Abstract
Kidney pericytes are progenitors of scar-forming interstitial myofibroblasts that appear after injury. The function of kidney pericytes as microvascular cells and how these cells detach from peritubular capillaries and migrate to the interstitial space, however, are poorly understood. Here, we used an unbiased approach to identify genes in kidney pericytes relevant to detachment and differentiation in response to injury in vivo, with a particular focus on genes regulating proteolytic activity and angiogenesis. Kidney pericytes rapidly activated expression of a disintegrin and metalloprotease with thrombospondin motifs-1 (ADAMTS1) and downregulated its inhibitor, tissue inhibitor of metalloproteinase 3 (TIMP3) in response to injury. Similarly to brain pericytes, kidney pericytes bound to and stabilized capillary tube networks in three-dimensional gels and inhibited metalloproteolytic activity and angiogenic signaling in endothelial cells. In contrast, myofibroblasts did not have these vascular stabilizing functions despite their derivation from kidney pericytes. Pericyte-derived TIMP3 stabilized and ADAMTS1 destabilized the capillary tubular networks. Furthermore, mice deficient in Timp3 had a spontaneous microvascular phenotype in the kidney resulting from overactivated pericytes and were more susceptible to injury-stimulated microvascular rarefaction with an exuberant fibrotic response. Taken together, these data support functions for kidney pericytes in microvascular stability, highlight central roles for regulators of extracellular proteolytic activity in capillary homoeostasis, and identify ADAMTS1 as a marker of activation of kidney pericytes.
Recent studies have identified pericytes of the peritubular capillaries of the kidney as a major precursor population of scar-forming myofibroblasts,1,2 and studies from embryonic development and cancer growth demonstrate vital angiogenic and vascular stabilizing functions for pericytes.3,4 Prompted by these studies, we sought to understand the role of kidney pericytes in homeostasis and the molecular mechanisms regulating pericyte attachment to capillaries in the kidney and detachment and migration from capillaries in response to injury.
Pericytes are mesenchyme-derived mural cells of capillaries that have processes embedded within capillary basement membrane and they may play a direct role in conjunction with endothelial cells (ECs) to stimulate vascular basement membrane matrix assembly.5,6 Each tissue bed has pericytes derived from organ-specified mesenchyme,7 and thus there are likely to be organ-specific differences in pericyte functions that are poorly understood. The direct interaction of pericyte processes with endothelium is believed to be the location of cell: cell signaling.5 Pericytes of the kidney derive during embryogenesis from a layer of embryonic cap metanephric mesenchyme lying outside of the mesenchyme that specifies epithelium. This mesenchyme activates the forkhead transcription factor Foxd1, specifically during embryonic specification.8 In normal adult kidney, pericytes are Foxd1-derived, PDGF receptor β (PDGFRβ+), CD73+, Coll1a1+, CD45-, α smooth muscle actin (αSMA-) cells.2,9
During angiogenesis in other organs, heparan sulfate–tethered PDGF-BB released by tip ECs is thought to signal via PDGFRβ to promote pericyte migration along the forming capillary and stable attachment to endothelial cells.10 However, signaling via pericyte PDGFRβ in formed kidney vessels, in response to microvascular injury, stimulates pericyte detachment, migration, and differentiation into myofibroblasts.9,11 Similarly, vascular endothelial growth factor A (VEGFA) signaling from pericytes to angioblasts in organ development is important in successful angiogenesis; however, in response to injury, expression of alternate VEGFA splice variant by kidney pericytes stimulates pericyte detachment and differentiation into myofibroblasts.9,12 Therefore, it is likely that multiple signaling pathways and extracellular interactions that are poorly explored are important in determining pericyte attachment, migration, and activation.
The kidney is particularly susceptible to ischemic and toxic injuries, due in part to the concentrating functions of the nephron and to its unusual vasculature. The kidney vasculature comprises a primary capillary network in the glomeruli specialized for filtration followed by a secondary capillary plexus that supplies the remainder of the kidney, resorbs solutes, and uptakes water being returned to the circulation. The specialized functions of this secondary capillary plexus, peritubular capillaries (PTCs), are facilitated by its low oxygen tension, with low flow. Moreover, the secondary plexus is at the vagaries of the glomerular plexus. Because the kidney cortex and medulla perform complex transport functions, they have significant oxygen and nutrient demands. Hence, the kidney is susceptible to ischemia if PTC flow/supply is insufficient. Subtle kidney ischemia due to loss of PTCs is widely believed to be a major cause of essential hypertension and a major driving force in the progression of CKD and in the failure of AKI to resolve.13–15
Previous studies, particularly in cancer growth and wound repair, have implicated proteinase-mediated degradation of the capillary basement membrane and surrounding extracellular matrix as critical for vascular instability and vascular regression (rarefaction).16,17 Moreover, proteinases and factors that regulate their activity are now recognized to serve as key regulators of cell migration and spreading in cancer biology and development, model systems that may be instructive in studying kidney injury.18–20
In this study, we investigated the role of kidney pericytes in vascular stabilization and the central role that pericytes play in regulating protease activity of the vasculature.
Results
Microarray Analyses of Pericytes in Early Injury Responses in Kidney Identify Enriched Pathways and Regulated Genes
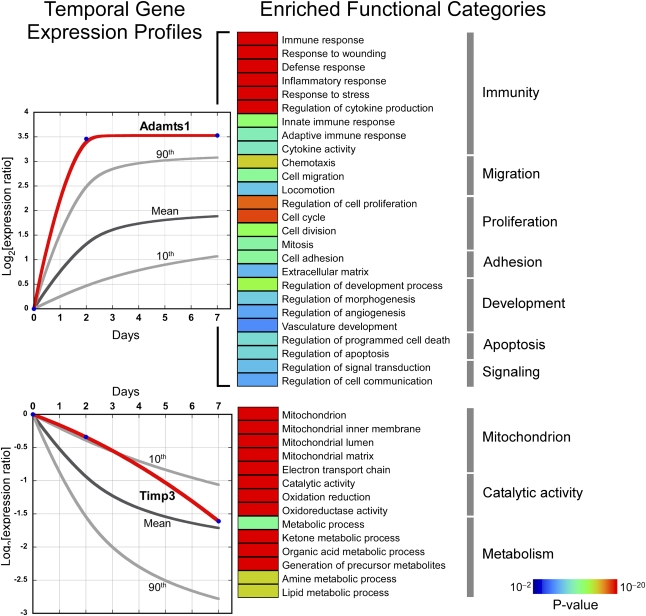
To explore mechanisms that orchestrate attachment and detachment of pericytes, we used an unbiased approach to identify regulated genes and activated pathways. Most biologic processes entail coordinated interaction among functionally coherent groups of genes known as modules.21 We postulated that pericyte differentiation is characterized by the temporal activation of distinct biologic modules and that key regulators of this process reside within these coherent pathways. We purified pericytes from normal Coll-GFP kidneys (day 0) by flow cytometry and at early time points after onset of injury (day 2 and day 7) in the murine unilateral ureteral obstruction (UUO) model in which pericyte detachment is well defined, using well established methods of cell purification1,22–24 (Supplemental Figure 1) and assessed their transcriptome by microarray analysis. The Coll-GFP reporter mouse used in this disease model specifically labels pericytes and myofibroblasts, and there are no significant fibrocytes contaminating the collection (Supplemental Figure 1).1,22–24 In addition to pericytes, small numbers of perivascular fibroblasts are collected because they also express Coll-GFP, but these represent <5% of all cells expressing GFP under regulation of the collagen1a1 promoter (Coll-GFP+ cells) in normal kidney.1 Principal component analysis of the transcriptomes revealed grouping of biologic replicates that corresponded to the time of injury, implying global perturbations in temporal gene expression as pericytes detach and differentiate into myofibroblasts (Supplemental Figure 2A). More than 800 genes were differentially expressed when control pericytes (day 0) were compared with postinjury myofibroblasts (days 2 and 7) (Supplemental Figure 2B and Supplemental Table 1). To elucidate the temporal patterns of gene expression and pathway activation in response to injury, we implemented a clustering algorithm optimized for analysis of short time series25 and identified two highly significant gene clusters—each mapping to very distinct functional categories (Figure 1). One cluster corresponded to upregulated genes and was highly enriched in processes involved in matrix production, cell proliferation, migration, innate immune responses, and chemotaxis. In addition, genes mapping to angiogenesis and vascular development were members of this upregulated group. The second cluster was composed of genes downregulated during injury, and was functionally enriched in processes involved in mitochondrial function, redox status, and metabolism. A small number of genes regulating extracellular matrix production and angiogenesis were also downregulated. A disintegrin and metalloproteinase with thrombospondin motif-1 (ADAMTS1) was highly significantly upregulated early after injury onset and among the 15 most upregulated genes early after injury in pericytes (Figure 1 and Supplemental Figure 2C). Moreover, it was a strong candidate effector molecule in regulating detachment and migration. ADAMTS1 has been reported to cleave capillary basement membrane proteins and inhibit angiogenesis and therefore may reasonably facilitate pericyte detachment.26,27 Tissue inhibitor of matrix metalloproteinase 3 (TIMP3), an endogenous inhibitor of ADAMTS1, is highly expressed at baseline in pericytes28 but is significantly downregulated as disease progresses (Figure 1 and Supplemental Figure 2C). TIMP3 has been demonstrated or implicated in silencing the activity of metalloproteinases including ADAM17 and some matrix metalloproteinases (MMPs) such as MMP14, and has been reported to regulate vasculogenesis and angiogenesis and to promote tissue regeneration after injury in the lung.29–31 Thus, the downregulation of TIMP3 as pericytes transition to myofibroblasts in vivo may have important consequences for the kidney microvasculature. Given the marked regulation of these two functionally related genes and candidacy as regulators of pericyte function in response to injury, we aimed to study their function in kidney pericytes.
Figure 1.
Global analysis of kidney pericyte transcriptome in response to UUO injury. Temporal changes in gene expression map to two distinct clusters characterized by progressively increasing or decreasing patterns. In each graph, the dark gray line corresponds to the average change in gene expression in response to injury relative to baseline expression (day 0) and the light gray lines identify the 90th and 10th percentiles of each group. The temporal expression patterns of two genes, Adamts1 and Timp3, are depicted with red lines. Gene members of each cluster underwent functional analysis based on Gene Ontology database annotations. Enriched functional categories among upregulated and downregulated genes are shown using color-coded bars corresponding to enrichment P values with the most highly significant shown in red and the least significant shown in dark blue.
Kidney Pericytes Prevent Capillary Tubular Network Collapse
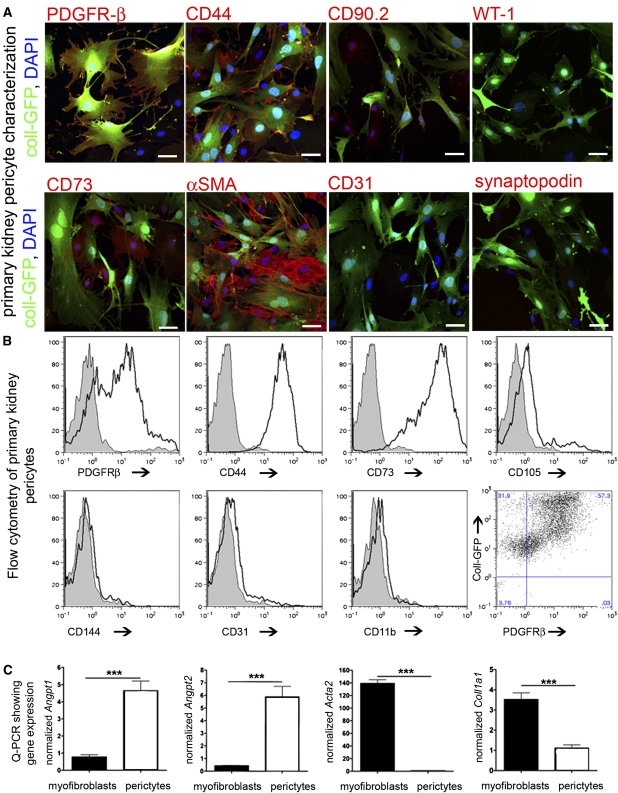
To study pericyte function, we generated primary pure cultures by magnetic bead immunoaffinity from mouse kidneys. Primary mouse pericytes expressed typical pericyte markers (Figure 2, A and B) and morphology, which share characteristics with bone marrow mesenchymal stromal cells and lacked epithelial, endothelial, and leukocyte markers and lacked the podocyte markers WT1 and synaptopodin.32,33 Compared with primary cultures of myofibroblasts, pericytes expressed more angiogenic factors including Angiopoietin-1 (Angpt1) and Angiopoietin-2 (Angpt2), fewer Collagen1a1, and intermediate filament transcripts.
Figure 2.
Characterization of primary kidney pericyte cultures. (A) Fluorescent confocal images of kidney pericyte cultures for typical pericyte markers and markers of endothelium or podocytes (bar=25 µm). (B) Histogram plots and dot plot from FACS analysis of primary kidney pericyte cultures for typical pericyte markers and markers of potential contaminating cells (gray areas are the isotype controls and white areas are the specific antibody). (C) Quantitative PCR for typical pericyte markers and myofibroblast markers in primary kidney pericyte compared with myofibroblast cultures (n=3–6/group; ***P<0.001).
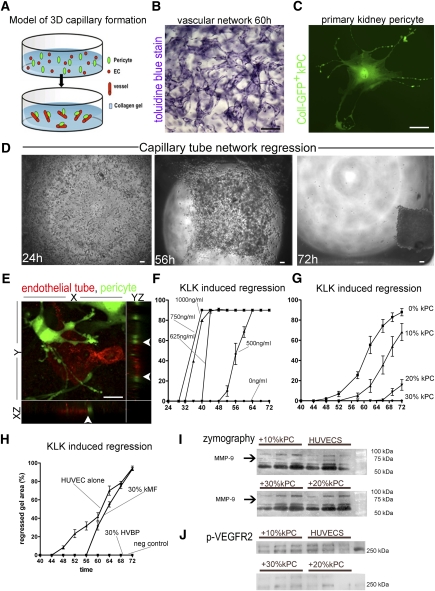
One of the defining features of pericytes is their ability to bind to and stabilize capillaries.12,34–36 We tested their capacity to stabilize a capillary tubular network formed by three-dimensional (3D) EC culture. Human ECs spontaneously form a capillary tube network in collagen gel (Figure 3, A–C). The capillary tube network can be destabilized and undergoes regression in the presence of coagulation proteases, which function by a number of mechanisms including activation of endothelial matrix metalloproteinases.37,38 Because the lumina of the forming capillary tubes do not contain any collagen gel, tube regression can be visualized by gel retraction (collapse) (Figure 3D). In 3D co-culture, mouse kidney pericytes bind to EC capillary tubes (Figure 3E and Supplemental Movie 1). Moreover, the cells have long processes that form invaginations on the tube abluminal surface similar to the peg and socket processes seen by electron microscopy.9,39 When EC capillary tubes were exposed to the serine proteinase kallikrein (KLK), both capillary tube regression and gel collapse were stimulated in a time- and concentration-dependent manner (Figure 3F). In the absence of KLK, EC capillary tubes did not regress. When kidney pericytes were added to the gel in a 10%/20% ratio (1 PC for every 10 ECs/2 PCs for every 10 ECs), the collapse of the gel in the presence of KLK was retarded (Figure 3G). When 30% (3 PCs for every 10 ECs) kidney pericytes were co-cultured, vascular regression was completely prevented and the gel did not collapse (Figure 3G). Similar observations were made when human vascular brain pericytes were substituted for kidney pericytes (Figure 3H); however, kidney myofibroblasts, which are derived in vivo from kidney pericytes, did not stabilize the capillary network (Figure 3H). Kidney pericytes suppressed the amount of active EC-derived MMP9 in the collagen gels as determined by gelatin zymography (Figure 3I), and suppressed activation (phosphorylation) at the EC-restricted vascular endothelial growth factor receptor 2 (VEGFR2) (Figure 3J) triggered by KLK. These observations indicate that kidney pericytes and brain pericytes have similar capacity to stabilize capillary tubes, and that pericytes lose vascular stabilizing functions when they differentiate into myofibroblasts.
Figure 3.
Kidney pericytes stabilize capillary tubes in a 3D gel assay. (A) Schema showing the addition of ECs (red) to gel in wells that spontaneously form capillary networks. Addition of pericytes to this assay permits migration and binding of pericytes to capillary tubes. (B) Toluidine blue–stained gel showing capillary tube network (ECs only) within the gel (bar=100 μm). (C) Kidney pericyte (GFP+) in culture (bar=25 μm). Note cell processes extending the length of several cell bodies. (D) Low-power light images of gels containing capillary tubes (ECs only). Under the influence of the coagulation cascade serine protease, KLK, endothelial tubes are destabilized in the gel and vessels become disorganized leading to progressive collapse of the gel (bar=100 μm). (E) Confocal image of 3D gel with YZ and XZ stacks showing capillary tube (red, CD34) and kidney pericyte (green, Coll-GFP). Note the attachment of kidney pericytes to the capillary tube and numerous processes attached to the capillary tube. Z stacks (arrowheads) show yellow color at points of direct interaction of pericyte processes with capillary tubes, suggestive of peg and socket junctions (bar=25 μm). (F) Dose-response curves measuring collapse of collagen gel (ECs only) induced by KLK. (G) Gel collapse curves induced by KLK (625 ng/ml) in the presence of increasing numbers of kidney pericytes. Note 30% kidney pericytes completely prevent gel collapse (n=12–16/timepoint). (H) Gel collapse curves in response to KLK (625 ng/ml) in the presence of 30% human vascular brain pericytes (HVBPs) or 30% mouse kidney myofibroblasts (kMF). (I) Gelatin zymography of supernatants from collagen gels in the presence of KLK showing multiple bands of gelatinase activity. Note that an approximately 72-kD band of activity, representing MMP9, is completely lost by the addition of 20% or 30% pericytes and the major approximately 60-kD band of MMP2 is attenuated by the presence of 30% pericytes. (J) Phosphoblot for proteins from the collagen gels of KLK-activated gels detecting phospho-VEGFR2. Note that kidney pericytes complete suppress signaling at VEGFR2.
TIMP3 and ADAMTS1 Are Regulated during Kidney Pericyte Detachment In Vivo
The microarray studies (Figure 1) indicated that Timp3 transcripts were highly expressed in kidney pericytes and downregulated in response to kidney injury. By contrast, among a number of metalloproteinases that were regulated in pericytes in response to injury (Table 1), Adamts1 transcripts were highly regulated early and most markedly. We postulated that the dynamic regulation of these kidney pericyte genes served as important determinants of the detachment of pericytes and vascular instability that occurs in response to kidney injury.1,9,40 To validate the microarray findings, we performed in situ hybridization and immunofluorescence studies to localize gene expression (Figure 4, A and B). Timp3 transcripts were readily seen in kidney pericytes of normal adult mouse kidney. Timp3 transcripts were detectable at day 2 after kidney injury onset in the interstitium. At day 7 after injury onset, the interstitium in which myofibroblasts are located was almost devoid of signal for Timp3 (Figure 4A). Timp3 expression was seen in some injured epithelium and also localized to podocytes, but not mesangium in the glomerulus (Figure 4A). Coll-GFP+ cells were purified from normal kidneys (pericytes) or kidneys day 7 after disease (myofibroblasts). Timp3 transcripts were downregulated in Coll-GFP+–purified myofibroblasts compared with Coll-GFP+ pericytes purified from normal kidneys (Figure 4C). Consistent with these observations, primary cultures of kidney pericytes generated higher levels of TIMP3 protein compared with primary cultures of myofibroblasts (Figure 4D). By contrast, in situ hybridization of normal adult kidney did not detect any transcripts for Adamts1 (Figure 4A). However, by day 2 after kidney injury, Adamts1 was readily detected both interstitial cells and epithelial cells, and it was seen in epithelium and to a lesser extent in interstitial cells on day 7 (Figure 4A). Immunostaining of kidney tissues with antibodies against ADAMTS1 revealed a similar pattern (Figure 4B) with expression in the vast majority (83%±5.6%) of Coll-GFP+ cells at day 2 after injury onset and 96%±6.1% after day 7. ADAMTS1 protein was also clearly detected in epithelial cells, predominantly at the apical surface in the region of the brush border. As expected, when kidney pericytes or myofibroblasts were purified from normal or diseased (day 2) kidneys, respectively, Adamts1 transcripts were highly upregulated (Figure 4E) in the myofibroblasts. In keeping with this observation, primary cultures of pericytes expressed much lower levels of Adamts1 transcript compared with primary myofibroblasts (Figure 4F). To determine the relationship between Timp3 transcripts and protein, immunoblots of whole kidney lysates confirmed the downregulation of TIMP3 protein levels in mouse kidney in response to disease (day 2) and cultured myofibroblasts expressed lower levels of TIMP3 protein compared with pericytes (Figure 4G).
Table 1.
Quantitative PCR of metalloproteinases and inhibitors regulated in pericytes during detachment at day 0, day 2, and day 7 after UUO injury
| Metalloproteinases and Inhibitors | Day 0 | Day 2 after Injury Onset | Day7 after Injury Onset |
|---|---|---|---|
| Adamts-1 | 0.8958 | 9.8249 | 10.3441a |
| Adamts-2 | 0.4564 | 0.6883 | 6.42167b |
| Adamts-4 | 0.2562 | 1.3983 | 1.94952 |
| Adamts-5 | 0.2412 | 0.4097 | 0.94389 |
| Mmp2 | 2.85 | 2.58 | 2.87 |
| Mmp7 | 0.33 | 0.87 | 1.48a |
| Mmp9 | 2.14 | 3.03 | 4.33b |
| Mmp11 | 1.0536 | 2.1585 | 7.43393 |
| Mmp14 | 4.92 | 12.96 | 22.00 |
| Mmp23 | 0.7695 | 0.8386 | 8.73210 |
| Timp-3 | 137.4332 | 66.172 | 45.0715a |
| Timp-1 | 0.1280 | 8.1431 | 26.6397a |
P<0.01.
P<0.05.
Figure 4.
Characterization of TIMP3 and ADAMTS1 expression in pericytes, in vivo and in vitro. (A) In situ hybridization of kidney sections from normal kidney (day 0) and on day 2 and day 7 after injury onset (disease) for Timp3, Adamts1, and control epithelial marker Ngfa. Positive-stained pericytes and interstitial cells are shown (arrows) and positive-stained epithelial cells are shown (arrowheads) (bar=50 µm). (B) Split panels showing immunostaining for Adamts1 in normal kidneys (day 0) and on day 2 and day 7 after injury kidney sections from Coll-GFP mice. Coll-GFP+ pericytes do not express Adamts1 (arrows) but early after injury onset (day 2) pericytes and cellular processes (arrowheads) now express Adamts1. Kidney tubule expression is also increased. (C and D) Quantitative PCR for Timp3 comparing (C) Coll-GFP+ purified pericytes (normal kidney) with Coll-GFP+ purified myofibroblasts (disease kidney day 2) or (D) primary pericyte cultures with primary myofibroblast cultures. (E and F) Quantitative PCR for Adamts1 comparing (E) Coll-GFP+ purified pericytes (normal kidney) with Coll-GFP+ purified myofibroblasts (disease kidney day 2) or (F) primary pericyte cultures with primary myofibroblast cultures. (G) Western blot showing expression of TIMP3 (upper blot) in normal kidney compared with diseased kidney (day 7) or (lower blot) pericyte cultures compared with myofibroblasts. Note that the 24-kD Timp3 band is expressed at higher levels in pericytes (n=3–5/group; *P< 0.05, ***P<0.001).
ADAMTS1 Destabilizes and TIMP3 Stabilizes Capillary Tubular Networks
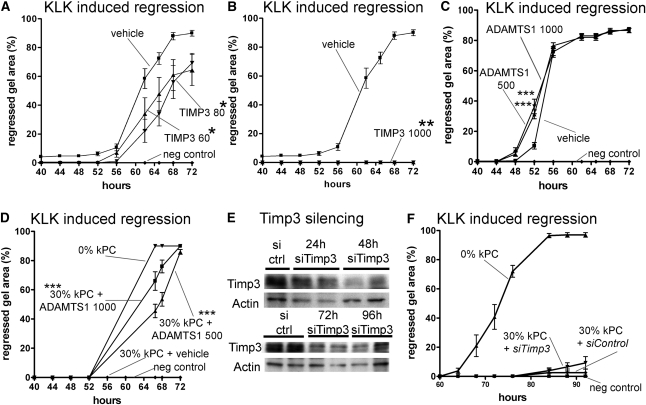
TIMP3 has multiple biologic activities that may contribute to vascular stability. Foremost, it inhibits activity of a number of metalloproteinases including ADAMTS1 and endothelial MMPs. In addition, in high concentrations, it may regulate signaling directly at endothelial VEGFR2.28,29,41To explore the role of TIMP3 in vascular integrity further, we applied recombinant TIMP3 to the capillary tube regression assay. In lower concentrations, TIMP3 retarded capillary tube regression; however, at higher concentrations, it completely blocked capillary tube regression (Figure 5, A and B). Thus, TIMP3 can completely replace pericyte function (Figure 3) in this assay. By contrast, application of recombinant ADAMTS1 to the vascular assay in the absence of pericytes weakly accelerated capillary tube regression in the presence of KLK (Figure 5C), but did not trigger regression (not shown). Much more strikingly, however, is the finding that kidney pericytes were no longer able to stabilize capillary tubes in the capillary tube regression assay in the presence of recombinant ADAMTS1 (Figure 5D). These findings indicate that ADAMTS1 was sufficient to overcome the vascular stabilizing functions of pericytes (Figure 5D). To test specifically whether TIMP3 in pericytes stabilizes capillary tubes, TIMP3 protein expression was silenced using specific small interfering RNA (siRNA) (Figure 5E). Using sequential silencing methods, a >70% reduction in protein level was achieved in pericytes. Whereas pericytes with control gene silencing completely stabilized capillary tubes, pericytes with TIMP3 silencing showed a nonsignificant, weakly impaired capacity to stabilize capillary tubes during KLK-mediated regression (Figure 5F), in keeping with pericyte-derived TIMP3 playing a direct role in vascular stability.
Figure 5.
Characterization of TIMP3 and ADAMTS1 functions on capillary tube stability in a 3D tube assay. (A and B) Graph of KLK-induced capillary tube regression (ECs only) showing (A) retardation of collapse in the presence of low concentrations of recombinant TIMP3 and (B) complete prevention of collapse (ECs only) in the presence of high concentrations of recombinant TIMP3 (doses stated in ng/ml). (C) Graph of KLK-induced capillary tube regression (ECs only) showing modest acceleration by recombinant ADAMTS1 (dose stated in ng/ml). (D) Graph of KLK-induced capillary tube regression in the presence of kidney pericytes and recombinant ADAMTS1. Thirty percent pericyte addition is no longer able to stabilize capillaries when ADAMTS1 is present (doses stated in ng/ml). (E) Western blots showing Timp3 levels in pericytes at time points after introduction of siRNA by transfection. (F) Graph of KLK-induced capillary tube regression in the presence of 30% kidney pericytes after Timp3 silencing or mock silencing (n=12–16/timepoint; *P<0.05, **P<0.01, ***P<0.001).
TIMP3 Mutant Mice Have a Kidney Microvascular Phenotype
Our findings suggested that TIMP3 may play a role in vivo in regulating vascular stability in the kidney and perhaps more broadly in pericyte function. Timp3-deficient mice survive into adult life but have been noted to have microvascular defects in the retina and in cancer growth, and also fail to resolve lung inflammation after injury. Furthermore, recent reports suggest that Timp3-deficient mice have kidney fibrosis.31,42,43 We therefore investigated the microvasculature of normal Timp3−/− kidneys, the response of the kidney microvasculature to acute ischemia reperfusion injury (IRI), and the function of pericytes in these vascular responses. Adult Timp3−/− mice were smaller than littermate controls but had normal renal function and no albuminuria (Table 2). The microvascular density of kidney PTCs of adult mice, assessed by two different methods, was increased with areas of abnormal capillaries (Figure 6, A and E and Supplemental Figure 3) and a greater number of PTC ECs were in the cell cycle (Figure 6F). There were discrete expansions of Pdgfrβ+, CD73+, αSMA− microvascular pericytes in PTC areas and also αSMA+ myofibroblasts in the Timp3−/− kidneys (Figure 6B) not seen in Timp3+/+ kidneys. Small increases of interstitial fibrillar collagen deposition were detected in normal Timp3−/− adult kidneys (Figure 6, C, H, and I), confirming previous reports.42 Furthermore, unlike pericytes of wild-type normal kidneys, pericytes of Timp3−/− normal kidneys expressed ADAMTS1 (Figure 6, D, J, and L). In addition to the interstitial changes, there were mild changes to glomeruli of Timp3−/− normal kidneys comprising mild mesangial hypercellularity and matrix expansion (not shown). Given that the major site of expression of Timp3 in the kidney is the pericyte, these findings collectively suggest that Timp3−/− pericytes in vivo are more activated.
Table 2.
Characterization of Timp3-deficient mice
| Timp3+/+ Male 12 wks | Timp3−/− Male 12 wks | |
|---|---|---|
| Weight (g) | 29.9±0.9a | 24.7±0.8 |
| Albuminuria | ND | ND |
ND, not detected.
P<0.01.
Figure 6.
TIMP3 deficiency in vivo predisposes to microvascular instability of kidney peritubular capillaries due to overactivated pericytes. (A) Confocal images showing microvascular density and pericyte coverage of normal and day 5 post-IRI kidneys. (B) Confocal images showing areas of expanded pericytes (Pdfgfrß+) in normal kidneys of Timp3−/− mice, some of which express αSMA (arrows) and some of which do not (arrowheads). Five days after IRI, the pericyte/myofibroblasts (Pdgfrβ+) show greater expansion and higher levels of αSMA, particularly in Timp3−/− kidneys. (C) Low-power images of Sirius red–stained kidneys. (D) Fluorescence images of Adamts1. Note that normal Timp3−/− kidneys have pericytes that express Adamts1 (arrowheads). (E through K) Graphs showing quantification of (E) vascular density, (F) endothelial cell proliferation, (G) pericytes, (H) myofibroblasts, and (I) fibrosis in interstitial (J) Adamts1+ cells. (K) Western blot showing αSMA and Pdgfrβ expression in kidneys from Timp3−/− and Timp3+/+ mice. (L) Western blot quantifying Adamts1 in kidney lysates after IRI. (M) Graphs showing primary pericyte cultures undergoing migration in a scratch closure assay, proliferation, and quantitative PCR expression of Acta2. (N and O) Images of (N) Evans blue–labeled mouse skin and sectioned kidneys and (O) Evans blue quantification indicating a microvascular leak 5 days after IRI. (P) pVEGFR2 expression in normal plus day 5 post-IRI kidneys (bar=50 µm; n=6–8/group; *P<0.05, **P<0.01, ***P<0.001). g, glomerulus; a, arteriole.
To determine whether Timp3−/− pericytes were intrinsically overactivated as opposed to activated in response to vascular changes, we studied primary cultures of pericytes in proliferation and migration assays (Figure 6M) and expression of αSMA. Timp3−/− pericytes showed enhanced αSMA expression, increased proliferation, and enhanced motility, in keeping with an overactivated phenotype. Enhanced migration by Timp3−/− pericytes was cell specific because cultured Timp3−/− macrophages migrated normally (not shown).
To test the effect of injury responses in the microvasculature, we used a mild kidney IRI model that has a well recognized microvascular injury, rarefaction, followed by incomplete angiogenesis.9,40,44 We observed that ischemic injury resulted in a greater rarefaction of the vasculature in Timp3−/− kidneys compared with wild-type kidneys in this model (Figure 6, A and E), and IRI stimulated more ECs into the cell cycle in Timp3−/− kidneys (Figure 6F). In concert with this observation, there was excessive expansion of pericyte/myofibroblast population (Figure 6, B, G, H, and K) after IRI. Using the αSMA marker to define pericyte transition to myofibroblasts, there was increased expansion of these myofibroblasts, excessive expression of αSMA in response to injury, and increased interstitial fibrosis in Timp3−/− kidneys compared with Timp3+/+ kidneys by day 5 after IRI (Figure 6, C, I, and K). After kidney IRI, there was a persistent microvascular leak in Timp3−/− kidneys, as detected by Evans blue dye assay (Figure 6, N and O) and this leak extended to vascular beds outside of the kidney, suggesting that generalized microvascular injury responses in these mice results in persistent increase in microvascular permeability. The enhanced microvascular leak suggests that pericyte functions were more compromised in Timp3−/− mice compared with Timp3+/+ mice after vascular injury. Consistent with this compromised pericyte function, phosphorylated VEGFR2 was increased in the injured Timp3−/− kidneys compared with injured Timp3+/+ kidneys indicative of destabilized microvasculature surrounded by overactivated pericytes (Figure 6P).9,12 Because these studies indicated that the injury responses in Timp3−/− kidneys resulted in deleterious microvascular responses, we tested whether acute IRI resulted in greater functional deficiency. Twenty four hours after 21 minutes of kidney IRI, Timp3−/− mice (n=6) had plasma creatinine levels of 0.49±1.2 mg/dl, whereas Timp3+/+ mice (n=6) had plasma creatinine levels of 0.31±0.03 mg/dl (P=0.06). Mice exposed to sham surgery had creatinine levels of 0.17±0.03 and 0.15±0.02 mg/dl, respectively.
Discussion
These studies describe novel vascular stabilizing functions for kidney pericytes, and identify genes involved in regulation of pericyte detachment and activation. Specifically, they identify the protease regulator TIMP3 and one of its targets, ADAMTS1, as highly regulated kidney pericyte genes that play roles in microvascular stability of the peritubular capillaries. Moreover, ADAMTS1 expression is a marker of activated pericytes.
Tissue ischemia is increasingly believed to be a central problem in failure to regenerate the kidney normally after injury, a driving force for progressive organ dysfunction after injury, and a cause for hypertension and possibly cardiovascular disease.45 Central to kidney tissue ischemia is loss of the microvasculature, which is also known as capillary rarefaction. The recent identification of a relatively large population of pericytes in the cortex and medulla of normal kidney and the observation that they detach from capillaries in response to injury, migrate into the interstitial space, and transition to scar-forming myofibroblasts have raised the possibility that chronic diseases in the kidney are a state of functional pericyte deficiency. Studies have suggested that injury of the kidney is a state of vascular instability that can easily lead to rarefaction despite endothelial proliferation and that factors that prevent pericyte detachment can prevent this instability.9,11 The fact that the findings presented here reveal kidney pericytes to have a similar capacity to pericytes from other organs to prevent microvascular instability ex vivo strongly suggests that interventions that will promote pericyte attachment in the kidney will lead to preservation of capillaries. In keeping with the hypothesis that chronic diseases have functional pericyte deficiency due to pericyte transition to myofibroblasts, kidney myofibroblasts were unable to stabilize vessels in the capillary tube assay.
The microarray analysis indicated that there is a marked phenotypic change in pericytes when they become myofibroblasts. The analysis we performed was based on collecting cells that express Coll-GFP. We previously characterized these cells in the kidney and showed that they are pericytes. We identified very few circulating fibrocytes (leukocytes that produce collagen matrix) in this kidney injury model and our purification method excludes all leukocytes.1,2,9,39 We previously identified perivascular fibroblasts around arterioles of the kidney that also express Coll-GFP and expand to become myofibroblasts. These cells were also purified along with pericytes because there is currently no marker described to distinguish them. Although the microarray analysis requires validation due to potential contamination from other kidney cells that may have been inadvertently collected during the flow cytometric sorting procedure, it is striking that activated pericytes are involved in innate immune responses, chemotaxis, and represent a major source of cytokines. This is highly relevant because recent studies that target the pericyte directly by blocking PDGFRβ or PDGFRα signaling in the kidney not only attenuate fibrosis and capillary rarefaction but also markedly attenuate the inflammatory response.11 Therefore, it is likely that activated pericytes are a major contributing cell to the inflammatory response. Further studies are required to understand whether this response can be specifically targeted in the kidney.
Our microarray studies identified Timp3 as a pericyte factor that is lost during pericyte transition to myofibroblasts, and our in vitro and in vivo studies showed TIMP3 to function as a molecular factor promoting microvascular stability. Although TIMP3 is an inhibitor of metalloproteinases, it is also a pleiotropic, extracellular matrix–anchored molecule that is able to inhibit a number of enzymes that breakdown capillary basement membrane, including ADAMTS1, and other matrix components. In addition, TIMP3 is able to specifically inhibit signaling at the endothelial receptor VEGFR2, initiated by all VEGFA isoforms. Interestingly, TIMP2 is also capable of inhibiting VEGFR2 signaling in a manner independent of its proteinase inhibitor activity, and previous work demonstrated a role for both pericyte-derived TIMP3 and EC-derived TIMP2 in capillary tube stabilization.16 In recent studies, we identified excessive signaling in response to injury at VEGFR2, particularly in response to dysangiogenic VEGFA isoforms, released by activated pericytes/myofibroblasts in the kidney, as a major factor in microvascular instability.9,11 Therefore, “dialing down” the degree of signaling at this receptor may be desirable. Our studies in vitro and in vivo indicate that kidney pericytes and TIMP3 function to limit endothelial VEGFR2 signaling potentially by both direct and indirect mechanisms. Timp3 mutation in humans is associated with retinal microvascular disease and Timp3-deficient mice exhibit increased dysangiogenesis (increased angiogenesis with leaky, poorly functioning vessels) in growth of some tumors and increased angiogenesis in growth of others.29,46–49 Consistent with these findings, we identified dysangiogenesis in the kidney after injury when Timp3 was mutated, particularly in response to microvascular injury, and showed that pericytes are more activated and exhibit excessive migration and proliferation. Although further studies are required, measurement of plasma creatinine in acute injury of Timp3-deficient kidneys suggests that TIMP3 may serve to protect kidneys from acute injury, as we previously showed for the lung.31 Further studies are required to understand whether TIMP3 regulates pericyte properties through regulating MMPs or through regulating cleavage of other factors such as semaphorins.29 The finding that TIMP3 can mimic pericytes in vascular stabilization in vitro but that TIMP3 silencing had a weak effect on pericyte function in vascular stabilization in vitro was surprising but may be instructive. Timp3-deficient mice are born healthy and have a subtle microvascular phenotype only. Therefore, it may not be surprising that only a modest defect was seen in the short vascular stabilization assay. These findings also indicate that pericytes rely on factors other than TIMP3 to stabilize capillary tubes. These other factors are currently unknown. In the capillary tube stabilization assay, pericytes inhibit MMP production primarily from ECs, but the signal that mediates this inhibition is unknown. Although TIMP3 may be a factor in this regulation, other factors must be contributing. TIMP3-deficient pericytes have a number of functional abnormalities, including enhanced migration proliferation and activated phenotype as judged by αSMA expression and ADAMTS1 expression. It is unclear how TIMP3 regulates all of these processes, but these studies suggest that TIMP3 must be able to affect transcription by either direct or indirect means.
Although a number of metalloproteinases including ADAMTS2 and MMP11 are activated in the microvasculature in response to injury, Adamts1 was most remarkably regulated in pericytes of the kidney. ADAMTS1, like TIMP3, is highly tethered to the extracellular matrix and acts locally. We identified ADAMTS1 regulation in epithelial cells, as well as pericytes, in the kidney. ADAMTS1 expression in kidney development was previously reported.44,50 Because ADAMTS1 acts locally, the significance of epithelial expression of ADAMTS1, particularly, at the brush border proximal tubule, is unclear. However, it is likely that pericyte expression of ADAMTS1 early after injury exerts activity at the pericyte-endothelial interface. ADAMTS1 has been reported to cleave capillary basement membrane components, including aggrecan and versican; to play an anti-angiogenic role in both in vivo and in vitro angiogenesis assays; and to regulate migratory capacity of a number of cells through cleavage of a number of substrates, including syndecans and semaphorins. It is therefore a protease that may mediate detachment of pericytes. Moreover, it selectively binds to and sequesters VEGFA164 but not dysangiogenic VEGFA isomers.51 We recently showed that when pericytes detach from capillaries in the kidney, they switch from VEGF164 to VEGF120 and VEGF188 production. The latter two VEGFA forms contribute to unstable angiogenesis. Therefore, it is likely that ADAMTS1 also promotes unstable angiogenesis by regulating VEGFR2-mediated signaling. In keeping with this, Timp3-deficient mice show overexpression of ADAMTS1 in pericytes, have increased but dysfunctional vascularity under physiologic conditions, and have increased vessel rarefaction after injury compared with littermate controls. Our studies predict therefore that ADAMTS1 blockade would beneficially affect the kidney by limiting pericyte detachment after injury, promoting vessel stability, and reducing interstitial fibrosis. Adamts1 mutant mice have microvascular defects as well as a severe kidney developmental phenotype resembling hydroureter.52,53 The severe hydroureter phenotype renders them unsuitable for study of pericyte function in response to injury. One possible explanation for the developmental kidney phenotype is impaired migration of kidney stroma during nephrogenesis leading to impaired nephron lengthening and papilla formation.53 Further studies will be required to determine whether Adamts1 is a targetable gene in kidney injury to prevent pericyte detachment.
In summary, kidney pericytes stabilize capillaries ex vivo and detach from capillaries in interstitial disease, and pericyte factors TIMP3 and ADAMTS1 are key regulators of these pericyte functions in vivo.
Concise Methods
Materials
All reagents were from Sigma Aldrich, all plastic ware was from BD Falcon, and all media were from Cellgro unless otherwise stated.
Animals
Coll1α1-GFP (Coll-GFP) transgenic mice were generated and validated as previously described on the C57BL6 background.1,9,23 Timp3−/− gene–targeted homozygous mice and littermate controls were generated as previously described31,43 and maintained on the C57BL6 background.
Animal Models of Kidney Injury
UUO and unilateral and bilateral kidney IRI models were performed in adult mice (aged 10–12 weeks) as previously described.1,22,23 The ischemic time was 21 minutes. In some experiments, BrdU (50 µg/g) or Evans blue dye (1%) was injected intraperitoneally or intravenously, respectively, 1 hour before euthanasia. All animal experiments were performed under protocols approved by the Animal Resources and Comparative Medicine Committee of Harvard University and the Department of Comparative Medicine at the University of Washington.
Pericyte or Myofibroblast Isolation from Kidney
For pericyte isolation from normal kidney of C57BL6 WT or Coll-GFP mice, the kidney was decapsulated, diced, and then incubated at 37°C for 30 minutes with Liberase (0.5 mg/ml; Roche) and DNase (100 U/ml; Roche) in DMEM/F12. Digestion was curtailed by the addition of 10% FCS to the single cell suspension. The suspension was filtered (40 μm) several times, centrifuged (300×g, 5 minutes), and resuspended in MACS (Miltenyi Biotech, Bergisch Gladbach, Germany) buffer or 1% BSA buffer containing EDTA. In some experiments, cells were incubated with anti-rabbit Pdgfrβ polyclonal antibodies generated against a segment of the full-length receptor54 (800 ng per kidney on ice) for 15 minutes. After washing, cells were incubated with goat anti-rabbit IgG microbeads (Miltenyi Biotech) (15 minutes at 4°C) and resuspended and positive selection was performed by MACS magnetic bead separation. Selected cells were resuspended in RLT buffer (Qiagen) or cultured in DMEM/F12 with 10% FBS and 1% penicillin/streptomycin and 1% ITS, in six-well plates precoated with gelatin. Primary cultured cells used in all experiments were between passages 2 and 5. For other purposes, RNA was isolated using the RNeasy Mini Kit (Qiagen). To note, this purification does not distinguish pericytes from perivascular fibroblasts. In other experiments, single cells from Coll-GFP mice were separated by green fluorescent protein fluorescence by FACS Aria cell sorting, directly into RLT buffer for RNA analysis or into medium (cell culture). In these experiments, kidney single cell suspensions were also incubated with anti-CD45-APC, anti-CD31-PE, anti-F4/80-APC,or anti-Tim1 antibodies (eBioscience) as described1,22–24 to exclude these cell populations from contaminating the Coll-GFP cells. To purify kidney myofibroblasts, single cell preparation and purification were achieved as described above but kidneys from day 2 or day 7 after UUO were used.
Cell Culture
Human umbilical cord ECs (HUVECs) (American Type Culture Collection) were used from P3 to P6 and grown with endothelial cell basal medium (American Type Culture Collection) including one Endothelial Cell Growth Kit BBE (American Type Culture Collection) per 500 ml medium. Human brain pericytes (ScienCell) were cultured in DMEM (Cellgro) containing 10% FCS (Invitrogen) and used from P3 to P6. Primary mouse kidney pericytes (see below) were cultured on 0.1% gelatin-coated T75 flasks (BD Falcon) or six-well plates (BD Falcon) and used from P2 to P5. Mouse kidney–derived myofibroblasts were established as previously described1,9 and were maintained under the same conditions as pericyte cultures and used from P3 to P7.
Three-Dimensional Vascular Tube Regression Assay
ECs were placed in 3D vascular tube regression assays following a modified protocol of Saunders et al.30,37 HUVECs (106 cells/ml) were suspended in 3.75 mg/ml of rat tail collagen type I (4°C) (Invitrogen), transferred to a 37°C incubator in which polymerization occurred, and were allowed to form 3D networks in the presence of VEGF165 (40 ng/ml) and FGF2 (40 ng/ml) (Peprotech),38 in 384-well plates or 96-well 1/2 plates. For quantitative analysis of gel contraction, 15 μl of ECs alone or ECs and varying amounts of primary kidney PCs (as indicated) in collagen matrix were placed in 384-well plates (BD Falcon) (n>10/condition). Addition of medium with or without human plasma KLK (Enzyme Research Laboratories) denoted the starting point of the assay. Cultures were monitored every 4 hours for gel contraction. Upon initiation of gel contraction, the regressed area was quantified using an ocular grid. In every experiment, at least one condition (n=5–10) with HUVECs in collagen matrix without the addition of KLK was used as an internal control. In some experiments, recombinant mouse Adamts1 or recombinant human TIMP3 (R&D Systems) was applied to the media. For experiments in which rmAdamts1 was applied, HUVECs were allowed to form tubes for 24 hours before addition of KLK and recombinant protein. Toluidine blue staining of gels was performed as described previously.30 Conditioned media or whole gels were collected to examine differential protein expression at various time points and were analyzed by SDS-PAGE gels and Western blotting or zymography.
Pericyte Migration and Proliferation Assays
Forty-eight-well plates (BD Falcon) were coated with 0.1% gelatin for 2 hours at 37°C. Cells were seeded with the same density of cells per well and incubated with DMEM/F12 10% FCS until confluent. A scratch “wound” was performed using a 200-µl pipet tip, wells were washed twice with PBS, and DMEM/F12 with 2% FCS was applied. At pre-fixed points along the scratches, photomicrographs were performed at 0 hours and 12 hours after initialization of the wound. Migration of cells and quantification of the wound area and its closure after 12 hours were analyzed using ImageJ software. To quantify proliferation, 105 Timp3−/− and Timp3+/+ pericytes were seeded onto gelatin-coated glass 1.5-cm diameter cover slips (n=9 cover slips/group). BrdU (Sigma) (10 μM/ml) was applied to the medium and incubated for 3 hours. Cells were fixed in acid ethanol, washed, and incubated with 2M HCL, then 0.1M sodium borate, and then 0.2% Triton in 3% BSA. After additional washing, cells were incubated with an anti-BrdU FITC antibody (1:200; eBioscience) in 3% BSA and mounted with Vectashield and 4',6-diamidino-2-phenylindole (DAPI) (Vector Laboratories), and BrdU+ nuclei were quantified in 15 random fields per coverslip.
Gene Silencing in Primary Kidney Pericytes with siRNA
siRNA for Timp3 and control siRNA (Santa Cruz Biotechnology) were resuspended in universal buffer and stored (−80°C, 10 μM). For efficient transfection of primary kidney pericytes, cells were washed, trypsinized, and harvested. A single cell suspension was generated and cells were distributed into a six-well plate with 200,000 cells/well. siRNA (final concentration of 100 nM/well) was resuspended in serum-free medium, vortexed, and incubated for 10 minutes at room temperature with HiPerfect Transfection Reagent (Qiagen). Medium containing siRNA was added dropwise to the wells and plates were swirled gently. Cells were incubated under normal conditions for 3 hours, followed by substitution of additional culture medium. After 48 hours, cells were analyzed for gene silencing. In experiments in which TIMP3 was silenced, cells were transfected again with siRNA as described above 48 hours after initial transfection to achieve higher levels of gene silencing from 72 to 96 hours.
Microarray Experiments
Kidney pericytes or kidney myofibroblasts were purified from 12-week-old adult kidneys of Coll-GFP mice that had no surgery (pericytes, day 0, n=2) or had undergone UUO surgery 2 days (n=2) or 7 days (n=1) previously (myofibroblasts) by generating single cell preparation and FACS sorting into RLT buffer as described in the section above. RNA was purified by the RNeasy method (Qiagen). The quality of RNA was tested by a Agilent Bioanalyzer and high-quality samples underwent RNA amplification by in vitro transcription methods at Harvard University Core Microarray Biotechnology Center. cRNA samples were hybridized to CodeLink mouse whole-genome microarrays using the manufacturer’s protocols (Applied Microarrays) and scanned using Axon GenePix scanner (Molecular Devices). Image processing, background subtraction, and median-based normalization of probe set intensities were performed using CodeLink Expression Analysis Software (Applied Microarrays).
Microarray Data Analyses
Principal component analysis using the entire transcriptome of pericytes and myofibroblasts was performed based on the covariance matrix of normalized gene expression data55 (Supplemental Figure 2A). Temporal profiles of gene expression (day 0, day 2, and day 7) were produced using Short Time-series Expression Miner clustering—an algorithm developed for analyzing short time series microarray data.25 Replicate gene expression values were averaged at day 0 and day 2. Highly significant clusters (P values ranging from 5.4×10−8 to 5.9×10−199) were merged if they displayed similar patterns, resulting in two distinct temporal profiles (Figure 1). Functional analysis of the gene members of these two clusters was performed using the Database for Annotation, Visualization, and Integrated Discovery, a web-based program based on the Gene Ontology database.56 To demonstrate differential gene expression between pericytes (day 0, no surgery) versus myofibroblasts (day 2 and day 7 after UUO surgery), we applied a Bayesian implementation of the parametric t test57 and corrected for multiple comparisons using the Q value method.58 A Q value <0.01 was chosen for identifying significant differential expression, and genes meeting this cutoff underwent two-dimensional hierarchical clustering using Pearson’s correlation (Supplemental Figure 2B and Supplemental Table 1).55
Quantitative Analyses
We used Western blot analysis to quantify protein concentrations in tissues. Total cellular protein extracted using radioimmunoprecipitation assay buffer containing Complete Mini Enzyme Inhibitor Cocktail (Roche Diagnostics) from whole tissue, cells, or supernatants or collagen gel from wells of the vascular stabilization assay. Equal protein amounts were subjected to SDS-PAGE and Western blotting using previously described methods.59 The following primary antibodies were used to detect the specific protein: anti-TIMP3-C-terminus (rabbit 1:100; Millipore), anti-ADAMTS-1 (rabbit 1:100; Santa Cruz Biotechnology), anti-β-actin (goat 1:1000; Santa Cruz Biotechnology), and anti-phospho-VEGFR2 and anti-PDGFRβ (rabbit 1:1000). To measure MMP activity after regression in 3D cultures treated with KLK, we used a 10% gelatin zymogram ready gel (BioRad) and loaded supernatant of regressed gels for electrophoresis under nonreducing conditions. Protein amounts were normalized by the Bradford method. After electrophoresis, gels were washed twice in a 2.5% Triton X-100 including 0.02% NaN3 buffer for 20 minutes, switched to an incubation buffer (50 mM Tris HCl pH 8.0, % mM CaCl2, 0.02% NaN3) for 10 minutes and incubated in fresh incubation buffer at 37°C for 24 to 48 hours. After incubation, gels were fixed and stained in fresh Coomassie blue solution and destained in MeOH:AcOH:H2O (5:1:4) followed by 10% AcOH. Gels were scanned and MMP activity was measured by densitometry of destained areas.
Total RNA was extracted using RNeasy Mini Kit. Purity was determined by the A260/A280 ratio. cDNA was synthesized using Iscript (BioRad). Conventional and quantitative PCR was performed using previously described methods.1 The specific primer pairs used for PCR are listed in Supplemental Table 2.
In Situ Hybridization
Riboprobes were designed by linearization pCytoMegaloVirus sport6 vectors containing the specific cDNA for Timp3, Adamts1, or Ngf alpha (control) (Invitrogen) via restriction enzyme digestion. RNA probes were then generated using a DIG labeling kit (Roche) containing Sp6 or T7 polymerases to generate probes from the 3′ end. Probe sizes were confirmed by RNA agarose gel electrophoresis and digoxigenin incorporation was determined by membrane spotting. Hybridizations were performed following methods adapted from in situ hybridization in kidney embryos.60 In brief, paraformaldehyde (PFA) fixed kidney cryosections (8 μm) were used within 48 hours, postfixed, proteinase-K digested, postfixed again, acetylated, and then incubated with 750 ng/ml riboprobes at 68°C for 16 hours in hybridization buffer (ENZ-33808; Enzo Life Sciences). Sections were digested by RNase, and then washed again with solutions of increasing stringency. After incubation with anti-digoxigenin antibody (Roche) for 2 hours at room temperature, sections were washed and developed with nitro blue tetrazolium chloride (Roche) for up to 24 hours. Color reactions were stopped with fixation and sections were mounted with ProLong Gold (Invitrogen).
Tissue Preparation, Histology, and Analyses
Mouse kidneys were flushed with ice-cold PBS to remove blood, and were harvested and fixed with paraformaldehyde, l-lysine, periodate solution for 2 hours before cryopreservation for immunofluorescence studies or with 10% formalin solution for 12 to 16 hours for paraffin sections.61 Mouse kidneys for in situ hybridization were fixed overnight with 4% PFA before cryopreservation.60 Human kidney biopsies were either prepared for electron microscopy (EM) by standard methods9 or fixed with paraformaldehyde, l-lysine, periodate solution for 1 hour, followed by cryopreservation.61 Human or mouse cryosections (7 µm) for immune fluorescence studies were blocked in 10% normal serum and then incubated with primary antibody (2 hours at room temperature or 16 hours at 4°C) in blocking serum using the following primary antibodies for mouse kidneys: anti-ADAMTS1 (rabbit 1:100; Santa Cruz Biotechnology), anti-PDGFRβ (rabbit 1:400 collaborator), anti-CD31 (rat 1:250; R&D Systems), and anti-αSMA-cy3 (mouse 1:250; Sigma Aldrich). Anti-mouse PDGFRβ antibodies were generated and characterized as previously described.54 The following antibodies were used for human kidneys: anti-CD34 (1:250 Ab30375; Abcam) (1:300; BD Biosciences), anti-PDGFRβ (1:250; Cell Signaling), and anti-αSMA-Cy3 (mouse 1:250; Sigma Aldrich). As necessary, sections were incubated with appropriate affinity purified secondary Cy3-conjugated antibodies (1:500; Jackson Immunoresearch) for 1 hour at room temperature, postfixed in 1% PFA for 2 minutes, and mounted with Vectashield plus DAPI or ProLong Gold plus DAPI. Quantification of vascular density was performed as previously described,40 and morphometry of Sirius red stain or other fluorescent labels was performed as previously reported.1,22–24 In some studies, extravasated Evans blue dye (1%) was detected in whole kidney measuring absorbance.62 Digital images were captured by confocal microscopy. Sirius red staining or periodic acid–Schiff staining were performed on 3-µm paraffin sections as previously described.63 Positive cells or area of positive stain/fluorescence in images were quantified as previously described.1,38
Statistical Analyses
Comparisons of two groups were performed using the t test, whereas several groups were analyzed by one-way ANOVA followed by multiple comparison testing, using GraphPad Prism software. Groups were considered significant if P values were <0.05. Error bars indicate SEM.
Disclosures
J.S.D. is on the scientific advisory board for Promedior Inc and Regulus Therapeutics, has stock options with Promedior Inc, and has consulted for Amira Pharamceuticals, Gilead, Abbott Pharmaceuticals, Takeda Pharmaceuticals, and Boehringer Ingelheim.
Acknowledgments
We thank Dr. Li Li Hsiao (Harvard Medical School, MA), Dr. Roderick Jensen (Virginia Tech, VA), and Michael J. Lombardi (Microarray Biotechnology Center, Harvard Medical School) for assistance with microarrays; Dr. William Parks and Dr. Yujiro Kida (University of Washington, WA) and Dr. Anastasia Sacharidou (University of Missouri, MI) for advice and assistance with the vascular assay, respectively; Dr. Rama Khokha (University of Toronto, Toronto, ON, Canada) for providing the Timp3−/− mice; and the Lynne and Mike Garvey Microscopy Suite at South Lake Union for microscopy support. We also thank Dr. Herman Haller for ongoing support and advice. The Duffield laboratory is funded by the National Institutes of Health (Grants DK73299, DK84077, and DK87389), University of Washington, Genzyme (Research in Progress Grant), and the Nephcure Foundation, as well as by a research agreement with Regulus Therapeutics. C.S. was partly funded by Medizinische Hochschule, Hannover, Germany.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011080851/-/DCSupplemental.
See related editorial, “Activated Pericytes and the Inhibition of Renal Vascular Stability: Obstacles for Kidney Repair,” on pages 767–769.
References
- 1.Lin SL, Kisseleva T, Brenner DA, Duffield JS: Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173: 1617–1627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS: Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin LE, Hemo I, Keshet E: A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 125: 1591–1598, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E: Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest 103: 159–165, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armulik A, Abramsson A, Betsholtz C: Endothelial/pericyte interactions. Circ Res 97: 512–523, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C: Pericytes regulate the blood-brain barrier. Nature 468: 557–561, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Majesky MW, Dong XR, Hoglund V, Mahoney WM, Jr, Daum G: The adventitia: A dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol 31: 1530–1539, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levinson RS, Batourina E, Choi C, Vorontchikhina M, Kitajewski J, Mendelsohn CL: Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development 132: 529–539, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Lin SL, Chang FC, Schrimpf C, Chen YT, Wu CF, Wu VC, Chiang WC, Kuhnert F, Kuo CJ, Chen YM, Wu KD, Tsai TJ, Duffield JS: Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol 178: 911–923, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stratman AN, Schwindt AE, Malotte KM, Davis GE: Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood 116: 4720–4730, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL, Chiang WC, Shen J, Chen YM, Wu KD, Tsai TJ, Duffield JS, Lin SL: Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int 80: 1170–1181, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS, Angle N, Cheresh DA: A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature 456: 809–813, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wühl E, Schaefer F: Managing kidney disease with blood-pressure control. Nat Rev Nephrol 7: 434–444, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Jung YJ, Kim DH, Lee AS, Lee S, Kang KP, Lee SY, Jang KY, Sung MJ, Park SK, Kim W: Peritubular capillary preservation with COMP-angiopoietin-1 decreases ischemia-reperfusion-induced acute kidney injury. Am J Physiol Renal Physiol 297: F952–F960, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Rabelink TJ, Wijewickrama DC, de Koning EJ: Peritubular endothelium: The Achilles heel of the kidney? Kidney Int 72: 926–930, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Davis GE, Saunders WB: Molecular balance of capillary tube formation versus regression in wound repair: Role of matrix metalloproteinases and their inhibitors. J Investig Dermatol Symp Proc 11: 44–56, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Bausch D, Pausch T, Krauss T, Hopt UT, Fernandez-del-Castillo C, Warshaw AL, Thayer SP, Keck T: Neutrophil granulocyte derived MMP-9 is a VEGF independent functional component of the angiogenic switch in pancreatic ductal adenocarcinoma. Angiogenesis 14: 235–243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darmanin S, Chen J, Zhao S, Cui H, Shirkoohi R, Kubo N, Kuge Y, Tamaki N, Nakagawa K, Hamada J, Moriuchi T, Kobayashi M: All-trans retinoic acid enhances murine dendritic cell migration to draining lymph nodes via the balance of matrix metalloproteinases and their inhibitors. J Immunol 179: 4616–4625, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P: MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: Differential regulation by inflammatory cytokines. Blood 109: 4055–4063, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Vigetti D, Moretto P, Viola M, Genasetti A, Rizzi M, Karousou E, Pallotti F, De Luca G, Passi A: Matrix metalloproteinase 2 and tissue inhibitors of metalloproteinases regulate human aortic smooth muscle cell migration during in vitro aging. FASEB J 20: 1118–1130, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Hartwell LH, Hopfield JJ, Leibler S, Murray AW: From molecular to modular cell biology. Nature 402[Suppl]: C47–C52, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS: Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci USA 107: 4194–4199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castaño AP, Lin SL, Surowy T, Nowlin BT, Turlapati SA, Patel T, Singh A, Li S, Lupher ML, Jr, Duffield JS: Serum amyloid P inhibits fibrosis through Fc gamma R-dependent monocyte-macrophage regulation in vivo. Sci Transl Med 1: 5ra13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin SL, Castaño AP, Nowlin BT, Lupher ML, Jr, Duffield JS: Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol 183: 6733–6743, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Ernst J, Bar-Joseph Z: STEM: A tool for the analysis of short time series gene expression data. BMC Bioinformatics 7: 191, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jönsson-Rylander AC, Nilsson T, Fritsche-Danielson R, Hammarström A, Behrendt M, Andersson JO, Lindgren K, Andersson AK, Wallbrandt P, Rosengren B, Brodin P, Thelin A, Westin A, Hurt-Camejo E, Lee-Søgaard CH: Role of ADAMTS-1 in atherosclerosis: Remodeling of carotid artery, immunohistochemistry, and proteolysis of versican. Arterioscler Thromb Vasc Biol 25: 180–185, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Vázquez F, Hastings G, Ortega MA, Lane TF, Oikemus S, Lombardo M, Iruela-Arispe ML: METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J Biol Chem 274: 23349–23357, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez-Manzaneque JC, Westling J, Thai SN, Luque A, Knauper V, Murphy G, Sandy JD, Iruela-Arispe ML: ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun 293: 501–508, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B: A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): Inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med 9: 407–415, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE: Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol 175: 179–191, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gill SE, Huizar I, Bench EM, Sussman SW, Wang Y, Khokha R, Parks WC: Tissue inhibitor of metalloproteinases 3 regulates resolution of inflammation following acute lung injury. Am J Pathol 176: 64–73, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai X, Lin Y, Friedrich CC, Neville C, Pomerantseva I, Sundback CA, Zhang Z, Vacanti JP, Hauschka PV, Grottkau BE: Bone marrow derived pluripotent cells are pericytes which contribute to vascularization. Stem Cell Rev 5: 437–445, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B: A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Hayden MR, Karuparthi PR, Habibi J, Lastra G, Patel K, Wasekar C, Manrique CM, Ozerdem U, Stas S, Sowers JR: Ultrastructure of islet microcirculation, pericytes and the islet exocrine interface in the HIP rat model of diabetes. Exp Biol Med (Maywood) 233: 1109–1123, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caruso RA, Fedele F, Finocchiaro G, Pizzi G, Nunnari M, Gitto G, Fabiano V, Parisi A, Venuti A: Ultrastructural descriptions of pericyte/endothelium peg-socket interdigitations in the microvasculature of human gastric carcinomas. Anticancer Res 29: 449–453, 2009 [PubMed] [Google Scholar]
- 36.Suzuki K, Masawa N, Sakata N, Takatama M: Pathologic evidence of microvascular rarefaction in the brain of renal hypertensive rats. J Stroke Cerebrovasc Dis 12: 8–16, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Saunders WB, Bayless KJ, Davis GE: MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J Cell Sci 118: 2325–2340, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Davis GE, Pintar Allen KA, Salazar R, Maxwell SA: Matrix metalloproteinase-1 and -9 activation by plasmin regulates a novel endothelial cell-mediated mechanism of collagen gel contraction and capillary tube regression in three-dimensional collagen matrices. J Cell Sci 114: 917–930, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Schrimpf C, Duffield JS: Mechanisms of fibrosis: The role of the pericyte. Curr Opin Nephrol Hypertens 20: 297–305, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Li B, Cohen A, Hudson TE, Motlagh D, Amrani DL, Duffield JS: Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation 121: 2211–2220, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim NH, Kashiwagi M, Visse R, Jones J, Enghild JJ, Brew K, Nagase H: Reactive-site mutants of N-TIMP-3 that selectively inhibit ADAMTS-4 and ADAMTS-5: Biological and structural implications. Biochem J 431: 113–122, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kassiri Z, Oudit GY, Kandalam V, Awad A, Wang X, Ziou X, Maeda N, Herzenberg AM, Scholey JW: Loss of TIMP3 enhances interstitial nephritis and fibrosis. J Am Soc Nephrol 20: 1223–1235, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janssen A, Hoellenriegel J, Fogarasi M, Schrewe H, Seeliger M, Tamm E, Ohlmann A, May CA, Weber BH, Stöhr H: Abnormal vessel formation in the choroid of mice lacking tissue inhibitor of metalloprotease-3. Invest Ophthalmol Vis Sci 49: 2812–2822, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Basile DP, Fredrich K, Chelladurai B, Leonard EC, Parrish AR: Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Renal Physiol 294: F928–F936, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Ronco C, House AA, Haapio M: Cardiorenal syndrome: Refining the definition of a complex symbiosis gone wrong. Intensive Care Med 34: 957–962, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Anania MC, Sensi M, Radaelli E, Miranda C, Vizioli MG, Pagliardini S, Favini E, Cleris L, Supino R, Formelli F, Borrello MG, Pierotti MA, Greco A: TIMP3 regulates migration, invasion and in vivo tumorigenicity of thyroid tumor cells. Oncogene 30: 3011–3023, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Kornfeld JW, Meder S, Wohlberg M, Friedrich RE, Rau T, Riethdorf L, Löning T, Pantel K, Riethdorf S: Overexpression of TACE and TIMP3 mRNA in head and neck cancer: Association with tumour development and progression. Br J Cancer 104: 138–145, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song B, Wang C, Liu J, Wang X, Lv L, Wei L, Xie L, Zheng Y, Song X: MicroRNA-21 regulates breast cancer invasion partly by targeting tissue inhibitor of metalloproteinase 3 expression. J Exp Clin Cancer Res 29: 29, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qi JH, Dai G, Luthert P, Chaurasia S, Hollyfield J, Weber BH, Stöhr H, Anand-Apte B: S156C mutation in tissue inhibitor of metalloproteinases-3 induces increased angiogenesis. J Biol Chem 284: 19927–19936, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamura A, Sakai Y, Ohata C, Komurasaki T: Expression and significance of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-1 in an animal model of renal interstitial fibrosis induced by unilateral ureteral obstruction. Exp Toxicol Pathol 59: 1–7, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Luque A, Carpizo DR, Iruela-Arispe ML: ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J Biol Chem 278: 23656–23665, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Mittaz L, Ricardo S, Martinez G, Kola I, Kelly DJ, Little MH, Hertzog PJ, Pritchard MA: Neonatal calyceal dilation and renal fibrosis resulting from loss of Adamts-1 in mouse kidney is due to a developmental dysgenesis. Nephrol Dial Transplant 20: 419–423, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Mittaz L, Russell DL, Wilson T, Brasted M, Tkalcevic J, Salamonsen LA, Hertzog PJ, Pritchard MA: Adamts-1 is essential for the development and function of the urogenital system. Biol Reprod 70: 1096–1105, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB: NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn 222: 218–227, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J: TM4: A free, open-source system for microarray data management and analysis. Biotechniques 34: 374–378, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Huang W, Sherman BT, Lempicki RA: Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Baldi P, Long AD: A Bayesian framework for the analysis of microarray expression data: Regularized t -test and statistical inferences of gene changes. Bioinformatics 17: 509–519, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Storey JD, Tibshirani R: Statistical significance for genomewide studies. Proc Natl Acad Sci USA 100: 9440–9445, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin SL, Chen RH, Chen YM, Chiang WC, Lai CF, Wu KD, Tsai TJ: Pentoxifylline attenuates tubulointerstitial fibrosis by blocking Smad3/4-activated transcription and profibrogenic effects of connective tissue growth factor. J Am Soc Nephrol 16: 2702–2713, 2005 [DOI] [PubMed] [Google Scholar]
- 60.McMahon AP, Aronow BJ, Davidson DR, Davies JA, Gaido KW, Grimmond S, Lessard JL, Little MH, Potter SS, Wilder EL, Zhang P, GUDMAP project : GUDMAP: The genitourinary developmental molecular anatomy project. J Am Soc Nephrol 19: 667–671, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Brenes F, Harris S, Paz MO, Petrovic LM, Scheuer PJ: PLP fixation for combined routine histology and immunocytochemistry of liver biopsies. J Clin Pathol 39: 459–463, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.David S, Ghosh CC, Kümpers P, Shushakova N, Van Slyke P, Khankin EV, Karumanchi SA, Dumont D, Parikh SM: Effects of a synthetic PEG-ylated Tie-2 agonist peptide on endotoxemic lung injury and mortality. Am J Physiol Lung Cell Mol Physiol 300: L851–L862, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP: Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest 115: 56–65, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]