Abstract
Frequent hemodialysis improves cardiovascular surrogates and quality-of-life indicators, but its effect on survival remains unclear. We used a matched-cohort design to assess relative mortality in daily home hemodialysis and thrice-weekly in-center hemodialysis patients between 2005 and 2008. We matched 1873 home hemodialysis patients with 9365 in-center patients (i.e., 1:5 ratio) selected from the prevalent population in the US Renal Data System database. Matching variables included first date of follow-up, demographic characteristics, and measures of disease severity. The cumulative incidence of death was 19.2% and 21.7% in the home hemodialysis and in-center patients, respectively. In the intention-to-treat analysis, home hemodialysis associated with a 13% lower risk for all-cause mortality than in-center hemodialysis (hazard ratio [HR], 0.87; 95% confidence interval [95% CI], 0.78–0.97). Cause-specific mortality HRs were 0.92 (95% CI, 0.78–1.09) for cardiovascular disease, 1.13 (95% CI, 0.84–1.53) for infection, 0.63 (95% CI, 0.41–0.95) for cachexia/dialysis withdrawal, 1.06 (95% CI, 0.81–1.37) for other specified cause, and 0.59 (95% CI, 0.44–0.79) for unknown cause. Findings were similar using as-treated analyses. We did not detect statistically significant evidence of heterogeneity of treatment effects in subgroup analyses. In summary, these data suggest that relative to thrice-weekly in-center hemodialysis, daily home hemodialysis associates with modest improvements in survival. Continued surveillance should strengthen inference about causes of mortality and determine whether treatment effects are homogeneous throughout the dialysis population.
Thrice-weekly in-center hemodialysis has been the dominant renal replacement therapy in the United States for >30 years.1 Challenges associated with it include excess fluid accumulation, dysregulated bone and mineral metabolism, vascular access infection, and anemia secondary to blood loss and hemolysis.1–3 More frequent hemodialysis has been suggested to approximate continuous glomerular filtration; improvements in BP and phosphorus clearance have frequently been observed.4–6 Whether such mechanisms result in lower mortality risk is unknown, as observational studies that have reported improved survival with more frequent dialysis were relatively small and/or incompletely adjusted for selection bias.7–11
The popularity of home hemodialysis has recently risen in the United States. According to the ESRD Network Organizations Program, the number of home hemodialysis patients increased from 1481 at the end of 2004 to 4836 at the end of 2009.12 Although the periodicity of daily home hemodialysis (DHHD) may be efficacious, in-home treatment requires increased attention to hygiene techniques for vascular access and equipment maintenance. In this study, we assessed the relative mortality of DHHD and matched thrice-weekly in-center patients between 2005 and 2008. DHHD patients initiated use of the NxStage System One between 2005 and 2007. Because the NxStage System One is the only portable hemodialysis machine approved by the US Food and Drug Administration for in-home treatment, its users represent the largest subset of home hemodialysis patients. Matched in-center patients were identified from the US Renal Data System (USRDS) database; matching factors included first date of follow-up, demographic characteristics, and disease severity measures. Despite matching, the possibility of unmeasured confounding persists, as in all observational comparisons of treatments.
Results
Descriptive Analyses
The study cohort included 1873 DHHD patients, of whom 1586 (84.7%) had Medicare as the primary payer during the 3 months preceding NxStage System One use initiation. Counts of new DHHD patients were 202 (10.8%), 619 (33.0%), and 1052 (56.2%) in 2005, 2006, and 2007, respectively. Characteristics of DHHD patients, matched thrice-weekly in-center patients, and all thrice-weekly in-center patients alive on January 1, 2007, are displayed in Table 1. Compared with the entire in-center population, DHHD patients were younger (mean age 52.2 versus 62.6 years), more likely to be wait-listed for kidney transplant (35.0% versus 14.2%), less likely to have congestive heart failure (26.9% versus 44.6%), and less likely to be black (26.5% versus 38.8%). DHHD patients also accumulated fewer hospital days (mean 2.3 versus 4.0) and had longer ESRD duration (5.5 versus 4.0 years). After matching, characteristics of DHHD and in-center patients were generally balanced (standardized differences <7%), although an excess of dually eligible Medicare/Medicaid beneficiaries persisted in the in-center population (prevalence, 35.4% versus 23.2%). Regarding the cumulative number of factors by which DHHD patients were successfully matched with in-center patients, 34.3%, 50.3%, and 91.3% of DHHD patients were matched according to 17, at least 15, and at least 8 factors, respectively.
Table 1.
Characteristics of daily home hemodialysis patients, matched thrice-weekly in-center hemodialysis patients, and all thrice-weekly in-center hemodialysis patients alive on January 1, 2007
| Characteristics | DHHD | Matched In-Center | Standardized Differencea | All In-Centerb | Standardized Differencec |
|---|---|---|---|---|---|
| Sample size (n) | 1873 | 9365 | 262,249 | ||
| Aged (yr) | |||||
| mean (SD) | 52.2 (14.8) | 53.2 (14.7) | 6.8 | 62.6 (15.2) | 69.1 |
| median | 52.3 | 53.1 | 64.0 | ||
| Race (%) | |||||
| black | 26.5 | 28.3 | 4.1 | 38.8 | 26.4 |
| other | 73.5 | 71.7 | 4.1 | 61.2 | 26.4 |
| Sex (%) | |||||
| women | 35.8 | 37.7 | 3.9 | 45.3 | 19.5 |
| men | 64.2 | 62.3 | 3.9 | 54.7 | 19.5 |
| Primary ESRD cause (%) | |||||
| diabetes | 27.3 | 30.3 | 6.7 | 44.1 | 35.7 |
| hypertension | 19.3 | 20.6 | 3.4 | 28.9 | 22.7 |
| GN or cystic kidney disease | 30.3 | 28.4 | 4.3 | 12.6 | 44.1 |
| other or unknown | 23.1 | 20.7 | 5.9 | 14.4 | 22.6 |
| ESRD duration (yr)d | |||||
| mean (SD) | 5.5 (6.3) | 5.1 (5.8) | 5.7 | 4.0 (4.5) | 26.2 |
| median | 3.2 | 3.3 | 2.7 | ||
| BMI (kg/m2)d | |||||
| mean (SD) | 28.1 (7.0) | 27.9 (6.7) | 2.4 | 27.5 (7.0) | 8.1 |
| median | 26.9 | 26.6 | 26.3 | ||
| Comorbid conditions (%)e | |||||
| atherosclerotic heart disease | 24.0 | 22.7 | 3.2 | 34.6 | 23.4 |
| cerebrovascular disease | 8.3 | 8.1 | 1.0 | 16.4 | 24.7 |
| congestive heart failure | 26.9 | 27.1 | 0.4 | 44.6 | 37.6 |
| peripheral vascular disease | 20.9 | 20.5 | 0.9 | 32.0 | 25.3 |
| other cardiovascular disease | 20.0 | 17.9 | 5.2 | 25.8 | 14.0 |
| cancer | 9.1 | 7.3 | 6.7 | 8.7 | 1.2 |
| diabetes | 40.6 | 42.1 | 2.9 | 59.0 | 37.3 |
| Dual Medicare/Medicaid eligibility (%)d | 23.2 | 35.4 | 27.0 | 42.4 | 41.8 |
| Cumulative EPO dose (1000s IU)f | |||||
| mean (SD) | 184.0 (237.4) | 180.6 (226.6) | 1.5 | 198.9 (227.0) | 6.4 |
| median | 110.7 | 113.1 | 132.6 | ||
| Cumulative hospital daysf | |||||
| mean (SD) | 2.3 (7.7) | 2.3 (7.7) | 0.0 | 4.0 (9.6) | 18.9 |
| median | 0.0 | 0.0 | 0.0 | ||
| Transplant waitlist registrationd (%) | 35.0 | 34.8 | 0.5 | 14.2 | 49.7 |
EPO, epoetin alfa.
Difference between DHHD and matched in-center patients, in percentage of 1 SD.
Alive on January 1, 2007, with Medicare as primary payer or recent ESRD.
Difference between DHHD and in-center patients alive on January 1, 2007, in percentage of 1 SD. Differences <10% indicate similarity.
On index date.
During the 6 months preceding the index date or as indicated on the Medical Evidence Report.
During the 3 months preceding the index date.
Survival Analyses
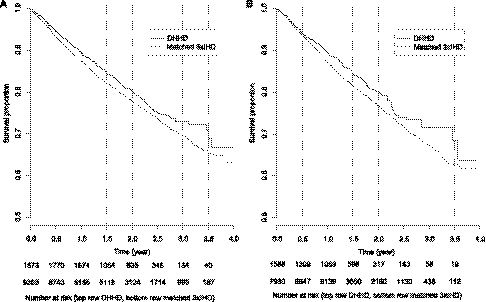
In intention-to-treat analysis, 360 (cumulative incidence, 19.2%) and 2033 (21.7%) deaths occurred in DHHD and matched in-center patients, respectively. Corresponding mortality rates were 110 and 127 deaths per 1000 patient-years, with mean follow-up of 1.8 and 1.7 years, respectively. Survival estimates are displayed in Figure 1A. Survival percentages in DHHD versus in-center patients were 89.4% versus 87.4% at 1 year, 80.1% versus 77.8% at 2 years, and 72.9% versus 69.8% at 3 years. The difference in survival estimates was significant (log-rank test P=0.01). In as-treated analysis of Medicare primary payer patients, 240 (15.1%) and 1653 (20.8%) deaths occurred in DHHD and matched in-center patients, respectively. Corresponding mortality rates were 112 and 137 deaths per 1000 patient-years, with mean follow-up of 1.4 and 1.5 years, respectively. Survival estimates are displayed in Figure 1B. Survival percentages for DHHD versus in-center patients were 89.4% versus 87.3% at 1 year, 80.0% versus 76.7% at 2 years, and 71.7% versus 67.6% at 3 years. The difference in survival estimates was likewise significant (P=0.01).
Figure 1.
Kaplan–Meier estimates of survival for daily home hemodialysis (DHHD) and matched thrice-weekly in-center hemodialysis (3xIHD) patients, by analytical approach. (A) Intention-to-treat analysis. (B) As-treated analysis.
Relative hazards of death for DHHD versus matched in-center patients from unadjusted Cox regression are displayed in Table 2. In intention-to-treat analysis of all-cause mortality, the mortality hazard ratio (HR) for DHHD versus in-center patients was 0.87 (95% confidence interval [CI], 0.78–0.97). Cause-specific mortality HRs were 0.92 (95% CI, 0.78–1.09) for cardiovascular disease (percentage of all deaths in in-center patients, 43.0%), 1.13 (95% CI, 0.84–1.53) for infection (11.1%), 0.63 (95% CI, 0.41–0.95) for cachexia/dialysis withdrawal (9.6%), 1.06 (95% CI, 0.81–1.37) for other specified cause (15.8%), and 0.59 (95% CI, 0.44–0.79) for unknown cause (20.5%). The all-cause mortality HR shifted from 0.88 to 0.95 in the first 2 years of follow-up, but the trend was not significant (P=0.97). Findings were insensitive to model-based adjustment of matching factors, as the only imbalanced factor (dual eligibility) was unassociated with risk of death (HR, 0.98). In as-treated analysis, the mortality HR for DHHD versus in-center patients was 0.82 (95% CI, 0.72–0.94). Cause-specific mortality HRs were 0.83 (95% CI, 0.67–1.01) for cardiovascular disease (percentage of all deaths in in-center patients, 43.9%), 1.17 (95% CI, 0.83–1.66) for infection (11.1%), 0.70 (95% CI, 0.44–1.11) for cachexia/dialysis withdrawal (9.9%), 1.19 (95% CI, 0.88–1.61) for other specified cause (14.6%), and 0.41 (95% CI, 0.28–0.62) for unknown cause (20.6%). The all-cause mortality HR shifted from 0.77 to 0.89 in the first 2 years of follow-up, but the trend was not significant (P=0.78).
Table 2.
Relative hazards of death for daily home hemodialysis patients in intention-to-treat and as-treated analyses
| Intention to Treat | As Treated | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| All-cause mortality | 0.87 (0.78–0.97) | 0.01 | 0.82 (0.72–0.94) | <0.01 |
| Cause-specific mortality | ||||
| cardiovascular disease | 0.92 (0.78–1.09) | 0.34 | 0.83 (0.67–1.01) | 0.06 |
| infection | 1.13 (0.84–1.53) | 0.41 | 1.17 (0.83–1.66) | 0.38 |
| cachexia/dialysis withdrawal | 0.63 (0.41–0.95) | 0.03 | 0.70 (0.44–1.11) | 0.13 |
| other specified cause | 1.06 (0.81–1.37) | 0.69 | 1.19 (0.88–1.61) | 0.25 |
| unknown cause | 0.59 (0.44–0.79) | <0.01 | 0.41 (0.28–0.62) | <0.01 |
| Interval-specific mortality (mo) | ||||
| 1–6 | 0.88 (0.78–0.98) | 0.02 | 0.77 (0.68–0.89) | <0.01 |
| 7–12 | 0.89 (0.78–1.02) | 0.10 | 0.75 (0.63–0.89) | <0.01 |
| 13–18 | 0.92 (0.78–1.09) | 0.32 | 0.81 (0.65–1.01) | 0.06 |
| 19–24 | 0.95 (0.76–1.20) | 0.69 | 0.89 (0.66–1.21) | 0.45 |
| ≥25 | 0.92 (0.66–1.28) | 0.61 | 0.95 (0.62–1.47) | 0.82 |
Referent: matched thrice-weekly in-center patients.
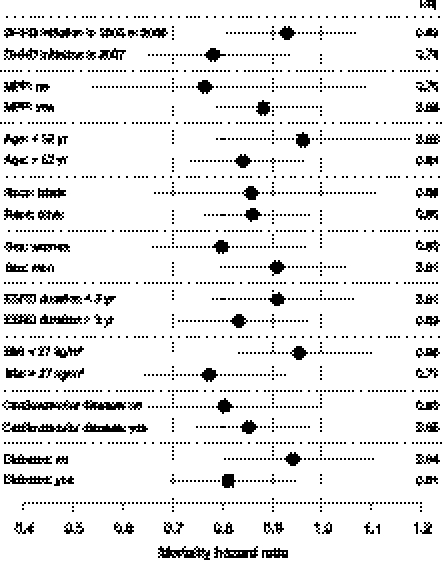
All-cause mortality HRs for DHHD versus matched in-center patients in intention-to-treat analysis are displayed across strata in Figure 2. All HRs were <1. Among the nine pairs of strata, the largest intra-pair differences in HRs were for body mass index (BMI) (0.96 with BMI <27 kg/m2 versus 0.77 with BMI >27 kg/m2), year of index date (0.93 with DHHD initiation in 2005 or 2006 versus 0.78 with DHHD initiation in 2007), and diabetes (0.94 without diabetes versus 0.81 with diabetes). However, none of the nine intra-pair differences was significantly different from null.
Figure 2.
Relative hazards of death for daily home hemodialysis patients in intention-to-treat analysis, by strata (referent: matched thrice-weekly in-center hemodialysis patients). HRs are displayed by dark circles and 95% CIs by solid lines. Cardiovascular disease was defined by any of atherosclerotic heart disease, cerebrovascular disease, congestive heart failure, peripheral vascular disease, or other cardiovascular disease. MPP, Medicare as primary payer.
Discontinuation Analyses
Regarding discontinuation of follow-up before the earlier of death or December 31, 2008, for Medicare primary payer patients, 626 (39.5%) and 1355 (17.1%) events occurred in DHHD and matched in-center patients, respectively. Reasons for discontinuation were change in dialytic modality (percentage of all DHHD versus matched in-center patients, 26.4% versus 2.8%), kidney transplant (10.2% versus 10.8%), and cessation of Medicare primary payer status (2.9% versus 3.5%). Reason-specific discontinuation HRs for DHHD versus matched in-center patients were 10.4 (95% CI, 8.9–12.3) for change in dialytic modality, 1.06 (95% CI, 0.89–1.25) for kidney transplant, and 0.92 (95% CI, 0.68–1.26) for cessation of Medicare primary payer status. In DHHD patients, significant predictors of change in dialytic modality (P<0.05) included ESRD duration at DHHD initiation (HR, 0.98 per year), dual Medicare/Medicaid eligibility (HR, 1.67), and any hospitalization during the 3 months preceding DHHD initiation (HR, 1.51). Of DHHD patients who changed dialytic modality, 96.9% initiated in-center hemodialysis and 3.1% initiated peritoneal dialysis. After DHHD cessation, the intention-to-treat mortality rate was 160 deaths per 1000 patient-years.
Sensitivity Analyses
As a sensitivity analysis, we considered robustness of the intention-to-treat all-cause mortality HR for DHHD versus matched in-center patients (0.87), with respect to adjustment for a hypothetical unmeasured binary factor U (e.g., “physical and mental capacity to self-dialyze”), stipulated to be independently associated with risk of death. The sensitivity analysis provides a theoretical answer to a practical question: “How imbalanced must an unmeasured risk factor for death be to account for the observed mortality reduction?” Findings are displayed in Table 3. In all cases, we assumed that U was present in all DHHD patients. If the HR for presence versus absence of U were 0.9, then the HR for DHHD versus in-center hemodialysis would remain <1, regardless of the prevalence of U in matched in-center patients. However, if the HR for presence versus absence of U were 0.8, 0.7, 0.6, or 0.5, then the HR for DHHD versus in-center hemodialysis would exceed 1 if prevalence of U in matched in-center patients were ≤40%, 65%, 77%, or 85%, respectively.
Table 3.
Relative hazards of death for daily home hemodialysis patients in intention-to-treat analysis, after adjustment for a hypothetical unmeasured binary factor Ua
| HRb for U | Prevalence (%) of U in Matched Thrice-Weekly In-Center Hemodialysis Patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 90 | 80 | 70 | 60 | 50 | 40 | 30 | 20 | 10 | 0 | |
| 0.90 | 0.87 | 0.88 | 0.89 | 0.90 | 0.91 | 0.92 | 0.93 | 0.94 | 0.95 | 0.96 | 0.97 |
| 0.80 | 0.87 | 0.89 | 0.91 | 0.94 | 0.96 | 0.98 | 1.00 | 1.02 | 1.04 | 1.07 | 1.09 |
| 0.70 | 0.87 | 0.91 | 0.94 | 0.98 | 1.02 | 1.06 | 1.09 | 1.13 | 1.17 | 1.21 | 1.24 |
| 0.60 | 0.87 | 0.93 | 0.99 | 1.04 | 1.10 | 1.16 | 1.22 | 1.28 | 1.33 | 1.39 | 1.45 |
| 0.50 | 0.87 | 0.96 | 1.04 | 1.13 | 1.22 | 1.31 | 1.39 | 1.48 | 1.57 | 1.65 | 1.74 |
Referent: matched thrice-weekly in-center hemodialysis patients.
The prevalence of U was assumed to be 100% in daily home hemodialysis patients.
The independent effect of U (e.g., yes versus no) on risk of death.
Discussion
In this study, we compared mortality of DHHD patients who initiated renal replacement therapy with the NxStage System One from 2005 to 2007 with matched thrice-weekly in-center hemodialysis patients. Matching factors included first date of follow-up, demographic characteristics, and measures of disease severity. Although DHHD patients were considerably younger with less comorbidity than the entire population of in-center patients, matching successfully balanced 16 of 17 measured factors, thereby yielding improved estimates of relative mortality between DHHD and in-center hemodialysis. We found that risk of death for DHHD patients was 13% and 18% lower in intention-to-treat and as-treated analyses, respectively, although relative risks varied considerably across causes of death. Relative risks did not vary significantly during follow-up or across patient strata; however, the sample size afforded limited power to detect heterogeneity of treatment effects.
Existing evidence of a beneficial effect of frequent hemodialysis on risk of death is weak. The Frequent Hemodialysis Network (FHN) trial recently found a large beneficial effect of six versus three in-center hemodialysis sessions per week on the composite endpoint of change in left-ventricular mass and death (HR, 0.61).4 With only 14 deaths in the trial (five and nine in patients who were randomized to six and three sessions per week, respectively), the difference was primarily a function of greater left-ventricular mass regression in patients who dialyzed frequently. Left-ventricular mass is a strong predictor of cardiovascular risk in dialysis patients, but unlike in the hypertensive population, regression does not necessarily portend improved outcomes; harm associated with complete correction of anemia is a case in point.13–17 Even if left-ventricular mass regression were to reduce the risk of major adverse cardiovascular events, the magnitude of mortality risk reduction would likely be <39%.18
In addition, nonrandomized studies of frequent hemodialysis may elicit skepticism, because selection of appropriate controls is not straightforward. Published estimates of mortality risk reductions with daily hemodialysis range from 36% to 66% in as-treated analysis (Table 4).7–11 All except one of these studies used the adjustment technique of indirect standardization to derive standardized mortality ratios, with the United States in-center population as the referent. Although this statistical approach is theoretically adequate, it is not well suited for high-dimensional adjustment. Lack of adjustment for confounding factors beyond age, race, sex, primary ESRD cause, and ESRD duration limits the value of findings from these studies; as a frame of reference, the intention-to-treat all-cause mortality HR in this study would have been 0.73 if we had included only age, race, sex, and primary ESRD cause as matching factors. Just as peritoneal dialysis patients in the United States have less comorbidity than hemodialysis patients, DHHD patients in this study were markedly healthier than their counterparts in the entire in-center population, with less cardiovascular disease, fewer hospital days, and a greater likelihood of being wait-listed for transplant.19
Table 4.
Published estimates of mortality risk reductions with frequent dialysis
| Reference | Modality | Sample Size | Mortality Ratea | Adjustment Technique | Relative Risk (95% CI) |
|---|---|---|---|---|---|
| Johansen et al.7 | NHD | 94 | 74 | Propensity score matchingb | 0.36 (0.22–0.61) |
| SDHD | 43 | 91 | Propensity score matchingb | 0.64 (0.31–1.31) | |
| Miller et al.8 | SDHD | 190 | 69 | Indirect standardizationc | 0.39 (0.22–0.64) |
| Kjellstrand et al.9 | SDHD | 415 | 84 | Indirect standardizationd | 0.34 (0.20–0.54) |
| Agar et al.10 | NHD | 72 | 65 | Indirect standardizatione | 0.42 (0.15–0.91) |
| Blagg et al.11 | SDHD | 117 | 76 | Indirect standardizationf | 0.39 (0.19–0.51) |
NHD, nocturnal hemodialysis; SDHD, short daily hemodialysis.
Per 1000 patient-years.
Scored by age, race, sex, primary ESRD cause, ESRD duration, BMI, number of cardiovascular comorbid conditions, Medicaid status, and cumulative admissions and days for all-cause, cardiovascular-related, and vascular access-related hospitalizations during a maximum of 12 months preceding follow-up.
Standardized by age, race, and sex.
Standardized by age and primary ESRD cause.
Standardized by age, race, sex, primary ESRD cause, and ESRD duration.
Standardized by age, race, sex, and primary ESRD cause.
The lower risk of death associated with DHHD in this study was primarily due to reduced risks of death from cardiovascular disease and unknown causes, because these accounted for nearly two of every three deaths in matched in-center hemodialysis patients. Frequent hemodialysis typically reduces predialysis systolic BP, primarily because of decreased extracellular fluid volume.4,5 Less severe hypertension generally reduces risk of cardiovascular morbidity, but lack of BP targeting trials in dialysis patients limits conclusions about the importance of this effect.20 Despite mixed findings in observational studies, the FHN trial definitively showed that frequent hemodialysis decreased predialysis serum phosphorus, of which higher levels are associated with increased coronary vessel and valvular calcification.4,21 In addition, frequent hemodialysis should reduce predialysis serum potassium, possibly reducing risk of cardiovascular mortality attributable to hyperkalemia.22
The reduction in unspecified causes of death is likely multifactorial. Importantly, cause of death is unknown when the ESRD Death Notification form is not submitted; thus, the observed reduction may merely represent further risk reductions in death due to cardiovascular disease and cachexia/dialysis withdrawal. Findings from previous studies are compatible with the hypothesis that daily hemodialysis improves Kidney Disease Quality of Life scores, which have been correlated with mortality risk.23 In the FHN trial, the physical health composite score improved significantly with frequent hemodialysis.4 In the ongoing Following Rehabilitation, Economics, and Everyday Dialysis Outcome Measurements study, daily hemodialysis patients had fewer depressive symptoms, better sleep quality, and shorter postdialysis recovery time.24,25 Depression severity has been identified as a predictor of dialysis withdrawal26; risk of death from dialysis withdrawal was markedly lower in DHHD patients in this study. In addition, frequent hemodialysis typically reduces intensity of pharmacological treatment, with decreased use of antihypertensive agents and phosphate binders.4,27,28 Given the pill burden on ESRD patients, fewer medications might be more efficacious, insofar as adherence may improve.29
More frequent dialysis may confer some risk. In the FHN trial, relative hazards of time to first (HR, 1.71) and multiple (HR, 1.35) vascular access interventions were higher in patients who were randomized to six sessions per week.4 We found increased risk of infectious mortality in DHHD. Although the association lacked statistical significance, it is likely an important safety signal. Home dialysis requires diligent antibacterial practices to prevent infection of dialysis equipment and the vascular access site. The latter is salient because arteriovenous fistulas have been identified as the predominant method of vascular access in frequent hemodialysis patients.30 Daily cannulation of fistulas may increase risk of access infections, particularly in patients who use the buttonhole technique.31 That DHHD patients dialyze at home raises the possibility that acute infections may not be identified by providers until they spread, resulting in septicemia or cardiac infection. Future studies should probe morbidity (e.g., resulting in hospitalization), as well as the roles of vascular access techniques in infectious mortality and depression in deaths after dialysis withdrawal.
Relative risks did not vary significantly during follow-up or across patient strata, but we observed some potentially important variation. Interval analysis indicated attenuation of the DHHD mortality advantage during the first 2 years of follow-up. This pattern may reflect growing fatigue with the daily dialysis regimen, as reported in a prospective study of five daily in-center hemodialysis patients.32 However, estimates of relative survival in later intervals mostly rely on follow-up data from patients who initiated DHHD in 2005 and 2006. We found that survival was better for patients who initiated DHHD in 2007 than for their predecessors, suggesting serial improvement in patient training and technique execution. Interestingly, we also found that patients with diabetes and higher BMI (itself correlated with diabetes) benefited relatively more from DHHD. In an analysis of 22 daily hemodialysis patients with mean treatment duration of almost 5 years, hyperinsulinemia was rare, suggesting improved insulin sensitivity.33 Because insulin resistance may lead to hypertension and dyslipidemia, one could speculate that patients with diabetes and/or obesity gain greater potential benefit from frequent dialysis.
This study has two major limitations. First, we used matching in lieu of randomization. We achieved balance in almost all matching factors, and sensitivity analysis showed that only a strong unmeasured confounder (e.g., with HR=0.8 and prevalence difference ≥60%) could alone undo the observed association of DHHD with mortality. However, this analysis does not eliminate the possibility that many weaker unmeasured confounders could collectively undo the association. Uncontrolled differences in disease severity (as measured by serum concentrations), educational attainment, social support, and socioeconomic status may underlie observed survival differences.34 Second, approximately 1 in 4 DHHD patients switched to in-center hemodialysis during follow-up. The high rate of DHHD cessation limits the capability of intention-to-treat analysis to discern the clinical effects of DHHD treatment. However, as-treated analysis is not necessarily superior, because sicker patients may be more likely to cease DHHD, thereby biasing HR estimates. To limit bias, we followed patients for 2 months after DHHD cessation.
This study also has four minor limitations. First, we used Medicare claims to identify measures of disease severity. Misclassification of comorbid conditions is likely. However, highly important matching factors, including age, hospital days, and epoetin alfa dose, were measured with minimal error. Second, because cause of death is unknown in 10%–20% of cases, estimates of cause-specific mortality HRs must be interpreted with caution. Third, we estimated the association of DHHD with mortality in a relatively young and healthy subset of the ESRD population. DHHD patients likely had some social support. The findings in this study may not apply to thrice-weekly in-center patients without the physical and mental capacity and social support to effectively dialyze at home. Finally, we did not have data regarding dialysis adequacy, dose, or frequency during follow-up.
Frequent hemodialysis in general and DHHD in particular may confer benefits on ESRD patients. The FHN trial and this study together suggest that frequent hemodialysis improves cardiovascular health, resulting in a modest improvement in survival. Because of possible safety concerns regarding infection with DHHD, continued surveillance and improvements in patient education are warranted. As with peritoneal dialysis, patients and providers should carefully consider the benefits and harms of DHHD, as well as its feasibility, whereas policymakers should design incentives that maximize its use in clinically appropriate subgroups.
Concise Methods
Protection of Human Participants
DHHD patient data were obtained through a data licensing agreement between NxStage Medical Inc (Lawrence, MA) and the Chronic Disease Research Group. Data were linked to the USRDS database by USRDS staff under a data use agreement with the National Institute of Diabetes and Digestive and Kidney Diseases; the agreement stipulated confidentiality rules and security regulations. Study approval was obtained from the Hennepin County Medical Center Human Subjects Research Committee (Minneapolis, MN).
Study Cohort
DHHD patients were identified from a registry of NxStage System One users maintained by NxStage Medical Inc. The registry included 2816 records, and each record included personal identifiers (first name, last name, date of birth), sex, beginning and ending dates of NxStage System One use, and the prescribed number of dialysis runs per week. Beginning dates ranged from January 1, 2005, to December 31, 2007, and ending dates (as applicable) occurred as late as December 31, 2008. Using personal identifiers and sex, we linked 2802 records (99.5%) to data in the USRDS database. We identified 2723 patients, including 2645 with 1 record, 77 with 2 records, and 1 with 3 records. Of patients with multiple records, 32 had replicate records, aside from name spelling differences; 9 had pairs of records indicating transfers between home dialysis programs with no interruption in DHHD; 18 had pairs indicating transfers between programs with possible interruptions of no longer than 1 month; and 19 had pairs indicating interruptions of longer than 1 month. For patients with possible interruptions of no longer than 1 month, we ignored gaps in treatment and merged the record pairs. For patients with interruptions of longer than 1 month, we retained only the first record. For specificity, we included patients with five or six prescribed dialysis sessions per week (n=2617). Finally, we required Medicare primary payer status during the 3 months preceding NxStage System One use initiation, or beginning of renal replacement therapy during the 6 months preceding use initiation (n=1873). These conditions allowed us to use data from the Medical Evidence Report (Centers for Medicare and Medicaid form CMS-2728) and Medicare claims as temporally proximal measures of baseline health status.
The source cohort of thrice-weekly in-center patients included all patients who were treated with in-center hemodialysis between January 1, 2005, and December 31, 2007 (n=637,109).
Data Elements
For each DHHD patient, we identified age, race, sex, primary ESRD cause, ESRD duration, BMI, atherosclerotic heart disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, other cardiovascular disease, cancer, diabetes, concurrent Medicare and Medicaid enrollment (dual eligibility), cumulative epoetin alfa dose, cumulative hospital days, and kidney transplant waiting list registration. Age, ESRD duration, dual eligibility, and waiting list registration were defined at NxStage System One use initiation (index date). BMI was ascertained from the final outpatient dialysis claim during the 3 months preceding the index date, or in lieu of claims with BMI data, from the Medical Evidence Report. In patients with Medicare as primary payer, epoetin alfa dose and hospital days were measured from outpatient and inpatient claims, respectively, during the 3 months preceding the index date; in patients without Medicare as primary payer, dose and days were unknown. Comorbid conditions were defined from the Medical Evidence Report and claims during the 6 months preceding the index date; in claims, we required at least one inpatient or two outpatient claims with relevant diagnosis codes to define disease.35 Dual eligibility is a coarse measure of socioeconomic status, as Medicaid eligibility requires very low annual income and assets.
For each in-center patient, we iteratively identified all aforementioned factors with respect to each day of hemodialysis between January 1, 2005, and December 31, 2007. Also on each day, we determined Medicare primary payer status during the 3 preceding months, and if Medicare was not the primary payer, whether renal replacement therapy began during the 6 preceding months.
Matching Algorithm
For each DHHD patient, we selected 5 thrice-weekly in-center patients with matching characteristics. To do so, we initially retained only in-center patients who underwent hemodialysis on the index date of the DHHD patient. Next, if the DHHD patient had Medicare as primary payer, we retained the subset of in-center patients with Medicare as primary payer; if not, we retained the subset of non-Medicare primary payer patients who began renal replacement therapy during the 6 preceding months. Subsequently, we iteratively winnowed the set of in-center candidate matches according to an ordering of aforementioned factors and factor-specific definitions of sufficient similarity (Table 5). The ordering was determined from survival analysis of all in-center patients alive on January 1, 2007, with follow-up until the earlier of death or December 31, 2008. We fit a Cox proportional hazards regression with all factors, with stratification by Medicare primary payer status. We also fit each model with one factor removed, and calculated the deviance of the model from the model with all factors. The ordering represents a descending list of deviance estimates.
Table 5.
Ordering of matching factors and definitions of sufficient similarity
| Order | Factor | Definition of Sufficient Similaritya |
|---|---|---|
| 1 | Ageb | AD <9.9 years |
| 2 | Cumulative hospital daysc | (1) If DHHD patient did not have MPP, factor is not applicable |
| (2) If DHHD patient had MPP and accumulated 0 days, AD=0 days | ||
| (3) If DHHD patient had MPP and accumulated >0 days, AD ≤3 days | ||
| 3 | Cumulative EPO dosec | (1) If DHHD patient did not have MPP, factor is not applicable |
| (2) If DHHD patient had MPP and accumulated 0 units, AD=0 units | ||
| (3) If DHHD patient had MPP and accumulated >0 units, AD <104,400 units | ||
| 4 | Body mass indexb | AD <4.6 kg/m2 |
| 5 | Transplant waitlist registrationb | Equality |
| 6 | Congestive heart failured | Equality |
| 7 | ESRD durationb | (1) If DHHD patient had ESRD duration <1 year, AD <0.2 years |
| (2) If DHHD patient had ESRD duration ≥1 year, AD <4.3 years | ||
| 8 | Race | Equality |
| 9 | Cancerd | Equality |
| 10 | Primary ESRD cause | Equality |
| 11 | Cerebrovascular diseased | Equality |
| 12 | Peripheral vascular diseased | Equality |
| 13 | Other cardiovascular diseased | Equality |
| 14 | Diabetesd | Equality |
| 15 | Atherosclerotic heart diseased | Equality |
| 16 | Sex | Equality |
| 17 | Dual Medicare/Medicaid eligibilityb | Equality |
AD, absolute difference; EPO, epoetin alfa; MPP, Medicare as primary payer.
Sufficient similarity was defined by equality in categorical factors and differences no greater than one-third of a SD in continuous factors, with SDs estimated in DHHD patients.
On index date.
During the 3 months preceding the index date.
During the 6 months preceding the index date or as indicated on the Medical Evidence Report.
If any iterative winnowing of candidate matches resulted in <5 candidates, the algorithm was reversed 1 factor, and 5 candidates were randomly selected. If execution of the entire algorithm resulted in >5 candidates, 5 were randomly selected.
Statistical Analyses
We calculated tabular summaries of measured factors in DHHD patients, all in-center patients alive on January 1, 2007, and matched in-center patients. We assessed match quality with standardized differences; differences <10% indicate similarity.36 We calculated survival estimates with the Kaplan–Meier method. We used both intention-to-treat and as-treated analyses. In the former, we followed patients from the index date of the DHHD patient in the matched cluster to the earlier of death or December 31, 2008. In the latter, we followed patients from the index date to the earlier of death, 2 months after change of dialytic modality (from DHHD to in-center hemodialysis or peritoneal dialysis, or from in-center hemodialysis to peritoneal dialysis), kidney transplant, cessation of Medicare as primary payer, or December 31, 2008; the 2-month extension after change of dialytic modality effectively assigned deaths and survival time to the first modality. As-treated analysis was restricted to Medicare primary payer patients to ensure ascertainment of modality changes. We compared survival with Cox regression, with and without model-based adjustment for factors already included in the matching algorithm. We compared all-cause and cause-specific survival with cause ascertained from the Death Notification form (Centers for Medicare and Medicaid form CMS-2746). We also assessed whether effects of DHHD versus in-center hemodialysis on survival varied by follow-up interval. In intention-to-treat analysis, we compared survival within nine strata defined by matching factors. In Medicare primary payer patients, we compared times to change in dialytic modality, kidney transplant, and cessation of Medicare primary payer status with Cox regression, and in the subset of DHHD patients, we identified predictors of time to change in dialytic modality with multivariate Cox regression. Finally, we assessed the sensitivity of the intention-to-treat all-cause mortality HR to adjustment for an unmeasured factor, as described by Lin et al.37
Disclosures
D.T.G. has provided consulting services to DaVita Clinical Research and A.J.C. to NxStage. The other authors report no conflicts of interest.
Acknowledgments
The authors thank Chronic Disease Research Group colleagues Shane Nygaard for manuscript preparation and Nan Booth, MSW, MPH, ELS, for manuscript editing.
This work was supported by a grant from NxStage Medical Inc, Lawrence, Massachusetts.
The interpretation and reporting of these data are the responsibility of the authors, who retain final authority over manuscript content.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “New Observational Data Demonstrate that Mortality Is Lower in Patients Receiving More Frequent Dialysis,” on pages 770–773.
References
- 1.US Renal Data System: USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010 [Google Scholar]
- 2.Arneson TJ, Liu J, Qiu Y, Gilbertson DT, Foley RN, Collins AJ: Hospital treatment for fluid overload in the Medicare hemodialysis population. Clin J Am Soc Nephrol 5: 1054–1063, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Block GA: Prevalence and clinical consequences of elevated Ca x P product in hemodialysis patients. Clin Nephrol 54: 318–324, 2000 [PubMed] [Google Scholar]
- 4.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS, FHN Trial Group : In-center hemodialysis six times per week versus three times per week. N Engl J Med 363: 2287–2300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suri RS, Nesrallah GE, Mainra R, Garg AX, Lindsay RM, Greene T, Daugirdas JT: Daily hemodialysis: A systematic review. Clin J Am Soc Nephrol 1: 33–42, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Walsh M, Culleton B, Tonelli M, Manns B: A systematic review of the effect of nocturnal hemodialysis on blood pressure, left ventricular hypertrophy, anemia, mineral metabolism, and health-related quality of life. Kidney Int 67: 1500–1508, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Johansen KL, Zhang R, Huang Y, Chen SC, Blagg CR, Goldfarb-Rumyantzev AS, Hoy CD, Lockridge RS, Jr, Miller BW, Eggers PW, Kutner NG: Survival and hospitalization among patients using nocturnal and short daily compared to conventional hemodialysis: A USRDS study. Kidney Int 76: 984–990, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller BW, Collins AJ, Finkelstein FO, Glickman JD, Hull AR, Kraus MA, McCarthy J, Spry LA, Jaber BL; FREEDOM Group: Daily hemodialysis (DHD) is associated with lower than expected mortality [Abstract]. J Am Soc Nephrol 20: 192A, 2009 [Google Scholar]
- 9.Kjellstrand CM, Buoncristiani U, Ting G, Traeger J, Piccoli GB, Sibai-Galland R, Young BA, Blagg CR: Short daily haemodialysis: Survival in 415 patients treated for 1006 patient-years. Nephrol Dial Transplant 23: 3283–3289, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Agar JW, Wilson S, Van Eps C, Hawley C, Blagg CR: Comparing the relative survival of an Australian nocturnal home HD cohort with a matched USRDS conventional HD cohort using standardized mortality ratios [Abstract] J Am Soc Nephrol 18: 513A, 2007 [Google Scholar]
- 11.Blagg CR, Kjellstrand CM, Ting GO, Young BA: Comparison of survival between short-daily hemodialysis and conventional hemodialysis using the standardized mortality ratio. Hemodial Int 10: 371–374, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Centers for Medicare and Medicaid Services : End Stage Renal Disease Network Organization Program 2009 Summary Annual Report, Baltimore, MD, CMS, 2011 [Google Scholar]
- 13.Paoletti E, Specchia C, Di Maio G, Bellino D, Damasio B, Cassottana P, Cannella G: The worsening of left ventricular hypertrophy is the strongest predictor of sudden cardiac death in haemodialysis patients: A 10 year survey. Nephrol Dial Transplant 19: 1829–1834, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Stack AG: Determinants of modality selection among incident US dialysis patients: Results from a national study. J Am Soc Nephrol 13: 1279–1287, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Devereux RB, Dahlöf B, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris KE, Edelman JM, Wachtell K: Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: The Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation 110: 1456–1462, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Parfrey PS, Lauve M, Latremouille-Viau D, Lefebvre P: Erythropoietin therapy and left ventricular mass index in CKD and ESRD patients: A meta-analysis. Clin J Am Soc Nephrol 4: 755–762, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Ioannidis JP, Panagiotou OA: Comparison of effect sizes associated with biomarkers reported in highly cited individual articles and in subsequent meta-analyses. JAMA 305: 2200–2210, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ: Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol 21: 499–506, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saint-Remy A, Krzesinski JM: Optimal blood pressure level and best measurement procedure in hemodialysis patients. Vasc Health Risk Manag 1: 235–244, 2005 [PMC free article] [PubMed] [Google Scholar]
- 21.Kalpakian MA, Mehrotra R: Vascular calcification and disordered mineral metabolism in dialysis patients. Semin Dial 20: 139–143, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Kovesdy CP, Regidor DL, Mehrotra R, Jing J, McAllister CJ, Greenland S, Kopple JD, Kalantar-Zadeh K: Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol 2: 999–1007, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Mapes DL, Bragg-Gresham JL, Bommer J, Fukuhara S, McKevitt P, Wikström B, Lopes AA: Health-related quality of life in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 44[Suppl 2]: 54–60, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Jaber BL, Schiller B, Burkart JM, Daoui R, Kraus MA, Lee Y, Miller BW, Teitelbaum I, Williams AW, Finkelstein FO, FREEDOM Study Group : Impact of short daily hemodialysis on restless legs symptoms and sleep disturbances. Clin J Am Soc Nephrol 6: 1049–1056, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaber BL, Lee Y, Collins AJ, Hull AR, Kraus MA, McCarthy J, Miller BW, Spry L, Finkelstein FO, FREEDOM Study Group : Effect of daily hemodialysis on depressive symptoms and postdialysis recovery time: Interim report from the FREEDOM (Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements) Study. Am J Kidney Dis 56: 531–539, 2010 [DOI] [PubMed] [Google Scholar]
- 26.McDade-Montez EA, Christensen AJ, Cvengros JA, Lawton WJ: The role of depression symptoms in dialysis withdrawal. Health Psychol 25: 198–204, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Jaber BL, Collins AJ, Finkelstein FO, Glickman JD, Hull AR, Kraus MA, McCarthy J, Miller BW, Spry LA; FREEDOM Study Group: Daily hemodialysis (DHD) reduces the need for anti-hypertensive medications [Abstract] J Am Soc Nephrol 20: SA-PO2461, 2009 [Google Scholar]
- 28.Achinger SG, Ayus JC: The role of daily dialysis in the control of hyperphosphatemia. Kidney Int Suppl 95: S28–S32, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Manley HJ, Wang S, Nissenson AR: Medication non-adherence predicts hospitalization rate and healthcare costs in hemodialysis (HD) patients [Abstract] J Am Soc Nephrol 21: F-PO1207, 2010 [Google Scholar]
- 30.Shurraw S, Zimmerman D: Vascular access complications in daily dialysis: A systematic review of the literature. Minerva Urol Nefrol 57: 151–163, 2005 [PubMed] [Google Scholar]
- 31.Chow J, Rayment G, San Miguel S, Gilbert M: A randomised controlled trial of buttonhole cannulation for the prevention of fistula access complications. J Ren Care 37: 85–93, 2011 [DOI] [PubMed] [Google Scholar]
- 32.André MB, Rembold SM, Pereira CM, Lugon JR: Prospective evaluation of an in-center daily hemodialysis program: Results of two years of treatment. Am J Nephrol 22: 473–479, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Pinciaroli AR: Hormonal changes in daily hemodialysis. Semin Dial 12: 455–461, 1999 [Google Scholar]
- 34.Harwood L, Clark AM: Understanding health decisions using critical realism: Home-dialysis decision-making during chronic kidney disease. Nurs Inq 19: 29–38, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM: Identifying persons with diabetes using Medicare claims data. Am J Med Qual 14: 270–277, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Austin PC: A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med 27: 2037–2049, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Lin DY, Psaty BM, Kronmal RA: Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics 54: 948–963, 1998 [PubMed] [Google Scholar]