Abstract
Human immunodeficiency virus drug resistance (HIVDR) in cohorts of patients initiating antiretroviral therapy (ART) at clinics in Chennai and Mumbai, India, was assessed following World Health Organization (WHO) guidelines. Twelve months after ART initiation, 75% and 64.6% of participants at the Chennai and Mumbai clinics, respectively, achieved viral load suppression of <1000 copies/mL (HIVDR prevention). HIVDR at initiation of ART (P <.05) and 12-month CD4 cell counts <200 cells/μL (P <.05) were associated with HIVDR at 12 months. HIVDR prevention exceeded WHO guidelines (≥70%) at the Chennai clinic but was below the target in Mumbai due to high rates of loss to follow-up. Findings highlight the need for defaulter tracing and scale-up of routine viral load testing to identify patients failing first-line ART.
At the end of 2009, 33.3 million people worldwide were living with human immunodeficiency virus (HIV) infection [1], including an estimated 2.3 million in India [2]. Additionally, an estimated 5.2 million people in low- and middle-income countries were receiving antiretroviral therapy (ART) for HIV [3].
India began ART scale-up in 2004 [2], and as of January 2011, approximately 393 632 HIV-infected adults and children were receiving ART at 293 centers throughout the country [4]. In the context of ART scale-up and maintaining large numbers of patients on ART, emergence of HIV drug resistance (HIVDR) is inevitable. HIVDR and associated treatment failure pose major challenges to successful global ART scale-up and necessitate surveillance of acquired HIVDR in populations receiving ART as well as identification of ART program practices that can be optimized to minimize HIVDR emergence [5]. In India, there are few reports on ART efficacy or HIVDR [6–11].
The World Health Organization (WHO) developed a population-level HIVDR assessment and prevention strategy that includes standardized surveys for assessing acquired HIVDR and associated program factors at sentinel ART clinics [12]. The National AIDS Research Institute, in collaboration with the National AIDS Control Organization and WHO, implemented a survey of acquired HIVDR at the ART center at the Government Hospital of Thoracic Medicine, Tambaram, Chennai, and at the ART center at J. J. Hospital, Mumbai. Both clinics have provided ART free of charge since 2004. The aim of this survey was to pilot the WHO survey of acquired HIVDR at 2 ART clinics for the purpose of identifying patient and program predictors of HIVDR in order to inform national recommendations on effective management of HIVDR at these and other ART clinics in India.
METHODS
A prospective survey of acquired HIVDR was adapted from WHO generic recommendations and then implemented [12]. Per WHO guidance, an effective sample size of 96 permits estimation of clinic-level HIVDR prevention (as defined by viral load [VL] <1000 copies/mL) 12 months after initiation of ART [12]. To achieve the necessary sample size, 150 patients initiating ART at the Chennai clinic and 148 patients initiating ART at the Mumbai clinic were enrolled. Enrollment occurred in March–April 2008 in Chennai and November–December 2007 in Mumbai. Patients were evaluated prior to ART initiation and after 12 months of ART. Patients who died or transferred to other ART clinics were excluded from the endpoint prevalence analysis, and only data from the first consecutive 96 patients with evaluable endpoints were analyzed. The ART centers at J. J. Hospital and Government Hospital of Thoracic Medicine were chosen because they had been functional for >4 years, maintained the necessary patient records, and had adequate facilities for specimen collection, processing, storage, and shipping. This survey was approved by the ethics committees of both hospitals and the institutional ethics committee of the National AIDS Research Institute.
Survey Participants
HIV-infected individuals ≥18 years initiating first-line ART on or after the survey start date who agreed to participate after providing written informed consent were enrolled consecutively. Patients with previous exposures to antiretroviral (ARV) drugs were included, with the exception of patients who had previously started and stopped first-line ART and individuals transferring from another ART clinic on a 3-drug first-line regimen. All enrolled patients initiated standard first-line ART [13]. Patients received either a combinations of zidovudine/stavudine, lamivudine, and nevirapine or efavirenz. Survey participants were assigned unique HIVDR survey codes. Plasma specimens were collected from all individuals on the day of ART initiation (baseline) for HIVDR genotyping. At 12 months, plasma specimens were collected from all patients still on first-line ART for HIV RNA and HIVDR testing (if VL ≥1000 copies/mL). The following survey endpoints were assessed: on first-line ART at 12 months, death, stop, transfer out, or loss to follow-up. CD4 testing was performed at ART initiation and at 6-month intervals. The following survey endpoints were defined:
HIVDR prevention: HIV RNA <1000 copies/mL after 12 months of ART. The WHO target for HIVDR prevention is ≥70% VL suppression 12 months after ART initiation [12].
Detected HIVDR: HIV RNA ≥1000 copies/mL at 12 months and ≥1 HIVDR mutation as defined by the Stanford HIVDR database [14].
Possible HIVDR: patients who stopped ART during the 12 months after initiation, patients lost to follow-up and patients with HIV RNA ≥1000 copies/mL at 12 months and no detected HIVDR.
Laboratory Analysis and Data Collection
Blood specimens were processed for plasma separation at the ART clinics. Specimens were stored at −20°C and transported to the National AIDS Research Institute, Pune, on dry ice. HIV VL was analyzed using an Amplicor HIV monitor (MWP 1.5) and/or a COBAS Amplicor HIV monitor kit version 1.5 (COBAS 1.5; Roche Diagnostics, Branchburg, New Jersey). HIVDR genotyping was performed using ViroSeq version 2.0 (Abbott, Wiesbaden, Germany) and sequencing was performed using an ABI 3100 Genetic Analyzer according to the manufacturer’s instructions. HIVDR was defined as the presence of 1 or more resistance mutations causing low-, intermediate-, or high-level resistance per the Stanford HIVDR database [14]. Patient demographic and clinical data were abstracted from medical records, and adherence to ART was estimated at survey endpoint using a 30-day visual analogue scale (VAS).
Statistical Analysis
The prevalence of HIVDR prevention (VL suppression) at 12 months and prevalence of detected HIVDR was estimated with 95% binomial exact confidence interval. Association of patient factors with HIVDR at 12 months was tested using Pearson χ2 test and Fisher exact test. Odds ratios (ORs) and adjusted ORs in univariate and multivariate analyses were calculated using a forward logistic regression method. Patient factors analyzed were age, sex, baseline and endpoint CD4 count, weight at baseline and endpoint, WHO clinical stage at ART initiation, adherence (VAS), previous ARV exposure, presence of HIVDR at baseline, ART regimen type, and regimen substitution. All statistical analyses were performed with SPSS software version 15.
RESULTS
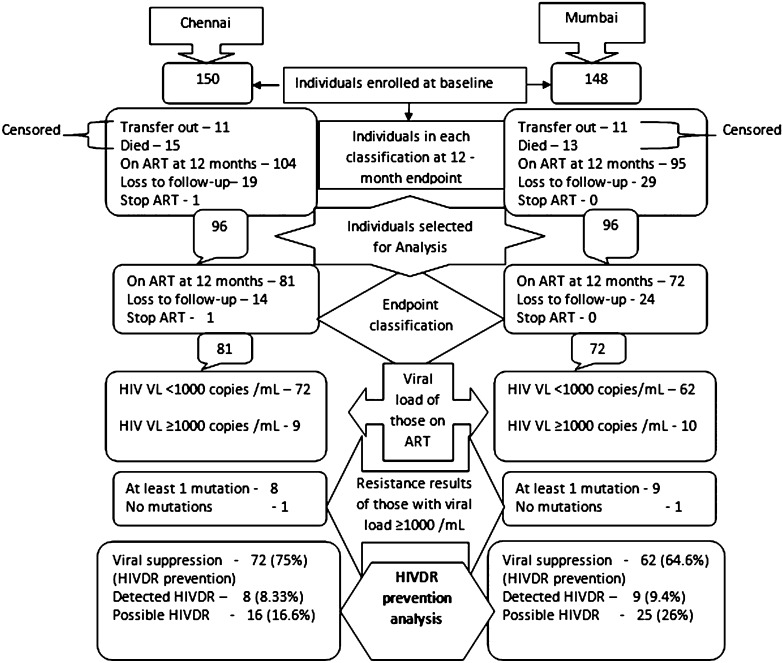
A total of 150 patients were enrolled at the Chennai clinic and 148 patients were enrolled at the Mumbai clinic. After 12 months at the Chennai clinic, 15 patients had transferred out, 11 died, 19 were lost to follow-up, 1 stopped ART, and 104 were still on first-line ART. At the Mumbai clinic, 11 had transferred out, 13 died, 29 were lost to follow-up, and 95 were still on first-line ART. No patient at either clinic switched to second-line ART. Patients who transferred out or died were censored from analyses. Among the remaining patients, the first consecutive 96 with the following endpoints were analyzed: (1) on ART at 12 months, (2) lost to follow-up, and (3) stopped. An overview of survey design and results is found in Figure 1. Among the first 96 patients analyzed at 12 months, 81 (84.4%) were on first-line ART at the Chennai clinic and 72 (75%) were on first-line ART at the Mumbai clinic. Fourteen (14.6%) were lost to follow-up at the Chennai clinic and 24 (25%) were lost to follow-up at the Mumbai clinic. Seventy-two (75%) from Chennai and 62 (64.6%) from Mumbai achieved VL suppression. Among those on ART at 12 months, 72 of 81 (89%) and 62 of 72 (86%) achieved virologic suppression while 9 (11%) and 10 (14%) patients experienced virologic failure at the Chennai and Mumbai clinics, respectively. Among those with virological failure, 89% (8 of 9) had detected HIVDR at the Chennai clinic and 90% (9 of 10) had detected HIVDR at the Mumbai clinic. Baseline characteristics of the 96 patients analyzed at endpoint and the baseline/endpoint characteristics of patients with endpoint VL and/or HIVDR results are shown in Table 1.
Figure 1.
Survey design and results from 2 antiretroviral therapy clinics, Chennai and Mumbai, India. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; HIVDR, HIV drug resistance; VL, viral load.
Table 1.
Baseline and Endpoint Characteristics of Survey Participants
| Characteristic | Baseline (n = 96)a | Baseline (n = 81)b Chennai | Endpoint (n = 81)c | Baseline (n = 96)d Mumbai | Baseline (n = 72)e | Endpoint (n = 72)f |
| Sex | ||||||
| Male | 61 (63.5) | 50 (61.72) | Same as baseline | 60 (62.5) | 44 (61.1) | Same as baseline |
| Female | 34 (35.5) | 31 (38.28) | Same as baseline | 36 (37.5) | 28 (38.9) | Same as baseline |
| Transgender | 1 (1.0) | None | Same as baseline | |||
| Age, y, median (IQR) | 36 (33–40) | 36 (33–40) | Same as baseline | 37 (32–41) | 39 (32–42) | Same as baseline |
| Weight, kg (female), median (IQR) | 45 (39.75–53.5) | 45 (39–53) | 46 (42–55) | 40 (35–44) | 40.5 (38.25–44) | 45 (40–48.75) |
| Weight, kg (male), median (IQR) | 54 (47.25–60) | 55.5 (48.75–61) | 60 (53–67.25) | 50 (45–55) | 50 (44.25–56.75) | 54 (48–60) |
| CD4+ cell count (cells/μL) | ||||||
| <200 | 68 (70.8) | 56 (69.1) | 7 (8.6) | 50 (52.1) | 32 (44.4) | 9 (12.5) |
| 201–350 | 28 (29.2) | 25 (30.9) | 27 (33.3) | 38 (39.6) | 35 (48.6) | 30 (41.7) |
| ≥351 | None | None | 47 (58.0) | 8 (8.3) | 5 (6.9) | 33 (45.8) |
| WHO clinical stage at baseline | ||||||
| Stage 1 | 60 (62.5) | 48 (66.6) | NA | |||
| Stage 2 | 4 (4.2) | 4 (5.6) | NA | |||
| Stage 3 | 58 (60.4) | 50 (61.7) | NA | 17 (17.7) | 10 (13.9) | NA |
| Stage 4 | 37 (39.6) | 31 (38.3) | NA | 15 (15.6) | 10 (13.9) | NA |
| Previous ARV exposure | 3 (3.0) | 2 (2.5) | NA | 13 (13.5) | 10 (13.9) | NA |
| ART regimen | ||||||
| ZDV+3TC+NVP | 50 (52.1) | 48 (59.3 | 38 (46.9) | 43 (44.8) | 35 (48.6) | 28 (38.9) |
| d4T+3TC+EFV | 15 (15.6) | 12 (14.8) | 2 (2.5) | 19 (19.8) | 12 (16.7) | 18 (25.0) |
| d4T+3TC+NVP | 22 (22.9) | 16 (19.8) | 38 (46.9) | 25 (26.0) | 19 (26.4) | 15 (20.8) |
| ZDV+3TC+EFV | 9 (9.4) | 5 (6.2) | 3 (3.7) | 9 (9.4) | 6 (8.3) | 11 (15.3) |
| HIVDR | 5 (6.2) | 5 (6.7) | 8 (9.9) | 10 (10.4) | 6 (8.3) | 9 (12.5) |
| HIV RNA (copies/mL) | ||||||
| <1000 (viral load suppression) | NA | NA | 72 (88.9) | NA | NA | 62 (86.1) |
| ≥1000 (virologic failure) | NA | NA | 9 (11.1) | NA | NA | 10 (13.8) |
| Drug adherenceg | ||||||
| <95% | NA | NA | 8 (9.9) | NA | NA | 10 (13.8) |
| ≥95% | NA | NA | 72 (88.8) | NA | NA | 62 (86.1) |
| Missing data | NA | NA | 1 |
Data are no. (%) of patients, unless otherwise indicated.
Abbreviations: 3TC, lamivudine; ART, antiretroviral therapy; ARV, antiretroviral; d4T, stavudine; EFV, efavirenz; HIV, human immunodeficiency virus type 1; HIVDR, HIV drug resistance; IQR, interquartile range; NA, not applicable; NVP, nevirapine; WHO, World Health Organization; ZDV, zidovudine.
Baseline characteristics of the first consecutive eligible 96 patients from Chennai included in the analysis.
Baseline characteristics of the 81 patients from Chennai on first-line ART after 12-month endpoint.
Endpoint characteristics of the 81 patients from Chennai on first-line ART after 12-month endpoint.
Baseline characteristics of the first consecutive eligible 96 patients from Mumbai included in the analysis.
Baseline characteristics of the 72 patients from Mumbai on first-line ART after 12-month endpoint.
Endpoint characteristics of the 72 patients from Mumbai on first-line ART after 12-month endpoint.
ART adherence assessed by 30-d visual analogue scale at 12-mo endpoint.
Genotypic HIV Resistance Mutations
At baseline, 1 or more resistance mutations were detected in 5 of 96 (5.2%) patients at the Chennai clinic and 10 of 96 (10.4%) patients at the Mumbai clinic.
Baseline HIVDR at Chennai
At the Chennai clinic, 3 of 5 patients had the nucleoside reverse transcriptase inhibitor (NRTI) mutation M184V and 2 of 5 had at least 1 thymidine analogue mutation (TAM). One patient had the protease inhibitor (PI) mutation M46L.
Baseline HIVDR at Mumbai
At the Mumbai clinic, 5 of 10 patients had the NRTI mutation M184V or M184I and 3 of 10 had at least 1 TAM. Most patients (9 of 10) had high-level resistance to nevirapine conferred by K103 N/S, G190A, Y181C/I/V, or Y188H/L/Y. No PI mutations were detected.
Overall HIVDR Survey Outcomes
Following WHO guidelines, HIVDR prevention was estimated to have occurred in 72 of 96 patients (75%; 95% confidence interval [CI], 65.1%–83.3%) at the Chennai clinic and 62 of 96 patients (64.6%; 95% CI, 54.2%–74.1%) at the Mumbai clinic. Possible HIVDR (VL ≥1000 copies/mL and no detected HIVDR + lost to follow-up + stopped) was 16.6% (16 of 96 patients) at Chennai and 26% (25 of 96 patients) at Mumbai. Detected HIVDR was present in 8 of 96 patients (8.3%; 95% CI, 3.7%–15.8%) of endpoint specimens from Chennai and 9 of 96 patients (9.4%; 95% CI, 4.4%–17.1%) of endpoint specimens from Mumbai.
Endpoint HIVDR at Chennai
At endpoint, the most commonly detected NRTI mutation was M184V (6 of 8 patients) and the most commonly detected nonnucleoside reverse transcriptase inhibitor (NNRTI) mutation was K103N (6 of 8 patients). Two of 8 patients had ≥1 TAM (Supplementary Table 1).
Endpoint HIVDR at Mumbai
At endpoint, the most frequently observed NRTI mutation was M184V (8 of 9 patients). K103N was observed in 3 of 9 and G190A/E in 3 of 9 patients; 4 of 9 patients had ≥1 TAM (Supplementary Table 1).
Factors Associated With Genotypic Drug Resistance
In multivariate analyses, the presence of baseline HIVDR was found to be independently associated with HIVDR at the 12-month endpoint in both the Chennai and Mumbai clinics (OR, 71.0 [95% CI, 6.4–791.8] and OR, 98.2 [95% CI, 5.5–1746.9], respectively), whereas a CD4 count ≤200 cells/μL measured at the visit closest to the 12-month endpoint was found to be associated with HIVDR at 12 months at the Chennai clinic only (OR, 9.7 [95% CI, 1.03–91.4]) (Supplementary Table 2). In contrast, age, sex, weight at baseline and endpoint, self-reported adherence, baseline CD4 count, WHO stage at baseline, ART regimen at endpoint, and regimen substitutions were not associated with HIVDR at the 12-month endpoint at either clinic.
DISCUSSION
The availability and widespread use of ART in high-income countries has significantly reduced AIDS-related morbidity and mortality. Rapid ART scale-up in resource-limited settings (RLSs) has achieved the same benefit. However, concern remains regarding the emergence of significant population-level HIVDR, which could threaten treatment outcomes. Importantly, the lack of routine individual VL and HIVDR testing need not limit optimization of patient care in RLSs. An important public health strategy includes population-level surveillance of acquired HIVDR and associated ART program factors. As part of its national HIVDR prevention and assessment strategy, India has implemented WHO global guidelines for the assessment of acquired HIVDR.
In this survey, the WHO method of assessing acquired HIVDR was implemented at 2 ART clinics in India. The WHO target for HIVDR prevention at the population level, defined by VL suppression 12 months after initiation of ART, is ≥70% [12]. Although the proportion of patients with VL suppression observed in this survey (75% for Chennai and 64.6% for Mumbai) was lower than that observed in studies from other countries where different methodologies were used [15–20], the 75% suppression rate estimated for Chennai does exceed the WHO suggested target of ≥70% VL suppression 12 months after ART initiation. However, the 64.6% VL suppression rate estimated for the Mumbai clinic fell short of the WHO target because of the high rate of loss to follow-up (25%) [12]. Notably, the VL suppression rate among those on ART at 12 months for the Chennai and Mumbai clinics was 88.8% (72 of 81) and 86% (62 of 72), respectively, which is comparable to cohorts described in other countries [15–22]. Reasons for high rates of loss to follow-up at the Mumbai clinic include a substantial proportion of patients who are migrants with no permanent address and inadequate human resources available for patient tracing. Additionally, some patients classified as lost to follow-up at both clinics may in fact have died or transferred care to another facility without transfer of records. The low proportion of individuals with detected HIVDR 12 months after ART initiation is comparable to documented prevalence estimates in other RLSs [17, 22]. Although the proportion of patients failing first-line ART with HIVDR at 12 months was low, in a country as large as India with 393 632 individuals receiving ART, a substantial number of patients failing first-line ART may have HIVDR.
Surprisingly, of 5 patients from the Chennai clinic who had detected HIVDR at baseline, 1 had VL suppression at the 12-month endpoint. Only 2 of 5 patients (20%) with baseline HIVDR had self-reported previous ARV exposures. Interestingly, at the Mumbai clinic, of 10 patients with HIVDR at baseline, 2 with baseline NNRTI resistance had suppressed VL at 12 months and 4 were lost to follow-up. Moreover, only 3 of 10 patients with resistance mutations at baseline at the Mumbai clinic reported previous ARV exposure. This baseline HIVDR could represent transmitted HIVDR or it may be that patients had not accurately reported previous ARV exposures during their interview.
The mutation patterns observed in this survey (Supplementary Table 1) are consistent with findings published in other studies from India and other regions [6–8, 10, 11, 23, 24]. The overall low number of patients with ≥1 TAM at endpoint suggests that currently available second-line ART in India (tenofovir/zidovudine + lamivudine + lopinavir/ritonavir) is likely to be effective in the majority of patients failing with HIVDR at 12 months and supports the need for routine VL monitoring to identify early virological failure. The overall success in achieving VL suppression in this survey may be attributable to several factors including the provision of free ART to all eligible patients [25], standard ART prescribing following national guidelines, overall high levels of adherence, and low levels of baseline HIVDR.
This survey does have limitations. Its short duration and small sample size preclude long-term assessment of first-line regimen efficacy. Additionally, because only 1 VL test was performed at 12 months, the duration of virological failure could not be estimated and selection of HIVDR could not be observed. This is important because the time point at which HIVDR genotyping was performed may have affected the detected level of resistance or mutation pattern, which is known to increase in complexity over time in patients maintained on failing regimens [26]. Additionally, adherence was assessed by 30-day VAS, which may have been subject to reporting bias and not on-time pill pickup or medication possession ratio, which may have provided a more accurate population-level estimate of pill-taking behavior [27]. Finally, some participants classified as lost to follow-up may have in fact died or transferred to another ART clinic without a transfer of records. Misclassification of deaths or transfers out as lost to follow-up likely played an important role in our findings and warrants attention.
As ART is scaled up in India, standardized programmatic assessment of program factors such as rates of loss to follow-up at all ART clinics will become increasingly important. Operational research to define reasons for loss to follow-up, the development of targeted interventions to minimize loss to follow-up, and support defaulter-tracing mechanisms are needed. Specifically, ART clinics such as J. J. Hospital in Mumbai may benefit from additional human resources to support patient adherence and defaulter tracing. However, it should be acknowledged that the ART clinics assessed in this survey are located in large urban hospitals with strong health delivery systems; therefore, findings cannot be generalized to other ART clinics in India.
Results demonstrate the successful implementation of a WHO survey of acquired HIVDR in India and further strengthen the consistent observation [15, 16, 19] that high levels of VL suppression can be achieved in RLSs. Additionally, the survey highlights the need for routine VL testing to identify virological failure in individuals receiving first-line ART prior to selection of multiple TAMs, as concluded in recent reviews [23, 28]. Finally, findings underscore the need for affordable second-line ART and mechanisms to minimize loss to follow-up among patients receiving ART in India’s national program.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
We acknowledge the support provided by the staff of the Government Hospital of Thoracic Medicine, Tambaram, Chennai, and J. J. Hospital, Mumbai, especially Ms Manisha Bhutkar, the counselor at J. J. Hospital. We also acknowledge the support provided by Dr Partha Haldar from SEARO, World Health Organization, New Delhi, for the study.
Disclaimer.
The conclusions and opinions expressed in this article are those of the authors and do not reflect those of the WHO or the Centers for Disease Control and Prevention.
Financial support.
This work was supported by funds from the WHO, South East Asia Regional Office; the National AIDS Control Organization, India; the Indian Council of Medical Research, India; and the National Institutes of Health (NIH K23 AI074423-05 to M. R. J.).
Supplement sponsorship.
This article was published as part of a supplement entitled “The World Health Organization HIV Drug Resistance Prevention and Assessment Strategy: Global, Regional, and Country Progress,” sponsored by The Bill & Melinda Gates Foundation (38180).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2010. Available at: http://www.unaids.org/globalreport/Global_report.htm. Accessed 7 March 2011.
- 2. National AIDS Control Organization, Department of AIDS Control, Ministry of Health and Family Welfare. Government of India. Annual report 2009–10. Available at: http://www.nacoonline.org. Accessed 28 October 2010.
- 3. World Health Organization, UNAIDS, UNICEF. Towards universal access, scaling up priority HIV/AIDS interventions in the health sector: progress report summary 2010. Available at: http://www.who.int/. Accessed 28 October 2010.
- 4. National AIDS Control Organization, Department of AIDS Control, Ministry of Health and Family Welfare. Government of India. List of ART centres as of January 2011. Available at: http://www.nacoonline.org. Accessed 7 June 2011.
- 5.Gilks CF, Crowley S, Ekpini R, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–10. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 6.Deshpande A, Jauvin V, Magnin N, et al. Resistance mutations in subtype C HIV type 1 isolates from Indian patients of Mumbai receiving NRTIs plus NNRTIs and experiencing a treatment failure: resistance to AR. AIDS Res Hum Retroviruses. 2007;23:335–40. doi: 10.1089/aid.2006.0183. [DOI] [PubMed] [Google Scholar]
- 7.Sen S, Tripathy SP, Patil AA, Chimanpure VM, Paranjape RS. High prevalence of human immunodeficiency virus type 1 drug resistance mutations in antiretroviral treatment-experienced patients from Pune, India. AIDS Res Hum Retroviruses. 2007;23:1303–8. doi: 10.1089/aid.2007.0090. [DOI] [PubMed] [Google Scholar]
- 8.Sen S, Tripathy SP, Chimanpure VM, Patil AA, Bagul RD, Paranjape RS. Human immunodeficiency virus type 1 drug resistance mutations in peripheral blood mononuclear cell proviral DNA among antiretroviral treatment-naive and treatment-experienced patients from Pune, India. AIDS Res Hum Retroviruses. 2007;23:489–97. doi: 10.1089/aid.2006.0221. [DOI] [PubMed] [Google Scholar]
- 9.Choudhury SD, Chaudhury AK, Kalra R, et al. Antiretroviral drug resistance mutations in the reverse transcriptase gene of HIV-1 isolates from northern Indian patients: a follow-up study. Arch Virol. 2010;155:563–9. doi: 10.1007/s00705-010-0605-4. [DOI] [PubMed] [Google Scholar]
- 10.Vidya M, Saravanan S, Uma S, et al. Genotypic HIV type-1 drug resistance among patients with immunological failure to first-line antiretroviral therapy in south India. Antivir Ther. 2009;14:1005–9. doi: 10.3851/IMP1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta A, Saple DG, Nadkarni G, et al. One-, two-, and three-class resistance among HIV-infected patients on antiretroviral therapy in private care clinics: Mumbai, India. AIDS Res Hum Retroviruses. 2010;26:25–31. doi: 10.1089/aid.2009.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan MR, Bennett DE, Bertagnolio S, Gilks CF, Sutherland D. World Health Organization surveys to monitor HIV drug resistance prevention and associated factors in sentinel antiretroviral treatment sites. Antivir Ther. 2008;13:15–23. [PubMed] [Google Scholar]
- 13.National AIDS Control Organization, Department of AIDS Control, Ministry of Health and Family Welfare, Government of India. Antiretroviral therapy guidelines for HIV-infected adults and adolescents including post-exposure prophylaxis. 2007. Available at: http://www.nacoonline.org. Accessed 28 October 2010. [Google Scholar]
- 14.Liu TF, Shafer RW. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis. 2006;42:1608–18. doi: 10.1086/503914. Epub 2006 Apr 28. Available at: http://hivdb.stanford.edu/. Accessed 21 and 28 November 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coetzee D, Hildebrand K, Boulle A, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–95. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 16.Johannessen A, Naman E, Kivuyo SL, et al. Virological efficacy and emergence of drug resistance in adults on antiretroviral treatment in rural Tanzania. BMC Infect Dis. 2009;9:108. doi: 10.1186/1471-2334-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wester CW, Kim S, Bussmann H, et al. Initial response to highly active antiretroviral therapy in HIV-1C–infected adults in a public sector treatment program in Botswana. J Acquir Immune Defic Syndr. 2005;40:336–43. doi: 10.1097/01.qai.0000159668.80207.5b. [DOI] [PubMed] [Google Scholar]
- 18.Tam LW, Hogg RS, Yip B, Montaner JS, Harrigan PR, Brumme CJ. Performance of a World Health Organization first-line regimen (stavudine/lamivudine/nevirapine) in antiretroviral-naive individuals in a Western setting. HIV Med. 2007;8:267–70. doi: 10.1111/j.1468-1293.2007.00463.x. [DOI] [PubMed] [Google Scholar]
- 19.Bussmann H, Wester CW, Thomas A, et al. Response to zidovudine/didanosine-containing combination antiretroviral therapy among HIV-1 subtype C–infected adults in Botswana: two-year outcomes from a randomized clinical trial. J Acquir Immune Defic Syndr. 2009;51:37–46. doi: 10.1097/QAI.0b013e31819ff102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrido C, Zahonero N, Fernandes D, et al. Subtype variability, virological response and drug resistance assessed on dried blood spots collected from HIV patients on antiretroviral therapy in Angola. J Antimicrob Chemother. 2008;61:694–8. doi: 10.1093/jac/dkm515. [DOI] [PubMed] [Google Scholar]
- 21.Hanson DL, Adje–Toure´ C, Talla-Nzussouo N, et al. HIV type 1 drug resistance in adults receiving highly active antiretroviral therapy in Abidjan, Cote d’Ivoire. AIDS Res Hum Retroviruses. 2009;25:489–95. doi: 10.1089/aid.2008.0273. [DOI] [PubMed] [Google Scholar]
- 22.Wang Xia, Yang Liting, Li Huiqin, et al. Factors associated with HIV virologic failure among patients on HAART for one year at three sentinel surveillance sites in China. Curr HIV Res. 2011;9:103–11. doi: 10.2174/157016211795569122. [DOI] [PubMed] [Google Scholar]
- 23.Hosseinipoura MC, Oosterhoutc JJG, Weigel R, et al. The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. AIDS. 2009;23:1127–34. doi: 10.1097/QAD.0b013e32832ac34e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marconi VC, Sunpath H, Lu Z, et al. Prevalence of HIV-1 drug resistance after failure of a first highly active antiretroviral regimen in KwaZulu Natal, South Africa. Clin Infect Dis. 2008;46:1589–97. doi: 10.1086/587109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivers LC, Kendrick D, Doucette K, et al. Efficacy of antiretroviral therapy programs in resource-poor settings: a meta-analysis of the published literature. Clin Infect Dis. 2005;41:217–24. doi: 10.1086/431199. [DOI] [PubMed] [Google Scholar]
- 26.Kantor R, Shafer RW, Follansbee S, et al. Evolution of resistance to drugs in HIV-1 infected patients failing antiretroviral therapy. AIDS. 2004;18:1503–11. doi: 10.1097/01.aids.0000131358.29586.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMahon JH, Jordan MR, Kelley K, et al. Pharmacy adherence measures to assess adherence to antiretroviral therapy: review of the literature and implications for treatment monitoring. Clin Infect Dis. 2011;52:493–506. doi: 10.1093/cid/ciq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta RK, Hill A, Sawyer AW, et al. Virological monitoring and resistance to first-line highly active antiretroviral therapy in adults infected with HIV-1 treated under WHO guidelines: a systematic review and meta-analysis. Lancet Infect Dis. 2009;9:409–17. doi: 10.1016/S1473-3099(09)70136-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.