Abstract
The HIV drug resistance (HIVDR) prevention and assessment strategy, developed by the World Health Organization (WHO) in partnership with HIVResNet, includes monitoring of HIVDR early warning indicators, surveys to assess acquired and transmitted HIVDR, and development of an accredited HIVDR genotyping laboratory network to support survey implementation in resource-limited settings. As of June 2011, 52 countries had implemented at least 1 element of the strategy, and 27 laboratories had been accredited. As access to antiretrovirals expands under the WHO/Joint United Nations Programme on HIV/AIDS Treatment 2.0 initiative, it is essential to strengthen HIVDR surveillance efforts in the face of increasing concern about HIVDR emergence and transmission.
In 2010, 6.6 million adults and children in low- and middle-income countries received antiretroviral therapy (ART), representing a 22-fold increase from 2001 [1]. Successful ART scale-up in resource-limited settings (RLSs) was realized using a public health approach, including the use of standard protocols and simplified patient monitoring. The Joint United Nations Programme on HIV/AIDS and the World Health Organization (WHO) launched the Treatment 2.0 initiative in 2010, which is aimed at sustaining universal access to treatment and maximizing the preventive benefits of ART [2]. The treatment of millions of human immunodeficiency virus (HIV)–infected patients will inevitably be accompanied by the emergence and transmission of HIV drug resistance (HIVDR); moreover, the use of antiretroviral (ARV) drugs for prevention has the potential to increase the prevalence of HIVDR.
In high-income countries, HIVDR testing of individual patients is used to tailor regimen selection and predict treatment response. However, in most, if not all RLSs, HIVDR testing is neither routinely available nor recommended for individual patient management. Even with recent technological advancements, it is unlikely that HIVDR testing for patient care will be routinely available for millions of patients in the near future. Furthermore, the limited availability of alternative regimens restricts treatment change based on test results [3]. HIVDR remains a threat to the long-term effectiveness of ARV treatment [4–8]. The absence of accessible HIVDR testing for individual patient care in RLSs necessitates that efforts be intensified to optimize population-based ARV treatment and to minimize HIVDR [9]. Unless carefully monitored and contained, HIVDR has the potential to reduce the efficacy of standard ART regimens in a high proportion of patients in RLSs.
WORLD HEALTH ORGANIZATION HIVDR STRATEGY
The WHO, in collaboration with HIVResNet, an advisory body of international experts from >50 institutions, has led global efforts for the prevention and assessment of HIVDR and has developed a standardized, minimum-resource, population-based strategy, which provides comparable data over time and across regions [10]. The strategy consists of 3 main assessment elements: HIVDR early warning indicators (EWIs) and surveys of acquired and transmitted HIVDR [10–12] for pediatric and adult populations. Additionally, the strategy includes the development of a network of HIVDR testing (genotyping) laboratories that support public health surveillance [13].
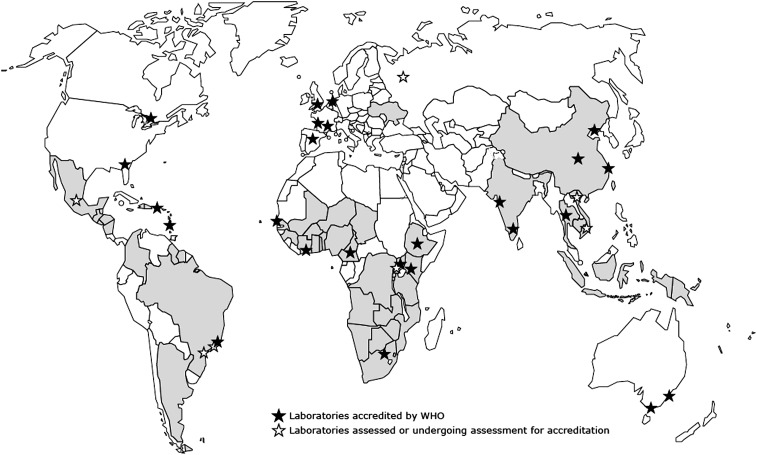
As of June 2011, 52 countries had implemented 1 or more elements of WHO’s HIVDR prevention and assessment strategy, and 27 laboratories had become members of the WHO HIVDR laboratory network [14] (Figure 1). The purpose of this supplement is to update the reader on global, regional, and country-level results generated by the strategy, as well as resulting public health actions. Additionally, this supplement reports a new surveillance method to assess HIVDR in children aged <18 months [15] and provides an update on the WHO external quality assurance process for genotyping, as well as WHO-supported operational research on the use of dried blood spots as specimens for HIVDR testing [16, 17].
Figure 1.
Countries implementing 1 or more elements of the World Health Organization human immunodeficiency virus drug resistance prevention and assessment strategy, June 2011. This map is an approximation of actual country borders. Abbreviation: WHO, World Health Organization.
HIVDR EARLY WARNING INDICATORS
The foundation of the global HIVDR prevention and assessment strategy is the annual monitoring of HIVDR EWIs at all ART clinics or representative clinics [10]. HIVDR EWIs assess prescribing practices, rates of retention, losses to follow-up, population-level adherence to ART, drug supply continuity, and virological suppression, which have been shown to be major predictors of HIV disease progression, death, and HIVDR [18–26]. Each EWI is associated with a suggested clinic-level target. The WHO HIVDR EWIs and their associated targets are listed in Table 1 of Bennett et al (in this supplement [27]). EWI results provide clinic and program managers with data about how their clinics perform compared with international targets aimed at preventing emergence of HIVDR [10]. To date, EWIs have been monitored in 50 countries, assessing > 2000 clinics [27]. This supplement contains the first global EWI report with data obtained from 50 countries in 5 regions: Africa, Latin America and the Caribbean, Southeast Asia, the Western Pacific, and Eastern Europe. One regional report comes from Latin America and the Caribbean. A report comes from PharmAccess, which has implemented EWIs in 6 countries; additionally, 4 country reports come from China, Papua New Guinea, Vietnam, and Zimbabwe [27–33]. Although these data represent early experiences, common themes have emerged. The rate of patients lost to follow-up, rate of patient retention on first-line ART at 12 months, and patient adherence as measured by on-time pill pickup often fall below suggested targets and merit concern and investigation. Additionally, EWI monitoring in several countries has highlighted weaknesses in patient information systems that have resulted in the inability to monitor specific EWIs. Reported weaknesses in current data systems include incomplete records, missing data, use of nonstandardized records, and intraclinic and interclinic variability in data recording. As a result of EWI monitoring, several countries have taken steps to strengthen their information systems [27–31, 34, 35].
SURVEYS OF ACQUIRED HIVDR
WHO prospective surveys of acquired HIVDR are performed at sentinel ART clinics and supplement EWI data by estimating the prevalence and patterns of HIVDR in adult and pediatric populations experiencing ART failure [11]. At each sentinel survey clinic, a cohort is formed of patients initiating first-line ART. HIVDR genotyping is performed on patients initiating ART, and HIV RNA quantification is performed at the time that treatment is switched to second-line or 12 months after ART initiation for patients remaining on first-line treatment. In patients with detectable virus (>1000 copies/mL), genotyping is performed to characterize drug resistance mutations. Other survey endpoints include loss to follow-up, death, ART stop, or transfer to another ART clinic.
As of June 2011, 51 surveys of acquired HIVDR had been performed in 13 countries. The results from 12 surveys performed in 4 countries are published in this supplement [36–40]. The overall prevalence of HIVDR prior to the initiation of ART remains relatively low, and the predicted level of susceptibility observed among patients with HIVDR at 12 months suggests that the nucleoside reverse transcriptase inhibitor component of currently recommended second-line regimens, in combination with a boosted protease inhibitor, is likely to be effective for the majority surveyed [14]. Notably, in surveys published in this supplement, patient resistance to nonnucleoside reverse transcriptase inhibitors at the time of first-line ART initiation and treatment interruption are factors shown to predict virological failure at 12 months [36–39]. Additionally, surveys have concluded that improved adherence, especially among young adults, and improved defaulter tracing mechanisms may lead to improved rates of virological suppression and HIVDR prevention [36–39].
SURVEYS OF TRANSMITTED HIVDR
WHO TDR surveys use truncated sequential sampling and small sample sizes of ≤47 to classify TDR as low (<5%), high (>15%), or moderate (5%–15%) in populations likely to have been recently infected [12, 41]. Where possible, these surveys use remnant specimens and data from regularly performed serosurveys that estimate HIV prevalence, which are already in place in many RLSs. Results contribute to ART policy decisions, including guidelines on ART regimens and HIV prophylaxis.
As of June 2011, 53 TDR surveys have been performed in 22 countries [27]. Rates of TDR remain low in most areas and populations assessed using WHO-recommended methods [14]. However, as ART rollout continues, increased rates of TDR may occur. Recent publications, including reports published in this supplement, document moderate (5%–15%) levels of TDR in specific geographical regions [42–47]. These reports merit attention, warrant concern, and underscore the importance of routine TDR surveillance as well as addressing and preventing HIVDR at both national program and clinic levels.
NEW DIRECTIONS
Although monitoring of HIVDR EWIs are very important, their uptake has generally been limited to pilot experiences. Therefore, in 2011, WHO partnered with the Centers for Disease Control and Prevention (CDC) and the US President’s Emergency Plan for AIDS Relief (PEPFAR) to initiate a process that would update and simplify EWI guidance in order to facilitate monitoring at larger numbers of ART clinics. Simplifications of definitions and abstraction procedures are currently being considered that should facilitate integration into existing program monitoring and evaluation processes.
Additionally, in 2011, a less resource-intensive, cross-sectional survey using lot quality assurance sampling was developed to estimate acquired HIVDR. This cross-sectional survey should enable rapid generation of HIVDR data from large numbers of ART clinics using significantly smaller sample sizes than the current prospective method. Furthermore, this method will provide more comprehensive datasets describing HIVDR in adult and pediatric patients experiencing ART failure at both 12 and >24 months after treatment initiation. WHO and CDC/PEPFAR will pilot this new method in several countries in 2012, and the results of the pilot experience will be used to inform future global surveillance guidance.
As prevention of mother-to-child transmission is scaled up, fewer children are expected to become infected with HIV, but for those who do, a substantial proportion will be expected to harbor drug-resistant virus. Assessments of HIVDR in children <18 months of age are performed to guide population-level, first-line treatment in children. WHO and the CDC have developed a new surveillance protocol that uses remnant specimens from early infant diagnosis to make nationally representative statements about HIVDR in this population. This generic protocol is described in this supplement, and it will be piloted in Sub-Saharan Africa in 2012 by WHO and CDC/PEPFAR [15].
In conclusion, under the Treatment 2.0 initiative [2], millions of infected people will initiate or be maintained on ART. Additionally, many may be exposed to prevention strategies using ARVs. Now more than ever, greater funding, infrastructure and political will are urgently required to sustain and expand global HIVDR surveillance efforts. Without the benefit of cumulative standardized HIVDR surveillance data and a commitment on the part of international organizations, national governments, ART programs, funders, and implementing partners to identify and address programmatic challenges associated with HIVDR, we risk suboptimal population-level responses to current and future ART regimens over the next decade.
Notes
Acknowledgments.
The authors sincerely acknowledge The Bill & Melinda Gates Foundation, which has generously funded the WHO Global HIVDR Strategy since 2007. The authors acknowledge the governments of Canada, Italy, and Spain, which have provided significant financial contributions leading to the success of WHO’s HIVDR prevention and assessment strategy. The authors acknowledge the contributions of the US CDC, PEPFAR, PharmAccess, Treat Asia, and the Agence National de Recherche sur le SIDA et les Hépatites for their strong support as implementing partners. The authors acknowledge the valuable contribution of Mark Myatt, Brixton Health, UK, to the development of the epidemiological and statistical methods used in the strategy. The authors acknowledge the contribution of Jesper Kjaer, Copenhagen HIV Program.
Disclaimer.
Some authors are staff members of WHO or the CDC. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions or stated policies of WHO or the CDC.
Financial support.
This work was supported by grants from the National Institutes of Health (K23 AI074423-05 to M. R. J. and 5T32AI007438-19 to S. Y. H.) and the Lifespan/Tufts/Brown Center for AIDS Research (P30AI4853 to J. H. M.). The research leading to these results has received partial funding from the European Community's Seventh Framework Programme (FP7/2007–2013) under the project “Collaborative HIV and Anti-HIV Drug Resistance Network (CHAIN)” (grant agreement No. 223131).
Supplement sponsorship.
This article was published as part of a supplement entitled “The World Health Organization HIV Drug Resistance Prevention and Assessment Strategy: Global, Regional, and Country Progress,” sponsored by The Bill & Melinda Gates Foundation (38180).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) AIDS at 30: nations at the crossroads. Geneva, Switzerland: UNAIDS; 2011. Available at: http://www.unaids.org/unaids_resources/aidsat30/aids-at-30.pdf. Accessed 23 December 2011. [Google Scholar]
- 2.World Health Organization/UNAIDS. The treatment 2.0 framework for action: catalysing the next phase of treatment, care, and support. Available at: http://data.unaids.org/pub/Outlook/2010/20100713_outlook_treatment2_0_en.pdf. Accessed 15 December 2011. [Google Scholar]
- 3.Mills EJ, Nachega JB. A wake-up call for global access to salvage HIV drug regimens. Lancet. 2007;370:1885–7. doi: 10.1016/S0140-6736(07)61790-5. [DOI] [PubMed] [Google Scholar]
- 4.Wainberg MA, Zaharatos GJ, Brenner BG. Development of antiretroviral drug resistance. N Engl J Med. 2011;365:637–46. doi: 10.1056/NEJMra1004180. [DOI] [PubMed] [Google Scholar]
- 5.Little SJ, Holte S, Routy JP, et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med. 2002;347:385–94. doi: 10.1056/NEJMoa013552. [DOI] [PubMed] [Google Scholar]
- 6.Grant RM, Hecht FM, Warmerdam M, et al. Time trends in primary HIV-1 drug resistance among recently infected persons. JAMA. 2002;288:181–8. doi: 10.1001/jama.288.2.181. [DOI] [PubMed] [Google Scholar]
- 7.Violin M, Cozz-Lepri A, Velleca R, et al. Risk of failure in patients with 215 HIV-1 revertants starting their first thymidine analogue containing highly active antiretroviral therapy. AIDS. 2004;18:227–35. doi: 10.1097/00002030-200401230-00012. [DOI] [PubMed] [Google Scholar]
- 8.Kuritzkes DR, Lalama CM, Ribaudo HJ, et al. Preexisting resistance to nonnucleoside reverse-transcriptase inhibitors predicts virologic failure of an efavirenz-based regimen in treatment naive HIV-1 infected subjects. J Infect Dis. 2008;197:867–70. doi: 10.1086/528802. [DOI] [PubMed] [Google Scholar]
- 9.Jordan MR. Assessments of HIV drug resistance mutations in resource-limited settings. Clin Infect Dis. 2011;52:1058–60. doi: 10.1093/cid/cir093. [DOI] [PubMed] [Google Scholar]
- 10.Bennett DE, Bertagnolio S, Sutherland D, Gilks CF. The World Health Organization’s global strategy for prevention and assessment of HIV drug resistance. Antivir Ther. 2008;13(Suppl 2):1–13. [PubMed] [Google Scholar]
- 11.Jordan MR, Bennett DE, Bertagnolio S, Gilks CF, Sutherland D. World Health Organization surveys to monitor HIV drug resistance prevention and associated factors in sentinel antiretroviral treatment sites. Antivir Ther. 2008;13(Suppl 2):15–23. [PubMed] [Google Scholar]
- 12.Bennett DE, Myatt M, Bertagnolio S, Sutherland D, Gilks CF. Recommendations for surveillance of transmitted HIV drug resistance in countries scaling up antiretroviral treatment. Antivir Ther. 2008;13(Suppl 2):25–36. [PubMed] [Google Scholar]
- 13.Bertagnolio S, Derdelinckx I, Parker M, et al. World Health Organization/HIVResNet drug resistance laboratory strategy. Antivir Ther. 2008;13(Suppl 2):49–57. [PubMed] [Google Scholar]
- 14.Jordan MR, Parkin N, Bertagnolio S. Surveillance of transmitted and acquired HIV drug resistance using World Health Organization survey methods in resource-limited settings. Antivir Ther. 2011;16(Suppl 1):A41. [Google Scholar]
- 15.Bertagnolio S, Penazzato M, Jordan MR, Persaud D, Mofenson LM, Bennett DE. World Health Organization generic protocol to assess drug-resistant HIV among children <18 months of age and newly diagnosed with HIV in resource-limited countries. Clin Infect Dis. 2012;54(Suppl 4):S254–60. doi: 10.1093/cid/cis003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parkin N, Bremer J, Bertagnolio S. Genotyping external quality assurance in the World Health Organization HIV Drug Resistance Laboratory Network during 2007–2010. Clin Infect Dis. 2012;54(Suppl 4):S266–72. doi: 10.1093/cid/cir992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkin N, De Mendoza C, Schuurman R, et al. the WHO DBS working group. Evaluation of in-house genotyping assay performance using dried blood spot specimens in the global WHO laboratory network. Clin Infect Dis. 2012;54(Suppl 4):S273–9. doi: 10.1093/cid/cir982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146:564–73. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 19.Harrigan PR, Hogg RS, Dong WW, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005;191:339–47. doi: 10.1086/427192. [DOI] [PubMed] [Google Scholar]
- 20.Oyugi JH, Byakika-Tusiime J, Ragland K, et al. Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. AIDS. 2007;21:965–71. doi: 10.1097/QAD.0b013e32802e6bfa. [DOI] [PubMed] [Google Scholar]
- 21.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–3. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 22.Hogg RS, Heath K, Bangsberg D, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16:1051–8. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 23.Nachega JB, Hislop M, Dowdy DW, et al. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr. 2006;43:78–84. doi: 10.1097/01.qai.0000225015.43266.46. [DOI] [PubMed] [Google Scholar]
- 24.Ford N, Darder M, Spelman T, Maclean E, Mills E, Boulle A. Early adherence to antiretroviral medication as a predictor of long-term HIV virological suppression: five-year follow-up of an observational cohort. PLoS One. 2010;5:e10460. doi: 10.1371/journal.pone.0010460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nachega JB, Marconi VC, van Zyl GU, et al. HIV treatment adherence, drug resistance, virologic failure: evolving concepts. Infect Disord Drug Targets. 2011;11:167–74. doi: 10.2174/187152611795589663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palombi L, Marazzi MC, Guidotti G, et al. DREAM Program. Incidence and predictors of death, retention, and switch to second-line regimens in antiretroviral-treated patients in sub-Saharan African sites with comprehensive monitoring availability. Clin Infect Dis. 2009;48:115–22. doi: 10.1086/593312. [DOI] [PubMed] [Google Scholar]
- 27.Bennett DE, Jordan MR, Bertagnolio S, et al. HIV drug resistance early warning indicators: summary report from 50 countries. Clin Infect Dis. 2012;54(Suppl 4):S280–9. doi: 10.1093/cid/cis207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jack N, Ravasi G, Schrooten W, Sutherland D, Ghidinelli M, Del Riego A. Implementing HIV drug resistance early warning indicators in the Caribbean. Clin Infect Dis. 2012;54(Suppl 4):S290–3. doi: 10.1093/cid/cir1044. [DOI] [PubMed] [Google Scholar]
- 29.Ye M, Zhang F, Li H, et al. Monitoring HIV drug resistance using early warning indicators in China: results from pilot survey conducted in 2008. Clin Infect Dis. 2012;54(Suppl 4):S300–2. doi: 10.1093/cid/cir1018. [DOI] [PubMed] [Google Scholar]
- 30.Daoni E, Kitur U, Parunga A, Ndenzako F, Lloyd A, Yu D. Experience in piloting HIV drug resistance early warning indicators to improve the antiretroviral program in Papua New Guinea. Clin Infect Dis. 2012;54(Suppl 4):S303–5. doi: 10.1093/cid/cir994. [DOI] [PubMed] [Google Scholar]
- 31.Nhan DT, Nguyen TMT, Hoa DO, et al. Combining cohort analysis and monitoring of HIV drug resistance early warning indicators to assess antiretroviral therapy service in Vietnam. Clin Infect Dis. 2012;54(Suppl 4):S306–12. doi: 10.1093/cid/cir1045. [DOI] [PubMed] [Google Scholar]
- 32.Dzangare J, Gonese E, Mugurungi O, et al. Monitoring of early warning indicators for HIV drug resistance in antiretroviral therapy clinics in Zimbabwe. Clin Infect Dis. 2012;54(Suppl 4):S313–6. doi: 10.1093/cid/cir1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigaloff KCE, Hamers RL, Menke J, et al. The PharmAccess African Studies to Evaluate Resistance (PASER) Early warning indicators for population-based monitoring of HIV drug resistance in six African countries. Clin Infect Dis. 2012;54(Suppl 4):S294–9. doi: 10.1093/cid/cir1015. [DOI] [PubMed] [Google Scholar]
- 34.Hong SY, Jonas A, Dumeni E, et al. Population-based monitoring of HIV drug resistance in Namibia with early warning indicators. J Acquir Immune Defic Syndr. 2010;55:27–31. doi: 10.1097/QAI.0b013e3181f5376d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hedt BL, Wadonda-Kabondo N, Makombe S, et al. Early warning indicators for HIV drug resistance in Malawi. Antiviral Ther. 2008;13(Suppl 2):69–75. [PubMed] [Google Scholar]
- 36.Wadonda-Kabondo N, Hedt BL, Van Oosterhout JJ, et al. A retrospective survey of HIV drug resistance among patients 1 year after initiation of antiretroviral therapy at four clinics in Malawi. Clin Infect Dis. 2012;54(Suppl 4):S355–61. doi: 10.1093/cid/cis004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wadonda-Kabondo N, Bennett DE, Van Oosterhout JJ, et al. Prevalence of HIV-1 drug resistance before and 1 year after treatment initiation in 4 sites in the Malawi antiretroviral therapy program. Clin Infect Dis. 2012;54(Suppl 4):S324–7. doi: 10.1093/cid/cir987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaz P, Augusto O, Billa D, et al. Surveillance of HIV drug resistance in children receiving antiretroviral therapy: a pilot study of the World Health Organization generic protocol in Maputo, Mozambique. Clin Infect Dis. 2012;54(Suppl 4):S369–74. doi: 10.1093/cid/cis006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hingankar NK, Thorat SR, Deshpande A, et al. Initial virologic response and HIV drug resistance among HIV-1 infected individuals initiating first-line antiretroviral therapy at 2 clinics in Chennai and Mumbai, India. Clin Infect Dis. 2012;54(Suppl 4):S348–54. doi: 10.1093/cid/cis005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ugbena R, Aberle-grasse J, Diallo K, et al. Virological response and HIV-1 drug resistance 12 months after antiretroviral therapy initiation at 2 clinics in Nigeria. Clin Infect Dis. 2012;54:1187–95. doi: 10.1093/cid/cir1064. [DOI] [PubMed] [Google Scholar]
- 41.Myatt M, Bennett DE. A novel sequential sampling technique for the surveillance of transmitted HIV drug resistance by cross-sectional survey for use in low resource settings. Antivir Ther. 2008;13(Suppl 2):37–48. [PubMed] [Google Scholar]
- 42.Somda A, Sangare L, Soro M, et al. Surveillance of transmitted drug-resistant HIV among young pregnant women in Ouagadougou, Burkina Faso. Clin Infect Dis. 2012;54(Suppl 4):S317–9. doi: 10.1093/cid/cir988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunt GM, Ledwaba J, Bassoon AE, et al. Surveillance of transmitted HIV-1 drug resistance in Gauteng and KwaZulu-Natal provinces, South Africa from 2005–2009. Clin Infect Dis. 2012;54(Suppl 4):S334–8. doi: 10.1093/cid/cir1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lingjie L, Hui X, Yonghui D, et al. Surveys of transmitted HIV drug resistance in several geographic regions in China, 2008–2009. Clin Infect Dis. 2012;54(Suppl 4):S320–3. doi: 10.1093/cid/cir1016. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen BD, Bui TH, Wagar N, et al. Surveillance of transmitted HIV drug resistance using matched plasma and dried blood spot specimens from voluntary counseling and testing sites in Ho Chi Minh City, Vietnam, 2007–2008. Clin Infect Dis. 2012;54(Suppl 4):S343–7. doi: 10.1093/cid/cir1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sigaloff KC, Mandaliya KN, Hamers RL, et al. High prevalence of transmitted antiretroviral drug resistance among newly HIV-1 diagnosed adults in Mombasa, Kenya. [published online ahead of print 2 February 2012] AIDS Res Hum Retroviruses 2012. doi: 10.1089/AID.2011.0348. [DOI] [PubMed] [Google Scholar]
- 47.Hamers RL, Wallis CL, Kityo C, et al. PharmAccess African Studies to Evaluate Resistance (PASER) HIV-1 drug resistance in antiretroviral-naive individuals in sub-Saharan Africa after rollout of antiretroviral therapy: a multicentre observational study. Lancet Infect Dis. 2011;11:750–9. doi: 10.1016/S1473-3099(11)70149-9. Epub 2011 Jul 27. [DOI] [PubMed] [Google Scholar]