks after cessation of therapy, creatinine rose to 1.7 mg/dl with an sMAC level of 1.26 mg/L, prompting resumption of therapy.
Table 3.
Detailed results from subjects with dense deposit disease
| Week | DDD1 | DDD2 | DDD3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scr | UPCR | Salb | C3a | C4b | CH50c | Scr | UPCR | Salb | C3a | C4b | CH50c | Scr | UPCR | Salb | C3a | C4b | CH50c | |
| 0 | 1.8 | 0.326 | 4.6 | 35 | 20 | 32 | 2.1 | 2.413 | 3.6 | 27 | 35 | 44 | 1.2 | 5.933 | 4.2 | 67 | 28 | 49 |
| 4 | 1.5 | 0.287 | 4.5 | 17 | 15 | 0 | 2.2 | 4.279 | 3.6 | 25 | 28 | 1 | 1.2 | 10.580 | 2.9 | 122 | 35 | — |
| 8 | 1.6 | 0.148 | 4.7 | 19 | 20 | 0 | 2.3 | 4.876 | 3.6 | 40 | 46 | 1 | 1.6 | 5.710 | 3.8 | 124 | 31 | 4 |
| 12 | 1.5 | 0.317 | 4.6 | 21 | 19 | 1 | 1.9 | 4.152 | 3.3 | 27 | 33 | 1 | 1.6 | 2.480 | 4.1 | 119 | 36 | 6 |
| 16 | 1.4 | 0.240 | 4.4 | 22 | 19 | 0 | 2.1 | 5.607 | 3.0 | 28 | 31 | 2 | 1.6 | 2.829 | 4.5 | 106 | 35 | 2 |
| 20 | 1.5 | 0.176 | 4.6 | 17 | 17 | 0 | 2.2 | 5.580 | 3.1 | 33 | 40 | 5 | 1.4 | 3.189 | 4.0 | 105 | 28 | 2 |
| 24 | 1.4 | 0.290 | 4.3 | 23 | 21 | 1 | 2.5 | 4.948 | 3.0 | 25 | 35 | 3 | 1.6 | 1.417 | 4.1 | 115 | 33 | 1 |
| 28 | 1.3 | 0.442 | 4.5 | 24 | 21 | 1 | 2.2 | — | 3.4 | 29 | 33 | 1 | 1.6 | 0.897 | 4.3 | 101 | 29 | 0 |
| 32 | 1.3 | 0.663 | 4.2 | 25 | 22 | 0 | 2.4 | 7.707 | 3.1 | 34 | 32 | 4 | 1.4 | 1.270 | 4.2 | 89 | 24 | 1 |
| 36 | 1.5 | 0.592 | 4.2 | 23 | 20 | 4 | 2.8 | 6.167 | 3.2 | 29 | 36 | 1 | 1.8 | 1.391 | 4.6 | 96 | 30 | 3 |
| 40 | 1.4 | 0.845 | 4.1 | 23 | 25 | 1 | 2.9 | 4.786 | 3.0 | 31 | 27 | 2 | 1.7 | 1.373 | 4.2 | 95 | 32 | 1 |
| 44 | 1.4 | 0.381 | 4.1 | 20 | 20 | 1 | — | — | — | — | — | — | 1.5 | 1.917 | 3.9 | 112 | 29 | 4 |
| 48 | 1.3 | 0.548 | 4.0 | 20 | 18 | 0 | — | — | — | — | — | — | 1.6 | 0.214 | 4.2 | 105 | 29 | 3 |
| 52 | 1.4 | 0.586 | 4.2 | 23 | 22 | 2 | — | — | — | — | — | — | 1.7 | 1.763 | 4.0 | 93 | 28 | 1 |
DDD, dense deposit disease; Scr, serum creatinine (mg/dl); UPCR, urine protein to creatinine ratio (g/g); Salb, serum albumin (g/dl).
C3 reference range=83–177.
C4 reference range=16–47.
CH50 reference range=60–144.
Subject DDD2 (Table 3), despite effective inhibition of the terminal complement cascade, showed no clinical response to therapy, with both creatinine and proteinuria rising over 40 weeks of therapy. In the 5 years before initiating eculizumab therapy, his creatinine had slowly risen from 1.2 to 1.8 mg/dl, with proteinuria persistently above 4 g/d. Levels of sMAC were consistently normal (<0.30 mg/L) pretherapy and on therapy. Given lack of clinical response, this subject withdrew from the study and declined repeat biopsy and fundoscopic examination.
Subject DDD3 (Table 3) had recurrent disease diagnosed by biopsy 20 months after a living related transplant from an HLA-identical sibling. Serum creatinine during the first 4 weeks of therapy (1.2 mg/dl) was lower than his baseline of 1.5–1.7 mg/dl over the previous 12 months; this lower creatinine was felt to be a dilutional effect from anasarca (peak proteinuria was 10.6 g/g with albumin nadir of 2.9 g/dl). Pretreatment sMAC testing was unable to be performed because of inadequate specimen; by week 4, however, sMAC level was 0.06 mg/L (normal<0.30 mg/L), and it remained normal throughout the treatment course. After 1 year of treatment, proteinuria ranged from 0.2 to 1.9 g/g, with albumin consistently above 4.0 g/dl and no edema on exam. Renal function has remained stable. Repeat biopsy was notable for decreased mesangial proliferation and less extensive deposits on electron microscopy. Repeat fundoscopic examination after completion of therapy showed no qualitative changes in drusen deposition or autofluorescent pattern. Laboratory tests drawn 4 and 8 weeks after completion of therapy remained unchanged.
Subject C3GN1 (Table 4) began eculizumab while on prednisone (30 mg daily) and mycophenolate mofetil (MMF; 1000 mg two times per day), with recent dose escalations in both immunosuppressive medications in light of a clinical and histopathologic flare of disease 2 months before study entry: creatinine had risen from baseline 1.5 to 2.1 mg/dl, proteinuria had risen from 2.1 to 4.6 g/g, and biopsy showed a diffuse MPGN. Steroids were tapered off by week 16 of therapy, and MMF was tapered off by week 24 of therapy given stable renal function, declining proteinuria (from 2.3 to 0.5 g/g), and rising albumin (from 3.2 to 3.9 g/dl). However, over the following 24 weeks of therapy, creatinine rose to 2.4 mg/dl, proteinuria rose to 1.8 g/g, and albumin fell to 3.1 g/dl. Repeat biopsy at week 48 of therapy compared with pretreatment biopsy showed increased chronicity, with 85% of glomeruli globally sclerotic (increased from 50% in previous biopsy), and continuously active GN in the few open glomeruli with persistent membranoproliferative changes and large subendothelial deposits. Steroids and MMF were reintroduced at week 49, and the subject remains on MMF (1000 mg two times per day) with tapering doses of prednisone. Levels of sMAC were in the normal range pre-eculizumab therapy and throughout the course of therapy.
Table 4.
Detailed results from subjects with C3 glomerulonephritis
| Week | C3GN1 | C3GN2 | C3GN3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scr | UPCR | Salb | C3a | C4b | CH50c | Scr | UPCR | Salb | C3a | C4b | CH50c | Scr | UPCR | Salb | C3a | C4b | CH50c | |
| 0 | 1.6 | 2.279 | 3.2 | 80 | 16 | 96 | 1.7 | 4.455 | 3.4 | 41 | 38 | 0 | 1.8 | 0.078 | 4.3 | 28 | 14 | 30 |
| 4 | 1.6 | 1.202 | 3.1 | 88 | 21 | 0 | 1.8 | 2.184 | 4.2 | 37 | 42 | 0 | 2.0 | 0.080 | 5.2 | 30 | 13 | 1 |
| 8 | 1.7 | 1.310 | 3.9 | 113 | 29 | 0 | 1.8 | 1.529 | 4.2 | 32 | 32 | 0 | 1.3 | 0.133 | 5.2 | 43 | 25 | 1 |
| 12 | 1.8 | 1.141 | 3.7 | 56 | 14 | 0 | 1.8 | 2.161 | 3.8 | 36 | 47 | 0 | 1.5 | 0.099 | 4.7 | 26 | 15 | 0 |
| 16 | 1.6 | 1.230 | 3.7 | 90 | 25 | 1 | 1.6 | 3.167 | 4.4 | 28 | 32 | 0 | 1.4 | 0.085 | 5.3 | 29 | 16 | 1 |
| 20 | 1.7 | 1.538 | 3.8 | 78 | 22 | 2 | 1.7 | 3.371 | 4.2 | 27 | 35 | 0 | 1.4 | 0.112 | 4.8 | 41 | 26 | 0 |
| 24 | 1.8 | 0.520 | 3.9 | 89 | 23 | 0 | 1.7 | 2.441 | 4.2 | 31 | 35 | 0 | 1.5 | 0.091 | 5.3 | 28 | 17 | 0 |
| 28 | 1.6 | 2.000 | 3.2 | 61 | 17 | 0 | 1.6 | 2.777 | 3.7 | 22 | 30 | 0 | 1.5 | 0.098 | 5.1 | 24 | 15 | 2 |
| 32 | 1.9 | 2.288 | 3.5 | 64 | 22 | 0 | 1.8 | 5.542 | 3.7 | 37 | 32 | 0 | 1.6 | 0.095 | 4.9 | 30 | 16 | 0 |
| 36 | 2.2 | 2.034 | 3.6 | 66 | 21 | 0 | 1.7 | 5.721 | 3.4 | 30 | 35 | 0 | 1.4 | 0.138 | 4.8 | 42 | 24 | 4 |
| 40 | 2 | 2.197 | 3.5 | 84 | 35 | 0 | 1.8 | 8.000 | 3.4 | 36 | 33 | 3 | 1.6 | 0.085 | 4.6 | — | — | 0 |
| 44 | 2 | 1.638 | 3.6 | 51 | 25 | 0 | 1.7 | 4.274 | 3.2 | 30 | 26 | 0 | 1.4 | 0.090 | 4.9 | 37 | 19 | 1 |
| 48 | 2.4 | 1.837 | 3.1 | 63 | 33 | 1 | 1.8 | 4.788 | 3.1 | 45 | 44 | 0 | 1.4 | 0.086 | 5.1 | 39 | 18 | 0 |
| 52 | 2.3 | 1.483 | 3.8 | 43 | 26 | 1 | 2.3d | 3.797 | 3.1 | 39 | 34 | 0 | 1.4 | 0.097 | 5.0 | 31 | 11 | 0 |
C3GN, C3 glomerulonephritis; Scr, serum creatinine (mg/dl); UPCR, urine protein to creatinine ratio (g/g); Salb, serum albumin (g/dl).
C3 reference range=83–177.
C4 reference range=16–47.
CH50 reference range=60–144.
Repeat testing done 3 weeks after completion of eculizumab therapy showed creatinine of 1.9 mg/dl. The subject, as described in the text, subsequently had an elevation of creatinine to 8.4 mg/dl on routine laboratory tests done 7 weeks after completion of eculizumab; repeat biopsy showed active disease. He improved with resumption of therapy.
Subject C3GN2 (Table 4) had recurrent disease diagnosed by biopsy 4 months after a living related transplant from his mother when his proteinuria rose from 0.1 to 1.4 g/g. Over the next 8 months before initiating eculizumab therapy, his creatinine steadily rose to 1.7–1.9 mg/dl accompanied by rising proteinuria to nephrotic range (4.3 mg/g). During the 1-year course of therapy, during which the only modification to his immunosuppression was a lowering of his prednisone dose from 5 mg daily to 5 mg every other day alternated with 2.5 mg every other day, his creatinine, proteinuria, and serum albumin remained stable. Levels of sMAC, elevated before therapy (0.71 mg/L), dropped to and remained normal (<0.30 mg/L) for duration of treatment. Repeat biopsy showed less mesangial and endocapillary proliferation, with a significantly decreased mean number of inflammatory cells per glomerulus compared with the biopsy performed 1 year earlier. Repeat fundoscopic examination showed no change in drusen accumulation or autofluorescent pattern. His renal function remained stable for 3 weeks after eculizumab was discontinued. He missed a follow-up examination 3 weeks later, and 1 week after the missed appointment (i.e., 7 weeks after discontinuing eculizumab), he presented feeling well but with creatinine of 8.4 mg/dl. Repeat renal biopsy showed recurrent active C3GN with crescents. He was treated with plasma exchange, pulse steroids, and resumption of eculizumab with significant improvement in renal function (creatinine=3.8 mg/dl 9 weeks after restarting eculizumab).
Subject C3GN3 (Table 4) had recurrent disease diagnosed by biopsy 1 month after a living related transplant from his mother when his creatinine rose from a nadir of 1.3 to a peak of 2.2 mg/dl. He began eculizumab 2 months later, with a serum creatinine of 1.8 mg/dl and no proteinuria. During the 1-year course of therapy, steroids were tapered off, but he continued on stable doses of tacrolimus and MMF. His creatinine fell from a peak of 2.0 mg/dl to a new baseline of 1.4 mg/dl by completion of therapy. Baseline sMAC level was modestly elevated but fell to normal range by week 4. No significant decrease was detected between pre- and post-treatment biopsies, which both showed mild mesangial proliferation with no endocapillary proliferation or exudative features. Laboratory tests done 4 and 8 weeks after completing therapy were unchanged.
No adverse events, including no infections, were reported during the course of therapy for any subject.
Discussion
We present here our experience using eculizumab, a monoclonal antibody against C5, for the C3 glomerulopathies of DDD and C3GN. In this open-label, nonblinded proof of concept study, we have shown that eculizumab effectively inhibits the terminal complement cascade and is well tolerated, with no adverse events reported by the six treated subjects. The blockade of C5 and its downstream products translated to improvements for some but not all patients. Two patients showed improvements in serum creatinine (DDD1 and C3GN3), one patient had partial remission of nephrotic syndrome (DDD3), and one patient (C3GN2) had stable laboratory values but less proliferation on repeat biopsy. The two remaining patients, however, showed declining renal function during treatment. Baseline genetic screening coupled with functional assays of the complement pathway were together suggestive of response to treatment. In particular, normalization of elevated sMAC levels may be the best marker of eculizumab’s ability to improve clinical and histopathologic disease parameters. Our results suggest that eculizumab may be an appropriate treatment for a subgroup of patients with DDD and C3GN.
The variability in response to treatment in this study is not entirely unexpected based on our current understanding of the pathophysiology of these two diseases (2). Three familial studies, in particular, have provided unique insights into DDD and C3GN. These families include one family described by Martínez-Barricarte et al. (11) in which a mother and her two identical twin boys segregated a 2-amino acid deletion in MG7 of C3 (Δ923-924AspGly). This mutation makes C3 resistant to cleavage by C3 convertase; however, through the normal tick-over process, a hydrolyzed mutant C3 convertase forms that is resistant to factor H regulation but can cleave circulating wild-type C3. The persistent fluid-phase mutant C3 convertase activity in this family causes DDD exclusively from fluid-phase alternative pathway dysregulation without a contribution of the TCC. In this family, therefore, sMAC would not be elevated, and eculizumab would not be helpful.
A second familial example of C3 glomerulopathy was reported by Gale et al. (12) in two families from Cyprus segregating autosomal dominant microscopic hematuria. Renal biopsy was remarkable for C3GN, and a genome-wide linkage study localized a genetic abnormality to the CFH/CFHR region of chromosome 1q31-32. A novel CFHR5 fusion was identified that is less effective than native CFHR5 in associating with surface-bound C3b, suggesting a dominant-negative mechanism of action in controlling C3 and C5 convertase activity and highlighting the importance of CFHR5 in complement processing within the kidney; this role is also supported by the identification of CFHR5 mutations in some patients with DDD and C3GN (8).
The third family reported by Habbig et al. (13) included two siblings of consanguineous parentage with childhood onset hematuria and proteinuria. Serum C3 and CFB were decreased, although C4 was normal and C3d was increased. Prominent mesangial deposition of C3 and C5b-9 was noted on renal biopsy, and by electron microscopy, there were numerous osmiophilic mesangial deposits with intramembranous and subendothelial deposits, consistent with C3GN. Both children were homozygous for the deletion of a lysine at position 224 (CFH K244), leading to severely reduced cofactor, decay-accelerating activity, and C3b binding by the mutant factor H. In the second and third families described above, in which disease is associated with dysregulation of the C5 convertase and subsequent initiation of the TCC, eculizumab would be expected to have a beneficial response.
Based on these familial cases, C3 glomerulopathy seems to be a disease spectrum that is dependent on the level and degree of dysregulation of the alternative pathway and TCC. As such, in those patients with minimal dysregulation of the TCC, any benefit from eculizumab would be minimal. The degree of dysregulation of the TCC, in turn, can potentially be inferred from MAC testing in the serum and/or renal biopsy coupled with focused genetic screening. In our cohort, for example, DDD1 carried a p.Thr956Met mutation in short consensus repeat 16 of factor H. This variant has been reported in aHUS (aHUS mutation database is available at http://www.fh-hus.org) and based on its location, would be predicted to impact factor H-mediated control of the complement cascade on cell surfaces. The fact that the phenotype was DDD and not aHUS suggests the impact of other genetic variants, which has been shown in two patients carrying identical mutations in factor B that resulted in a DDD phenotype in one case and an aHUS phenotype in the other (14). One subject (C3GN3) carried a mutation in MCP (c.475+1G>A), which alters a splice donor site and is predicted to lead to a null allele. Although this splice mutation is novel, other splice site mutations in MCP are reported in patients with aHUS. These genetic findings may be relevant; these subjects both had elevated sMAC, and they were the only two subjects with serum creatinine that dropped while on treatment.
Dysregulation of the C3 convertase leading to fluid-phase consumption of C3 can also occur in the presence of stabilizing nephritic factors, which were identified in three subjects using a C3 convertase stabilizing assay with properdin and one subject by immunofixation electrophoresis (7). These IgG antibodies directly stabilize the C3 convertase and prevent its normal physiologic regulation. Notably, the most robust C3Nef activity was present in C3GN2, which may explain, in part, the limited clinical response to eculizumab despite restoring elevated sMAC levels to normal as well as the subject’s rapid decline in kidney function when therapy was discontinued. In theory, eculizumab could be offered to those patients with C3 glomerulopathies who have elevated sMAC levels—a recent series on causes of alternative pathway dysregulation in 32 patients with DDD suggests that approximately one-third of patients would fit this criteria (i.e., sMAC≥0.3 mg/L) (7)—with the best results expected in those patients with no (or weak) C3 nephritic factor activity.
As with other glomerular lesions, disease duration likely is another important predictor of response to therapy. The two subjects with renal function that worsened during therapy had very long histories of disease before eculizumab exposure (more than 27 years for DDD2 and more than 13 years for C3GN1), whereas the other subjects received the drug early in the disease course (range=0.5–25 months). Indeed, in appropriately screened patients, eculizumab may have its greatest use in transplant recipients with recurrent disease, employed either immediately after recognition of recurrence or, potentially, as prophylactic therapy initiated immediately post-transplantation, which has been done with aHUS (15). We cannot conclude whether the benefits of therapy experienced by our subjects will be sustained and whether continued therapy with the drug will be needed for such sustenance; thus far, two subjects have shown evidence of relapsing disease and have been restarted on therapy. Speculatively, longer duration of therapy may induce even greater improvements in our responders. Prior experience with eculizumab in treating PNH and aHUS has involved prolonged, often lifelong, courses of therapy (16,17), and additional research into the role of eculizumab in C3 glomerulopathies will need to address not only efficacy but also optimal duration of dosing.
In conclusion, we present here the first reported series of patients treated with eculizumab for the C3 glomerulopathies of DDD and C3GN. Our results suggest that the pathophysiological basis of the C3 glomerulopathies is more complex than the pathophysiological basis of either PNH or aHUS, both of which are now approved for treatment with eculizumab. However, our data are consistent with a response to therapy in some patients who may be identifiable by genetic and functional tests of the complement system. If additional patients with DDD and C3GN are treated with eculizumab, treatment should only be offered in the context of a thorough genetic and functional evaluation. In addition, there is a clear need for additional anticomplement therapies that offer the possibility of complement control at the level of the C3 convertase instead of C5.
Disclosures
This study was supported by an investigator-initiated study grant from Alexion Pharmaceuticals, the manufacturer of Soliris (eculizumab). The investigators were responsible for study design, implementation, data analysis, and manuscript preparation. G.B.A. has received consulting honoraria from Alexion Pharmaceuticals.
Acknowledgments
We are grateful to Angela Cha, Irma Orbe, and Melanie Foley for assistance with the conduct of this study at the Clinical Research Offices of the Division of Nephrology at Columbia University.
This research was supported, in part, by the Center for Glomerular Diseases at Columbia University and National Institutes of Health Grant DK074409 (to R.J.S.).
Portions of these data were presented in abstract form at the 2011 American Society of Nephrology Renal Week, November 8–13, 2011, Philadelphia, Pennsylvania.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Monoclonal Antibodies for the Treatment of the C3 Glomerulopathies,” on pages 704–706.
References
- 1.Fakhouri F, Frémeaux-Bacchi V, Noël LH, Cook HT, Pickering MC: C3 glomerulopathy: a new classification. Nat Rev Nephrol 6: 494–499, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Sethi S, Nester CM, Smith RJ: Membranoproliferative glomerulonephritis and C3 glomerulopathy: Resolving the confusion. Kidney Int 81: 434–441, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Servais A, Frémeaux-Bacchi V, Lequintrec M, Salomon R, Blouin J, Knebelmann B, Grünfeld JP, Lesavre P, Noël LH, Fakhouri F: Primary glomerulonephritis with isolated C3 deposits: A new entity which shares common genetic risk factors with haemolytic uraemic syndrome. J Med Genet 44: 193–199, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sethi S, Fervenza FC, Zhang Y, Nasr SH, Leung N, Vrana J, Cramer C, Nester CM, Smith RJ: Proliferative glomerulonephritis secondary to dysfunction of the alternative pathway of complement. Clin J Am Soc Nephrol 6: 1009–1017, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appel GB, Cook HT, Hageman G, Jennette JC, Kashgarian M, Kirschfink M, Lambris JD, Lanning L, Lutz HU, Meri S, Rose NR, Salant DJ, Sethi S, Smith RJ, Smoyer W, Tully HF, Tully SP, Walker P, Welsh M, Würzner R, Zipfel PF: Membranoproliferative glomerulonephritis type II (dense deposit disease): An update. J Am Soc Nephrol 16: 1392–1403, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Nasr SH, Valeri AM, Appel GB, Sherwinter J, Stokes MB, Said SM, Markowitz GS, D’Agati VD: Dense deposit disease: Clinicopathologic study of 32 pediatric and adult patients. Clin J Am Soc Nephrol 4: 22–32, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Meyer NC, Wang K, Nishimura C, Frees K, Jones M, Katz LM, Sethi S, Smith RJ: Causes of alternative pathway dysregulation in dense deposit disease. Clin J Am Soc Nephrol 7: 265–274, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abrera-Abeleda MA, Nishimura C, Smith JL, Sethi S, McRae JL, Murphy BF, Silvestri G, Skerka C, Józsi M, Zipfel PF, Hageman GS, Smith RJ: Variations in the complement regulatory genes factor H (CFH) and factor H related 5 (CFHR5) are associated with membranoproliferative glomerulonephritis type II (dense deposit disease). J Med Genet 43: 582–589, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maga TK, Nishimura CJ, Weaver AE, Frees KL, Smith RJ: Mutations in alternative pathway complement proteins in American patients with atypical hemolytic uremic syndrome. Hum Mutat 31: E1445–E1460, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Herlitz LC, Bomback AS, Markowitz GS, Stokes MB, Smith RN, Colvin RB, Appel GB, D'Agati VD: Renal biopsy findings after eculizumab treatment in dense deposit disease and C3 glomerulonephritis. J Am Soc Nephrol in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martínez-Barricarte R, Heurich M, Valdes-Cañedo F, Vazquez-Martul E, Torreira E, Montes T, Tortajada A, Pinto S, Lopez-Trascasa M, Morgan BP, Llorca O, Harris CL, Rodríguez de Córdoba S: Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J Clin Invest 120: 3702–3712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gale DP, de Jorge EG, Cook HT, Martinez-Barricarte R, Hadjisavvas A, McLean AG, Pusey CD, Pierides A, Kyriacou K, Athanasiou Y, Voskarides K, Deltas C, Palmer A, Frémeaux-Bacchi V, de Cordoba SR, Maxwell PH, Pickering MC: Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet 376: 794–801, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habbig S, Mihatsch MJ, Heinen S, Beck B, Emmel M, Skerka C, Kirschfink M, Hoppe B, Zipfel PF, Licht C: C3 deposition glomerulopathy due to a functional factor H defect. Kidney Int 75: 1230–1234, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Maga TK, Deluca A, Taylor KR, Scherer S, Scheetz TE, Smith RJH: Targeted-genomic capture and high-throughput sequencing for genetic testing and new gene discovery in atypical hemolytic uremic syndrome. Mol Immunol 48: 1666, 2011 [Google Scholar]
- 15.Nester C, Stewart Z, Myers D, Jetton J, Nair R, Reed A, Thomas C, Smith R, Brophy P: Pre-emptive eculizumab and plasmapheresis for renal transplant in atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol 6: 1488–1494, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker C: Eculizumab for paroxysmal nocturnal haemoglobinuria. Lancet 373: 759–767, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Köse O, Zimmerhackl LB, Jungraithmayr T, Mache C, Nürnberger J: New treatment options for atypical hemolytic uremic syndrome with the complement inhibitor eculizumab. Semin Thromb Hemost 36: 669–672, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Han DP, Sievers S: Extensive drusen in type I membranoproliferative glomerulonephritis. Arch Ophthalmol 127: 577–579, 2009 [DOI] [PubMed] [Google Scholar]