Abstract
Antioxidant therapy can protect against ischemic injury, but the inability to selectively target the kidney would require extremely high doses to achieve effective local concentrations of drug. Here, we developed a directed therapeutic that specifically targets an antioxidant to renal proximal tubule cells via the folate receptor. Because a local increase in superoxide contributes to renal ischemic injury, we created the folate-antioxidant conjugate 4-hydroxy-Tempo (tempol)-folate to target folate receptors, which are highly expressed in the proximal tubule. Dihydroethidium high-performance liquid chromatography demonstrated that conjugated tempol retained its efficacy to scavenge superoxide in proximal tubule cells. In a mouse model of renal ischemia-reperfusion injury, tempol-folate reduced renal superoxide levels more effectively than tempol alone. Furthermore, electron spin resonance revealed the successful targeting of the tempol-folate conjugate to the kidney and other tissues expressing folate receptors. Administration of tempol-folate protected the renal function of mice after ischemia-reperfusion injury and inhibited infiltration of macrophages. In conclusion, kidney-specific targeting of an antioxidant has therapeutic potential to prevent renal ischemic injury. Conjugation of other pharmaceuticals to folate may also facilitate the development of treatments for other kidney diseases.
Acute ischemic renal injury is a devastating clinical problem that significantly affects the US healthcare system, including 50% of intensive care patients, and is currently without effective treatment.1–3 Renal ischemia-reperfusion injury commonly occurs as a result of hemorrhage or hypotension followed by the reintroduction of oxygenated blood into hypoxic tissue, leading to a cascade of injurious events that can progress to ARF.2,3 Upon reperfusion of ischemic tissue, there is a decrease in mitochondrial ATP production and an increase in purine degradation, resulting in elevated xanthine oxidase levels.4,5 This sequence of events gives rise to extremely reactive free radicals, inflammation, and oxidation of lipids, proteins, and DNA, causing apoptosis and tubular cell death.6–11 As a result, vasoconstriction, vascular injury, tubular blockage, and reduced glomerular permeability occur, which can contribute to injury particularly in the proximal tubule, causing renal dysfunction.4,5,12
Antioxidant therapy has the potential to protect against ischemia-reperfusion injury. Previous studies have shown that, in high doses, the membrane permeable SOD mimetic 4-hydroxy-Tempo (tempol) and mito-TEMPO are beneficial in vitro when administered 12 hours before ischemia and in vivo models of renal ischemia-reperfusion injury when administered immediately before reperfusion.13,14 However, there is evidence that antioxidant therapy such as vitamin E administration can also have adverse, off-target effects, such as inhibiting the beneficial effects of simvastatin in patients with coronary disease, and has been associated with an increase in all-cause mortality.15–18 Delivery challenges such as the inability to selectively target the kidney necessitate the administration of excessively high antioxidant doses, thus risking unwanted effects.13,19,20 This lack of effective, targeted antioxidant therapy has limited the treatment of renal ischemic injury and ARF, and this study addresses this unmet clinical need.
A key feature of the kidney, pertinent to the design of this study, is the high density of folate receptors expressed in sites such as the proximal tubule that are severely affected by ischemic injury. Despite the proportionally high blood flow to the kidney, specific delivery of a therapeutic compound to the kidney has been limited.21–24 We designed a targeting strategy to deliver the SOD mimetic tempol to specific sites by making use of the selective expression of the folate receptor in the renal proximal tubules. Folic acid is an essential vitamin with a high affinity for the folate receptor, which maintains folate homeostasis.25,26 The selectively expressed folate receptor allows for passage of folate into the cell by encapsulation into clathrin-coated pits.27 Folate is absorbed by the kidney, predominantly in the proximal tubule, which fortuitously is a site particularly at risk during ischemia.28 The binding of folate to the folate receptor occurs at a relatively high affinity with half maximal binding as high as 12 nM in human proximal tubule cells, making it ideal for pharmacological targeting.25,26,29
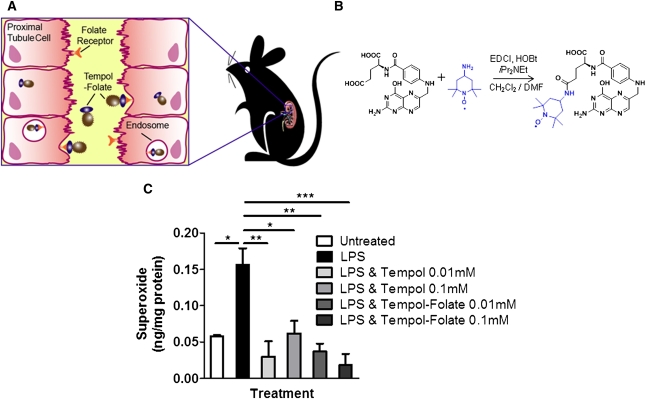
In this study, we synthesized a novel folate-antioxidant conjugate to selectively target the kidney, to enhance localized superoxide scavenging, and to prevent the development of ARF while avoiding the associated side effects of systemic antioxidant therapy.16–18,30,31 We hypothesize that the tempol-folate conjugate selectively targets the renal proximal tubule and protects from ischemic injury by way of scavenging reactive oxygen species, therefore preventing the cascade of events resulting in tubular dysfunction and ARF (Figure 1A).
Figure 1.
Conjugation of folic acid to the antioxidant tempol selectively targets the proximal tubule cells (HK-2) that express high levels of folate receptor, without altering its ability to scavenge superoxide. (A) Schematic showing how tempol-folate conjugate binds to tubule folate receptors on the epithelium-like layer of the proximal tubule cells, is taken up into endosomes, and released into the cell. (B) Structure and synthesis of tempol-folate conjugate. Tempol-folate can be readily synthesized from folic acid and 4-amino tempol via EDC coupling. (C) Superoxide concentration in cultured HK-2 cells treated with 0.1 μg/ml LPS endotoxin with or without 0.01–0.1 mM tempol or tempol-folate, measured by DHE-HPLC (n=3). *P<0.05, **P<0.01, and ***P<0.001 comparing the LPS-treated group with all other groups. Superoxide concentration was normalized to protein concentration.
Results
Tempol-Folate Scavenges Superoxide as Efficiently as Tempol
Tempol-folate was synthesized by coupling 4-amino tempol and folic acid via an amide bond, using ethylene carbodiimide coupling (EDC) (Figure 1B). To test the efficacy of the tempol-folate conjugate to scavenge superoxide, cultured human proximal tubule cells (HK-2) were stimulated with 0.1 μg/ml LPS endotoxin to stimulate superoxide production. Using dihydroethidium high-performance liquid chromatography (DHE-HPLC) we found that treatment with 0.1 mM and 0.01 mM tempol and tempol-folate conjugate effectively and equally inhibited elevated superoxide levels in HK-2 cells, therefore confirming that conjugation of tempol with folate did not alter the antioxidant efficacy of tempol (Figure 1C).
Tempol-Folate Uptake Is Enhanced in Cultured Renal Proximal Tubule Cells
The presence of folate receptor–α expression in the proximal tubule was demonstrated previously by Birn et al.25 Tempol-folate is designed to be membrane impermeable and therefore have low accumulation in nontarget organs. However, we hypothesized that it would accumulate in the kidney due to the high expression of the folate receptor and would subsequently be transported across the basolateral membrane into the kidney.27,32,33 The prevalence and specificity of the folate receptor in the proximal tubule confirms that it is an excellent target for therapeutic delivery to the kidney.
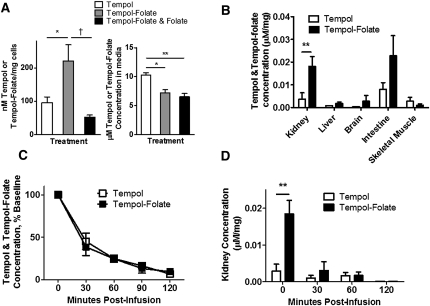
HK-2 cells have previously been shown to express folate receptor–α.34 To confirm the ability of the tempol-folate conjugate to enter the proximal tubule cell, we measured the concentration of tempol and tempol-folate in cultured HK-2 cells, media, and mouse tissue after administration of 1 μM of each compound. The unpaired electron present on tempol and tempol-folate gives a characteristic electron spin resonance (ESR) spectra, thus allowing measurement and quantification of free tempol or tempol-folate in plasma and tissue (Supplemental Figure 1, A and B).35 We recorded a linear relationship between ESR peak intensity and tempol and tempol-folate concentration (Supplemental Figure 1C). We observed a significantly higher concentration of tempol-folate in the cellular fraction of HK-2 cells compared with free tempol (Figure 2A), indicating enhanced uptake of tempol-folate into HK-2 cells, supporting our hypothesis that folate conjugation increases uptake of tempol-folate into proximal tubule cells. However, the concentration of tempol-folate in the media was not different than tempol concentration (Figure 2A). Tempol-folate levels in the cells were significantly reduced when administered with equimolar free folate but not different in the media, indicating competition for the folate receptor prevented tempol-folate from entering the cell (Figure 2A). Note that concentrations of tempol and tempol-folate in the cellular fraction were not at equilibrium with the media because the cells were washed and diluted in Krebs HEPES buffer (KHB) to measure tempol and tempol-folate concentration by ESR.
Figure 2.
Tempol-folate is selectively taken up by cultured HK-2 cells as well as folate receptor-rich mouse tissues and remains detectable in the plasma up to 120 minutes postinfusion. (A) Concentration of tempol and tempol-folate in HK-2 cells resuspended in KHB and media, quantified by ESR after 1-hour incubation with 1 μM tempol, tempol-folate, or tempol-folate with 1 μM folate. *P<0.05 and **P<0.01 comparing tempol with other groups; †P<0.05 comparing tempol-folate with combined tempol-folate and folate treatment (n=3 per group). (B) Relative tissue tempol and tempol-folate concentration quantified by ESR in mice after 48-hour intravenous infusion at 500 μg/kg per h. *P<0.05 between tempol and tempol-folate concentration (n=4–7). (C) Postinfusion tempol and tempol-folate plasma concentration measured by ESR. (D) Tempol and tempol-folate concentration in kidneys from mice receiving intravenous infusion of tempol or tempol-folate for 48 hours before withdrawal of infusion. **P<0.01 comparing tempol and tempol-folate concentration (n=2–11 per group). Data represent mean ± SEM.
Tempol-Folate Targets Tissues with High Folate Receptor Expression
Further supporting our hypothesis, in mice receiving 500 μg/kg per h tempol or tempol-folate by intravenous infusion, we observed a marked tissue accumulation of tempol-folate that differed dramatically from tempol, resulting in enhanced distribution to the kidney and intestine. Both the kidney and intestine exhibit high levels of folate receptor expression (Figure 2B).36,37 In contrast, skeletal muscle has low folate receptor expression and we observed no difference between tempol and tempol-folate concentration in the muscle.38 These data strongly indicate that in cells expressing high concentrations of folate receptor, such as the kidney, uptake of tempol-folate exceeded that of free tempol, demonstrating effective targeting of the tempol-folate conjugate.
To gain further insight into the pharmacokinetics of tempol and tempol-folate, we measured the concentrations of both tempol and tempol-folate by ESR in mouse plasma and kidney tissue, after a 48-hour intravenous infusion at 500 μg/kg per h (Figure 2, C and D). In vivo we observed a marked washout of both tempol and tempol-folate from the plasma to approximately 40% of baseline 30 minutes postinfusion (Figure 2C). Plasma tempol and tempol-folate levels fell similarly throughout 2 hours after discontinuation of the infusion and both tempol and tempol-folate were undetectable 4 hours postinfusion. In contrast, as a result of the targeted accumulation of tempol-folate, kidney levels of tempol-folate during the infusion were significantly higher than tempol alone; however, from 30 minutes postinfusion, renal tempol and tempol-folate levels were not different (Figure 2D). These data indicate that the accumulation of tempol-folate in folate receptor-rich organs such as the kidney allows for concentrated tissue antioxidant levels with continuous infusion.
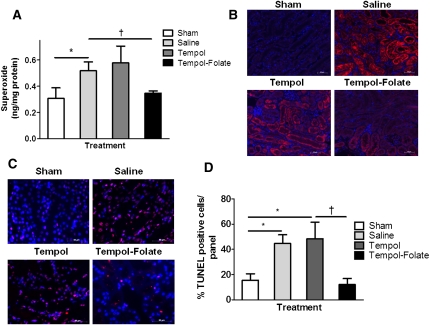
Tempol-Folate Scavenges Renal Superoxide More Efficiently Than Tempol and Prevents Apoptosis In Vivo
Elevations in superoxide can initiate functional and structural changes in the subsequent hours and days after renal ischemia, leading to decreased filtration and progression to ARF.4,5 To explore the ability of tempol-folate to scavenge oxidative metabolites in renal ischemia-reperfusion injury, we measured postischemic superoxide levels in mouse kidneys (Figure 3, A and B).4,39,40 Intrarenal intracellular superoxide concentration 2 hours postischemia measured by DHE-HPLC showed a significant elevation in the saline group compared with the sham, which was successfully lowered by tempol-folate but not by tempol alone (Figure 3A). Nitrotyrosine staining was also more intense in the saline-treated kidneys compared with the sham group 48 hours postischemia, confirming prolonged exposure of the kidney to elevated oxidative stress (Figure 3B). Tempol-folate treatment reduced postischemic nitrotyrosine staining compared with tempol-treated mice, further demonstrating that folate conjugation enhances the antioxidant efficacy of tempol in the kidney. Increased positive terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) staining was observed in kidney sections 2 hours postischemia (Supplemental Figure 2, A and B) and 48 hours postischemia (Figure 3, C and D), indicating amplified apoptosis as a result of excess superoxide.5 TUNEL-positive staining was not prevented by tempol treatment; however, apoptotic cells were less prevalent in the tempol-folate–treated kidneys. We propose that the protection from cellular apoptosis tempol-folate in the kidney was as a result of its enhanced superoxide scavenging ability.
Figure 3.
Tempol-folate treatment reduced postischemic superoxide levels and apoptosis in the kidney. (A) Quantification of whole kidney superoxide concentration 2 hours after ischemia measured by DHE-HPLC (n=4 per group). *P<0.05 comparing sham and saline-treated mice. †P<0.05 between saline- and tempol-folate–treated mice (n=4–5). Data represent mean ± SEM. (B) Representative nitrotyrosine immunostaining in 10-μm kidney sections 48 hours after ischemia. Magnification, ×20; scale bar, 50 μm. Nitrotyrosine stained red and nuclei stained blue. (C) Representative TUNEL staining in 10-μm kidney sections, 48 hours after ischemia. Magnification, ×40; scale bar, 20 μm. TUNEL-positive cells are stained red and nuclei are stained blue. (D) Quantification of percentage of TUNEL-positive cells. *P<0.05 comparing sham with saline- and tempol-treated mice. †P<0.05 between tempol and tempol-folate–treated mice (n=4–5).
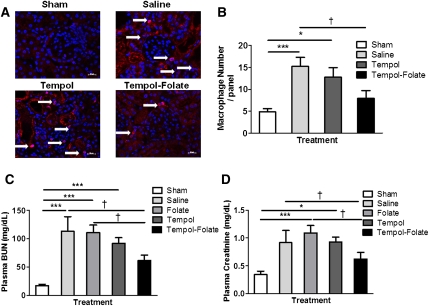
Tempol-Folate Ameliorates Postischemic Inflammation and Renal Injury In Vivo
Confirmation of postischemic inflammation was evident from the identification of macrophage infiltration in the kidney, primarily inside or close to the glomeruli and the area surrounding the tubules (Figure 4, A and B). There were a significantly greater number of macrophages present in kidneys of the saline- and tempol-treated mice compared with the sham-treated group. Tempol-folate treatment significantly reduced the number of infiltrating macrophages compared with saline-treated mice, indicating reduced inflammation that may be responsible for the reduced postischemic renal injury in this treatment group. Plasma BUN, a marker of proximal tubule function, displayed a significant increase postischemia in the saline-treated group compared with the sham group, confirming tubular injury (Figure 4C). Treatment with tempol or folate did little to ameliorate the increase in BUN. However, tempol-folate infusion prevented a significant increase in plasma BUN levels, indicating proximal tubule function was protected. Moreover, plasma creatinine concentration, a measure of GFR, was increased 48 hours postischemia, compared with sham levels (Figure 4D). This postichemic increase in plasma creatinine was also prevented by tempol-folate treatment compared with saline-treated mice.
Figure 4.
Renal inflammatory cell infiltration and proximal tubule injury was prevented by tempol-folate treatment. (A) Representative images of macrophage infiltration into the kidney measured 48 hours after ischemia in the four treatment groups. Arrows indicate red positively stained macrophages. Magnification, ×40; scale bar, 50 μm. Nuclei are stained blue. (B) Quantification of macrophage number in kidney sections, mean number per panel (n=10). ***P<0.001 between sham- and saline-treated groups. *P<0.05 between sham and tempol-treated groups. †P<0.05 between saline- and tempol-folate–treated groups. (C) BUN was measured 48 hours after 30 minutes bilateral renal ischemia-reperfusion. ***P<0.001 comparing values for plasma BUN in sham-treated mice compared with saline-, tempol-, and folate-treated mice. †P<0.05 between plasma BUN in saline- and folate- compared with tempol-folate–treated mice (n=7–14). (D) Plasma creatinine levels measured 48 hours after 30-minute bilateral renal ischemia-reperfusion. ***P<0.001 comparing values of plasma creatinine tempol and folate-treated mice with sham mice. †P<0.05 between saline- and folate- compared with tempol-folate–treated mice (n=7–14). Data represent mean ± SEM.
Discussion
The consequences of renal ischemia-reperfusion injury and subsequent ARF are associated with high morbidity and mortality.40,41 There is evidence that renal tubular damage and dysfunction associated with ischemic injury develop as a result of a net increase in renal oxidative metabolite concentration.5,14,42 Elevations in oxidants can initiate functional and structural changes in the renal proximal tubule, a site particularly at risk during ischemic injury that also expresses high concentrations of folate receptor for the reabsorption of filtered folate.5,26 These factors led us to design a targeted antioxidant therapeutic directed at specifically protecting the proximal tubule from postischemic oxidative damage and subsequently preventing renal dysfunction.
Folate is tolerant of structural substitution allowing for the potential conjugation with a wide range of compounds, thus providing an ideal delivery vehicle to selectively target the kidney. Previously, in cancer therapy, the folate receptor has been used successfully to deliver chemotherapeutic agents to tumors.41,42 However, this is the first time that the folate receptor has been used to specifically target kidney cells.33 We demonstrate that conjugating tempol with folate enabled us to selectively target the kidney allowing for a lower effective antioxidant dose, therefore potentially reducing possible side effects.16–18,30,31 We measured kidney superoxide levels using the current gold standard DHE-HPLC and nitrotyrosine staining as a marker of prolonged oxidative stress. We found that superoxide levels were comparably reduced in vitro by tempol and tempol-folate, confirming that folate conjugation did not impair the ability of tempol to scavenge superoxide. In contrast, we recorded significantly reduced renal superoxide levels in vivo with tempol-folate treatment compared with tempol treatment, indicating that targeted delivery by folate conjugation enhances the inhibition of superoxide in the kidney through efficient delivery.39
We used tempol as our antioxidant of choice because of its small size, solubility, low toxicity, and membrane permeability. However, other antioxidants with similar properties may be equally effective in protecting against renal ischemia-reperfusion injury. In previous studies in SOD1-deficient mice and rats administered tempol, a reduction in superoxide levels provided some protection from renal ischemia-reperfusion injury.10,13,20,43 In these studies, however, concentrations as high as 30 mg/kg per h of tempol were required to observe a beneficial effect on renal function.13 The high concentrations of antioxidants required to prevent renal injury could increase the likelihood of side effects, and subsequently limit their clinical use. These off- target effects may have also contributed to the failure of antioxidant therapy in recent clinical trials.17,43,44 It is with this in mind that we propose that a folate receptor targeted antioxidant therapy has great potential as a clinical therapy for renal ischemic injury.
We provide evidence of successful delivery and concentration of tempol-folate within the kidney. However, the precise movement of the tempol-folate conjugate within the postischemic kidney requires further investigation. We currently propose that the conjugate is taken up into the cell by the standard folate transport system by binding to the folate receptor followed by subsequent encapsulation into clathrin coated pits.32,33 The amide bond joining folate and tempol is stable during moderate changes in pH, increasing the likelihood of tempol remaining bound to folate in vivo.45 Tempol-folate is designed to be membrane impermeable and thus has low accumulation in nontarget organs; however, we propose that it accumulates in the kidney due to the high expression of the folate receptor and can then be actively transported across the basolateral membrane into the kidney.27,32,33 ESR was used to measure tempol concentration by detecting the resonance of the unpaired electron and therefore only provides information about the levels of unreacted tempol or tempol-folate in cells, plasma or tissue.35 Using this technique, the comparison between concentrations is equal and a fair estimate as to the active concentration of each compound in the biologic sample. Renal tempol-folate levels declined rapidly 30 minutes after removal of the intravenous infusion therefore administration of the compound would be necessary during or immediately after ischemia to protect the kidney from ischemic injury.
Renal function was significantly protected by tempol-folate as a result of enhanced scavenging of superoxide but no such protection was observed with free tempol treatment. We observed amelioration of markers of renal injury such as plasma creatinine and plasma BUN. We propose that this amelioration of renal function was as a result of the reduction in oxidative stress afforded by tempol-folate by way of reduced cell apoptosis and tubular injury. Elevations in free radical production have been shown to induce apoptosis and inflammatory cell infiltration.5,7 We observed marked renal macrophage infiltration 48 hours after ischemia that we propose was as a result of the elevated levels of reactive oxygen species and cell apoptosis.7 These infiltrated macrophages, most likely contributed to the reduced renal function by way of cytokine release and tubular obstruction.46,47 Treatment with the tempol-folate conjugate prevented this increase in reactive species, therefore reducing the stimulus for inflammatory cell infiltration, which was evident by a reduced macrophage number and preservation of renal tubular function.
In summary, this study provides compelling evidence that treatment with a tempol-folate conjugate effectively targets the kidney, prevents oxidative metabolite accumulation, and ameliorates postischemic acute renal injury. For the first time, we demonstrate successful targeted drug delivery to the kidney utilizing the folate receptor and propose that this targeting strategy may provide an ideal vehicle for the delivery of therapeutics in many forms of kidney disease. This strategy has the advantage of avoiding off-target effects and lowering the total dose required for renal protection. In addition, folate conjugation and folate receptor targeting could open the door to treatments aimed specifically at other organs expressing high concentrations of folate receptors such as the intestines.
Concise Methods
Synthesis of Tempol-Folate Conjugate
Tempol-folate conjugate was synthesized following the method described by Crich et al.45
HK-2 Cell Culture
HK-2 cells were purchased from ATCC (Manassas, VA) and cultured on 100-mm plates in keratinocyte serum-free media containing 25 mg bovine pituitary extract and 2.5 μg recombinant EGF (Invitrogen, Carlsbad, CA). For the comparison of superoxide inhibition ability of tempol- and tempol-folate–treated HK-2 cells were used at 80% confluence. HK-2 cells were treated with 0.1 μg/ml LPS endotoxin, (Sigma, St Louis, MO) and were incubated at 37°C for 4 hours. Next 0.01 mM and 0.1 mM tempol or tempol-folate was added to the cells and incubated for 1 hour at 37°C.
Tempol and Tempol-Folate Concentration Analysis by ESR
For tempol and tempol-folate uptake studies, HK-2 cells were incubated with 1 μM tempol, tempol-folate, or tempol-folate with 1 μM folate in PBS for 20 minutes at 37°C with agitation. Cell supernatant was collected, and the cells were washed twice in KHB and then resuspended in 100 μl KHB before analysis by ESR as described previously.35 In tissue analysis, tissue was homogenized in KHB and 100 μl of homogenate was used for analysis. Briefly, the concentration of nitroxide was determined from a calibration curve for intensity of the ESR signal of tempol at various known concentrations. Samples were scanned in 100-μl capillary tubes using EMX EPR spectrometer (Bruker Biospin Corp, Billerica, MA). Three characteristic peaks were observed in the tempol and tempol-folate–treated samples. The intensity of the first peak from peak to trough was measured and quantified by comparison with peak intensities obtained from standard solutions of tempol and tempol-folate. A linear relationship between peak intensity and concentration was observed. Concentrations were normalized to protein concentrations quantified by Bradford assay (Bio-Rad, Hercules, CA).
Measurement of Superoxide by DHE-HPLC
HK-2 cells cultured on 100-mm plates until 80% confluent then were treated with 0.1 and 0.01 mM tempol or tempol folate for 30 minutes before overnight incubation with 0.1 μg/ml LPS. Minimal cell death was observed with this treatment. Cells were then washed with KHB and incubated with 10 μM dihydroethidium in 1% DMSO in KHB for 20 minutes at 37°C. Cell supernatant was discarded, and the cells were washed twice with KHB and then scraped from the dish with 100 μl KHB and transferred to methanol to extract the superoxide specific product hydroxyethidium. Kidneys were harvested and cortical slices were taken and incubated in 10 mM dihydroethidium in 1% DMSO for 30 minutes. Cells or tissue were homogenized, filtered, and then separated into ethidium, hydroxyethidium, and dihydroethidium using HPLC (Beckman, Brea, CA) with a C-18 reverse-phase column (Nucleosil, 250, 4.5 mm; Sigma, St. Louis, MO).39 Superoxide concentrations were calculated with reference to standard samples and normalized to protein concentrations quantified by Bradford assay (Bio-Rad, Hercules, CA).
In Vivo Tempol and Tempol-Folate Administration
Tempol and tempol-folate were weighed and dissolved in saline. The solutions were loaded into 7-day osmotic mini-pumps attached to jugular catheters delivering 15 μM/h (Alzet, Cupertino, CA). Mini-pumps with catheters were incubated in saline at 37°C for 24 hours before implantation to reach a steady infusion rate. Mini-pumps were implanted before the renal ischemia and tempol or tempol-folate infusion into the jugular vein commenced immediately before ischemia and continued throughout the 48-hour reperfusion period.
Animals and Experimental Protocol
All animal experiments were carried out in accordance with Emory University Institutional Animal Care and Use Committee guidelines. Male, 11-week-old, C57bl/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were anesthetized using isoflurane with oxygen (0.5 L/h). The neck and back area were shaved and an incision was made to expose the right jugular vein. The jugular vein was cannulated with a jugular catheter attached to a 7-day mini-osmotic pump (Alzet, Cupertino, CA). The mini-osmotic pump delivered a dosage of 500 μg/kg per h tempol or tempol-folate per day. The pump was inserted under the skin at the nape of the neck and the skin was closed with sutures. Immediately after the implantation of the jugular catheter two flank incisions were made, the renal arteries were exposed, and nontraumatic micro-serrafines (Fine Science Tools, Foster City, CA) were placed on both renal arteries for 30 minutes. The serrafines were removed allowing the kidneys to be reperfused before suturing the incisions closed. After the completion of the surgery, mice received 1 μg buprenorphine in 100 μl saline subcutaneously and were allowed to recover for 2 hours for superoxide measurements or were allowed to recover from the anesthesia before being placed into metabolic cages for 48 hours reperfusion.48 Forty-eight hours after the bilateral renal ischemia, mice were euthanized and urine, plasma, and tissue samples were harvested for analysis of renal function. For superoxide measurements, mice were euthanized at an earlier time point at which kidneys were harvested 2 hours after ischemia-reperfusion.
Immunofluorescence
Kidneys were removed from the mice and incubated in 10% formalin for 24 hours then dehydrated and embedded in paraffin. Five-micrometer kidney sections were deparaffinized and rehydrated. For nitrotyrosine staining, 5-μm kidney sections were incubated with rabbit anti-mouse nitrotyrosine antibody (Millipore, MA) at 4°C overnight followed by goat anti-rabbit secondary antibody for 1 hour at room temperature. Slides were washed and then incubated with streptavidin conjugated quantum dot for 10 minutes. Slides were mounted using Vectasheild mounting media containing DAPI (Vector Labs, Burlingame, CA). Images were taken using a Zeiss Axioskop 2 microscope (Thornwood, NY) at ×40 magnification. For macrophage staining, 5-μm kidney sections were incubated with rabbit anti-mouse macrophage primary antibody (Cedarlane, Canada) at a 1:200 dilution for 1 hour, then goat anti-rabbit secondary 1:1000 for 1 hour. Slides were washed and then incubated with streptavidin conjugated quantum dot with 655 nM emission for 10 minutes. Slides were mounted using Vectasheild mounting media with DAPI (Vector Labs). Images were taken using a Zeiss Axioskop 2 microscope at ×40 magnification.
In Vitro Assay of Apoptosis
Apoptotic nuclei were detected using an in situ cell death detection kit or TUNEL stain according to the manufacturer’s protocol (Roche Applied Science, Indianapolis, IN). Briefly, 10-μm paraffin-embedded kidney sections were rehydrated and permeabilized in the microwave in 0.1% sodium citrate for 1 minute. Slides were rinsed in PBS then incubated with terminal deoxynucleotidyl transferase enzyme and tetramethyl-rhodamine-dUTP in buffer for 60 minutes at 37°C in the dark. Slides were rinsed then mounted with coverslips using Vectashield mounting media containing DAPI (Vector Labs). Images were photographed using Zeiss Axioskop 2 microscope at×40 magnification. The percentages of TUNEL-positive cells were counted manually by a blinded reviewer.
Statistical Analyses
All statistical analyses were carried out using GraphPad Prism software (La Jolla, CA) using means and SEMs. One-way ANOVA with Bonferroni and Dunn or Tukey’s post-test were used to compare treatment groups. P values <0.05 were considered significant.
Disclosures
The tempol-folate conjugate described in this study is contained in patent application number WO2011/037913 assigned to Emory University.
Acknowledgments
Plasma creatinine and BUN levels were measured by Dr. James Ritchie, Department of Pathology, Emory University.
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health, as a Program of Excellence in Nanotechnology Award (N01 HV-08234) to W.R.T.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011070711/-/DCSupplemental.
References
- 1.Devarajan P, Mishra J, Supavekin S, Patterson LT, Steven Potter S: Gene expression in early ischemic renal injury: Clues towards pathogenesis, biomarker discovery, and novel therapeutics. Mol Genet Metab 80: 365–376, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Shoskes DA: Nonimmunologic renal allograft injury and delayed graft function: Clinical strategies for prevention and treatment. Transplant Proc 32: 766–768, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Perico N, Cattaneo D, Sayegh MH, Remuzzi G: Delayed graft function in kidney transplantation. Lancet 364: 1814–1827, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Paller MS, Hoidal JR, Ferris TF: Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest 74: 1156–1164, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li C, Jackson RM: Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol 282: C227–C241, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, Fujita T, Brodsky S, Goligorsky MS: Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol 281: F948–F957, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Daemen MA, van ’t Veer C, Denecker G, Heemskerk VH, Wolfs TG, Clauss M, Vandenabeele P, Buurman WA: Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest 104: 541–549, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams P, Lopez H, Britt D, Chan C, Ezrin A, Hottendorf R: Characterization of renal ischemia-reperfusion injury in rats. J Pharmacol Toxicol Methods 37: 1–7, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Goligorsky MS: Whispers and shouts in the pathogenesis of acute renal ischaemia. Nephrol Dial Transplant 20: 261–266, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Yamanobe T, Okada F, Iuchi Y, Onuma K, Tomita Y, Fujii J: Deterioration of ischemia/reperfusion-induced acute renal failure in SOD1-deficient mice. Free Radic Res 41: 200–207, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P: Differential gene expression following early renal ischemia/reperfusion. Kidney Int 63: 1714–1724, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Snoeijs MG, Vink H, Voesten N, Christiaans MH, Daemen JW, Peppelenbosch AG, Tordoir JH, Peutz-Kootstra CJ, Buurman WA, Schurink GW, van Heurn LW: Acute ischemic injury to the renal microvasculature in human kidney transplantation. Am J Physiol Renal Physiol 299: F1134–F1140, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee PK, Cuzzocrea S, Brown PA, Zacharowski K, Stewart KN, Mota-Filipe H, Thiemermann C: Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int 58: 658–673, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Ye J, Li J, Yu Y, Wei Q, Deng W, Yu L: L-carnitine attenuates oxidant injury in HK-2 cells via ROS-mitochondria pathway. Regul Pept 161: 58–66, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Metz JM, Smith D, Mick R, Lustig R, Mitchell J, Cherakuri M, Glatstein E, Hahn SM: A phase I study of topical Tempol for the prevention of alopecia induced by whole brain radiotherapy. Clin Cancer Res 10: 6411–6417, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Waters DD, Alderman EL, Hsia J, Howard BV, Cobb FR, Rogers WJ, Ouyang P, Thompson P, Tardif JC, Higginson L, Bittner V, Steffes M, Gordon DJ, Proschan M, Younes N, Verter JI: Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: A randomized controlled trial. JAMA 288: 2432–2440, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E: Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 142: 37–46, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M: Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 106: 8665–8670, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatterjee PK: Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: A comprehensive review. Naunyn Schmiedebergs Arch Pharmacol 376: 1–43, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Fujii T, Takaoka M, Ohkita M, Matsumura Y: Tempol protects against ischemic acute renal failure by inhibiting renal noradrenaline overflow and endothelin-1 overproduction. Biol Pharm Bull 28: 641–645, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Deelman LE, Declèves AE, Rychak JJ, Sharma K: Targeted renal therapies through microbubbles and ultrasound. Adv Drug Deliv Rev 62: 1369–1377, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stokman G, Qin Y, Rácz Z, Hamar P, Price LS: Application of siRNA in targeting protein expression in kidney disease. Adv Drug Deliv Rev 62: 1378–1389, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E, Brafman A, Faerman A, Atkinson SJ, Thompson JD, Kalinski H, Skaliter R, Erlich S, Feinstein E: siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol 20: 1754–1764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, Zhao Z, Ganger M, Tow CY, Seshan SV: Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol 22: 1041–1052, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birn H, Spiegelstein O, Christensen EI, Finnell RH: Renal tubular reabsorption of folate mediated by folate binding protein 1. J Am Soc Nephrol 16: 608–615, 2005 [DOI] [PubMed] [Google Scholar]
- 26.da Costa M, Rothenberg SP, Sadasivan E, Regec A, Qian L: Folate deficiency reduces the GPI-anchored folate-binding protein in rat renal tubules. Am J Physiol Cell Physiol 278: C812–C821, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Birn H, Nielsen S, Christensen EI: Internalization and apical-to-basolateral transport of folate in rat kidney proximal tubule. Am J Physiol 272: F70–F78, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Sandoval RM, Kennedy MD, Low PS, Molitoris BA: Uptake and trafficking of fluorescent conjugates of folic acid in intact kidney determined using intravital two-photon microscopy. Am J Physiol Cell Physiol 287: C517–C526, 2004 [DOI] [PubMed] [Google Scholar]
- 29.McMartin KE, Morshed KM, Hazen-Martin DJ, Sens DA: Folate transport and binding by cultured human proximal tubule cells. Am J Physiol 263: F841–F848, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Hurrell RF: Influence of vegetable protein sources on trace element and mineral bioavailability. J Nutr 133: 2973S–2977S, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Dotan Y, Lichtenberg D, Pinchuk I: No evidence supports vitamin E indiscriminate supplementation. Biofactors 35: 469–473, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Elnakat H, Gonit M, Salazar MD, Zhang J, Basrur V, Gunning W, Kamen B, Ratnam M: Regulation of folate receptor internalization by protein kinase C alpha. Biochemistry 48: 8249–8260, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Zhao X, Li H, Lee RJ: Targeted drug delivery via folate receptors. Expert Opin Drug Deliv 5: 309–319, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Ashokkumar B, Mohammed ZM, Vaziri ND, Said HM: Effect of folate oversupplementation on folate uptake by human intestinal and renal epithelial cells. Am J Clin Nutr 86: 159–166, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK: Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 45: 1340–1351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP: Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem 338: 284–293, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Luo Z, Chen Y, Chen S, Welch WJ, Andresen BT, Jose PA, Wilcox CS: Comparison of inhibitors of superoxide generation in vascular smooth muscle cells. Br J Pharmacol 157: 935–943, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antony AC: Folate receptors. Annu Rev Nutr 16: 501–521, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Dikalov S, Griendling KK, Harrison DG: Measurement of reactive oxygen species in cardiovascular studies. Hypertension 49: 717–727, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paller MS: The cell biology of reperfusion injury in the kidney. J Investig Med 42: 632–639, 1994 [PubMed] [Google Scholar]
- 41.Thadhani R, Pascual M, Bonventre JV: Acute renal failure. N Engl J Med 334: 1448–1460, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Snoeijs MG, van Heurn LW, Buurman WA: Biological modulation of renal ischemia-reperfusion injury. Curr Opin Organ Transplant 15: 190–199, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Wilcox CS: Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther 126: 119–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinhubl SR: Why have antioxidants failed in clinical trials? Am J Cardiol 101[10A]: 14D–19D, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Crich D, Grant D, Bowers AA: Heterobivalent library expansion by “living radical” processes: Thiocarbonyl addition/elimination, and nitroxide-based reactions with fluorous deconvolution. J Am Chem Soc 129: 12106–12107, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG: Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang HS, Kim J, Park YK, Park KM: Infiltrated macrophages contribute to recovery after ischemic injury but not to ischemic preconditioning in kidneys. Transplantation 85: 447–455, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Kim J, Jung KJ, Park KM: Reactive oxygen species differently regulate renal tubular epithelial and interstitial cell proliferation after ischemia and reperfusion injury. Am J Physiol Renal Physiol 298: F1118–F1129, 2010 [DOI] [PubMed] [Google Scholar]