Abstract
In 2004, Malawi began scaling up its national antiretroviral therapy (ART) program. Because of limited treatment options, population-level surveillance of acquired human immunodeficiency virus drug resistance (HIVDR) is critical to ensuring long-term treatment success. The World Health Organization target for clinic-level HIVDR prevention at 12 months after ART initiation is ≥70%. In 2007, viral load and HIVDR genotyping was performed in a retrospective cohort of 596 patients at 4 ART clinics. Overall, HIVDR prevention (using viral load ≤400 copies/mL) was 72% (95% confidence interval [CI], 67%–77%; range by site, 60%–83%) and detected HIVDR was 3.4% (95% CI, 1.8%–5.8%; range by site, 2.5%–4.7%). Results demonstrate virological suppression and HIVDR consistent with previous reports from sub-Saharan Africa. High rates of attrition because of loss to follow-up were noted and merit attention.
In June 2004, Malawi began scaling up its national antiretroviral therapy (ART) program, rapidly increasing the number of patients initiated on ART from 13 183 in 2004 to 271 105 by December 2009. Individuals infected with human immunodeficiency virus (HIV) with World Health Organization (WHO) clinical stage III or IV disease or with a CD4 cell count <250/μL (increased from 200/μL in 2007) are eligible for free ART in Malawi. After initiation, patients visit ART clinics monthly for clinical monitoring and drug collection until they are stable, at which time visit frequency decreases to once every 2–3 months.
At the time this survey was conducted, 1 standard first-line ART regimen consisting of nevirapine (NVP) in combination with stavudine and lamivudine (3TC) and alternative first-line regimens containing efavirenz (EFV) and/or zidovudine (ZDV) were used within the national program. Additionally, 1 second-line combination of ZDV, 3TC, tenofovir (TDF), and lopinavir/ritonavir was available [1].
Because of the need for lifelong treatment and HIV’s high mutation rate, virological failure and emergence of HIV drug resistance (HIVDR) among populations receiving ART is inevitable. National treatment programs in sub-Saharan Africa have observed levels of virological failure varying between 14% and 53% within the first 6–24 months after treatment initiation [2–8]. Studies show that among individuals with virological failure receiving ART from the national ART programs, the presence of at least 1 HIVDR mutation varied widely from 44% to 100% [3–15].
At present, individual patient HIVDR testing is not feasible in Malawi due to its high cost and the lack of adequate laboratory infrastructure. Therefore, surveillance of population-level HIVDR and identification of ART program factors, which can be adjusted to minimize emergence of HIVDR and optimize treatment outcomes, are essential. In 2005, the Malawi Ministry of Health created an HIVDR Task Force and adopted the WHO strategy for assessing transmitted and acquired HIVDR and monitoring HIVDR early warning indicators (EWIs) in resource-limited countries [16–19]. In December 2006, Malawi completed its first survey of transmitted HIVDR that classified transmitted drug resistance in the capital Lilongwe as low (<5%) to nucleoside reverse transcriptase inhibitors, nonnucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors [20]. In 2007, Malawi's first HIVDR EWI report documented that most clinics monitored achieved WHO targets for relevant indicators except for patient retention on first-line ART at 12 months, which was achieved by less than half of clinics [21].
In this article we describe results from a retrospective survey of HIVDR performed in patients on ART at 4 clinics in Malawi. This survey was performed in 2007 on cohorts of patients initiating first-line ART 12 months previously.
METHODS
We used a retrospective method to assess acquired HIVDR and associated program factors in populations of patients on ART for 12 months. This retrospective methodology was endorsed by the WHO in 2006 for the HIVDR Monitoring Survey, although the later standard versions of the WHO protocol only include prospective cohort surveys [18]. The survey was approved by the National Health Sciences Research Committee of Malawi, and informed consent was obtained from survey participants who were alive and attended a 12-month clinic appointment falling between 11 and 15 months after ART initiation.
Clinics Surveyed
Four ART clinics in Malawi were selected for this survey (3 of them central hospitals): Queen Elizabeth Central Hospital (QECH), Blantyre; Mzuzu Central Hospital, Mzuzu; Lighthouse Clinic at Kamuzu Central Hospital, Lilongwe; and Thyolo District Hospital, Thyolo. These clinics were chosen because they were among the first ART clinics in Malawi, could provide sufficient sample sizes, had the capacity to identify selected patients during routine follow-up visits, and had facilities to collect and store plasma specimens on site.
Survey Participants and Procedures
In March 2007, we retrospectively identified cohorts of patients who had initiated first-line ART 12 months prior (in February 2006) at each of the 4 clinics. The target sample size for each clinic (n = 96) provided a 95% confidence interval ± 10% regardless of the cumulative incidence of viral load (VL) suppression, as per the WHO protocol [18]. The actual sample size was increased to accommodate for expected attrition due to death and transfers to other clinics (transfers out).
Different expected attrition rates based on previous reports at each site resulted in varying sample sizes (Mzuzu Hospital, n = 143; Lighthouse Clinic, n = 145; QECH, n = 148; and Thyolo Hospital, n = 160). Patients aged ≥15 years and initiating ART for the first time (not transferring in from another clinic) were consecutively sampled for the cohorts until the target sample size was reached. Sampling was independent of actual outcomes after treatment initiation.
For each patient, relevant demographic data were abstracted from medical records, including tuberculosis status, WHO clinical stage, CD4 cell count, VL at the start of ART, and ART regimen prescribed. Additionally, dates of clinical appointments, number of appointments with pill count completed, and number of appointments with a pill count and at least 95% adherence were abstracted to estimate adherence to prescribed ART. All patients were classified into one of the following survey endpoints: on first-line ART, stopped ART, switched to and on second-line ART, dead, transferred out, or lost to follow-up. As information on exposure to antiretroviral drugs prior to initiation of ART at the clinic was generally not available in routine medical records, a brief questionnaire was administered.
In 3 of the 4 clinics, CD4 cell counts were routinely performed 12 months after ART initiation. Plasma specimens for VL and HIVDR genotyping were prepared from remnant blood obtained from routine CD4 cell count specimens except at QECH, where routine CD4 counts were not performed during the survey period. At QECH a specimen was drawn for the sole purpose of this survey. Plasma specimens were stored on site at −80°C until transportation on ice packs for storage at the HIV reference laboratory at the Community Health Sciences Unit in Lilongwe. At the end of the survey, all specimens were shipped on dry ice to the National Institute for Communicable Diseases in Johannesburg, South Africa, for VL determination and HIVDR genotyping.
HIV RNA and HIVDR Genotyping
HIV RNA testing was performed using the Roche COBAS Amplicor automated system, per manufacture instructions. A previously described broadly sensitive in-house assay for HIV pol sequencing was used for genotypic resistance testing and performed on all specimens with HIV RNA >400 copies/mL [22].
Data Analysis
The total eligible population for analysis was defined as patients alive and on ART and from whom a useable specimen was collected or patients classified as lost to follow-up or who stopped treatment. Individuals who died, those who transferred out, and those without 12-month specimens or who had unusable specimens were excluded. Survey participants were classified into one of three 12-month HIVDR outcome classifications: (1) HIVDR prevention: on first-line ART and VL ≤400 copies/mL; (2) possible HIVDR: on ART with VL >400 copies/mL and no detected HIVDR, or classified as lost to follow-up or stopped treatment; (3) detected HIVDR: on ART with VL >400 copies/mL and detected mutations causing low-, intermediate-, or high-level resistance to ≥1 relevant drugs per the Stanford HIV database [23].
The WHO survey of acquired HIVDR defines VL suppression as ≤1000 copies/mL [18]; however, we chose a more stringent threshold of VL ≤400 copies/mL. Numerators and denominators of HIVDR outcomes are defined in Supplementary Table 1.
Patient characteristics were compared across clinics and outcome groups using analysis of variance, Fisher exact test, Wilcoxon rank-sum test, and k sample equality of medians test, as appropriate. All analyses were performed using Stata/MP software, version 11 (StataCorp).
RESULTS
A total of 596 individuals meeting survey inclusion criteria were identified as having consecutively initiated first-line ART at the 4 clinics, and all were classified into survey endpoints. Patient characteristics at time of ART initiation are summarized in Table 1. All patients at each of the 4 sites had initiated standard first-line ART; 60% of individuals were female, and the mean age of the cohort was 36.3 years (standard deviation, 9.5 years). Rates of tuberculosis infection within the last 2 years varied significantly by clinic, from 7% at Mzuzu Central Hospital to 24% at QECH (P < .01). WHO clinical stage at initiation also varied by clinic, with more patients initiating ART with WHO clinical stages I and II (18%) at Mzuzu Central Hospital compared with the other 3 sites (P < .01). CD4 counts prior to ART initiation were available for 73% (436 of 596) of patients sampled, ranging from 17% at QECH to 99.4% at Thyolo. Median CD4 at ART initiation was 116 (interquartile range [IQR], 54–203) cells/μL. Pretreatment VLs were only available from Mzuzu (median VL, 396 508 copies/mL; IQR, 117 540–976 800).
Table 1.
Baseline Demographic Characteristics by Site
| Characteristics | Mzuzu (n = 143a) | Lighthouse (n = 148b) | QECH (n = 145c) | Thyolo (n = 160d) | Total |
| Female | 79 (55%) | 96 (65%) | 88 (61%) | 95 (60%) | 358 (60%) |
| Mean age, years (SD) | 37.9 (10.2) | 35.6 (9.9) | 36.7 (8.7) | 35.2 (9.2) | 36.3 (9.5) |
| Tuberculosis (current or within past 2 years)e | 10 (7%) | 23 (16%) | 35 (24%) | 26 (16%) | 94 (16%) |
| WHO clinical stage I or IIe | 26 (18%) | 15 (10%) | 12 (8%) | 7 (4%) | 60 (10%) |
| WHO clinical stage III | 83 (58%) | 102 (69%) | 101 (71%) | 129 (81%) | 415 (70%) |
| WHO clinical stage IV | 34 (24%) | 30 (20%) | 30 (21%) | 24 (15%) | 118 (20%) |
| Median CD4 count (IQR) prior to ART initiation | 129 (94–202) | 114 (40–232) | 132 (90–199) | 100 (42–184) | 116 (54–203) |
| Median viral load (IQR) prior to ART initiation | 396 508 (177 540–976 800) | Not measured | Not measured | Not measured | 396 508 (177 540–976 800) |
| Previous ART exposure | |||||
| Questionnaire administered | 83 | 53 | 67 | 61 | 264 |
| PMTCT exposure prior to initiation | 1 (1%) | 5 (10%) | 0 (0%) | 3 (2%) | 9 (3%) |
| Other ART exposure prior to initiation | 0 (0%) | 1 (2%) | 4 (6%) | 0 (0%) | 5 (2%) |
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range; PMTCT, prevention of mother-to-child transmission; QECH, Queen Elizabeth Central Hospital; WHO, World Health Organization.
Baseline CD4 count missing for 18 patients; baseline viral loads missing for 3 patients.
WHO stage missing for 1 patient; baseline CD4 count missing for 21 patients.
WHO stage missing for 2 patients; baseline CD4 count only available for 25 patients.
Age and sex missing for 1 patient.
P < .01.
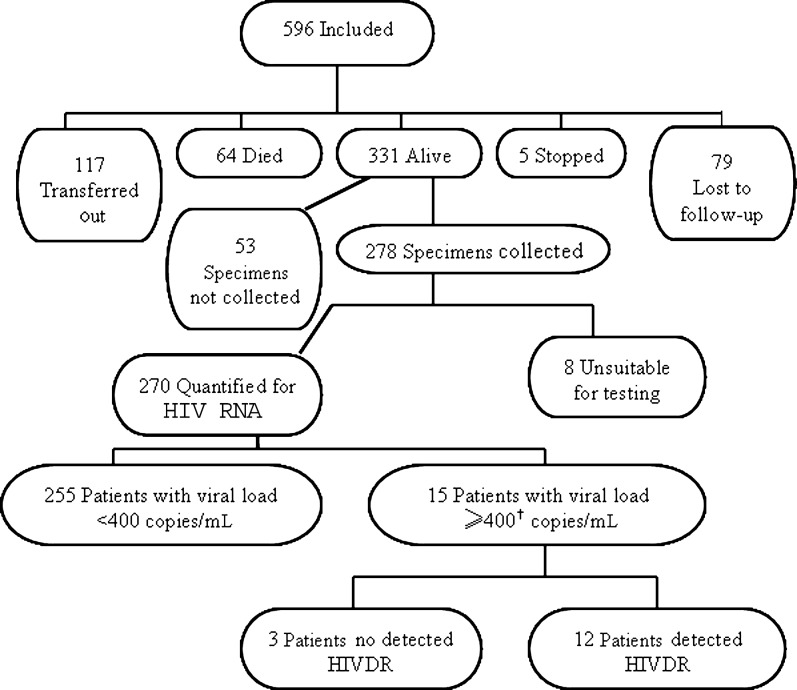
Figure 1 summarizes 12-month endpoints and HIVDR outcomes, and clinic-specific results are presented in Table 2. Of the 596 patients, 117 of 596 (20%) transferred to another site before the 12-month follow-up date. Overall, there was high early mortality with 64 deaths (11%) within the first year of ART. In total, 44 of 64 deaths (69%) occurred within the first 3 months after ART initiation. Five (0.8%) patients, all at Lighthouse Clinic, stopped treatment, and 79 (13%) were lost to follow-up during the first 12 months after ART initiation. Rates of loss to follow-up varied by site, ranging from 4% in Thyolo to 20% in Mzuzu. At the end of the first year of ART, 331 of 596 (56%) patients were alive and on first-line ART, ranging from 46% at Thyolo to 66% at QECH. No patient switched to the second-line ART during the course of the survey. First-line ART substitutions for reasons of toxicity or adverse effects did occur during the first 12 months for 12 patients: 2 at Mzuzu, 4 at Thyolo, and 6 at QECH.
Figure 1.
Survey overview. Only 1 patient had viral load of 400–1000 copies/mL; this specimen was genotyped and had detected human immunodeficiency virus drug resistance. Abbreviations: HIV, human immunodeficiency virus; HIVDR, HIV drug resistance.
Table 2.
Twelve-Month Survey Endpoints by Site
| Outcomes | Mzuzu (n = 143) | Lighthouse (n = 148) | QECH (n = 145) | Thyolo (n = 160) | Total (n = 596) |
| Dead | 12 (8%) | 17 (11%) | 17 (12%) | 18 (11%) | 64 (11%) |
| Lost to follow-up | 28 (20%) | 24 (16%) | 20 (14%) | 7 (4%) | 79 (13%) |
| Transferred out | 14 (10%) | 28 (19%) | 13 (9%) | 62 (39%) | 117 (20%) |
| Stopped ART | 0 (0%) | 5 (3%) | 0 (0%) | 0 (0%) | 5 (1%) |
| On first-line ART at 12 months | 89 (62%) | 74 (50%) | 95 (66%) | 73 (46%) | 331 (56%) |
| No specimen collected | 6 | 22 | 5 | 20 | 53 |
| If alive, specimen collected | 83/89 (93%) | 52/74 (70%) | 90/95 (95%) | 53/73 (73%) | 278/331 (84%) |
| Usable specimens | 77/83 (93%) | 51/52 (98%) | 90/90 (100%) | 52/53 (98%) | 270/278 (97%) |
| HIV RNA >400 copies/mLa | 6 | 3 | 3 | 3 | 15 |
| HIVDR detected | 5 | 2 | 3 | 2 | 12 |
| Total eligible population for analysis (total sample size excluding those who died, transferred, or had no usable specimen) | 105 | 80 | 110 | 59 | 354 |
| HIVDR prevention (VL ≤400 copies/mL) | 71 | 48 | 87 | 49 | 255 |
| 95% CI | 67.6% (57.8%–76.4%) | 60.0% (48.4%–70.8%) | 79.1% (70.2%–86.3%) | 83.1% (71.0%–91.6%) | 72.0% (67.0%–76.6%) |
| Possible HIVDR | 29 | 30 | 20 | 8 | 87 |
| Possible HIVDR rate (95% CI) | 27.6% (19.3%–37.2%) | 37.5% (26.9%–49.0%) | 18.2% (11.5%–26.7%) | 13.6% (6.0%–25.0%) | 24.6% (20.2%–29.4%) |
| Detected HIVDR | 5 | 2 | 3 | 2 | 12 |
| Detected HIVDR rate (95% CI) | 4.7% (1.6%–10.8%) | 2.5% (0.3%–8.7%) | 2.7% (0.6%–7.8%) | 3.4% (0.4%–11.7%) | 3.4% (1.8%–5.8%) |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; HIVDR, HIV drug resistance; QECH, Queen Elizabeth Central Hospital; VL, viral load.
Used more stringent cutoff of ≤400 copies/mL instead of ≤1000 copies/mL recommended by the World Health Organization.
Of the 331 patients alive and on ART after 12 months, 53 of 331 (16%) did not have specimens collected for VL testing and were subsequently excluded from analyses (Figure 1). Of the 278 patients with endpoint specimens, 264 completed the questionnaire about previous antiretroviral drug exposure. In total, 14 of 264 (5.3%) reported exposure to any antiretrovirals prior to initiating first-line ART at the clinic; 9 of 14 (64%) were women who had received NVP for the prevention of mother-to-child transmission (Table 1).
Of the specimens collected, 8 of 278 (2.9%) were not suitable for testing due to hemolysis or insufficient quantity; 255 of 270 (94.4%) with usable specimens 12 months after ART initiation had VL ≤400 copies/mL. Of the 15 of 270 (5.5%) patients with VL >400 copies/mL, 12 of 15 (80%) had at least 1 HIVDR mutation (Supplementary Table 2). All 12 had high-level resistance to NVP and varying levels of EFV resistance (42% high-, 17% intermediate-, and 42% low-level resistance) mainly due to the Y181C mutation. Nine (75%) patients had M184V conferring high-level resistance to 3TC, and 1 patient had K65R associated with didanosine and TDF resistance. All sequences clustered with HIV subtype C.
HIVDR prevention varied among clinics: 60% at Lighthouse, 68% at Mzuzu, 79% at QECH, and 83% at Thyolo. These differences were largely driven by the differences in loss to follow-up rates. Detected HIVDR was 3.4%, ranging from 2.5% to 4.7% by clinic. Possible HIVDR was considerably higher than detected resistance, ranging from 13.6% at Thyolo to 37.5% at the Lighthouse Clinic.
Factors associated with virological failure are presented in Supplementary Table 3. The only significant association was age at initiation of ART (30.7 in those with VL >400 copies/mL vs 37.4 years in those with VL ≤400 copies/mL; P < .05). We also compared those lost to follow-up or who stopped ART (n = 84) with those who died (n = 64). No differences were observed between the 2 groups with regard to sex (female, 54% vs 49%; P = .62), mean age (35.3 vs 35.8 years; P = .74), tuberculosis infection within the last 2 years (19% vs 20%; P >.99), and median VL at ART initiation (415 600 vs 428 000; P >.99). However, there were differences in WHO clinical stage at initiation (24% in stage IV for lost to follow-up/stopped patients and 45% for those who died; P < .05) and baseline CD4 (median baseline CD4 for lost to follow-up/stopped patients was 127 compared with 44 for those who died; P < .01). Median times from initiation of first-line ART to the outcomes of either death or loss to follow-up were 2 months (IQR, 1–5 months) and 6 months (IQR, 4–11 months) (P < .01), respectively.
DISCUSSION
Overall, we observed a low rate of detected HIVDR (3.4%) among adult patients in Malawi receiving ART for 12 months at 4 clinics in the national ART program. However, the rate of possible HIVDR was high (24.6%, varying per site from 13.6% to 37.5%), indicating that the true rate of resistance may be higher than detected. High possible HIVDR was driven by the relatively high rate of loss to follow-up. These patients were classified as possible HIVDR because if they were to reinitiate first-line ART, they would have a considerable chance of having HIVDR to 1 or more components of current first-line regimen due to treatment interruption [14]. If patients who were lost to follow-up were censored from the analysis, estimated rates of possible HIVDR decreased to 2.9% (range by site, 0–10.7%) with a slight increase in the rate of detected HIVDR (4.4%; range by site, 3.3%–6.5%). The most appropriate handling of loss to follow-up remains unclear, as evidence from Malawi suggests that a substantial proportion of individuals who default from care in the first 4 months of initiation have in fact died [24]. However, there was a significant difference in the baseline WHO clinical stage and CD4 counts, suggesting that these groups should be handled differently in the analysis.
Due to limited resources and lack of laboratory capacity, few ART clinics in Malawi offer routine VL monitoring to detect virological failure; thus most patients are monitored by clinical means only, sometimes with CD4 counts. In those who fulfill clinical and/or immunological failure criteria, VL measurement is required to confirm failure to minimize misclassification and inappropriate switch to second-line regimens. Individual HIVDR testing is widely used in high-income countries to determine optimal second-line regimens. However, the cost and complexity of HIVDR genotyping preclude its routine use in Malawi outside of surveillance and research settings. Furthermore, interpretation of individual HIVDR test results is often not straightforward and may be challenging, especially in peripheral clinics where primary care has been shifted to nurses or clinical officers who have not had training in HIVDR interpretation.
In this study, patients with virological failure and detected HIVDR had expected mutations to 3TC and NNRTIs (Supplementary Table 2). Interestingly, no thymidine analogue mutations were detected, suggesting fairly recent onset of virological failure [16]. Unlike in this survey, complex and extensive HIVDR has been observed in Malawian adults enrolled in the national ART program when ART failure is identified using clinical and immunological criteria [9]. However, such patients likely had virological failure for much longer periods of time before HIVDR genotyping than did patients in this survey.
It is noteworthy that only 3 of 15 (20%) patients with virological failure at 12 months had no detected HIVDR, suggesting high population-level adherence to ART among those retained on ART. This finding is juxtaposed to a similar WHO survey of acquired HIVDR conducted in Burundi, where the majority of patients experiencing virological failure at 12 months had no detected HIVDR [25]. The resulting public health response should be different in these 2 settings.
One strength of this survey of acquired HIVDR is that it was integrated into routine care delivery at each of the 4 clinics to obtain data reflecting real-life scenarios and to minimize burden on patients and clinic staff. However, because it was not set up as a research study, identifying the preselected patients at their 12-month appointments and collecting specimens from them was challenging. Of the 331 patients alive after 12 months of ART, 53 attended their 12-month appointment but were not included in the survey due to logistical challenges at the clinics. A further 8 had specimens that could not be amplified for genotyping. There were no significant differences in baseline characteristics between those for whom usable specimens were obtained and the 61 subjects that were excluded from analysis (data not shown), suggesting that missed or compromised specimens were not related to treatment outcomes. However, entirely excluding these from analysis potentially inflates the estimate of possible HIVDR. Additionally, because of the retrospective methodology, we could only explore the relationship between virological failure/HIVDR and routinely collected variables. Relevant factors such as on-time clinic attendance, on-time pill pickup, and HIVDR prior to ART initiation could not be assessed because data were not available in medical records and remnant pretreatment specimens were not available for HIVDR genotyping. Additionally, we could not assess virological suppression among patients transferring to other health centers, and the number transferring was large due to the national policy of stimulating decentralization of care. Finally, clinics monitored in this survey were selected because of their capacity to implement the survey and therefore are not intended to be representative of Malawi’s entire ART program.
CONCLUSIONS
This retrospective survey of acquired HIVDR among patients on ART for 12 months suggests that these 4 clinics are achieving high rates of VL suppression among patients retained on ART and low rates of detected HIVDR. However, possible HIVDR was high due to high rates of loss to follow-up. Routine population-level surveillance of acquired HIVDR and identification of associated program factors is critical to ensure long-term efficacy of currently available first- and second-line ART.
As a result of this survey and other program monitoring data, Malawi plans to implement operational research to define reasons for loss to follow-up to develop appropriately targeted interventions. Finally, although WHO, the US President’s Emergency Plan for AIDS Relief (PEPFAR), and the Centers for Disease Control and Prevention initially deprioritized retrospective surveillance of HIVDR as was implemented in Malawi, this method is likely less expensive and time-consuming than a prospective design, and we encourage reintroduction of this design. Recently, the WHO, along with PEPFAR, has developed a new cross-sectional survey to assess HIVDR at representative ART clinics within countries, which will be piloted in 2012 and may provide another feasible alternative to the prospective surveillance of acquired resistance.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
We acknowledge the participation of patients and staff at the 4 sites included in this survey, especially Hannock Tweya, Joseph Wu, Olesi Pasulani, and Dr Chrysanthus Foncha. At the National Institute for Communicable Diseases, we thank Dr Adrian Puren and staff for viral load testing, Johanna Ledwaba for genotyping, and Dr Visva Pillay for technical guidance related to human immunodeficiency virus (HIV) drug resistance; we also thank Mark Myatt, Brixton Health, United Kingdom.
Disclaimer.
The conclusions and opinions expressed in this article are those of the authors and do not reflect those of the World Health Organization (WHO) or the US Centers for Disease Control and Prevention.
Financial support.
This survey was supported by the WHO and the National Institutes of Health (grant NIH K23 AI074423-05 to M. R. J.). B. L. H. received funding from the National Institutes of Health (grant T32 AI007358) and was an American Schools of Public Health fellow during the implementation of the survey.
Supplement sponsorship.
This article was published as part of a supplement entitled “The World Health Organization HIV Drug Resistance Prevention and Assessment Strategy: Global, Regional, and Country Progress,” sponsored by The Bill & Melinda Gates Foundation (38180).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ministry of Health. Treatment of AIDS: guidelines for the use of antiviral therapy in Malawi. Lilongwe, Malawi: Ministry of Health; 2006. [Google Scholar]
- 2.Coetzee D, Hildebrand K, Boulle A, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–95. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 3.Barth RE, van der Loeff MF, Schaorman R, Hoepelman AI, Wensing AM. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2010;10:155–66. doi: 10.1016/S1473-3099(09)70328-7. [DOI] [PubMed] [Google Scholar]
- 4.Kamya MR, Mayanja-Kizza H, Kambugu A, et al. Predictors of long-term viral failure among Ugandan children and adults treated with antiretroviral therapy. JAIDS. 2007;46:187–93. doi: 10.1097/QAI.0b013e31814278c0. [DOI] [PubMed] [Google Scholar]
- 5.Marcelin AG, Jarrousse B, Derache A, et al. HIV drug resistance after the use of generic fixed-dose combination stavudine/lamivudine/nevirapine as standard first-line regimen. AIDS. 2007;21:2341–3. doi: 10.1097/QAD.0b013e328235a527. [DOI] [PubMed] [Google Scholar]
- 6.Maldonado F, Biot M, Roman F, et al. Viraemia and HIV-1 drug resistance mutations among patients receiving antiretroviral treatment in Mozambique. Trans R Soc Trop Med Hyg. 2008 doi: 10.1016/j.trstmh.2008.07.014. doi: 10.1016/j.trstmh.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Koyalta D, Charpentier C, Beassamda J, et al. High frequency of antiretroviral drug resistance among HIV-infected adults receiving first-line highly active antiretroviral therapy in N’Djamena, Chad. Clin Infect Dis. 2009;49:155–9. doi: 10.1086/599611. [DOI] [PubMed] [Google Scholar]
- 8.Kouanfack C, Montavon C, Laurent C, et al. Low levels of antiretroviral-resistant HIV infection at a routine clinic in Cameroon that uses the World Health Organization (WHO) public health approach to monitor antiretroviral treatment and adequacy with the WHO recommendation for second-line treatment. Clin Infect Dis. 2009;48:1318–22. doi: 10.1086/597779. [DOI] [PubMed] [Google Scholar]
- 9.Hosseinipour MC, van Oosterhout JJG, Weigel R, et al. The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. AIDS. 2009;23:1127–34. doi: 10.1097/QAD.0b013e32832ac34e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallis CL, Mellors JW, Venter WD, Sanne I, Stevens W. Varied patterns of HIV-1 drug resistance on failing first-line antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr. 2010;53:480–4. doi: 10.1097/QAI.0b013e3181bc478b. [DOI] [PubMed] [Google Scholar]
- 11.Marconi VC, Sunpath H, Lu Z, et al. Prevalence of HIV-1 drug resistance after failure of a first highly active antiretroviral regimen in KwaZulu Natal, South Africa. Clin Infect Dis. 2008;46:1589–97. doi: 10.1086/587109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puthanakit T, Jourdain G, Hongsiriwon P, et al. HIV-1 drug resistance mutations in children after failure of first-line nonnucleoside reverse transciptase inhibitor-based antiretroviral therapy. HIV Med. 2010 doi: 10.1111/j.1468-1293.2010.00828.x. doi: 10.1111/j.1468-1293.2010.00828.x. [DOI] [PubMed] [Google Scholar]
- 13.Sungkanuparph S, Manosuthi W, Kiertiburanakul S, Saekang N, Pairoj W, Chantratita W. Prevalence and risk factors for developing K65R mutations among HIV-1 infected patients who fail an initial regimen of fixed-dose combination of stavudine, lamivudine and nevirapine. J Clin Virol. 2008;41:310–3. doi: 10.1016/j.jcv.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Oyugi JH, Byakika-Tusiime J, Ragland K, et al. Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. AIDS. 2007;21:965–71. doi: 10.1097/QAD.0b013e32802e6bfa. [DOI] [PubMed] [Google Scholar]
- 15.Johannessen A, Naman E, Kivuyo SL, et al. Virological efficacy and emergence of drug resistance in adults on antiretroviral treatment in rural Tanzania. BMC Infect Dis. 2009;9:108. doi: 10.1186/1471-2334-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett DE, Bertagnolio S, Sutherland D, Gilks CF. The World Health Organization’s global strategy for prevention and assessment of HIV drug resistance. Antivir Ther. 2008;13(Suppl 2):1–13. [PubMed] [Google Scholar]
- 17.Bennett DE, Myatt M, Bertagnolio S, Sutherland D, Gilks CF. Recommendations for the surveillance of transmitted HIV drug resistance in countries scaling up antiretroviral treatment. Antivir Ther. 2008;13(Suppl 2):25–36. [PubMed] [Google Scholar]
- 18.Jordan MR, Bennett DE, Bertagnolio S, Gilks CF, Sutherland D. World Health Organization surveys to monitor HIV drug resistance prevention and associated factors in sentinel antiretroviral treatment sites. Antivir Ther. 2008;13(Suppl 2):15–23. [PubMed] [Google Scholar]
- 19. World Health Organization. HIV drug resistance early warning indicators: World Health Organization indicators to monitor HIV drug resistance prevention at antiretroviral treatment sites. Available at: www.who.int/hiv/topics/drugresistance/hiv_dr_early_warning_indicators.pdf. Accessed 15 July 2011.
- 20.Kamoto K, Aberle-Grasse J, on behalf of Members of the Malawi HIV Drug Resistance Task Force Surveillance of transmitted HIV drug resistance with the World Health Organization threshold survey method in Lilongwe, Malawi. Antivir Ther. 2008;13(Suppl 2):83–7. [PubMed] [Google Scholar]
- 21.Hedt BL, Wadonda-Kabondo N, Makombe S, et al. Early warning indicators for HIV drug resistance in Malawi. Antivir Ther. 2008;13(Suppl 2):69–75. [PubMed] [Google Scholar]
- 22.Pillay V, Ledwaba J, Hunt G, et al. Antiretroviral drug resistance surveillance among drug-naive HIV-1-infected individuals in Gauteng Province, South Africa in 2002 and 2004. Antivir Ther. 2008;13(Suppl 2):101–7. [PubMed] [Google Scholar]
- 23.Liu TF, Shafer RW. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis. 2006;42:1608–18. doi: 10.1086/503914. Epub 2006 April 28. Available at: http://hivdb.stanford.edu/. Accessed 14 December 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu JKL, Chen SCC, Wang KY, et al. True outcomes for patients on antiretroviral therapy who are “lost to follow-up” in Malawi. Bull World Health Organ. 2007;85:552–4. doi: 10.2471/BLT.06.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nzeyimana SD, Baramperanye E, Mouacha F, et al. Monitoring of HIV-1 drug resistance and associated programmatic factors in patients initiating antiretroviral therapy at two ART sites in Bujumbura, Burundi. Antivir Ther. 2010;15(Suppl 2):A153. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.