Abstract
Monitoring human immunodeficiency virus drug resistance (HIVDR) early warning indicators (EWIs) can help national antiretroviral treatment (ART) programs to identify clinic factors associated with HIVDR emergence and provide evidence to support national program and clinic-level adjustments, if necessary. World Health Organization–recommended HIVDR EWIs were monitored in Zimbabwe using routinely available data at selected ART clinics between 2007 and 2009. As Zimbabwe’s national ART coverage increases, improved ART information systems are required to strengthen routine national ART monitoring and evaluation and facilitate scale-up of HIVDR EWI monitoring. Attention should be paid to minimizing loss to follow-up, supporting adherence, and ensuring clinic-level drug supply continuity.
Zimbabwe has an estimated national human immunodeficiency virus (HIV) prevalence of 14.3%. Antiretroviral treatment (ART) scale-up started in 2004, and by the end of 2009 more than 220000 (37%) of the 600000 estimated people in need were receiving therapy [1]. At present, HIV care and ART services are being decentralized to primary care clinics in order to improve access to HIV care. As ART is scaled up and access expands, appropriate program monitoring should be instituted to optimize quality of care and minimize situations that favor the emergence of HIV drug resistance (HIVDR). Zimbabwe’s National HIV Drug Resistance Working Group, led by the Ministry of Health and Child Welfare, developed a population-based HIVDR prevention and assessment strategy that includes monitoring of World Health Organization (WHO)–recommended HIVDR early warning indicators (EWIs) [2]. Early warning indicators monitor factors associated with HIVDR using information routinely recorded in medical and pharmacy records, and results provide ART programs with an evidence base to take corrective action at clinic and national program levels to minimize the emergence of population-level HIVDR.
Based on data routinely collected in its existing medical and pharmacy records, Zimbabwe monitored the following EWIs: ART prescribing practices; proportion of patients lost to follow-up 12 months after ART initiation; proportion of patients still on appropriate first-line ART 12 months after initiation; on-time antiretroviral (ARV) drug pickup; on-time clinic appointment keeping; and ARV drug supply continuity.
Appropriate first-line ART regimens were defined as regimens listed in national ART guidelines or a regimen recommended by WHO 2006 treatment guidelines. Patients were classified as lost to follow-up if they did not pick up their ARVs or attend a clinic appointment within 90 days after the last scheduled ARV pickup or last scheduled clinic appointment. On-time drug pickups were defined as pickups that occurred before the previous ARVs would have run out if taken as prescribed. On-time appointment keeping was defined as attending an appointment ≤7 days after the appointment was scheduled to take place.
For each year under review, a number of ART clinics were selected for assessment based on geographic distribution, levels of service delivery, population served, and feasibility.
SAMPLING METHOD AND DATA ANALYSIS
For patients initiating ART in 2007 and 2008, a sample of eligible patients was chosen from a period of either 3 months or 6 months, depending on the clinic’s ART patient case load, in order to achieve a sample size of at least 100 individuals, following 2007 WHO EWI guidance [3].
For patients initiating ART in 2009, a minimum sample size for each clinic was determined based on the total number of patients initiating ART at the selected clinics permitting estimation of a 95% confidence interval plus or minus 7% [4]. Retrospective data were abstracted from records of patients consecutively initiating ART or consecutively picking up ARVs on or after 1 January of each year under review until the designated sample size was reached [4].
In all years, data for each indicator were directly recorded and analyzed using a standardized electronic EWI tool developed by WHO [5]. Abstracted data included ART initiation date, ART regimen prescribed, dates of scheduled and kept clinic appointments, dates of drug pickups, number of days of ARVs dispensed, and dates of deaths and transfers out, if applicable. Pharmacy stock cards were used to determine stockouts for each ARV routinely used at the clinic from 1 January to 31 December of each year. Reliability of data was checked by a review of 10% of records at all clinics and in some cases by use of other sources for the same data (eg, a pharmacy record was used to check reliability of a prescription date abstracted from the ART card).
Early warning indicators were piloted at 17 ART clinics in 2007 and monitored at 40 clinics in 2008. In 2009, because a revised WHO method [4] and electronic abstract tool [5] were used, only 24 clinics were monitored. Only 8 clinics participated in all 3 years of monitoring; thus, trends were assessed only in these 8 clinics.
RESULTS
Overall prescribing practices followed national guidelines in all sites across all years (Table 1). Although trends cannot be reliably calculated because different clinics were included in different years, the proportion of ART clinics meeting the WHO-recommended target for appropriate prescribing practices appeared to increase from 65% (11 of 17) in 2007 to 96% (23 of 24) in 2009. Over the same time period, the proportion of facilities meeting the minimum target of ≤20% for loss to follow-up declined from 70% to 33.3%, whereas the proportion of patients retained on appropriate first-line ART at 12 months did not differ significantly over the same period.
Table 1.
Percentage of Antiretroviral Treatment Clinics Monitored in 2007, 2008, and 2009 Achieving the World Health Organization Early Warning Indicator Targets
| ART Clinics Achieving the EWI Target, % |
||||
| Indicator | EWI Target for All Clinics, % | 2007 (n = 17) | 2008 (n = 40) | 2009 (n = 24) |
| Adult patients initiating ART who were initially prescribed an appropriate first-line ART regimena | 100 | 65 | 92.5 | 96 |
| Patients initiating ART at a site who were lost to follow-up 12 mo after initiation | ≤20 | 70 | 52.5 | 33.3 |
| Patients who were taking an appropriate first-line ART regimen 12 mo after ART initiation | ≥80 | 53 | 35 | 45.8 |
| Patients on ART who picked up ARV drugs on time | ≥70 | ND | ND | 80 (n = 10) |
| ART patients who attended clinical consultations on time | ≥90 | ND | ND | 80 (n = 10) |
| Months during a designated year with no ARV drug stockouts | 100 | ND | 57 | 22 (n = 18) |
Abbreviations: ART, antiretroviral treatment; ARV, antiretroviral; EWI, early warning indicator; ND, EWI not assessed.
Appropriate regimens were defined as regimens following national or World Health Organization first-line ART prescribing guidelines.
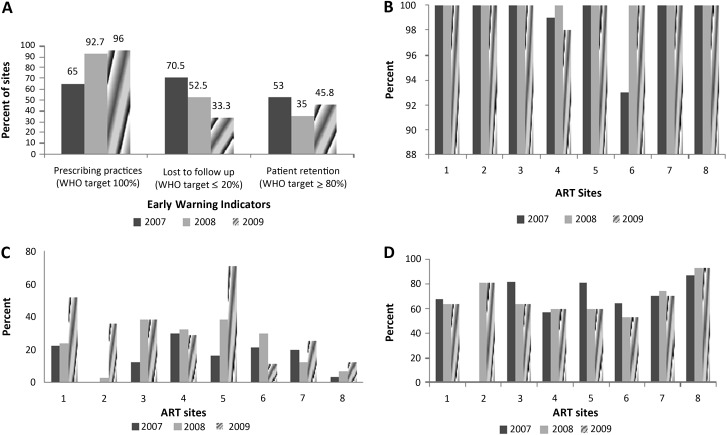
Of the 8 clinics where EWI data were collected consecutively in 2007, 2008, and 2009, prescribing practices improved over time (Figure1A and 1B). Only 1 clinic did not meet the prescribing practices target in 2007 and 2009, and the proportion of ART clinics achieving the minimum target for patient retention on first-line ARVs did not vary over time (Figure 1C). Rates of loss to follow-up generally increased over time at all 8 sites (Figure 1D).
Figure 1.
Percentage of antiretroviral treatment clinics achieving the World Health Organization early warning indicator targets: 8 clinics monitored in 2007, 2008 and 2009. A, Overall early warning indicators. B, Prescribing practices. C, Loss to follow-up. D, Patient retention. WHO, World Health Organization.
For cohorts initiating ART in 2007 and 2008, dates of ARV drug pickup were often found to be inconsistent when comparing individual patient ART cards and pharmacy registers, which made assessment of on-time ARV pickup particularly problematic. Additionally, ARV supply continuity could not be assessed at some clinics prior to 2009 due to incomplete pharmacy records.
DISCUSSION
The public health approach to ART scale-up in resource-limited settings involves the use of standardized and simplified treatment regimens consistent with international standards and appropriate to local circumstances [2]. Use of the public health approach for HIV treatment has led to substantial decline in AIDS-related morbidity and mortality in sub-Saharan Africa. However, HIVDR emergence remains a major concern for national ART programs; hence, strategies to prevent first-line ART failure are essential for the long-term success of ART in sub-Saharan Africa. The widespread use of fixed-dose combination ART in Zimbabwe has reduced pill burden and improved ART adherence.
At present, individual HIVDR testing is neither available nor recommended in Zimbabwe. However, the lack of individual HIVDR testing need not limit the optimization of HIV care and treatment. Care may be optimized and HIVDR minimized through the strengthening of healthcare systems and routine assessment of programmatic factors associated with HIVDR emergence [3, 6].
In Zimbabwe, prescribing practices generally follow the national guidelines. In some cases, low rates of patient retention on first-line therapy at 12 months and high rates of loss to follow-up may have been driven by poor record keeping or missing information. Nonetheless, detailed clinic-level investigations to determine reasons for high rates of loss to follow-up and low rates of retention on first-line ART at 12 months are urgently needed. An important indicator of population-level adherence to ART is the assessment of on-time ARV pickup, which has been shown to be associated with viral load suppression [6, 7]. However, prior to 2009, monitoring of on-time ARV pickups was not feasible because pharmacy records did not permit data abstraction and inconsistencies were observed between individual patient records and pharmacy dispensing logs.
Health system factors associated with failure to achieve EWI targets included inadequate clinic staff, poor record keeping, and long travel distances for patients. Additionally, labor-related population mobility was cited by some clinics, especially those close to international borders and in major cities, as a barrier to patient on-time drug pickup and patient tracing.
Evidence from other countries suggests that reducing financial burden to patients may improve access to and increase patient retention in care [8, 9]. High transport costs due to hyperinflation may have contributed to high patient loss to follow-up rates and subsequent low patient retention rates at clinics. Patients receiving ART from clinics with large catchment areas may have longer distances to travel and may have reduced access to services. Because of the economic situation, even patients with good ART adherence may encounter barriers to taking their ARVs consistently. Individuals who fail to access care or who discontinue ART have the greatest risk of disease progression and death [9] and constitute Zimbabwe’s highest HIV/AIDS-related public health priority. Providing ARVs for longer periods and decentralized service delivery may help to reduce frequency of patient visits and transportation costs.
Among the clinics monitored, loss to follow-up was recognized as a significant problem, and innovative ways of tracing patients through use of volunteers and community-based organizations should be considered on a large scale to minimize loss to follow-up. Additionally, supporting on-time clinic attendance and ARV pickups through mobile phone technologies and community-based treatment supporters and health workers should be explored. Importantly, the fact that many EWIs were difficult to monitor due to poor record keeping and missing information underscores the urgency of medical and pharmacy record strengthening and standardization. Additional resources are needed to optimize ART clinic record keeping and to increase local and national supervision to ensure successful scale-up of program-based monitoring and evaluation in the future.
CONCLUSIONS
As ART is scaled up, monitoring of situations known to favor emergence of HIVDR is critical to the success of Zimbabwe’s national ART program. Early warning indicator monitoring has informed the national program about strengths and limitations in existing medical and pharmacy record systems and has identified clinic-specific barriers to HIVDR prevention. Urgent public health actions include support for improved clinic record keeping and innovative solutions to minimize loss to follow-up and maximize on-time clinic appointment keeping and ARV pickups.
Notes
Acknowledgment.
The authors acknowledge Pierre Kariyo (WHO Regional Office for Africa) for reviewing the article.
Disclaimer.
The conclusions and opinions expressed in this article are those of the authors and do not reflect those of their respective organizations, including the Centers for Disease Control and Prevention, the US Department of Health and Human Services, and the WHO.
Financial support.
This work was supported by The Bill & Melinda Gates Foundation grant through the WHO (award 52227-GLOBAL); and the National Institutes of Health (K23 AI074423-05 to M. R. J.).
Supplement sponsorship.
This article was published as part of a supplement entitled “The World Health Organization HIV Drug Resistance Prevention and Assessment Strategy: Global, Regional, and Country Progress,” sponsored by The Bill & Melinda Gates Foundation (38180).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.WHO, UNAIDS, UNICEF. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Progress report, 2010. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 2.Bennett DE, Bertagnolio S, Sutherland D, et al. The World Health Organization’s global strategy for prevention and assessment of HIV drug resistance. Antivir Ther. 2008;13(Suppl 2):S1–S13. [PubMed] [Google Scholar]
- 3.World Health Organization. HIV drug resistance early warning indicators: WHO recommended set of indicators (2007) Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 4.World Health Organization. HIV drug resistance early warning indicators. Geneva, Switzerland: World Health Organization; 2010. pp. 1–45. Available at: http://www.who.int/hiv/topics/drugresistance/hiv_dr_early_warning_indicators.pdf. Accessed 12 January 2011. [Google Scholar]
- 5.World Health Organization. HIV drug resistance early warning indicator tools (English) Available at: http://www.who.int/hiv/topics/drugresistance/hiv_dr_tool_en.xls. Accessed 12 January 2010. [Google Scholar]
- 6.Jordan MR. Assessments of HIV drug resistance mutations in resource-limited settings. Clin Infect Dis. 2011;52:1053–7. doi: 10.1093/cid/cir093. [DOI] [PubMed] [Google Scholar]
- 7.El-Khatib Z, Katzenstein D, Marrone G, et al. Adherence to drug-refill is a useful early warning indicator of virologic and immunologic failure among HIV patients on first-line ART in South Africa. PLoS One. 2011;6:e17518. doi: 10.1371/journal.pone.0017518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maskew M, MacPhail P, Menezes C, Rubel D. Lost to follow up: contributing factors and challenges in South African patients on antiretroviral therapy. S Afr Med J. 2007;97:853–7. [PubMed] [Google Scholar]
- 9.Lucas GM, Chaisson RE, Moore RD. Survival in an urban HIV-1 clinic in the era of highly active antiretroviral therapy: a 5-year cohort study. J Acquir Immune Defic Syndr. 2003;33:321–8. doi: 10.1097/00126334-200307010-00005. [DOI] [PubMed] [Google Scholar]