Abstract
Stromal keratitis (SK) is a chronic immunopathological lesion of the eye caused by herpes simplex virus-1 (HSV-1) infection that can result in blindness. Since the inflammatory lesions are primarily orchestrated by Th1 cells, and to a lesser extent by Th17 cells, inhibiting their activity represents a useful form of therapy. In this report we evaluated the therapeutic potential of galectin-1, an endogenous lectin which in some autoimmune diseases was shown to suppress the functions of Th1 and Th17 cells. Treatment was begun at different times after ocular infection with HSV and the outcome was assessed clinically as well as for effects on various immune parameters. Treatment with recombinant galectin-1 (gal-1) significantly diminished SK lesion severity and the extent of corneal neovacularization. Treated mice had reduced numbers of IFN-γ and IL-17 producing CD4+ T cells, as well as neutrophil infiltration in the cornea. Furthermore, disease severity was greater in galectin-1 knockout mice compared with their wild type counterparts. The many effects of gal-1 treatment include reduction in the production of pro-inflammatory cytokines and chemokines, increased production of IL-10, and inhibitory effects on molecules involved in neovascularization. Our findings are the first to show that gal-1 treatment represents a useful approach to control lesion severity in a virally induced immunopathological disease.
Keywords: Galectin-1, Immunopathology, HSV-1, Herpes Stromal Keratitis
Introduction
Herpes simplex virus (HSV) infection of the eye is a chronic inflammatory reaction in the corneal stroma that impedes vision and can result in blindness (1, 2). The immunopathological lesion is thought to be primarily orchestrated by CD4+ T cells, particularly Th1 cells and Th17 cells to a lesser extent (3, 4, 5). Therefore either preventing the migration of these cells to the eye or diminishing their activity at the ocular sites represents a potentially valuable approach to reduce SK lesion severity. One approach could be to exploit the fact that the host itself produces several anti-inflammatory molecules that help to regulate aberrant inflammatory events. Galectins, a family of endogenous carbohydrate binding proteins have emerged as the key players in the regulation of immune responses (6). Galectin-1 (gal-1), a member of this family, has been shown to modulate immune responses by controlling the proliferation of effector T cells, blocking the production of pro-inflammatory cytokines, and increasing the production of IL-10, an anti-inflammatory cytokine (7). Notably, recent studies in vivo, demonstrate that gal-1 treatment suppresses Th1 mediated autoimmune disorders that include experimental autoimmune encephalomyelitis (EAE) (8), autoimmune diabetes (9), collagen induced arthritis (10), and experimental colitis (11) in mice. To our knowledge, the effect of gal-1 in controlling virus induced immunopathology is yet to be evaluated.
In this report we demonstrate the value of gal-1 against ocular disease caused by HSV-1 infection. The results show that gal-1 administration markedly diminished SK lesion severity and corneal neovascularization. We show that treatment with gal-1 decreased the influx of effector CD4+ T cells and other innate cells such as neutrophils, and the production of pro-inflammatory cytokines and molecules involved in ocular neovascularization, an essential component of SK pathogenesis. In addition, galectin-1 gene knockout mice show greater incidence of SK lesion severity suggesting an important role for endogenous gal-1 in controlling virus immunopathology. Thus, our results indicate that gal-1 therapy may represent a novel approach to control HSV-induced ocular immunopathological lesions, the most common infectious cause of human blindnessin the western world.
Materials and Methods
Mice and Virus
Female C57BL6 mice were purchased from Harlan Sprague-Dawley (Indianapolis, IN). Breeder pairs of galectin-1 knockout mice (Lgals−/−; GKO) mice were obtained from Jackson laboratories (Bar Harbor, ME) and additional mice were bred in the Walters Life Sciences animal facility at the University of Tennessee, Knoxville. The animals were housed in American Association of Laboratory Animal Care approved facilities at the University of Tennessee, Knoxville. All investigations followed guidelines of the Institutional Animal Care and Use Committee. HSV-l RE Tumpey strain was used in all procedures. Virus was grown in Vero cell monolayers (AmericanType Culture Collection, Manassas, VA), titrated, and stored in aliquots at −80°C until used.
Corneal HSV infection
Corneal infections of all mouse groups (6–10 week old) were conducted underdeep anesthesia with avertin (Tribromoethanol). The mice were scarified lightly on their corneas with a 27 gauge needle and a 3-μl drop containing 1 × 104 PFU of HSV-1 RE Tumpey was applied to the eye. In some experiments, animals were infected with a lower dose of virus (5×103PFU HSV-1 RE Tumpey) for comparing disease in GKO mice and control mice.
Detection of infectious virus on HSV infected corneas
Mouse corneas were swabbed with sterile swabs (Fisher HealthCare, USA) at 3, 6, and 8 days post infection. The swabs were then placed in 0.5 ml RPMI 1640 and frozen at 80°C until used for assays. Samples were added to confluent Vero cells, incubated for 75 min at 37°C, and overlaid with 1% methylcellulose. The cultures were incubated for 72 h, fixed with buffered formalin, stained with crystal violet, and viral cytopathic effect was examined.
Galectin-1 administration
Stable form of recombinant human galectin-1 (gal-1) was kindly provided by Dr. Toshiro Niki, GalPharma, Japan. Mice were given gal-1 intra peritonealy (i.p.) at a dose of 100μg/mice daily once from day 3 until day 12 post infection for treatments starting early (pre-clinical phase) or was administered daily once from day 6 to day 12 for treatment starting late (clinical phase). The vehicle control group received an equal volume of phosphate buffered saline (PBS). In some experiments, 10μg of gal-1 (in 10μl volume) was injected subconjuctivally into the eye on days 6, 8, 10 and 12 post infection using Hamilton syringe and 33 gauge needle (Hamilton, Reno, NV). Control animals were injected with 10ul of PBS subconjuctivally into the eye. The dose of gal-1 used was based on our preliminary studies and previous observations (9).
Clinical observations
The eyes were examined on different days after infection for the development of clinical lesions by slit-lamp biomicroscopy (Kawa Co., Nagoya, Japan), and the clinical severity of keratitis of individually scored mice was recorded by a blinded observer. The scoring system was as follows: 0, normal cornea; +1, mild corneal haze; +2, moderate corneal opacity or scarring; +3, severe corneal opacity but iris visible; +4, opaque cornea and corneal ulcer; +5, corneal rupture and necrotizing stromal keratitis. The severity of angiogenesis was recorded as described previously (12). In reference to the angiogenic scoring system, the method relied on quantifying the degree of neovessel formation based on three primary parameters: 1) the circumferential extent of neovessels (as the angiogenic response is not uniformly circumferential in all cases); 2) the centripetal growth of the longest vessels in each quadrant of the circle; and 3) the longest neovesselin each quadrant was identified and graded between 0 (no neovessel) and 4 (neovessel in the corneal center) in increments of ~0.4mm (radius of the cornea is ~1.5 mm). According to this system, a grade of 4 for a given quadrant of the circle represents a centripetal growth of 1.5 mm toward the corneal center. The score of the four quadrants of the eye were then summed to derive the neovessel index (range, 0 to 16) for each eye at a given time point.
Immunohistochemical Staining
At the termination of the experiment (day 15 post infection), eyes were removed from control and gal-1 treated mice and snap- frozen in OCT compound (Miles, Elkhart, IN). Six-micron thick sections were cut and stained with hematoxylin and eosin.
Flow cytometric analysis
Single-cell suspensions were prepared from the cornea and draining cervical lymph nodes (DLNs) obtained from mice sacrificed at 15 days post infection. Cornea were excised, pooled group wise and digested with 60 U/ml Liberase (Roche Diagnostics) for 45 min at 37°C in a humidified atmosphere of 5% CO2. After incubation, the corneas were disrupted by grinding with a syringe plunger on a cell strainer and a single-cell suspension was made in complete RPMI 1640 medium. The single-cell suspensions were stained for different cell surface molecules for FACS analyses. All steps were performed at 4°C. Briefly, a total of 1 × 106 cells were first blocked with an unconjugated anti-CD32/CD16 mAb for 30 min in FACS buffer. After washing with FACS buffer, the cells were stained with respective fluorochrome-labeled Abs for 30 min. Finally, the cells were washed three times and re-suspended in 1% paraformaldehyde. To enumerate the number of IFN-γ and IL-17-producing T cells, intracellular cytokine staining was performed. In brief, cells were stimulated with anti-CD3 (3μg/ml) and anti-CD28 (1μg/ml) for 5 hrs in the presence of brefelidin A (5μg/ml) in U-bottom 96-well plates. After this period, cell surface staining was performed, followed by intracellular cytokine staining using a Cytofix/Cytoperm kit (BD Pharmingen) in accordance with the manufacturer’s recommendations. The antibodies used were anti-CD4 APC, anti-IFN-γ PE and IL-17 percp cy5.5. The fixed cells were re-suspended in 1% paraformaldehyde. The stained samples were acquired with a FACS Calibur (BD Biosciences) and the data were analyzed using the FlowJo software (Tree star Inc, Ashland, OR).
Protein quantification of corneal lysates by ELISA
The corneal samples were pooled group wise (5 corneas per sample) and homogenized using a tissue homogenizer (Pellet Pestle mortar; Kontes). For lymph node samples, 4 draining cervical lymph nodes were collected for each mouse sample and ELISA was performed on the homogenized sample. The concentrations of various cytokines and VEGF was measured by sandwich ELISA kits from eBioscience (IL-6, IL-10, IFN-γ, IL-17) and R&D (VEGF-A) as per the manufacturer’s instructions.
Quantitative PCR (QPCR)
At 15 days after ocular infection, the corneas were isolated, pooled group wise (5 corneas per sample), corneal cells were lysed and total mRNA was extracted using TRIzol LS reagent (Invitrogen). Total cDNA was made with 1 μg of RNA using oligo (dT) primer. Quantitative PCR was performed using SYBR Green PCR Master Mix (Applied Biosystem, Foster City, CA) with iQ5 real-time PCR detection system (Bio Rad, Hercules, CA). The expression levels of different molecules were normalized to β-actin using ΔCt calculation. Relative expression between control and experimental groups were calculated using the 2−ΔΔCt formula. The PCR primers used VEGF-A, MMP-2, MMP-9, CXCL1 and CCL2 were described previously (13, 14).
In vitro differentiation of CD4+ Foxp3+ regulatory T cells (Tregs)
Splenocytes isolated from DO11.10 Rag2−/− mice were used as the precursor population for induction of Foxp3 in CD4+ T cells as described before (15). Briefly, 5 × 105 of total splenocytes after RBC lysis and washings were cultured in 1 ml volume with previously optimized doses of plate-bound anti-CD3/anti-CD28 Ab (1μg), rhIL-2 (100 U/ml), and TGF-β (0–5 ng/ml) for 4 days at 37°C in a 5% CO2 incubator in 48-well plates. In some cultures in addition to IL-2, gal-1 either alone or various concentrations of gal-1 in combination with TGF-β (1ng) was added. After 4 days, cells were harvested, stained for Foxp3 expression and characterized phenotypically by flow cytometry.
Apoptosis Assay (Ex vivo)
Draining lymph node cells from HSV-1 infected B6 mice (day 15 post infection) were incubated for 5 h at 37° with different concentrations of recombinant gal-1 in the absence or the presence of 50mM lactose in 96-well flat-bottom plate in humidified incubator in the presence of 5% CO2 as described before (16). After the incubation period the cells were transferred to 96 well U bottom plate and the cells were stained for annexin-V and propidium iodide (PI) as per the manufacturer’s instructions (BD Biosciences). Additionally, cells were also co-stained for CD4. Stained cells were immediately acquired using FACS Caliber (BD).
Statistical analysis
Statistical significance was determined by Student’s t test unless otherwise mentioned. GraphPad (Prism 5) software was used for statistical analysis. A p value of ≤0.05 was regarded as a significant difference between groups *, p ≤0.05; **, p ≤ 0.005; ***, p ≤ 0.0005. Error bars represent mean ± S.E.M.
Results
Administration of gal-1 reduces the severity of SK lesions
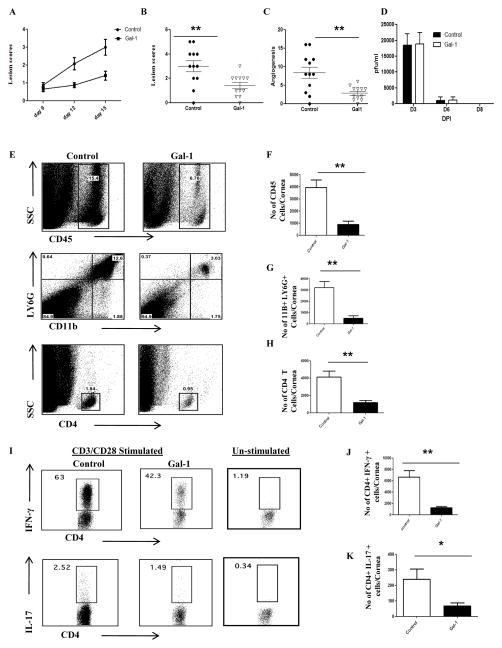
To evaluate if the administration of gal-1 could influence the severity of SK lesions, two types of experiments were performed in mice ocularly infected with HSV. Animals were either treated i.p. with gal-1 starting at day 3 (priming phase/pre-clinical) until day 12 post infection or were given the reagent injected i.p. starting at day 6 (effector phase/clinical phase) until day 12 post infection. Mice were sacrificed at day 15 post infection to evaluate different parameters. The results demonstrated that following systemic administration of gal-1 starting early (day 3 post infection), significant inhibition of SK lesions (p<0.005) was observed in the treated mice when compared with untreated controls (Fig.1A–B). Lesions were more severe in control mice with a higher percentage of infected eyes exhibiting a lesion score of ≥3 when compared with gal-1 treated mice. A similar pattern was observed with the extent of corneal neovascularization, wherein gal-1 treated mice showed significantly lower angiogenesis scores (Fig.1C, p<0.002). Almost similar virus titers were observed in the gal-1 treated (starting at day 3 post infection) and control animals and the virus was completely cleared from the eyes by day 8 post infection (1D). The experiments were terminated on day 15 post infection and corneas were pooled and digested with liberase to measure various immune parameters by flow cytometry. As shown in figures 1E–H, the numbers of total leukocytes (CD45+ cells), neutrophils (CD11b+Ly6G+) and CD4+ T cells were significantly lower in the corneal samples obtained from gal-1 treated mice when compared with control mice. Additionally the numbers of CD4+ T cells producing IFN-γ and IL-17 were significantly reduced in gal-1 treated mice compared to controls (Fig. 1I–K).
Figure 1. Effect of gal-1 treatment started during the pre-clinical phase (day 3 post infection) on SK severity and cellular infiltration.
C57BL/6 mice infected with 1×104 PFU of HSV-1 RE were given gal-1 i.p. once daily starting from day 3 until day 12 post infection. The disease severity and immune parameters were evaluated at day 15 post infection. A. Disease progression is shown. B–C. SK lesion severity and angiogenesis at day 15 post infection are shown. D. At the indicated time points, eyes of HSV infected mice were swabbed with a sterile swab and assayed for infectious virus by standard plaque assay. E. Three corneas were pooled per sample, group wise and digested by liberase and stained with antibodies. Representative histograms show percentage of leukocytes (CD45+), neutrophils (11b+ Ly6G+) and CD4+ T cells in the inflamed cornea of control and gal-1 treated animals at day 15 post infection. F–H. Average no. of (F) CD45+ cells, (G) 11b+ Gr1hi and (H) CD4+ T cells per cornea at indicated time point are shown. I. Representative plots show percentage of CD4+ cells producing IFN-γ or IL-17 following stimulation with CD3/CD28 in the cornea of infected animals. Plots shown were gated on CD4+ T cells. J–K. Average no. of CD4 cells producing (J) IFN-γ and (K) IL-17 in the cornea are shown. The level of significance was determined by Student’s t test (unpaired). Error bars represent mean ± S.E.M. n = 4–5 corneas. n= 6–7 mice per group. Experiments were repeated at least three times.
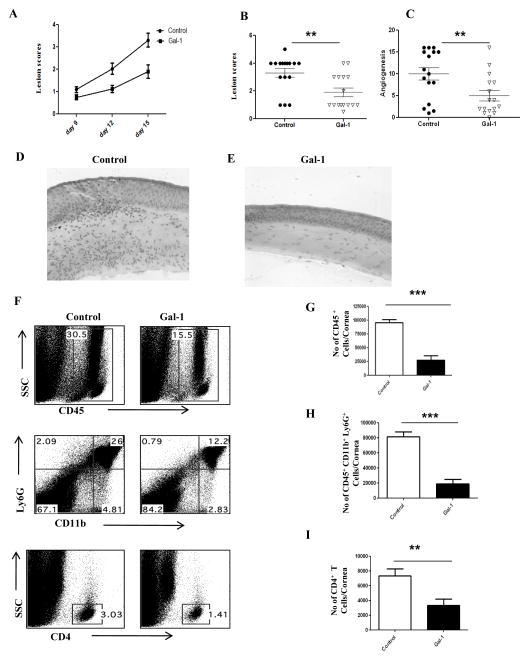
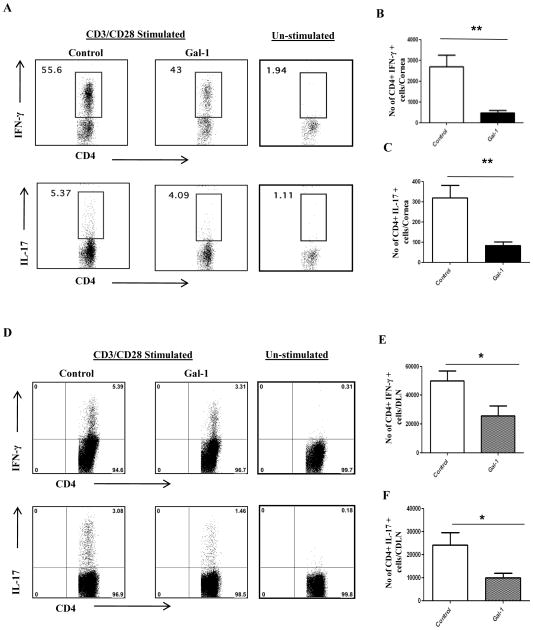
We next examined whether or not gal-1 treatment of mice has any effect on the disease progression if treatment is begun in the clinical phase, i.e. 6 days post infection, a stage when replicating virus is mostly cleared on the HSV infected corneas. As shown in figure 2A–C, similar reduction in lesion severity was observed when gal-1 treatment was begun at day 6 post infection. The results demonstrate that SK lesions and the extent of corneal neovacularization were suppressed in animals treated with gal-1 even when treatment was started at day 6 post infection (Fig. 2A–C). Immuno-histochemical analyses showed that cornea from untreated mice were inflamed and contained a massive infiltrate of inflammatory cells compared with gal-1 treated mice (Fig.2D–E). The numbers of infiltrating leukocytes and PMNs into the infected cornea were reduced in the mice treated with gal-1 compared to untreated control mice (Fig. 2F–H). In addition, the numbers of CD4+ T cells were reduced in gal-1 treated animals (Fig. 2I). Also, the numbers of CD4+ T cells producing IFN-γ or IL-17 cells were significantly reduced in the corneas obtained from treated mice compared to controls (Fig. 3A–C). Furthermore, our results also demonstrated that gal-1 treatment significantly reduced the numbers of Th1 and Th17 cells in the draining lymph nodes of treated mice compared to control mice (Fig. 3D–F). These studies show that administration of gal-1 early or late diminishes HSV-1 induced corneal immunopathology and leukocyte infiltration.
Figure 2. Effect of gal-1 treatment started during the clinical phase (day 6 post infection) on SK severity and cellular infiltration.
C57BL/6 mice infected with 1×104 PFU of HSV-1 RE were given gal-1 i.p. once daily starting from day 6 until day 12 post infection The disease severity and immune parameters were analyzed at day 15. A. Disease progression is shown. B–C. SK lesion severity and angiogenesis at day 15 post infection are shown. D–E. Mice were terminated at day 15 post infection, and eyes were processed for cryosection. H&E staining was performed on 6-μm sections. The figure shows photographs of the sections taken at ×20 magnification. F. Three corneas were pooled per sample, group wise and digested by liberase and stained with antibodies. Representative histograms show percentage of leukocytes (CD45+), neutrophils (11b+ Ly6G+) and CD4+ T cells in the inflamed cornea of control and gal-1 treated animals at day 15. G–H. Average no. of (G) CD45+ cells, (H) 11b+ Gr1hi and (I) CD4+ T cells per cornea at indicated time point are shown. Experiments were repeated at least three times. The level of significance was determined by Student’s t test (unpaired). Error bars represent mean ± S.E.M. n = 5 corneas. n= 8–10 mice per group.
Figure 3. Gal-1 administration during clinical phase (6 days post infection) reduces Th1 and Th17 cell responses in the cornea and draining lymph nodes.
C57BL/6 mice infected with 1×104 PFU of HSV-1 RE were given gal-1 i.p. once daily starting from day 6 until day 12 post infection. Th1 and Th17 cell responses were analyzed in the corneas and draining cervical lymph nodes at day 15 post infection. (A) Three corneas were pooled per sample, group wise and digested by liberase and single cell suspensions were stained with antibodies. n = 6 corneas. n= 8–10 mice per group Representative plots show percentage of CD4+ T cells producing IFN-γ or IL-17 following stimulation with CD3/CD28 in the cornea of infected animals. Plots shown were gated on CD4+ T cells. B–C. Average no. of CD4 cells producing (B) IFN-γ and (C) IL-17 in the cornea are shown. (D) Representative FACS plots show percentage of CD4+ T cells producing IFN-γ or IL-17 following stimulation with CD3/CD28 in the draining lymph nodes of infected animals. Plots shown were gated on CD4+ T cells. E–F. Average no. of CD4+ T cells producing (E) IFN-γ and (F) IL-17 in the draining lymph nodes are shown. n= 3–4 mice/group. Experiments were repeated at least three times and the level of significance was determined by Student’s t test (unpaired). Error bars represent mean ± S.E.M.
One of the drawbacks of systemic therapy to control a local inflammatory disease is that the overall immune response is compromised. A desirable approach would be to treat the disease locally. Therefore we tested the therapeutic potential of local administration of gal-1. In animals given gal-1 locally starting on day 6 post infection (clinical phase), SK lesions were reduced by almost 2-fold on average compared with controls, with these differences being significant (Fig. 4A–B, p <0.009). The extent of neovascularization was also significantly reduced in the mice treated sub-conjunctively with gal-1 (Fig. 4C, p<0.02). The numbers of total leukocytes (CD45+ cells), PMN and CD4+ T cells recovered from corneas were significantly reduced in the treated groups compared with untreated controls (Fig. 4D–G.). Additionally, the frequencies and numbers of CD4+ T cells producing either IFN-γ or IL-17 were reduced 5–6-fold as a consequence of local gal-1 treatment (Fig. 4H–J). From these studies it is evident that one of the possible mechanisms by which gal-1 acts is by suppressing the recruitment of PMNs and CD4+ T cells into the HSV infected cornea. Notably, these studies show that gal-1 negatively regulates Th1 and Th17 cells, which are considered to be the chief mediators of SK immunopathology. Taken together, our results demonstrated that exogenous administration of gal-1 either systemically or locally diminished the severity of SK lesion and the extent of corneal neovascularization.
Figure 4. Effect of local administration of gal-1 on SK severity and cellular infiltration.
C57BL/6 mice infected with 1×104 PFU of HSV-1 RE were given gal-1 sub-conjunctively on day 6, 8, 10 and 12 post infection. The disease severity and immune parameters were analyzed at day 15 post infection. A. Disease progression is shown. B–C. SK lesion severity and angiogenesis at day 15 are shown. D. Three corneas were pooled per sample, group wise and digested by liberase and cell suspensions were stained with antibodies. Representative histograms show percentage of leukocytes (CD45+), neutrophils (11b+Gr1hi) and CD4+ T cells in the inflamed cornea of control and gal-1 treated animals at day 15. E–G. Average no. of (E) CD45+ cells, (F) 11b+ Gr1hi and (G) CD4+ T cells per cornea at indicated time point are shown. H. Representative plots show percentage of CD4+ T cells producing IFN-γ or IL-17 following stimulation with CD3/CD28 in the corneas of infected animals. Plots shown were gated on CD4+ T cells. I–J. Average no. of CD4+ T cells producing (I) IFN-γ and (J) IL-17 in the cornea are shown. Experiments were repeated at least three times and the level of significance was determined by Student’s t test (unpaired). Error bars represent mean ± S.E.M. n = 5 corneas. n= 5–6 mice per group
Effect of gal-1 treatment on cytokines, chemokines and angiogenic factors involved in SK
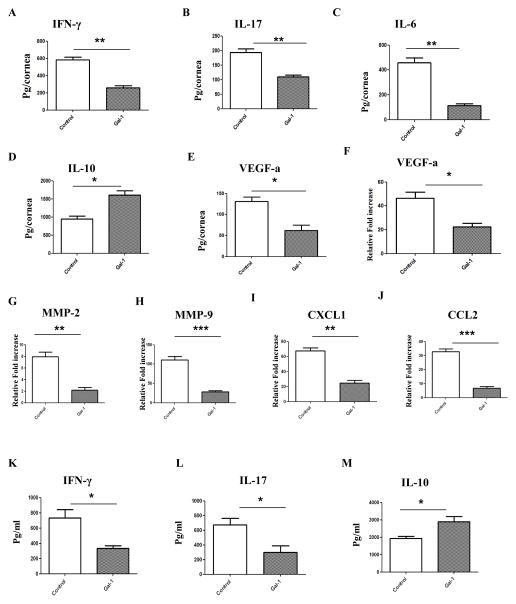
Pooled corneal extracts were collected on day 15 post infection from control and gal-1 treated (started 6 day post infection) animals and sample pools were analyzed for IFN-γ, IL-17, IL-6, IL-10 and VEGF-A protein levels using ELISA. The data show that gal-1 treatment reduced the production of IFN-γ by two fold in the treated mice compared to control mice (Fig. 5A). The levels of IL-17 were reduced by an average of 43% in the gal-1 treated group when compared to the control group (Fig. 5B). Importantly the levels of IL-6, a key pro-inflammatory cytokine involved in SK pathology (17), were reduced by 4 fold in gal-1 treated mice compared to the control group (Fig. 5C). Consistent with previous findings (18), gal-1 treatment induced production of IL-10 which was on average 40% higher when compared with control animals (Fig. 5D). Also, the levels of VEGF-A were reduced around 50% in the corneas from gal-1 treated animals compared to untreated control mice (Fig. 5E). Besides VEGF-A, we also examined the expression of MMP-2 and MMP-9, molecules that are also associated with the neovascularization process in the eye (19). The qPCR data demonstrated that levels of VEGF-A were reduced 2 fold, MMP-2 were reduced by 4 fold and MMP-9 by 6 fold in corneal tissues obtained from gal-1 treated group when compared to the control group (Fig. 5F–H). Also, the expression of two important chemokines involved in neutrophil and monocyte migration, CXCL1 and CCL-2, were found to be reduced in the corneal samples from gal-1 treated mice (Fig. 5I–J). CXCL1 was reduced by 3 fold and CCL2 was reduced by 5 fold in corneas obtained from gal-1 treated animals compared with untreated control mice. In addition, draining lymph node samples from HSV infected gal-1 treated and control mice were analyzed for IFN-γ, IL-17 and IL-10 protein levels by ELISA. As shown in Figure 5K-L, the levels of IFN-γ and IL-17 were significantly reduced in gal-1 treated mice compared to control animals. The levels of IL-10 were found to be higher in the draining lymph node samples obtained from gal-1 treated mice (Fig. 5M).
Figure 5. Effect of gal-1 treatment on inflammatory and angiogenic factors in the corneas and draining lymph nodes of HSV-1 infected animals.
C57BL/6 mice infected with 1×104 PFU of HSV-1 RE were given gal-1 i.p. once daily starting from day 6 until day 12 post infection. Mice were sacrificed at day 15 post infection and corneal and lymph node extracts were collected for measuring inflammatory factors using either sandwich ELISA or QPCR. (A) IFN- γ (B) IL-17 (C) IL-6 (D) (D) IL-10 and (E) VEGF-A protein levels in three different pooled corneal samples each consisting of five cornea in control and gal-1 treated animals were quantified using ELISA (n=3 samples/group). (F–J) Q PCR was used measure the expression of pro-angiogenic factors (F) VEGF-A (G) MMP-2, (H) MMP-9 and chemokines (I) CXCL1 and (J) CCL2 in pooled corneal samples each consisting of five cornea in control and gal-1 treated animals (n=3 samples/group). (K–M) ELISA was used to measure (K) IFN-γ (L) IL-17 and (M) IL-10 protein levels in draining lymph node samples obtained from control and gal-1 treated mice at day 15 post infection (n= 3 mice). The level of significance was determined by Student’s t test (unpaired). Error bars represent mean ± S.E.M.
Galectin-1 knockout mice show increased HSK related immunopathology
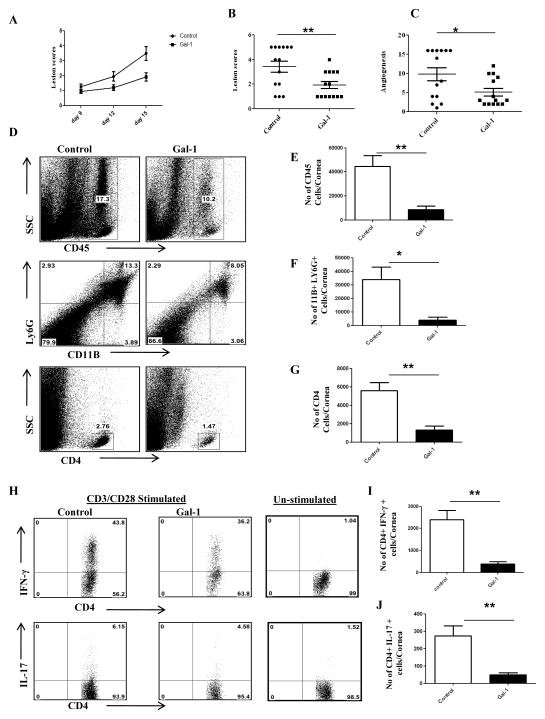
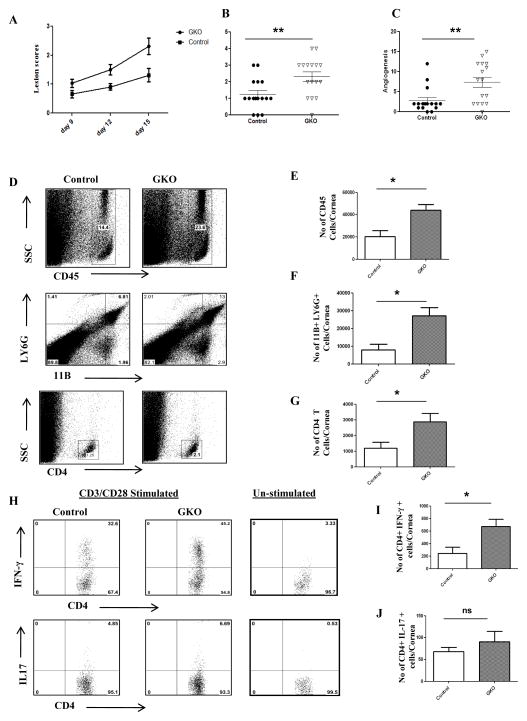
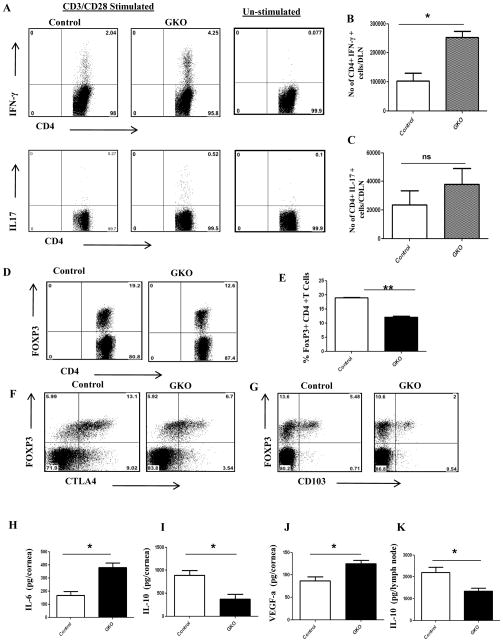
To further investigate the role of endogenous gal-1 in vivo in a virally induced immunopathological disease, we infected galectin-1 knockout mice (GKO) and control mice (B6) ocularly with HSV-1. We observed significant differences in the disease severity, with GKO mice showing greater incidence as compared to control mice (Fig. 6A–C). SK Lesions were more severe in GKO mice with a higher percentage of infected eyes exhibiting a lesion score of ≥3 when compared with control mice (Fig. 6A). A similar increase was observed with the extent of corneal neovascularization, where GKO mice showed significantly higher angiogenesis scores (Fig. 6C, p<0.004). The results show that the numbers of total leukocytes, neutrophils and CD4+ T cells were significantly higher in the infected corneas obtained from GKO mice compared to the control group (Fig. 6D–G). In addition, the cornea from GKO mice had a significantly higher number of IFN-γ producing CD4+ T cells (Fig. 6H–I). Although greater numbers of IL-17 producing CD4+ were present in the corneas of GKO mice, the differences were not significant (Fig. 6H, J). Furthermore, draining lymph node samples from GKO mice had a higher number of IFN-γ producing CD4+ T cells than control mice (Fig. 7A–C). The proportion of regulatory T cells was significantly lower in GKO mice (Fig. 7D–E). Notably the percentage of CD4+ FoxP3+ cells expressing functional markers CD103 or CTLA-4 was lower in GKO mice compared to control mice (Fig. 7F–G). Additionally, pooled corneal extracts and draining lymph node samples were collected at day 15 post infection from control and GKO animals and sample pools were examined for IL-6, IL-10 and VEGF-A protein levels using ELISA. Our data showed that the levels of IL-6 were increased by 2 fold in GKO mice compared to control mice (Fig. 7H). Importantly, the levels of IL-10 were reduced by 2 fold in the corneas obtained from GKO animals compared to control animals (Fig. 7J). Also, we found higher levels of VEGF-A in the corneas from GKO animals compared to control mice (Fig. 7J). A similar reduction in IL-10 levels was observed in draining lymph node samples obtained from GKO mice compared to control mice (Fig. 7K).
Figure 6. GKO mice exhibit increased severity of disease.
C57BL/6 or GKO mice infected with 5×103 PFU of HSV-1 and disease severity and immune parameters were analyzed at day 15 post infection. A. Disease progression is shown. B–C. SK lesion severity and angiogenesis at day 15 post infection are shown. D. Representative histograms show percentage of leukocytes (CD45+), neutrophils (11b+Gr1hi) and CD4+ T cells in the inflamed cornea of control and gal-1 treated animals at day 15 post infection. E–G. Average no. of (E) CD45+ cells, (F) 11b+ Gr1hi and (G) CD4+ T cells per cornea at indicated time point are shown. H. Representative plots show percentage of CD4+ T cells producing IFN-γ or IL-17 following stimulation with CD3/CD28 in the corneas of infected animals. Plots shown were gated on CD4+ T cells. I–J. Average no. of CD4+ T cells producing (I) IFN-γ and (J) IL-17 in the cornea are shown. Experiments were repeated two times and the level of significance was determined by Student’s t test (unpaired). Error bars represent mean ± S.E.M. n = 4 corneas. n= 7–8 mice per group
Figure 7. HSV-1 infected GKO mice have higher numbers of Th1 and Th17 cells and a lower proportion of regulatory T cells in the draining lymph nodes.
C57BL/6 or GKO mice infected with 5×103 PFU of HSV-1 and disease severity and immune parameters were analyzed at day 15 post infection. A. Representative plots show percentage of CD4+ T cells producing IFN-γ or IL-17 following stimulation with CD3/CD28 in the infected animals. Plots shown were gated on CD4+ T cells. B–C, Average no. of CD4+ T cells producing (B) IFN-γ and (C) IL-17 in the draining lymph nodes are shown. (D–F) Histograms show frequencies of (D) regulatory T cells (CD4+Foxp3+), (E) CD103+ Foxp3 cells and (F) CTLA+ Foxp3 cells. Plots shown were gated on CD4+ T cells. n=3–4 mice/group. Experiments were repeated two times. () Mice were sacrificed at day 15 post infection and corneal and lymph node extracts were collected for measuring inflammatory factors using either sandwich ELISA. (H) IL-6 (I) IL-10 and (J) VEGF-A protein levels in three different pooled corneal samples each consisting of five cornea/group in control and GKO animals were quantified using ELISA. (K) IL-10 protein levels in draining lymph node samples (4 lymph nodes/sample) obtained from control and GKO mice at day 15 post infection. The level of significance was determined by Student’s t test (unpaired). Error bars represent mean ± S.E.M.
Galectin-1 induces regulatory T cells synergistically with TGF-β in vitro
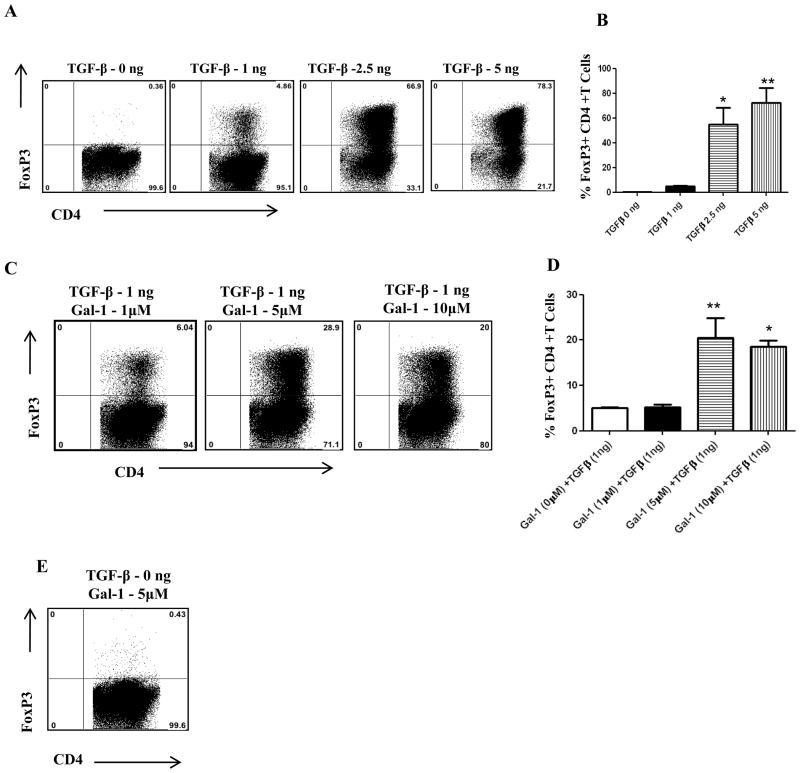
As GKO mice show lower frequencies of Tregs, we hypothesized that gal-1 may have a role in the expansion of Tregs. Therefore, to examine the possible effects of gal-1 on the induction of Foxp3+ Treg cells in vitro, splenocytes obtained from DO11.10 RAG−/− animals (that lack Tregs) were stimulated in vitro with plate-bound anti-CD3/CD28 in the presence or absence of TGF-β (Fig.8A–B). Addition of gal-1 along with sub-optimal concentration of TGF-β caused the induction of Foxp3 CD4+ T cells synergistically. As shown in figure 8C–D, the percentage of Foxp3 expressing cells increased in the presence of galectin-1. However, gal-1 when added alone did not induce Foxp3+ T cells (Fig. 8E). These results show that gal-1 in combination with TGF-β promotes Foxp3+ T cell expansion.
Figure 8. Gal-1 synergistically induces regulatory T cells in the presence of TGF-β.
Splenocytes isolated from DO11.10 TCR transgenic RAG−/− mice were stimulated for 96 hrs with plate-bound anti-CD3/CD28 (1 μg/ml each), 10ng/ml interleukin-2 (IL-2) in the presence of All histograms shown were gated on CD4+ T cells. (A) Representative plots show Foxp3+ CD4+ T cells at various concentrations of TGF-β (B) Percentage of Foxp3+ CD4+ T cells at different concentrations of TGF-β (C) Representative plots show Foxp3+ CD4+ T cells at various concentrations of gal-1 and sub-optimal concentration of TGF-β (1ng). (D) Percentage of Foxp3+ CD4+ T cells at different concentrations of gal-1 and sub-optimal concentration of TGF-β (1ng). (E) Gal-1 (5 μM) was added to some cultures in the absence of TGF-β as indicated. CD4+ T cells expressing Foxp3 were measured by intracellular staining. The level of significance was determined using one-way anova using Turkey’s multiple comparison test. Error bars represent mean ± S.E.M. These results are representative of three different experiments.
Galectin-1 causes apoptosis of CD4+ T cells isolated from HSV-1 infected mice in vitro
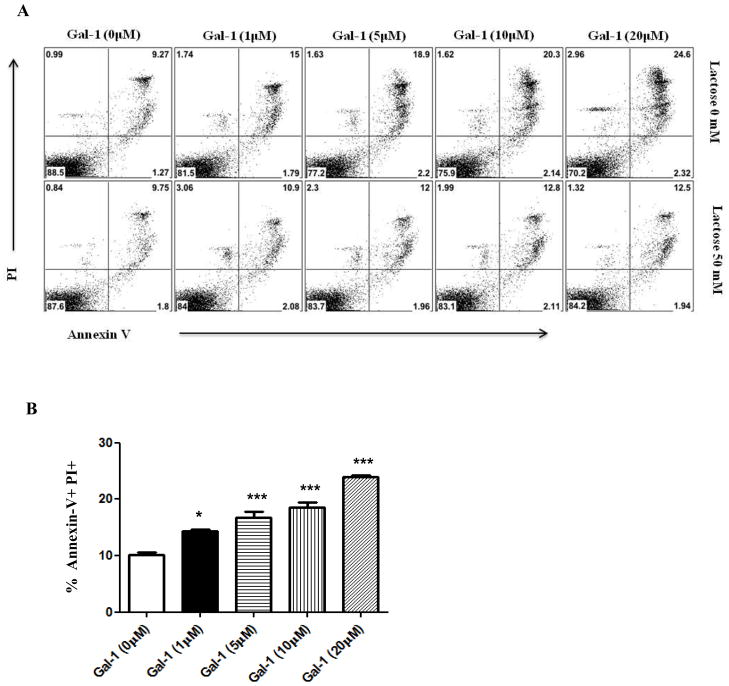
Prior studies suggest that one of the mechanisms by which gal-1 acts is through the selective apoptosis of activated CD4+ T cells (20). Therefore, we investigated whether CD4+ T cells isolated from HSV infected mice are susceptible to killing by gal-1. For this purpose, draining lymph node cells from HSV infected mice were exposed galectin-1 for 5 hrs. Our results demonstrated that CD4+ T cells as a consequence of HSV infection in vivo could be induced to undergo apoptosis when exposed to gal-1 in vitro (Fig. 9A–B)(Supplementary Fig.1). Addition of lactose, a competitive inhibitor to the culture medium reduced gal-1 mediated apoptosis of CD4+ T cells (Fig. 9A, lower panel).
Figure 9. Gal-1 promotes apoptosis of CD4 T cells.
Cells isolated from draining lymph nodes of HSV-1 infected mice (day 15 post infection) were incubated without gal-1 (control) and different concentrations of gal-1, with or without 50mM lactose for a period of 5 hours. After gal-1 exposure, cells were stained with PI, FITC-annexin V and APC- CD4. A–B. Plots shown were gated on CD4+ T cells (A) Representative histograms show Annexin V+ PI+ CD4+ T cells (B) Percentage of Annexin V+ PI+ CD4+ T cells. The experiments were repeated two times. The level of significance was determined using one-way anova using Turkey’s multiple comparison test. Error bars represent mean ± S.E.M.
Discussion
Controlling chronic immunoinflammatory lesions in the eye such as those caused by ocular HSV-1 infection remains a major therapeutic challenge. These SK lesions are thought to be primarily orchestrated by CD4+ T cells of the Th1 subtype with some involvement of Th17 CD4+ T cells (3, 4, 5). One therapeutic strategy would be to suppress the function of effector CD4+ T cell subsets and the events they orchestrate, as we demonstrate can be achieved by gal-1 treatment. The beneficial effect of gal-1 was the consequence of multiple mechanisms. These included apoptotic effects on pro-inflammatory T cells, reduced recruitment of tissue-damaging neutrophils, inhibitory effects on inflammatory cytokine and chemokine production, an increased production of the anti-inflammatory IL-10, as well as an inhibitory effect on the corneal neovascularization needed for inflammatory cells to access the corneal stroma. Our results indicate that gal-1 treatment may represent a useful approach to control HSV-induced ocular lesions, the most common infectious cause of blindness in the western world (2).
Although others have already shown the potential of gal-1 therapy in models of autoimmunity (9, 10, 11), this study is the first to document the useful modulatory effects of gal-1 in an infectious model of inflammatory disease. We show that early therapy was highly effective to diminish lesions of SK, and that even when the treatment was commenced at the stage when virus had been mostly cleared and early lesions begun, was also therapeutically useful. As was initially reported using gal-1 to control autoimmunity, gal-1 could effectively inhibit the participation of the inflammatory CD4+ T cells that orchestrate the lesions of SK. As a consequence, the number of such effector cells and the concentration of their inflammatory products such as IFN-γ and IL-17 and chemokines such as KC were significantly reduced by gal-1 treatment. In contrast mice unable to produce gal-1, because of gene knockout, experienced more severe SK lesions than normal HSV infected animals. The effect of gal-1 on inflammatory T cells was likely a direct one (20, 21, 22), which could be demonstrated in vitro with apoptosis studies. Fewer cells producing cytokines and chemokines would also explain why levels of such molecules were diminished in treated animals. Furthermore, treated mice had increased levels of the anti-inflammatory cytokine IL-10 which could have been derived from the Treg population (7, 18, 23). Moreover, others have shown that gal-1 may drive the differentiation of dendritic cells towards a regulatory phenotype, and capable of favoring Treg modulation of Th1 and Th17 cells (18, 24).
As we and others have shown (25), the tissue damage evident in SK appears to be mediated mainly by non-specific inflammatory cells, particularly neutrophils that are recruited to the corneal stroma. One major outcome of gal-1 therapy was a marked reduction of neutrophil numbers presumably due to a reduction in levels of chemokines such as KC. A critical secondary effect of diminishing neutrophil infiltration was effect on neovascularization. Thus PMN may act as a source of VEGF, a major angiogenic factor, as well molecules such as metalloproteinases that facilitate corneal neovascularization (19, 26, 27, 28). In addition to inhibiting CV via effects on neutrophil recruitment, gal-1 could be mediating anti-angiogenesis through its inhibitory effects on pro-angiogenic cytokines such as IL-6 and IL-17A, both of which may act to cause VEGF production (5, 17, 29, 30, 31).
In conclusion, gal-1, a normal host molecule may be acting to modulate the severity of viral immunoinflammatory lesions. In the absence of gal-1, lesions were more severe. It is also evident that the application of additional gal-1 particularly early in the disease process, was beneficial and reduced the extent of viral immunoinflammatory lesions. The latter effect occurred because gal-1 could diminish both the effector T cells that orchestrate lesions and inhibit the participation of inflammatory cells mainly responsible for tissue damage. Therefore, gal-1 treatment could represent a useful approach to manage the severity of inflammatory disease caused by an infectious agent.
Supplementary Material
Acknowledgments
This study was supported by National Institute of Allergy and Infectious Diseases Grant AI 063365 and National Institutes of Health Grant EY 005093.
We thank Dr. Linda Baum, Dr. Niki Toshiro, Mabel Pang, Tamara Veiga Parga, Fernanda Giminez and Sid Bhela for their assistance.
References
- 1.Pepose JD. Herpes simplex virus disease: Anterior segment of the eye. In: Pepose JS, Holland GN, Whlhelmus KR, editors. Ocular Infection and Immunity. Mosby; St. Louis: 1996. p. 27. [Google Scholar]
- 2.Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Niemialtowski MG, Rouse BT. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J Immunol. 1992;149:3035–3039. [PubMed] [Google Scholar]
- 4.Hendricks RL, Tumpey TM, Finnegan A. IFN-gamma and IL-2 are protective in the skin but pathologic in the corneas of HSV-1-infected mice. J Immunol. 1992;149:3023–3028. [PubMed] [Google Scholar]
- 5.Suryawanshi A, Veiga-Parga T, Rajasagi NK, Reddy PBJ, Sehrawat S, Sharma S, Rouse BT. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J Immunol. 2011;187:1919–1930. doi: 10.4049/jimmunol.1100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–52. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- 7.Rabinovich GA, Ilarregui JM. Conveying glycan information into T-cell homeostatic programs: a challenging role for galectin-1 in inflammatory and tumor microenvironments. Immunol Rev. 2009;230:144–59. doi: 10.1111/j.1600-065X.2009.00787.x. [DOI] [PubMed] [Google Scholar]
- 8.Offner H, Celnik B, Bringman TS, Casentini-Borocz D, Nedwin GE, Vandenbark AA. Recombinant human -galactoside binding lectin suppresses clinical and histological signs of experimental autoimmune encephalomyelitis. J Neuroimmunol. 1990;28:177–184. doi: 10.1016/0165-5728(90)90032-i. [DOI] [PubMed] [Google Scholar]
- 9.Perone MJ, Bertera S, Shufesky WJ, Divito SJ, Montecalvo A, Mathers AR, Larregina AT, Pang M, Seth N, Wucherpfennig KW, Trucco M, Baum LG, Morelli AE. Suppression of Autoimmune Diabetes by Soluble Galectin-1. J Immunol. 2009;182:2641–2653. doi: 10.4049/jimmunol.0800839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabinovich GA, Daly G, Dreja H, Tailor H, Riera CM, Hirabayashi J, Chernajovsky Y. Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J Exp Med. 1999;190:385–398. doi: 10.1084/jem.190.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santucci L, Fiorucci S, Rubinstein N, Mencarelli A, Palazzetti B, Federici B, Rabinovich GA, Morelli A. Galectin-1 suppresses experimental colitis in mice. Gastroenterology. 2003;24:1381–1394. doi: 10.1016/s0016-5085(03)00267-1. [DOI] [PubMed] [Google Scholar]
- 12.Kim B, Sarangi PP, Lee Y, Deshpande S, Lee S, Rouse BT. Depletion of MCP-1 increases development of herpetic stromal keratitis by innate immune modulation. J Leukocyte Biol. 2006;80:1405–1415. doi: 10.1189/jlb.0406295. [DOI] [PubMed] [Google Scholar]
- 13.Rajasagi NK, Reddy PBJ, Suryawanshi A, Mulik S, Gjorstrup P, Rouse BT. Controlling Herpes Simplex Virus-Induced Ocular Inflammatory Lesions with the Lipid-Derived Mediator Resolvin E1. J Immunol. 2011;186:1735–1746. doi: 10.4049/jimmunol.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulik S, Sharma S, Suryawanshi A, Veiga-Parga T, Reddy PBJ, Rajasagi NK, Rouse BT. Activation of Endothelial Roundabout Receptor 4 Reduces the Severity of Virus-Induced Keratitis. J Immunol. 2011;186:7195–7204. doi: 10.4049/jimmunol.1100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YC, Kim KK, Shevach EM. Simvastatin induces Foxp3+ T regulatory cells by modulation of transforming growth factor-beta signal transduction. Immunology. 2010;130:484–93. doi: 10.1111/j.1365-2567.2010.03269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motran CC, Molinder KM, Liu SD, Poirier F, Miceli MC. Galectin-1 functions as a Th2 cytokine that selectively induces Th1 apoptosis and promotes Th2 function. Eur J Immunol. 2008;38:3015–27. doi: 10.1002/eji.200838295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biswas PS, Banerjee K, Kinchington PR, Rouse BT. Involvement of IL-6 in the paracrine production of VEGF in ocular HSV-1 infection. Exp Eye Res. 2006;82:46–54. doi: 10.1016/j.exer.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, Tirado I, Markert UR, Klapp BF, Poirier F, Szekeres-Bartho J, Rabinovich GA, Arck PC. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13:1450–7. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Zheng M, Kim B, Rouse BT. Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus. J Clin Invest. 2002;110:1105–11. doi: 10.1172/JCI15755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378(6558):736–9. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 21.Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD, Zwirner NW, Poirier F, Riley EM, Baum LG, Rabinovich GA. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–34. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 22.Stowell SR, Qian Y, Karmakar S, Koyama NS, Dias-Baruffi M, Leffler H, McEver RP, Cummings RD. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J Immunol. 2008;180:3091–102. doi: 10.4049/jimmunol.180.5.3091. [DOI] [PubMed] [Google Scholar]
- 23.Toscano MA, Commodaro AG, Ilarregui JM, Bianco GA, Liberman A, Serra HM, Hirabayashi J, Rizzo LV, Rabinovich GA. Galectin-1 suppresses autoimmune retinal disease by promoting concomitant Th2- and T regulatory-mediated anti-inflammatory responses. J Immunol. 2006;176:6323–6332. doi: 10.4049/jimmunol.176.10.6323. [DOI] [PubMed] [Google Scholar]
- 24.Ilarregui JM, Croci DO, Bianco GA, Toscano MA, Salatino M, Vermeulen ME, Geffner JR, Rabinovich GA. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009;10:981–91. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- 25.Thomas J, Gangappa S, Kanangat S, Rouse BT. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J Immunol. 1997;158:1383–91. [PubMed] [Google Scholar]
- 26.Penn JS, Madhan A, Caldwell RB, Bartoli M, Caldwell RW, Harnett ME. Vascular endothelial growth factor in eye disease. Progr Retinal Eye Res. 2008;27:331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong Y, Koh DR. Neutrophils promote inflammatory angiogenesis via release of preformed VEGF in an in vivo corneal model. Cell Tissue Res. 2010;339:437–48. doi: 10.1007/s00441-009-0908-5. [DOI] [PubMed] [Google Scholar]
- 28.Zheng M, Deshpande S, Lee S, Ferrara N, Rouse BT. Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J Virol. 2001;75:9828–35. doi: 10.1128/JVI.75.20.9828-9835.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fenton RR, Molesworth-Kenyon S, Oakes JE, Lausch RN. Linkage of IL-6 with neutrophil chemoattractant expression in virus-induced ocular inflammation. Invest Ophthalmol Vis Sci. 2002;43:737–43. [PubMed] [Google Scholar]
- 30.Pickens SR, Volin MV, Mandelin AM, Kolls JK, Pope RM, Shahrara S. IL-17 contributes to angiogenesis in rheumatoid arthritis. J Immunol. 2010;184:3233–41. doi: 10.4049/jimmunol.0903271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banerjee K, Biswas PS, Kim B, Lee S, Rouse BT. CXCR2−/− mice show enhanced susceptibility to herpetic stromal keratitis: a role for IL-6-induced neovascularization. J Immunol. 2004;172:1237–45. doi: 10.4049/jimmunol.172.2.1237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.