Abstract
Increased use of nonnucleoside reverse transcriptase inhibitors (NNRTIs) in pregnant and breastfeeding women will result in fewer children infected with human immunodeficiency virus (HIV). However, among children infected despite prevention of mother-to-child transmission (PMTCT), a substantial proportion will acquire NNRTI-resistant HIV, potentially compromising response to NNRTI-based antiretroviral therapy (ART). In countries scaling up PMTCT and pediatric ART programs, it is crucial to assess the proportion of young children with drug-resistant HIV to improve health outcomes and support national and global decision making on optimal selection of pediatric first-line ART. This article summarizes a new World Health Organization surveillance protocol to assess resistance using remnant dried blood spot specimens from a representative sample of children aged <18 months being tested for early infant diagnosis.
As of December 2010, 3.4 million children aged <15 years were estimated to be living with human immunodeficiency virus (HIV). In 2010, an estimated 390 000 (range, 340 000–450 000) children aged <15 years were newly infected with HIV, and 250000 (range, 150000–360000) died from AIDS-related diseases [1].
The World Health Organization (WHO) and the US President’s Emergency Plan for AIDS Relief support countries to scale up services for the prevention of mother-to-child transmission (PMTCT) of HIV. In 2010, 48% (range, 44%–54%) of pregnant women living with HIV in low- and middle-income countries received the most effective regimens for PMTCT (excluding single-dose nevirapine [NVP]) [1].
Current WHO PMTCT guidelines [2] recommend that, in addition to women requiring antiretroviral therapy (ART) for their own health, pregnant women who do not require ART should be initiated on a prophylactic regimen as early as 14 weeks’ gestation. Infants born to women receiving ART either for their own health or as prophylaxis should also receive standard prophylaxis with daily zidovudine (ZDV) or NVP started at birth and continued to 4–6 weeks regardless of breastfeeding. For breastfed infants of mothers not receiving ART, daily NVP prophylaxis should be started at birth and continued until 1 week after stopping breastfeeding.
As recommended by WHO, infants known to be exposed to HIV should receive a diagnostic HIV polymerase chain reaction (PCR) test at 4–6 weeks of age using dried blood spots (DBSs), or at the earliest opportunity thereafter. For breastfed children, repeat HIV testing 6 weeks after cessation of breastfeeding (when the child is <18 months old) is recommended [3]. Additionally, WHO recommends that all HIV-infected infants and children aged ≤24 months start ART at time of diagnosis [3]. The public health approach to global ART scale-up is based on use of 2 nucleoside reverse transcriptase inhibitors (NRTIs) in combination with a nonnucleoside reverse transcriptase inhibitor (NNRTI). This antiretroviral (ARV) drug combination is widely available, relatively inexpensive, and available in generic formulations and coformulated tablets for pediatric populations.
WHO recommends that children with previous exposure to an NNRTI, either because their mothers received an NNRTI-based regimen during pregnancy, labor and delivery, or breastfeeding, or because they received an NNRTI directly, should initiate an ART regimen that includes the protease inhibitor lopinavir/ritonavir (LPV/r) [3].
The implementation of pediatric ART with ritonavir-boosted protease inhibitor (PI) regimens, which are commonly reserved for second-line ART, presents a number of challenges that limit ART success, including limited availability of PIs, relatively high cost, poor palatability, need for cold chain supply, interaction with rifampicin used for treatment of tuberculosis coinfections, and association with long-term metabolic complications [4–6]. In addition, a large proportion of children starting ART do not have documentation of previous PMTCT exposure and therefore are blindly initiated on a standard NVP-containing regimen [3]. Although NNRTI-based PMTCT regimens reduce HIV infection risk in infants and children, the increased risk of acquiring drug-resistant HIV infection in children who become infected despite PMTCT, particularly with extended NVP prophylaxis [7], poses a challenge.
In children, NNRTI-resistant HIV can be selected by exposure to NNRTIs used for maternal ART or child prophylaxis in the antenatal, intrapartum, and postpartum periods (including during breastfeeding). Furthermore, primary infection with NNRTI-resistant virus through mother-to-child transmission in utero, peripartum, and via breastfeeding is documented [8]. A meta-analysis [9] of 7 studies of HIV-exposed infants who became infected despite NVP PMTCT showed an overall prevalence of NNRTI HIV drug resistance (HIVDR) in 52.6% of infants (N = 201; 95% confidence interval [CI], 37.7%–67.0%) at 4–8 weeks following NVP exposure using standard HIV genotyping assays. The risk of acquiring NNRTI-resistant virus is increased further when NVP is given daily for prophylaxis against transmission while breastfeeding.
Infants who received 6-week extended-dose NVP during breastfeeding were significantly more likely to carry NNRTI resistance mutations detected by standard genotyping than those who received single-dose NVP (sd-NVP) [7, 10]. NNRTI resistance was reduced to 16.5% when NVP prophylaxis was combined with ZDV or ZDV plus lamivudine (3TC). The addition of extended ZDV to extended NVP prophylaxis also reduces the risk of NVP resistance for infants infected in utero [11]. A negative correlation between the level of detected NVP resistance and infant age is reported (P < .001) [12], which may have implications for timing of reuse of NVP for ART in infants with prior NNRTI exposure [13, 14].
Concerns have been raised for infants exposed to NRTI prophylaxis or whose mothers are receiving NRTIs for ART and are breastfeeding. In the Pediatric AIDS Clinical Trials Group Protocol (PACTG) 076 study [15] in which NRTIs were used for PMTCT, no NRTI resistance was detected in infected infants. However, the PACTG185 study [16] showed high ZDV resistance prevalence among infected infants whose mothers had ZDV PMTCT. In the Stopping Infection from Mother-to-Child via Breastfeeding in Africa study infants, prophylaxis with 3TC for prevention of breast milk transmission resulted in acquisition of the M184V/I mutation in 69% of infants, which was no longer detectable 5 months after discontinuation of 3TC [17]. The Kisumu Breastfeeding Study, a single-arm open-label PMTCT trial, assessed the safety and efficacy of ZDV, 3TC, and either NVP or nelfinavir given to HIV-infected women from 34 weeks’ gestation through 6 months of breastfeeding. The study evaluated the emergence of maternal ARV-associated resistance among 32 HIV-infected breastfed infants. Genotypic resistance was detected among 9 of 9 (100%) and 7 of 15 (47%) infected infants whose mothers were receiving nelfinavir and NVP, respectively [18]. The commonest mutations conferred resistance to 3TC (M184V) and NVP (K103N). No major PI mutations were detected [18]. The pattern of mutations detected suggests that drug resistance most likely arose through exposure of the infants to low levels of ARV drugs in breast milk rather than through mother-to-child transmission of drug-resistant virus. Given the limited availability of alternative drugs for infants in resource-limited settings, the authors suggest that provision of the standard WHO-recommended first-line NRTI backbone, which includes 3TC, with enhanced monitoring of the infant to ensure virologic suppression, could be considered. Such an approach should reduce both illness and morbidity among infants who become HIV positive through breastfeeding. Multiclass resistance mutations to both NNRTI and NRTI were detected in 11 of 37 breastfeeding infants whose mothers initiated NNRTI-based ART for their own health postpartum. The infants had received either sd-NVP or extended NVP prophylaxis [19, 20]. Initiation of maternal ART within 14 weeks of delivery was associated with detection of multiclass HIVDR in breastfeeding infants [21].
Selection of HIVDR may be due to low levels of ARV exposure to the infant or child through breastfeeding, although studies are limited. NVP levels in breast milk were found to be measurable for up to 16 days after maternal sd-NVP [22]. Although NNRTI exposure in breast milk may prevent infection, it may also select for drug-resistant virus, depending on the level to which the child is exposed. Suboptimal levels of NNRTIs have been documented in breast milk [23–24], but in the Kisumu Breastfeeding Study, NVP and 3TC were detected in breastfeeding infants at levels sufficient to suppress virus replication. Drug-resistant HIV may also be transmitted through breast milk; NNRTI-resistant virus has been detected in breast milk of women who received intrapartum sd-NVP [25]. The risk of NNRTI resistance transmission is reduced when sd-NVP prophylaxis in women is combined with a 7-day course of ZDV/3TC (“tail”) [26].
Selection of drug-resistant HIV during PMTCT has important implications for ART in children in whom prophylaxis fails. In an initial study in a small number of infants, sd-NVP–exposed infants were significantly more likely to fail ART when started on NVP-based regimens [27, 28]. More recently, the PACTG P1060 trial [29], a large randomized controlled trial conducted in 6 African countries, comparing an LPV/r-based ART regimen to a NVP-based ART regimen in HIV-infected children between 6 and 36 months of age who previously received sd-NVP for PMTCT, showed that LPV/r-based ART was superior to NVP-based ART in preventing virologic failure/discontinuation of ART (odds ratio [OR],18.6; P = .02). In this study, baseline NVP resistance was detected in 12% (18 of 148) of children studied and predicted ART failure in the NVP arm (P = .02 for the interaction between treatment and baseline NNRTI resistance).
NVP may be used in ART for sd-NVP–exposed children if the NVP-based ART is introduced after HIV replication is initially controlled with LPV/r-based therapy according to the Nevirapine Resistance Study (NEVEREST) [13]. In modified intent-to-treat analyses, more children who switched to NVP maintained HIV RNA <50 copies/mL through week 24 postrandomization compared with those continuing LPV/r (65.6% vs 49.5%; P = .02). However, fewer children in the switch group than in the control group maintained HIV RNA <1000 copies/mL (84.9% vs 96.8%; P = .007). Similar findings were observed at week 156 postswitch by Kuhn [30]. Moorthy et al [14] showed that levels at which NNRTI drug resistance mutations were present in pretreatment plasma (≥25%, and therefore detected with standard clinical genotyping assays) at the start of LPV/r-based ART in the infants in the NEVEREST study was predictive of ART failure with use of NVP-based ART.
In summary, scale-up of PMTCT efforts following WHO guidelines should result in a decrease in pediatric HIV infection. However, the increased use of ARVs for prophylaxis in children and pregnant/breastfeeding women or as part of ART regimens in women will lead to substantial increases in the proportion of children infected despite PMTCT, according to numerous studies [7–11]. Response to NNRTI-based first-line ART in HIV-infected children is compromised by prior exposure to NNRTIs for PMTCT and for maternal health, particularly in infants initiating ART immediately after diagnosis. Despite the revised 2010 treatment guidelines advocating LVP/r-based ART as the regimen of choice for HIV-infected children with prior NNRTI exposure, in many countries NNRTI-exposed children are still initiated on NNRTI-based regimens. Reasons include cost and availability of PIs and, in many instances, absence of adequate documentation of previous PMTCT exposures to justify initiation with PI-based regimens.
In settings scaling up PMTCT and pediatric ART programs, it is important to assess the proportion of children with HIVDR and therefore at risk for premature virological failure due to previously selected drug-resistant virus.
Despite the clear need, surveillance to assess “real-world” HIVDR among HIV-infected children aged <18 months has not been implemented to date on a large scale due to cost and logistical constraints. However, scale-up of early infant diagnosis (EID) using DBSs [31–34] provides a unique surveillance opportunity to test remnant specimens for drug resistance.
The WHO, HIVResNet, and the Centers for Disease Control and Prevention developed a surveillance method to assess HIVDR to specific ARVs among children <18 months of age and newly diagnosed with HIV in resource-limited countries. This surveillance activity is designed to be integrated into national surveillance strategies and repeated over time to capture evolving drug resistance scenarios as PMTCT regimens change and coverage improves. Results from these surveys will support decision making on optimal selection of pediatric ART regimens.
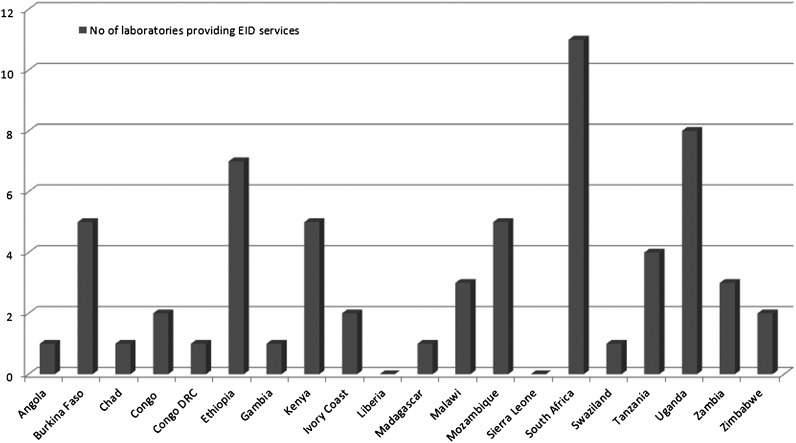
Because the survey uses remnant DBSs and routinely collected information from children being tested for EID, we evaluated survey feasibility and retrieved information to support development of survey methods by assessing laboratory capacity for EID through a questionnaire, which was sent to 20 African countries in 2010. Among the 14 that responded, only 2 countries (Liberia and Sierra Leone) reported no EID capacity. In the remaining 18 countries, between 1 and 11 laboratories provide EID (Figure 1). In total, 93% of the countries reported that basic demographic data such as age of the child, sex, date of birth, and date and site of specimen collection are recorded on the laboratory requisition accompanying the DBSs to the EID laboratory; however, only 43% recorded PMCTC exposure (unpublished data, WHO).
Figure 1.
Laboratories providing early infant diagnosis (EID) services in 20 African countries (May 2010).
METHODS
Because EID methods using DBSs are being implemented in many resource-limited countries and because of the suitability of DBSs for HIVDR genotyping [35–37], DBS is the specimen type selected for this survey. The survey method is retrospective and uses remnant DBSs from HIV-infected children aged <18 months stored at EID laboratories. Demographic and clinical information are abstracted from laboratory requisition accompanying DBSs. Patient variables are presented in Table 1.
Table 1.
Required and Optional Survey Variables
| Required variables: |
| 1. Date of birth (if not available, age of child in months at time of blood draw) |
| 2. Sex |
| 3. Site name where DBS was collected |
| 4. Site type where DBS was collected |
| 5. Date of DBS collection |
| 6. Date DBS frozen at −20°C or −70°C |
| 7. Child receiving ART (not PMTCT) at time of specimen collection (yes/no) |
| 8. Date of PCR testing |
| 9. Date of genotyping |
| Optional variables: |
| 1. Exposure to breastfeeding at time of specimen collection |
| 2. Name of ARV drugs received by mother antepartum/intrapartum/postpartum/during breastfeeding |
| 3. Name of ARV drugs received by infant/child postpartum/during breastfeeding |
Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral; DBS, dried blood spot; PCR, polymerase chain reaction; PMTCT, prevention of mother-to-child transmission.
DBSs collected for EID and genotyped will originate from routine follow-up of HIV-exposed children through PMTCT programs, maternal and child health clinics, or antenatal clinics; HIV testing of symptomatic children presenting to maternal and child health clinics, hospitals, or other medical facilities; and testing of children in provider-initiated testing and counseling sites or voluntary counseling and testing sites.
Because surveillance is conducted retrospectively using remnant DBSs, specimens must have been stored and handled according to WHO recommendations [38]. In countries where DBSs have not been stored according to WHO guidelines, specimen shipment and storage may need to be adjusted prior to survey implementation.
HIVDR Testing
The relevant portions of the reverse transcriptase region of the pol gene of HIV will be sequenced from DBSs using standard sequencing methods following standard WHO methods.
Survey Inclusion Criteria
Participant inclusion/exclusion criteria are described in Table 2.
Table 2.
Participant Inclusion and Exclusion Criteria
| Inclusion criteria: |
| 1. DBS tested HIV positive by PCR from a child <18 months of age |
| 2. If DBS for PCR is collected from a child at different time points, these should be clearly labeled with a unique ID so that the child is not counted more than twice. The most recent DBS specimen from the child is selected for genotyping. |
| 3. At least 1 viable remnant spot is available (2–4 DBSs optimal) |
| 4. From time of blood draw, DBS has been stored no longer than 14 days at ambient temperature, then stored at −20°C or −80°C with no thawing before genotyping. |
| Exclusion criteria: |
| 1. DBS from children ≥18 months of age |
| 2. Child is receiving ≥3 ARV drugs for the purpose of treatment of HIV at time of specimen collection |
Abbreviations: ARV, antiretroviral; DBS, dried blood spot; HIV, human immunodeficiency virus; PCR, polymerase chain reaction.
Sample Size, Laboratory, and Specimen Selection
In some resource-limited settings, all EID DBSs are tested by 1 national laboratory, whereas other countries have many EID laboratories. When possible, all laboratories performing HIV EID will participate in pediatric HIVDR surveillance and will contribute to the overall sample. If only a subset of laboratories participate, a sample of those will be selected based on probability proportional to size cluster sampling. In each participating laboratory, eligible DBSs will be sampled using simple random sampling without replacement.
The sample size calculation is based on the assumption that the true HIVDR prevalence is 50% and with CIs of ± 7%. Prevalence of 50% is the most conservative assumption, yielding the largest sample size and most precise CIs. Sequence amplification success from DBSs is assumed to be 80%. Using the normal approximation to a binomial distribution in PASS 2008 software (http://www.ncss.com), a 95% CI of 14% (±7%) for a prevalence of 50% requires a sample size of 196. Because the amplification rate is expected to be 80%, the effective sample size is 196/0.8 = 245. In countries where there is only 1 EID laboratory, DBSs from eligible children are selected until a sample size of 245 is reached. In countries where DBSs are obtained from >1 laboratory, the laboratories are assumed to be representative of geographic regions and will be treated as strata.
In countries conducting this survey for the first time with no background information on variation in HIVDR prevalence among different EID laboratories, a design effect of 2, which represents the ratio of variance from a stratified sample to the variance of a simple random sample, is applied. Therefore, in countries with >1 laboratory contributing specimens, the final effective sample size for the country will be 245 × 2 = 490 (protocol available at www.who.int/hiv/drugresistance/).
Ethical Considerations
Remnant DBS specimens will be tested anonymously and no personal identifiers will be abstracted; a “nonresearch” waiver will be requested from institutional review boards/ethics review committees.
Statistical Analysis
The prevalence of HIVDR mutations leading to a classification of high, intermediate, or low levels of HIVDR by drug and drug class as determined by the Stanford algorithm [39] will be determined.
HIVDR prevalence will be estimated with 95% CIs based on exposure to PMTCT (yes/none/unknown). If sample sizes are sufficient and patient data are available, separate analyses will be performed evaluating the association of HIVDR with specific PMTCT regimens.
DISCUSSION
Despite the revised 2010 WHO recommendations, in many countries, children are started on NNRTI-based regimens regardless of previous NNRTI exposure, because of cost and feasibility. It is important to assess the proportion of children who carry mutations potentially associated with NNRTI-based regimen failure. Even in countries where PI-based regimens are offered to children with documented exposure to NNRTI, PMTCT NNRTI exposure may not be routinely recorded or may be incorrectly reported as “none” or “unknown” and children may be inappropriately started on NNRTI-based regimens.
NNRTI resistance in children is often found at higher rates in observational studies as opposed to clinical trials. Explanations include insufficiently strict methodology for accurate evaluations, varying periods of exposure, or real-world conditions such as suboptimal adherence to ARVs. It is important to perform HIVDR surveillance and to monitor the implementation of the PMTCT program in the field to support optimal pediatric ART strategies. This survey will provide descriptive evidence of HIVDR in HIV-infected children and may provide information on association of different PMTCT ARV exposures and HIVDR, which will inform future WHO PMTCT ARV guidelines. The survey requires minimal infrastructures, resources, and personnel and is designed to be easily implemented, making use of remnant specimens and patient information routinely captured and transferred to EID laboratories. The simple design of this survey should encourage countries to implement it at regular intervals in order to monitor changes in HIVDR prevalence over time.
This protocol does, however, have limitations. First, in many countries, documentation of infant and child PMTCT exposures on laboratory forms accompanying DBSs for EID testing is incomplete, thus limiting ability to test association between ARV exposure and HIVDR emergence. Second, in many settings DBSs may not be properly collected, transported, or stored, thus lowering amplification efficiency, which may lead to over- or underestimation of resistance. Third, if EID coverage at the national level is not high, survey results may have limited generalizability to HIV-infected children aged <18 months in that country. Additionally, if EID coverage at the national level varies among sites or between geographic regions that offer different PMTCT regimens, overall HIVDR prevalence may be over- or underestimated.
Because of these limitations, countries already planning to prospectively and routinely measure rates of early mother-to-child transmission of HIV at 6 weeks postpartum and to follow up infants who were positive by enzyme-linked immunosorbent assay but negative by PCR for 18 months to determine whether the child becomes infected may consider integrating HIVDR surveillance as a nested study [40–42]. However, countries that are not planning to engage in such PMTCT effectiveness studies are strongly encouraged to implement this protocol, which provides relevant data by using a simple and low-cost approach.
CONCLUSIONS
Increased use of NNRTIs in pregnant and breastfeeding women will result in fewer HIV-infected children. However, among children infected despite PMTCT, a substantial proportion will have NNRTI-resistant virus, potentially compromising response to NNRTI-based ART. Expansion of PMTCT options may lead to changing patterns and prevalences of HIVDR in children infected when PMTCT regimens fail; this protocol will provide countries with data to monitor these changes in infants aged <18 months.
Additionally, the survey will provide information about HIVDR risks related to pretreatment ARV exposures and provide insight into HIVDR consequences of real-world PMTCT implementaion.
Finally, this survey provides an opportunity to map use of PMTCT and its record-keeping system and supports corrective actions, if necessary. If information regarding previous ARV experience is reported as “unknown” for many children and high levels of HIVDR are detected in this group, recommendations such as targeted early virologic monitoring (where feasible) and baseline genotypic testing (if possible) may be explored. Overall, the evaluation of HIVDR prevalence to specific ARVs will support decision making about pediatric ART guidelines.
Notes
Acknowledgments.
This protocol was developed and endorsed by the WHO HIVResNet Pediatric advisory panel, whose members included pediatricians and HIV experts from a number of institutions. We particularly acknowledge the contributions of Dr Elaine Abrams, Dr Louise Kuhn, Dr Nigel Rollins, Dr Gert van Zyl, Dr Carol Worrell, and Dr Nathan Shaffer.
Disclaimer.
The conclusions and opinions expressed in this article are those of the authors and do not reflect those of their respective organizations, including the Centers for Disease Control and Prevention, the National Institutes of Health, the US Department of Health and Human Services, and the World Health Organization.
Financial support.
This work was supported by The Bill & Melinda Gates Foundation (grant 38180) and the National Institutes of Health (NIH K23 AI074423-05 to M. R. J.). The research leading to these results has received part funding from the European Community's Seventh Framework Programme (FP7/2007-2013) under the project “Collaborative HIV and Anti-HIV Drug Resistance Network”–grant agreement n° 223131.
Supplement sponsorship.
This article was published as part of a supplement entitled “The World Health Organization HIV Drug Resistance Prevention and Assessment Strategy: Global, Regional, and Country Progress,” sponsored by The Bill & Melinda Gates Foundation (38180).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization, Joint United Nations Program on HIV/AIDS, UNICEF. Global HIV/AIDS response. Epidemic update and health sector progress towards universal access. Geneva, Switzerland: World Health Organization; 2011. Available at: http://www.who.int/hiv/pub/progress_report2011/en/index.html. Accessed 7 December 2011. [Google Scholar]
- 2.World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infections in infants. Geneva, Switzerland: World Health Organization; 2010. pp. 1–106. Available at: http://www.who.int/hiv/pub/mtct/antiretroviral2010/en/index.html. Accessed 13 June 2011. [Google Scholar]
- 3.World Health Organization. Antiretroviral therapy in infants and children: towards universal access. Recommendations for a public health approach: 2010 revision. Geneva, Switzerland: World Health Organization; 2010. Available at: http://www.who.int/hiv/pub/paediatric/infants2010/en/. Accessed 13 June 2011. [PubMed] [Google Scholar]
- 4.Ergun-Longmire B, Lin-Su K, Dunn AM, et al. Effects of protease inhibitors on glucose tolerance, lipid metabolism, and body composition in children and adolescents infected with human immunodeficiency virus. Endocr Pract. 2006;12:514–21. doi: 10.4158/EP.12.5.514. [DOI] [PubMed] [Google Scholar]
- 5.Haubrich RH, Riddler SA, DiRienzo AG, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS. 2009;23:1109–18. doi: 10.1097/QAD.0b013e32832b4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lainka E, Oezbek S, Falck M, Ndagijimana J, Niehues T. Marked dyslipidemia in human immunodeficiency virus-infected children on protease inhibitor-containing antiretroviral therapy. Pediatrics. 2002;110:e56. doi: 10.1542/peds.110.5.e56. [DOI] [PubMed] [Google Scholar]
- 7.Moorthy A, Gupta A, Bhosale R, et al. Nevirapine resistance and breast milk HIV transmission: effects of single and extended-dose nevirapine prophylaxis in subtype C HIV-infected infants. PLoS One. 2009;4:e4096. doi: 10.1371/journal.pone.0004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Persaud D, Palumbo P, Ziemniak C, et al. Early archiving and predominance of nonnucleoside reverse transcriptase inhibitor-resistant HIV-1 among recently infected infants born in the United States. J Infect Dis. 2007;195:1402–10. doi: 10.1086/513871. [DOI] [PubMed] [Google Scholar]
- 9.Arrivé E, Newell ML, Ekouevi DK, et al. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol. 2007;36:1009–21. doi: 10.1093/ije/dym104. [DOI] [PubMed] [Google Scholar]
- 10.Church JD, Omer SB, Guay LA, et al. Analysis of nevirapine (NVP) resistance in Ugandan infants who were HIV-infected despite receiving single-dose (SD) NVP versus SD NVP plus daily NVP up to 6 weeks of age to prevent HIV vertical transmission. J Infect Dis. 2008;198:1075–82. doi: 10.1086/591503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lidstrom J, Li Q, Hoover DR, et al. Addition of extended zidovudine to extended nevirapine prophylaxis reduces nevirapine resistance in infants who were HIV-infected in utero. AIDS. 2010;24:381–6. doi: 10.1097/QAD.0b013e3283352ef1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor BS, Hunt G, Abrams EJ, et al. Rapid development of antiretroviral drug resistance mutations in HIV-infected children less than two years of age initiating protease inhibitor-based therapy in South Africa. AIDS Res Hum Retroviruses. 2011;27:965–56. doi: 10.1089/aid.2010.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coovadia A, Abrams EJ, Stehlau R, et al. Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial. JAMA. 2010;304:1082–90. doi: 10.1001/jama.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moorthy A, Kuhn L, Coovadia A, et al. Induction therapy with protease-inhibitors modifies the effect of nevirapine resistance on virologic response to nevirapine-based HAART in children. Clin Infect Dis. 2011;52:514–21. doi: 10.1093/cid/ciq161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McSherry GD, Shapiro DE, Coombs RW, et al. The effects of zidovudine in the subset of infants infected with human immunodeficiency virus type-1 (Pediatric AIDS Clinical Trials Group Protocol 076) J Pediatr. 1999;134:717–24. doi: 10.1016/s0022-3476(99)70287-8. [DOI] [PubMed] [Google Scholar]
- 16.Mofenson LM, Lambert JS, Stiem E, et al. Program and abstracts of the 13th International AIDS Conference. Durban, South Africa: 2000. Association of zidovuidine (ZDV) genotypic resistance with perinatal HIV transmission in women receiving ZDV in Pediatric Clinical Trials Group Protocol 185 [abstract 1229] [Google Scholar]
- 17.Giuliano M, Galluzzo CM, Germinario EA, et al. Selection of resistance mutations in children receiving prophylaxis with lamivudine or nevirapine for the prevention of postnatal transmission of HIV. J Acquir Immune Defic Syndr. 2006;42:131–3. doi: 10.1097/01.qai.0000209911.10071.18. [DOI] [PubMed] [Google Scholar]
- 18.Zeh C, Weidle PJ, Nafisa L, et al. HIV-1 drug resistance emergence among breastfeeding infants born to HIV-infected mothers during a single-arm trial of triple-antiretroviral prophylaxis for prevention of mother-to-child transmission: a secondary analysis. PLoS Med. 2011;8:e1000430. doi: 10.1371/journal.pmed.1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lidstrom J, Kumwenda N, Kafulafula G, et al. Program and abstracts of the 18th International HIV Drug Resistance Workshop. Fort Myers, Florida: Antiviral Therapy 2009; 14:A158: Antiretroviral treatment of HIV-infected women can induce multiclass drug resistance in their breast-feeding infants [abstract 135] Available at: http://www.informedhorizons.com/resistance2009/pdf/AbstractBook_RW2009_070209.pdf. Accessed 13 June 2011. [Google Scholar]
- 20.Lidstrom J, Guay L, Musoke P, et al. Program and abstracts of the 17th Conference on Retroviruses and Opportunistic Infections. San Francisco, California: CROI: 2010. Multi-class drug resistance arises frequently in HIV-infected breastfeeding infants whose mothers initiate HAART postpartum [oral abstract 920] Available at: http://www.retroconference.org/2010/Abstracts/38186.htm. Accessed 13 June 2011. [Google Scholar]
- 21.Fogel J, Li Q, Taha TE, et al. Initiation of antiretroviral treatment in women after delivery can induce multiclass drug resistance in breastfeeding HIV-infected infants. Clin Infect Dis. 2011;52:1069–76. doi: 10.1093/cid/cir008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunz A, Mugenyi K, Karcher H, et al. Program and abstracts of the 16th International AIDS Conference. Toronto, Canada: International AIDS Society: 2006. Nevirapine is detectable in breast milk up to 2 weeks after single dose [abstract TUPE0353] Available at: http://www.iasociety.org/Default.aspx?pageId=11&abstractId=2194851. Accessed 14 June 2011. [Google Scholar]
- 23.Mirochnick M, Thomas T, Capparelli E, et al. Antiretroviral concentrations in breast-feeding infants of mothers receiving highly active antiretroviral therapy. Antimicrob Agents Chemother. 2009;53:1170–6. doi: 10.1128/AAC.01117-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirochnick M, Best BM, Clarke DF. Antiretroviral pharmacology: special issues regarding pregnant women and neonates. Clin Perinatol. 2010;37:907–27. doi: 10.1016/j.clp.2010.08.006. xi. [DOI] [PubMed] [Google Scholar]
- 25.Lee EJ, Kantor R, Zijenah L, et al. Breast milk shedding of drug-resistant HIV-1 subtype C in women exposed to single-dose nevirapine. J Infect Dis. 2005;192:1260–4. doi: 10.1086/444424. [DOI] [PubMed] [Google Scholar]
- 26.Farr SL, Nelson JA, Ng'ombe TJ, et al. Addition of 7 days of zidovudine plus lamivudine to peripartum single-dose nevirapine effectively reduces nevirapine resistance postpartum in HIV-infected mothers in Malawi. J Acquir Immune Defic Syndr. 2010;54:515–23. doi: 10.1097/qai.0b013e3181e3a70e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lockman S, Shapiro RL, Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356:135–47. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 28.Lockman S, Smeaton L, Ogwu A, et al. Program and abstracts of the 16th Conference on Retroviruses and Opportunistic Infections. Montreal, Canada: CROI: 2009. Outcomes on nevirapine-based HAART following receipt of peripartum single-dose nevirapine or placebo, Botswana [poster 955] Available at: http://www.retroconference.org/2009/Abstracts/36058.htm. Accessed 13 June 2011. [Google Scholar]
- 29.Palumbo P, Lindsey JC, Hughes MD, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010;363:1510–20. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhn L, Coovadia A, Meyers T, et al. Program and abstracts of the 18th Conference on Retroviruses and Opportunistic Infections. Boston, Massachusetts: CROI: 2011. Long-term outcomes of switching children to NVP-based therapy after initial suppression with a PI-based regimen [oral abstract 128] Available at: http://www.retroconference.org/2011/Abstracts/41025.htm. Accessed 13 June 2011. [Google Scholar]
- 31.Creek T, Tanuri A, Smith M, et al. Early diagnosis of human immunodeficiency virus in infants using polymerase chain reaction on dried blood spots in Botswana’s national program for prevention of mother-to-child transmission. Pediatr Infect Dis J. 2008;27:22–6. doi: 10.1097/INF.0b013e3181469050. [DOI] [PubMed] [Google Scholar]
- 32.Cherutich P, Inwani I, Nduati R, Mbori-Ngacha D. Optimizing paediatric HIV care in Kenya: challenges in early infant diagnosis. Bull World Health Organ. 2008;86:155–60. doi: 10.2471/BLT.07.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciaranello AL, Park JE, Ramirez-Avila L, Freedberg KA, Walensky RP, Leroy V. Early infant HIV-1 diagnosis programs in resource limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC Med. 2011;9:59. doi: 10.1186/1741-7015-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lofgren SM, Morrissey AB, Chevallier CC, et al. Evaluation of a dried blood spot HIV-1 RNA program for early infant diagnosis and viral load monitoring at rural and remote healthcare facilities. AIDS. 2009;23:2459–66. doi: 10.1097/QAD.0b013e328331f702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertagnolio S, Parkin NT, Jordan M, Brooks J, Garcia-Lerma JG. Dried blood spots for HIV-1 drug resistance and viral load testing: a review of current knowledge and WHO efforts for global HIV drug resistance surveillance. AIDS Rev. 2010;12:195–208. [PubMed] [Google Scholar]
- 36.Buckton AJ. New methods for the surveillance of HIV drug resistance in the resource poor world. Curr Opin Infect Dis. 2008;12:653–8. doi: 10.1097/QCO.0b013e3283186d1a. [DOI] [PubMed] [Google Scholar]
- 37.Yang C, McNulty A, Diallo K, et al. Development and application of a broadly sensitive dried-blood-spot-based genotyping assay for global surveillance of HIV-1 drug resistance. J Clin Microbiol. 2010;48:3158–64. doi: 10.1128/JCM.00564-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization. WHO manual for HIV drug resistance testing using dried blood spot specimens. Geneva, Switzerland: World Health Organization; 2010. pp. 1–29. Available at: http://www.who.int/hiv/topics/drugresistance/dbs_protocol.pdf. Accessed 13 June 2011. [Google Scholar]
- 39.Liu TF, Shafer RW. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis. 2006;42:1608–18. doi: 10.1086/503914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rollins NC, Dedicoat M, Danaviah S, et al. Prevalence, incidence, and mother-to-child transmission of HIV-1 in rural South Africa. Lancet. 2002;360:389. doi: 10.1016/s0140-6736(02)09599-5. [DOI] [PubMed] [Google Scholar]
- 41.Rollins N, Little K, Mzolo S, Horwood C, Newell ML. Surveillance of mother-to-child transmission prevention programmes at immunization clinics: the case for universal screening. AIDS. 2007;21:1341–7. doi: 10.1097/QAD.0b013e32814db7d4. [DOI] [PubMed] [Google Scholar]
- 42.Rollins N, Mzolo S, Moodley T, Esterhuizen T, van Rooyen H. Universal HIV testing of infants at immunization clinics: an acceptable and feasible approach for early infant diagnosis in high HIV prevalence settings. AIDS. 2009;23:1851–7. doi: 10.1097/QAD.0b013e32832d84fd. [DOI] [PubMed] [Google Scholar]