Abstract
L-Tryptophan (tryptophan) is an essential amino acid in humans. It has important roles as a precursor of different bioactive compounds. Based on previous studies in which tryptophan has been shown to be present in fresh cherries, the aim of the present work was to analyze the tryptophan content of a Jerte Valley cherry-based product. A previously optimized method of analysis of tryptophan was used, ie, high-performance liquid chromatography with fluorescence detection (HPLC/FL). As expected, HPLC/FL technique permitted to detect and quantify the tryptophan content in a different matrix rather than fresh cherries. In fact, the Jerte Valley cherry-based product contained 69.54 ± 10.64 ppm of tryptophan, thereby showing that this product is a good source of tryptophan. In summary, it has been proven that the Jerte Valley cherry-based product is rich in tryptophan and may be indicated as a supply of this essential amino acid as well as having potential health benefits for conditions where tryptophan is necessary.
Keywords: cherry, tryptophan, HPLC/FL
Introduction
L-Tryptophan (tryptophan) is an essential amino acid, i.e., an amino acid that cannot be synthesized in the human body and must be supplied by the diet. It has an important role in protein biosynthesis and as a precursor of various biologically active compounds, including serotonin, melatonin, kynurenine and quinolinic acid. In addition, tryptophan is precursor to the coenzymes nicotinamide adenine dinucleotide (NAD) and NAD phosphate (NADP), and can replace niacin as an essential nutrient. Tryptophan has been widely used as an over-the-counter, natural remedy for depression, pain, insomnia, hyperactivity, and eating disorders,1 but both excessive intake and deficiency of tryptophan are detrimental to health.2
The health-promoting effects of plant foods and plant-derived beverages have been traditionally attributed to some chemical constituents present in various plant tissues. These bioactive compounds have long-term human health benefits due to their wide range of biological activities, acting as dietary therapeutics or nutraceuticals and, therefore, by virtue of their content, some plant foods and beverages can be considered as functional foods, i.e., products consumed as part of a normal diet that may provide health benefits beyond basic nutritional functions.3,4 The most bioactive plant substances are mainly secondary metabolites and natural antioxidants such as isoprenoids, phenylpropanoids and indoleamines, among others.
Cherries are an important source of phytochemicals and reportedly have important health-promoting qualities, including anti-inflammatory and antioxidant effects.5 Sweet cherries from the Jerte Valley (Cáceres, Extremadura, Spain) contain not only high concentrations of anthocyanin pigments and other phenolic compounds,6 but also substantial amounts of melatonin, serotonin7 and tryptophan,8 as recently re ported in seven different cultivars of these fruits: Bourlat, Navalinda, Van, Ambrunés, Pico Limón, Pico Negro, and Pico Colorado. Particularly, tryptophan has been detected and quantified by using high-performance liquid chromatography with fluorescence detection (HPLC/FL).8
In light of the previous findings, the aim of this study was to analyze the tryptophan content of a Jerte Valley cherry-based product, which may be potentially recommended as a supply of the essential amino acid, and to prove the reproducibility of the HPLC/FL technique when quantifying the content of tryptophan in a different matrix rather than fresh cherries.
Materials and Methods
Reagents
L-Tryptophan, HPLC grade methanol, barium hydroxide (Ba(OH)2) and acetic acid (CH3COOH), all of analytical grade, were obtained from Sigma-Aldrich (Barcelona, Spain). All aqueous solutions were prepared with ultrapure water purified with the MilliQ-system (Millipore-Waters, Milford, MA, USA).
Jerte Valley cherry-based product
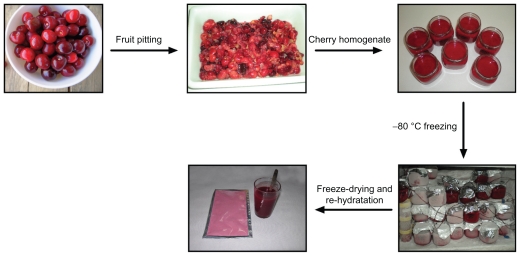
Every single dose of the Jerte Valley (Cáceres, Extremadura, Spain) cherry-based product (27.85 g) consisted of 18.85 g pitted, freeze-dried cherries (equivalent to 141 g fresh cherries) in equal parts of four Jerte Valley cherry cultivars (Bourlat, Navalinda, Pico Negro, and Pico Colorado), plus 7.5 g maltodextrin and 1.5 g ascorbic acid (Spanish patent no. ES 2342141 B1). Once it was freeze-dried, the Jerte Valley cherry product was ground to a powder (Diagram 1).
Diagram 1.
Cherry product was made with 141 g of fresh fruit in equal parts of four Jerte Valley cultivars: Bourlat, Navalinda, Pico Negro and Pico Colorado. Once pitted, 7.5 g of maltodextrin and 1.5 g of ascorbic acid were added to fresh fruit to obtain a cherry homogenate. Subsequently, the cherry homogenate was frozen at −80 °C, freeze-dried, and then ground to powder that can be easily dissolved in water.
Equipment
The HPLC system (Thermo, San José, CA, USA) was equipped with a solvent degasser SCM 1000, a pump P2000 and a fluorescence detector FL3000. The chromatographic analysis was performed using a ZORBAX Eclipse XDB-C18 column (4.6 × 150 mm i.d. column; 5 μm particle size) (Agilent Technologies, Santa Clara, CA, USA). The binary gradient system consisted of eluent A (methanol:acetic acid:water, 10:2:88, v:v:v) and eluent B (methanol). Before analysis, all eluents were filtered and degassed. The fluorescence was recorded at the optimal wavelength for tryptophan (λex = 284 nm and λem = 340 nm). The software used was Finnigan ChromQuest Chromatography Data System 4.2 (Thermo).
Standard solutions
Tryptophan stock standard solutions containing 200 μg/mL were prepared in MilliQ water, sonicated for 30 min for complete dissolution and stored at 4 °C for a maximum of one month. Successive dilutions of stock standard solutions were made with MilliQ water to obtain seven points of calibration curve (200, 100, 50, 25, 12.5, 6.25, 3.12 μg/mL). The calibration points were analyzed in triplicate with each point of the line corresponding to the average of three measures. The linearity of the calibration curve was checked and the regression equation obtained was: y = 398,899 + 126,293x (R2 = 0.998).
Sample preparation
To prepare cherry product for analysis by HPLC/FL, 1 g of the freeze-dried cherry product was accurately weighed and then 0.5 g of Ba(OH)2 plus 0.5 mL of distilled H2O were added.9 This mixture was gently shaken for 10 min and the hydrolysates obtained were heated in an oven at 100 °C for 12 h, and centrifuged for 15 min at 12,000 rpm afterwards. The supernatant was collected, filtered through filters (Albet™) of 0.45 micron pore and frozen at −28 °C. Upon analysis, the pH was adjusted to 5.5 by the addition of 1M CH3COOH and buffer CH3COOH/CH3COO−, measuring the pH with strips of pH Markus-Negel. Once adjusted, samples were vortexed for two minutes and injected (20 μL) in triplicate. Peak areas were correlated with the concentrations according to the calibration curve.
Results
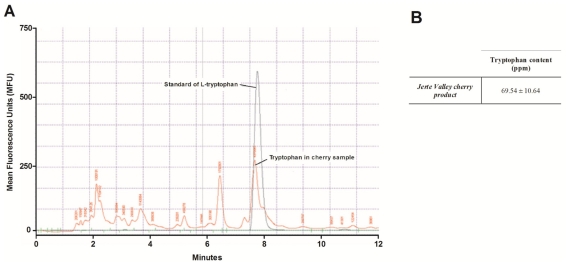
Chromatograms are shown in Figure 1. Peaks were observed at 7.9 ± 0.2 min with an average of three replicates, this retention time being consistent with that reported by Sánchez-Machado et al.10 The limit of detection (LOD) can be defined according to the ‘3s criterion’ (IUPAC, 1978) as the concentration of analyte that gives a net signal equal to three times the standard deviation of the blank, sb:
Figure 1.
HPLC chromatograms and tryptophan content. (A) Chromatograms of standard solution of tryptophan and a sample of cherry-based product are shown. The fluorescence was recorded at λex = 284 nm and λem = 340 nm, and was displayed as mean fluorescence units (MFU). (B) Tryptophan content (ppm) in the cherry-based product was expressed as mean ± SD.
where:
sb = standard deviation of signal of the blank.
b = slope calibration.
yc = critical value of the raw signal.
ȳb = half of the signs of the blank.
The limit of detection of our method was 0.017 μg/mL, a lower limit of detection than that previously described as 0.094 μg/mL.11
The limit of quantification (LOQ) is the concentration that achieves S/N = 10, and we can describe the calculation as follows:
where:
sb = standard deviation of signal of the blank.
b = slope calibration.
The limit of quantification of our method was 0.05 μg/mL.
Recently, Cubero et al,8 using the same methodology, reported that the concentrations of tryptophan in the different varieties used to formulate the Jerte Valley cherry-based product analyzed in the present work were, in decreasing order: 82.65 ± 4.29 ppm (Navalinda), 61.36 ± 5.81 ppm (Bourlat), 37.76 ± 2.43 ppm (Pico Negro), and 36.53 ± 9.84 ppm (Pico Colorado), the mean concentration of tryptophan for all four varieties being 54.57 ± 21.92 ppm. Interestingly, the content of tryptophan found in the Jerte Valley cherry-based product itself was 69.54 ± 10.64 ppm (Table 1), thus showing that this product is a good source of tryptophan.
Table 1A.
Tryptophan content of some fruits.
| Fruits | Tryptophan content (g/100 g edible portion) |
|---|---|
| Banana (Musa paradisiaca) | 0.009 |
| Strawberry (Fragaria vesca) | 0.005 |
| Tangerine (Citrus reticulata) | 0.002 |
| Pear (Pyrus communis) | 0.002 |
| Apple (Malus domestica) | 0.001 |
USDA National Nutrient Database.
Discussion
The cherry fruit is considered a nutrient dense food with a relatively low caloric content and a significant amount of important nutrients and bioactive food components.5 Although tryptophan present in cherries may function mainly in the plant’s anabolism as a precursor of both the hormone indol-3-acetic acid and the indolamines serotonin and melatonin, the Jerte Valley cherry-based product retained high amounts of tryptophan. In fact, tryptophan content in the cherry-based product was slightly higher (69.54 ± 10.64 ppm vs. 54.57 ± 21.92 ppm) than the average amount of tryptophan found in all four varieties used to produce the Jerte Valley cherry product. Compared to other sources of the amino acid, the tryptophan content (0.02 g of tryptophan) of one dose of the cherry-based product (18.85 g of freeze-dried product) is quite similar to that found in fruits, such as tangerines or pears (Table 1A). However, higher tryptophan levels have been reported in other foodstuffs like legumes, cereals or meat (Table 1B).
Table 1B.
Tryptophan content of some foodstuffs.
| Foodstuffs | Tryptophan content (g/100 g edible portion) |
|---|---|
| Chicken | 0.240 |
| Beef | 0.190 |
| Chickpeas | 0.185 |
| Lentils | 0.232 |
| Corn | 0.067 |
| Rice | 0.075 |
USDA National Nutrient Database.
Tryptophan has been proven to be involved in the regulation of several physiological processes. The therapeutic use of this amino acid for treatment of clinical disorders has concentrated primarily on increasing tryptophan intake. At this respect, numerous studies carried out in animals have corroborated the benefits of the supplementation of tryptophan in the diet, being involved in improvements in sleep-wake cycle and in the oxidative and inflammatory pathways.12–15 Likewise, it has been demonstrated that supplementing the diet with tryptophan-enriched foodstuffs, e.g., Jerte Valley cherries or cereals, enhanced the nocturnal rest both in healthy individuals (without sleep disturbances) and subjects with sleep disorders.16,17
Therefore, based on the current research, the Jerte Valley cherry-based product, which has been found to contain substantial amounts of tryptophan, may have a great number of potential health benefits and may be considered to be incorporated in a healthy diet. Furthermore, as Jerte Valley cherry is a short-lived fruit that is typically hand harvested from May to mid July, the elaboration of the cherry-based product make it possible to enjoy the potential benefits derived from the intake of this product throughout the year.
Tryptophan exhibits a strong native fluorescence that facilitates its detection without preliminary derivatization.10 Thus, tryptophan was identified by means of a HPLC/FL technique by comparing retention times between standard samples and prepared cherry samples after barytic hydrolysis, which has been regarded as one of the most appropriate methods for the release of tryptophan from protein, preventing its destruction.18 HPLC/FL has been previously used to assay the content of tryptophan in fresh fruits, including sweet cherries,8 and has been regarded as an efficient, fast, highly-reliable, not overly complicated method.19
In conclusion, it has been proven that the Jerte Valley cherry-based product contains high amounts of tryptophan and, consequently, it may be recommended as a supply of this essential amino acid available for consumption throughout the year.
Acknowledgments
This investigation was supported by Consejería de Economía, Comercio e Innovación-Fondo Social Europeo (Junta de Extremadura, PDT0A008) and Consejería de Empleo, Empresa e Innovación, Dirección General de Modernización e Innovación Tecnológica (Junta de Extremadura, AE-11-0102-4; Convenio Agrupación de Cooperativas Valle del Jerte-UEx no. 164/11). J. Espino was beneficiary of a grant from Ministerio de Ciencia e Innovación (AP2009-0753).
Footnotes
Author Contributions
Conceived and designed the experiments: ABR, SDP. Analysed the data: AFTD, JC, JIMM. Wrote the first draft of the manuscript: MG, JE. Contributed to the writing of the manuscript: MG, JE, CB. Agree with manuscript results and conclusions: AFTD, JC, JIMM. Jointly developed the structure and arguments for the paper: ABR, SDP, CB. Made critical revisions and approved final version: ABR, SDP. All authors reviewed and approved of the final manuscript.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Richard DM, Dawes MA, Mathias CW, Acheson A, Hill-Kapturczak N, Dougherty DM. L-tryptophan: Basic metabolic functions, behavioural research and therapeutic indications. Int J Tryptophan Res. 2009;2:45–60. doi: 10.4137/ijtr.s2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sainio EL, Puikki K, Young SN. L-Tryptophan: Biochemical, nutritional and pharmacological aspects. Amino Acids. 1996;10:21–47. doi: 10.1007/BF00806091. [DOI] [PubMed] [Google Scholar]
- 3.Heber D. Phytochemicals beyond antioxidation. J Nutr. 2004;134:3175S–6. doi: 10.1093/jn/134.11.3175S. [DOI] [PubMed] [Google Scholar]
- 4.Zhao J. Nutraceuticals, nutritional therapy, phytonutrients, and phytotherapy for improvement of human health: a perspective on plant biotechnology application. Recent Pat Biotechnol. 2007;1:75–97. doi: 10.2174/187220807779813893. [DOI] [PubMed] [Google Scholar]
- 5.McCune LM, Kubota C, Stendell-Hollis NR, Thomson CA. Cherries and health: a review. Crit Rev Food Sci Nutr. 2011;51:1–12. doi: 10.1080/10408390903001719. [DOI] [PubMed] [Google Scholar]
- 6.González-Gómez D, Lozano M, Fernández-León MF, Ayuso MC, Bernalte MJ, Rodríguez AB. Detection and quantification of melatonin and serotonin in eight Sweet Cherry cultivars (Prunus avium L.) Eur Food Res Technol. 2009;229:223–9. [Google Scholar]
- 7.González-Gómez D, Lozano M, Fernández-León MF, Bernalte MJ, Ayuso MC, Rodríguez AB. Sweet cherry phytochemicals: Identification and characterization by HPLC-DAD/ESI-MS in sweet-cherry cultivars grown in Valle del Jerte (Spain) J Food Compost Anal. 2010;23:533–9. [Google Scholar]
- 8.Cubero J, Toribio F, Garrido M, et al. Assays of the amino acid tryptophan in cherries by HPLC-Fluorescence. Food Anal Methods. 2010;3:36–9. [Google Scholar]
- 9.Alegría A, Barbera R, Farre R, Ferreres M, Lagarda MJ, Lopez JC. Isocratic high-performance liquid chromatographic determination of tryptophan in infant formulas. J Chromatogr A. 1996;721:83–8. doi: 10.1016/0021-9673(95)00629-x. [DOI] [PubMed] [Google Scholar]
- 10.Sánchez-Machado DI, Chavira-Willys B, López-Cervantes J. High-performance liquid chromatography with fluorescente detection for quantitation of tryptophan and tyrosine in a shrimp waste protein concentrate. J Chromatogr B. 2008;863:88–93. doi: 10.1016/j.jchromb.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Delgado-Andrade C, Rufián-Henares JA, Jiménez-Pérez S, Morales FJ. Tryptophan determination in milk-based ingredients and dried sport supplements by liquid chromatography with fluorescence detection. Food Chem. 2006;98:580–5. [Google Scholar]
- 12.Paredes SD, Barriga C, Reiter RJ, Rodríguez AB. Assessment of the potential role of tryptophan as the precursor of serotonin and melatonin for the aged sleep-wake cycle and immune function: Streptopelia risoria as a model. Int J Tryptophan Res. 2009;2:23–36. doi: 10.4137/ijtr.s1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paredes SD, Bejarano I, Terrón MP, Barriga C, Reiter RJ, Rodríguez AB. Melatonin and tryptophan counteract lipid peroxidation and modulate superoxide dismutase activity in ringdove heterophils in vivo. Effect of antigen-induced activation and age. AGE (Dordr) 2009;31:179–88. doi: 10.1007/s11357-009-9107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paredes SD, Marchena AM, Bejarano I, et al. Melatonin and tryptophan affect the activity-rest rhythm, core and peripheral temperatures, and interleukin levels in the ringdove: Changes with age. J Gerontol A Biol Sci Med Sci. 2009;64:340–50. doi: 10.1093/gerona/gln054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sánchez S, Paredes SD, Sánchez CL, Barriga C, Reiter RJ, Rodríguez AB. Tryptophan administration in rats enhances phagocytic function and reduces oxidative metabolism. Neuroendocrinol Lett. 2008;29:1026–32. [PubMed] [Google Scholar]
- 16.Garrido M, Paredes SD, Cubero J, et al. Jerte Valley-enriched diets improve nocturnal rest and increase 6-sulfatoxymelatonin and total antioxidant capacity in the urine of middle-aged and elderly humans. J Gerontol A Biol Sci Med Sci. 2010;65:909–14. doi: 10.1093/gerona/glq099. [DOI] [PubMed] [Google Scholar]
- 17.Cubero J, Chanclón B, Sánchez S, Rivero M, Rodríguez AB, Barriga C. Improving the quality of infant sleep through the inclusión at supper of cereals enriched with tryptophan, adenosine-5′-phosphate, and uridine-5′-phosphate. Nutr Neurosci. 2009;12:272–80. doi: 10.1179/147683009X423490. [DOI] [PubMed] [Google Scholar]
- 18.Landry J, Delhaye S, Viroben G. Tryptophan content of feedstuffs as determined from three procedures using chromatography of barytic hydrolyzates. J Agric Food Chem. 1988;36:51–2. [Google Scholar]
- 19.Hoenicke H, Simat JT, Steinhart H, Christoph N, Kohler HJ, Schwab A. Determination of free and conjugated indole-3-acetic acid, tryptophan and tryptophan metabolites in grape must and wine. J Agric Food Chem. 2001;49:5494–501. doi: 10.1021/jf010575v. [DOI] [PubMed] [Google Scholar]