Abstract
Renal failure, a major complication associated with multiple myeloma, is usually related to deposition of monoclonal immunoglobulin free light chains (FLCs) and directly contributes to morbidity and mortality in this disease. The present study focused on the cytotoxic effects of monoclonal FLCs. Human proximal tubular epithelial cells (HK-2) were examined after incubation with two human monoclonal FLCs (termed κ2 and λ3). Incubation of HK-2 cells for 24 and 48 hours with either FLCs at 1 mg/mL promoted activation of caspase-9 and caspase-3 and increased the rate of apoptosis. Because prior studies demonstrated that FLCs generated intracellular oxidative stress, our studies focused on the redox-sensitive mitogen-activated protein kinase kinase kinase known as apoptosis signal-regulating kinase 1 (ASK1). A time-dependent increase in phosphorylation of ASK1 at T845, indicating activation of this enzyme, was observed. Small interfering RNA designed to reduce ASK1 expression in HK-2 cells successfully decreased ASK1, which was confirmed by Western blot analysis. Incubation of ASK1-depleted HK-2 cells with the two FLCs prevented the increase in apoptosis while pretreating HK-2 cell with nontargeting small interfering RNA did not prevent FLCs-mediated apoptosis. The combined data demonstrate that monoclonal FLCs activated the intrinsic apoptotic pathway in renal epithelial cells by activation of ASK1.
A major function of proximal tubular epithelium is reabsorption of proteins that are present in glomerular ultrafiltrate. This process integrally involves the heteromeric receptor composed of megalin and cubilin.1–4 As low-molecular-weight proteins, immunoglobulin free light chains (FLCs) are filtered relatively freely and are presented to the proximal tubule. Unlike other low-molecular-weight proteins, however, monoclonal FLCs have high nephrotoxic potential.5–8 Batuman's laboratory in particular has demonstrated that monoclonal FLCs are directly cytotoxic, promoting apoptosis of proximal tubular cells. Apoptosis required endocytosis of the FLCs and subsequent activation of mitogen-activated protein (MAP) kinase pathways.9–12
A novel human protein kinase, apoptosis signal-regulating kinase 1 (ASK1, alias MAP3K5, MEKK5, and MAPKKK5) was cloned in 1996 and was found to function as a MAP kinase kinase kinase (MAP3K).13 This ubiquitously expressed MAP3K functions as an upstream activator of the c-Jun N-terminal kinase and p38 MAP kinase pathways.14,15 Overexpression of ASK1 promotes apoptosis specifically by inducing Bax translocation and cytochrome c release from mitochondria and activation of caspase-9 and caspase-3.16 ASK1 is inhibited by association with reduced cytoplasmic thioredoxin-1 and mitochondrial thioredoxin-2.17,18 Reactive oxygen species, particularly hydrogen peroxide, oxidize thioredoxin, releasing ASK1 and permitting phosphorylation at T845 and activation of this kinase, which results in apoptosis.19–22 ASK1 is also involved in promoting release of inflammatory molecules in ischemic events that include acute kidney injury.23,24 Intriguingly, protein kinase B (Akt) phosphorylates ASK1 at S83, which mitigates ASK1-mediated apoptosis.25 Thus, ASK1 is a highly regulated key element in stress-induced apoptosis.
The redox state of the cell modulates signal transduction activity and is a critical determinant of cell survival.26 Recently, endocytosis of monoclonal FLCs has been shown to generate intracellular oxidative stress sufficient to activate c-Src, the 60-kDa product of c-src, also known as pp60c-src, and the NF-κB pathway.27–29 We tested the hypothesis that monoclonal FLCs promotes apoptosis of renal epithelial cells through activation of ASK1.
Materials and Methods
Cells and Reagents
Human Immunoglobulin Monoclonal FLCs
Two unique monoclonal FLCs, one κ and the other λ, labeled κ2 and λ3, respectively, were purified using standard methods from the urine of patients who had multiple myeloma and light chain proteinuria.7 These patients had clinical evidence of renal damage, although renal biopsy was not performed. The FLCs were endotoxin-free (Limulus Amebocyte Lysate, QCL-1000; Lonza, Walkersville, MD) and observed to generate H2O2 and promote intracellular oxidative stress in human proximal tubular epithelial cells (HK-2) cells in culture.27
HK-2 Cells
HK-2 cells, which have previously been characterized,30 were obtained from the American Type Culture Collection (Manassas, VA). Monolayers of HK-2 cells were grown on six-well plates (Corning-Costar; Corning Incorporated Life Sciences, Lowell, MA) that were precoated with 5 μg/cm2 type 1 collagen (rat tail collagen type 1; Invitrogen Corporation, Carlsbad, CA), and incubated at 37°C with 5% CO2/95% air in keratinocyte serum-free medium (Gibco, Invitrogen Corporation, Carlsbad, CA) supplemented with recombinant human epidermal growth factor (5 ng/mL) and bovine pituitary extract (50 μg/mL). Medium was exchanged at 48-hour intervals, and cells were not used beyond 25 to 30 passages. In the present experiments, confluent cell monolayers were incubated at 37°C in medium containing a unique FLCs, 1 mg/mL, for 24 and 48 hours before study. This FLCs concentration was within the expected concentration range to which proximal tubular cells are exposed in patients with multiple myeloma.31
To suppress c-Src activity in HK-2 cells in some experiments, simultaneously with the addition of the FLCs, 4-amino-5-(4-chlorophenyl)−7-(tert-butyl)pyrazolo[3,4-d] pyramidine (PP2; EMD Biosciences, Gibbstown, NJ) was added to the medium in a final concentration of 10 μmol/L.32
Western Blot Analyses
After incubation, cells were lysed in radioimmunoprecipitation assay buffer containing a protease inhibitor cocktail (Complete; Roche, Indianapolis, IN) and clarified by centrifugation; lysates were then stored at −70°C until they were assayed. Total soluble proteins in lysates were determined with the BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL). Protein extracts (20 to 60 μg) were boiled for 3 minutes in Laemmli buffer and separated by 7% to 12% SDS-PAGE (BioRad Laboratories, Hercules, CA) before transfer onto polyvinylidene diflouride membranes. The membranes were blocked in 5% skim milk and incubated at 4°C overnight with one of the following primary antibodies: rabbit–anti-human polyclonal antibody to ASK1, phospho-ASK1 (T845), and phospho-ASK1 (S83); all were obtained from Cell Signaling Technology (Danvers, MA). Glyceraldehyde 3-phosphate dehydrogenase, determined using mouse anti-human glyceraldehyde 3-phosphate dehydrogenase (Abcam Inc., Cambridge, MA), served as a loading normalization control. Gels were developed in standard fashion using enhanced chemiluminescence (SuperSignal West Dura Chemiluminescent Substrate; Pierce Biotechnology), and densitometry was performed by Quantity One software (BioRad Laboratories).
Determination of Cytoplasmic Caspase-9 Activity and Concentration
Cytoplasmic caspase-9 activity was quantified with a fluorometric assay (Caspase-9 Activity Assay Kit; Calbiochem EMD Chemicals Inc., Darmstadt, Germany), following the protocol provided by the manufacturer. Briefly, caspase-9 activity was detected in cell lysates by using LEHD peptide substrate labeled with a fluorophore, 7-amino-4-trifluoromethyl coumarin. Cytoplasm was collected from pelleted cells using kit lysis buffer supplemented with protease inhibitors (Complete Protease Inhibitor tablets; Roche Diagnostics GmbH, Mannheim, Germany) and dithiothreitol (Sigma-Aldrich, St. Louis, MO). Lysates were added with substrate into a 96-well plate. The kit provided both a positive control, which consisted of frozen HL-60 cells previously cultured and treated by the vendor with 0.5 μg/mL actinomycin D for 19 hours to induce apoptosis, and a negative control that used the same cells also treated with a specific caspase-9 inhibitor. After incubation, caspase-9 activity was quantified with a fluorescent plate reader (Spectramax M2e Microplate Reader; Molecular Devices, Sunnyvale, CA) with an excitation of 400 nm and emission at 505 nm.
Cytoplasmic caspase-9 concentration was determined with an enzyme-linked immunosorbent assay (ELISA) (Human Caspase-9 ELISA; BioVendor Research and Diagnostic Products, Candler, NC), following the protocol provided by the manufacturer. Briefly, collected cells were pelleted and the pellets were resuspended in kit lysis buffer at a concentration of 5 × 106 cells/mL. The lysates were added to an antibody-coated 96-well plate and then incubated with the detection antibody at room temperature for 2 hours. Anti-rabbit horseradish peroxidase antibody was then added to all wells, followed by 3,3′,5,5′-tetramethylbenzidine substrate solution. Caspase-9 levels were quantified with a colorimetric plate reader (Molecular Devices Spectramax M2e reader) at 450 nm.
Human Active Caspase-3 Assay
Active caspase-3 was quantified by a sandwich ELISA [Human Caspase-3 (Active) ELISA kit; Invitrogen Corporation], following the protocol provided by the manufacturer. The capture antibody bound human caspase-3 and the specific active caspase-3 antibody served as the detection antibody. After addition of horseradish peroxidase–labeled anti-rabbit IgG and TMB substrate, active caspase-3 concentrations were quantified with a plate reader (SpectraMax M2e Microplate Reader, Molecular Devices) at 450 nm.
Silencing Apoptosis Signal-Regulation Kinase 1 (ASK1) Expression
RNA interference was accomplished by using small interfering RNA (siRNA) that targeted human ASK1, as reported by other investigators.33 RNA duplexes consisting of human ASK1 (MAP3K5)-specific sense and antisense RNA oligomers (NM-005923) were synthesized commercially; nontargeting siRNA #1 (D-001810) served as a control (all purchased from Dharmacon RNA Technologies, Lafayette, CO). HK-2 cells at 70% confluence were transfected using siRNA transfection reagent (DharmaFECT1; Dharmacon RNA Technologies) containing varying amounts (0 to 100 nmol/L) of siRNA. Preliminary experiments that used siTOX transfection control (Dharmacon RNA Technologies) determined the optimum exposure conditions that maximized transfection efficiency and minimized toxicity. ASK1 siRNA (50 nmol/L) was complexed with 2 μL of DharmaFECT1 in 200 μL total volume and then added to complete medium in a final volume of 1 mL for each well in a 12-well plate. After incubation in the transfection solution for 12 hours, the medium was replaced and incubation continued up to 48 hours. The cells were then incubated in medium containing 1 mg/mL of the FLCs (κ2 and λ3), for an additional 24 and 48 hours before study.
Flow Cytometry
The percentage of apoptotic cells in each population of HK-2 cells was determined by flow cytometry (model BD LSR II; BD Biosciences, San Jose, CA) and vital staining with the use of a kit (Mitochondrial Membrane Potential/Annexin V Apoptosis Kit V35116; Invitrogen Corporation). The kit contained recombinant annexin V conjugated to Alexa Fluor 488 and 1H,5H,11H,15H-xantheno[2,3,4-ij:5,6,7-i‘j’]diquinolizin-18-ium,9-[4(chloromethyl)phenyl]−2,3,6,7,12,13,16,17-octahydro-,chloride (MitoTracker Red). At the end of the incubation period, HK-2 cells, approximately 5 × 106 cells/mL, were stained according to manufacturer's instructions, by incubation in culture medium that contained 4 μL of 10 μmol/L MitoTracker Red for 30 minutes at 37°C in a mixture of 5% CO2 and 95% air. After washing in PBS, the cells were resuspended in 100 μL of annexin binding buffer with 5 μL of Alexa Fluor 488 annexin V. The cells were incubated for 15 minutes at room temperature in the dark, then diluted and immediately analyzed by flow cytometry.
Statistical Analysis
Data were expressed as mean ± SE. Significant differences among data sets were determined by analysis of variance followed by Tukey-Kramer multiple comparisons post hoc testing (InStat; GraphPad, San Diego, CA), where appropriate. A P value of <0.05 was assigned statistical significance.
Results
Human Monoclonal FLCs-Activated ASK1 in Renal Epithelial Cells
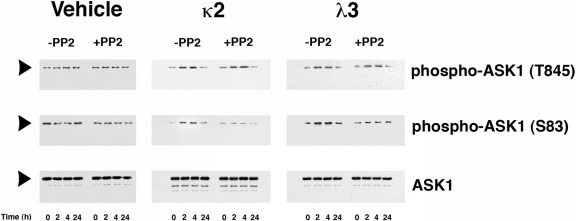
Incubation of HK-2 cells with κ2 and λ3 FLCs, 1 mg/mL, but not vehicle, promoted a time-dependent and sustained increase in phospho-ASK1 (T845) and phospho-ASK1 (S83), starting within 2 hours of exposure (Figure 1). The addition of PP2, a potent Src kinase inhibitor,32 to the medium did not inhibit phosphorylation at T845 but did inhibit phosphorylation at S83.
Figure 1.
Monoclonal FLCs (κ2 and λ3) activated ASK1 in proximal tubular epithelial cells. The first column shows the effect of vehicle treatment with and without PP2, a selective and potent inhibitor of Src kinases.32 The second and third columns show the effects of incubation of HK-2 cells with κ2 and λ3 over time. The top and middle rows of Western blot tests demonstrated phospho-ASK1 (T845) and phospho-ASK1 (S83), respectively, and the bottom represents total ASK1 protein. Both FLCs, 1 mg/mL, increased phospho-ASK1 (T845) in a time-dependent fashion. During incubation with FLCs, phospho-ASK1 (S83), an Akt-dependent event,25 also increased. Although PP2 had no effect on phosphorylation at T845, phosphorylation at S83 was inhibited, indicating participation by c-Src. The experiment was performed in duplicate with similar results.
Human Monoclonal FLCs Induce Apoptosis of Renal Epithelial Cells through Activation of ASK1
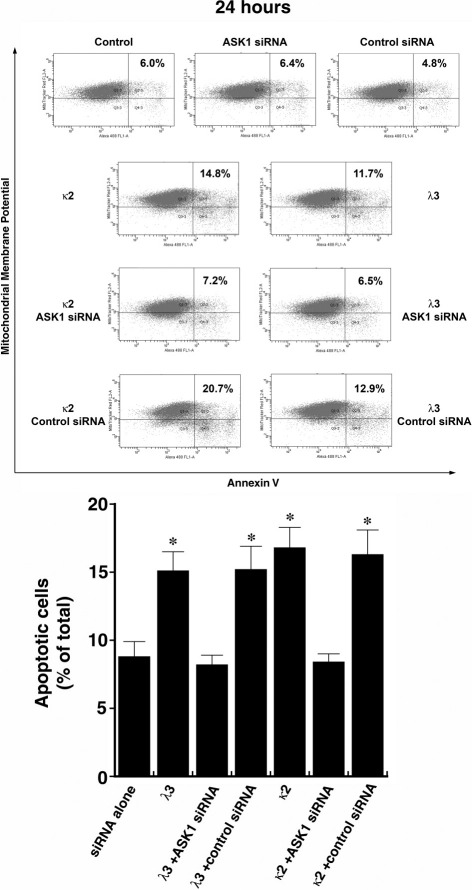
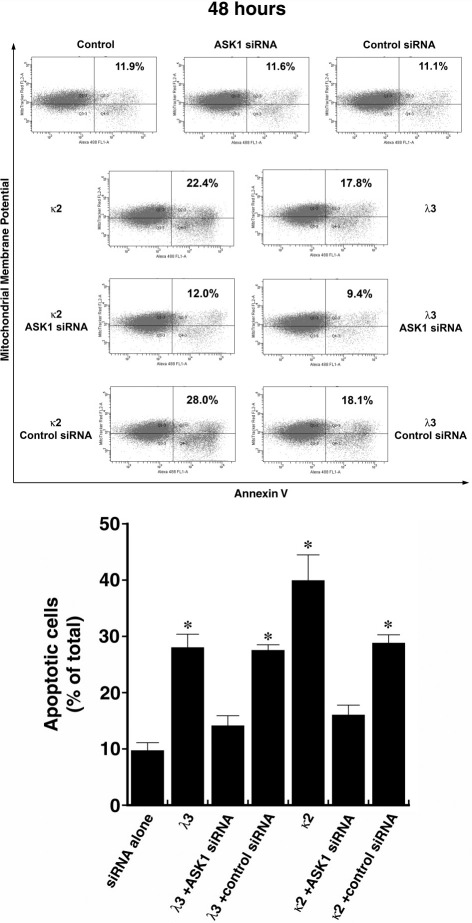
Initial experiments focused on reducing ASK1 levels using siRNA. ASK1 protein expression was prominent in untreated HK-2 cells (Figures 1 and 2) and was effectively reduced in HK-2 cells transfected with siRNA directed against ASK1; control (nontargeting) siRNA had no effect on ASK1 (Figure 2). Forty-eight hours after transfection, the cells were incubated in medium containing 1 mg/mL of the FLCs (κ2 and λ3), for an additional 24 hours before study (n = 8 to 10 experiments in each group). Apoptosis was detected using flow cytometry using MitoTracker Red and annexin V conjugated to Alexa Fluor 488. Incubation of HK-2 cells with κ2 and λ3 FLCs for 24 hours (Figure 3) and 48 hours (Figure 4) increased apoptosis rates. The κ FLCs produced an average 2.3-fold increase in apoptosis and the λ FLCs increased apoptosis by 1.9-fold over baseline levels. Although knockdown of ASK1 alone had no effect on baseline apoptosis rates, HK-2 cells with reduced ASK1 levels were protected from FLCs-induced apoptosis.
Figure 2.
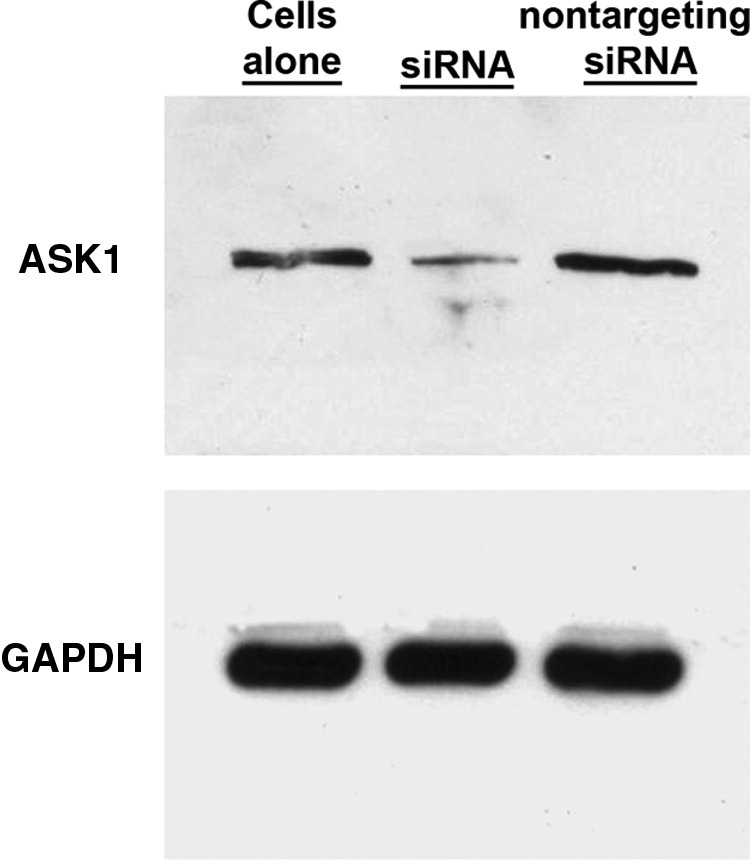
Western blot test showing the effect of siRNA that targeted human ASK1 and nontargeting siRNA, which served as a control. ASK1 protein was reduced by addition of siRNA that targeted ASK1 but not in cells exposed to the control (nontargeting) siRNA.
Figure 3.
Representative flow cytometry experiment (top) using cells incubated for 24 hours and then labeled with annexin V conjugated to Alexa Fluor 488 and MitoTracker Red before study. Apoptosis increased after exposure of HK-2 cells to the two monoclonal FLCs, but cells pretreated with siRNA that targeted ASK1 were protected from FLCs-induced apoptosis. The graph at the bottom represents a compilation of 8 to 10 experiments in each group. *P < 0.05 compared to siRNA alone and siRNA with λ3 and κ2.
Figure 4.
Representative flow cytometry experiment (top) using cells incubated for 48 hours and then labeled with annexin V conjugated to Alexa Fluor 488 and MitoTracker Red before study. An increase in apoptosis was again observed after exposure of HK-2 cells to the two monoclonal FLCs, but cells pretreated with siRNA that targeted ASK1 were protected from FLCs-induced apoptosis. Bottom: Compilation of 8 to 10 experiments in each group. *P < 0.05 compared to siRNA alone and siRNA with λ3 and κ2.
Human Monoclonal FLCs Promote Increased Active Caspase-9 and Active Caspase-3 in Renal Epithelial Cells
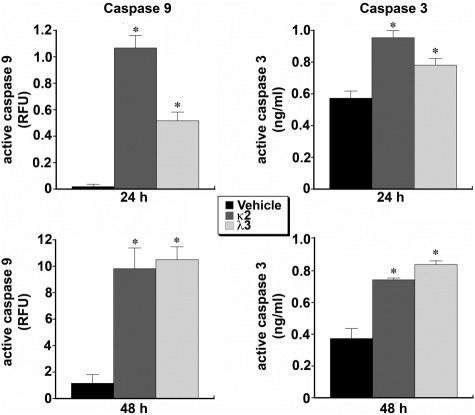
Cytoplasmic caspase-9 activity of HK-2 cells incubated with FLCs was quantified with a fluorometric assay. Both FLCs induced increases in cytoplasmic caspase-9 activity at 24 hours and 48 hours (n = 6 experiments in each group) (Figure 5). In addition, cytoplasmic caspase-9 protein levels were determined at 24 hours by a sandwich ELISA. Cytoplasmic caspase-9 levels increased (P < 0.05) after incubation in medium containing κ2 (172.0 ± 21.7 ng/mL) and λ3 (238.4 ± 30.1 ng/mL), compared to levels after incubation in medium alone (62.5 ± 6.6 ng/mL). Active caspase-3 was quantified by a sandwich ELISA after incubation with the monoclonal FLCs. Both FLCs increased active caspase-3 at 24 and 48 hours (Figure 5).
Figure 5.
Cytoplasmic caspase-9 (left) and caspase-3 (right) activities of HK-2 cells incubated with monoclonal FLCs for 24 and 48 hours. Compared to vehicle treatment alone (black bars), caspase-9 activity, which was quantified with a fluorometric assay, increased in HK-2 cells exposed to both monoclonal FLCs for 24 and 48 hours (n = 6 experiments in each group). Active caspase-3, which was quantified by a sandwich ELISA, also increased in HK-2 cells after incubation with the monoclonal FLCs for 24 and 48 hours (n = 6 experiments in each group). *P < 0.05 compared to vehicle-treated cells.
Discussion
The highly efficient protein reclamation system of the proximal tubule provides an important mechanism of conservation of amino acids and other essential molecules. For example, the daily production of polyclonal FLCs by the lymphoid system is approximately 500 mg; these low-molecular-weight proteins are filtered and subsequently endocytosed and catabolized by the proximal tubule, with less than 10 mg of polyclonal FLCs normally appearing in the urine.34 In multiple myeloma, circulating levels of FLCs increase substantially31 and can result in significant accumulation of these proteins within the proximal tubular epithelium. In this setting, monoclonal FLCs can injure the proximal tubule epithelium and produce clinical manifestations of renal failure.6–8,35–38 With the use of proximal tubular cells in culture, Batuman's laboratory demonstrated that monoclonal FLCs promote apoptosis.10,12 The mechanism of induction of apoptosis, however, has not been clarified. The present series of experiments demonstrated that human monoclonal FLCs induced apoptosis by activating the intrinsic apoptotic pathway through ASK1. This mechanism is unique and differs from the recently described activation in renal tubular cells of the intrinsic apoptotic pathway by albumin through Protein Kinase C-δ.39
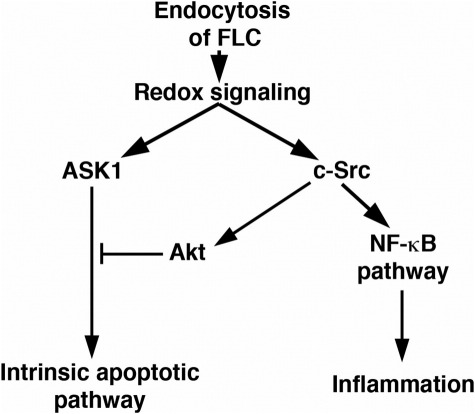
Previous studies showed that some FLCs, including the two monoclonal proteins used in the present experiments, but not all FLCs, generated reactive oxygen species, especially H2O2, in amounts sufficient to activate c-Src.28,29 In turn, c-Src activated the canonical and atypical NF-κB pathways, which increased production of monocyte chemoattractant protein-1 and IL-6.29 Mice treated with monoclonal FLCs demonstrated enhanced renal production of monocyte chemoattractant protein-1 and tumor necrosis factor-α before clinical evidence of renal failure.9,40 Monoclonal FLCs-induced production of H2O2 was also cytotoxic, and cell injury was prevented by 1,3-dimethyl-2-thiourea, a cell-permeable scavenger of reactive oxygen species including H2O2, demonstrating the important role for oxidative stress in the initiation of injury.27 These findings, combined with the present studies, established proximal tubular cell metabolism of monoclonal FLCs activated a proapoptotic intrinsic (mitochondrial) pathway initiated by ASK1 as well as prosurvival, proinflammatory pathways from activation of c-Src (Figure 6). As a MAP3K, however, ASK1 activation promotes not only apoptosis but also the production of proinflammatory molecules that include monocyte chemoattractant protein-124,41 and transforming growth factor-β.42 In the present studies, phosphorylation of ASK1 at S83, which inhibits the proapoptotic effect of ASK1 and is an Akt-dependent event,25 was inhibited by PP2, demonstrating that c-Src participated in the activation of Akt, as it does in other models.43 Thus, activation of c-Src appears to be a critical factor in promoting cell survival at the cost of producing a proinflammatory state in the kidney. The overall cellular effects of exposure to monoclonal FLCs include both an increase in apoptosis and increased production of important chemokines and cytokines that include monocyte chemoattractant protein-1 and IL-6.
Figure 6.
Simplified schematic representing the effect of monoclonal FLCs on function of proximal tubular epithelial cells. Endocytosis of monoclonal FLCs generated intracellular oxidative stress,28,29 which activated ASK1. ASK1 activates the intrinsic apoptotic pathway and thereby promoted apoptosis. In addition, monoclonal FLCs activated c-Src, which promoted a proinflammatory environment through activation of the canonical and atypical NF-κB pathways, but also promoted a prosurvival signal through activation of Akt and down-regulation of ASK1 activity through phosphorylation of ASK1 at S83. Thus, activation of c-Src may serve as a defense against cell death but promotes an inflammatory response. In addition to the interrelatedness of these pathways depicted in the schematic, NF-κB generates proinflammatory signals and can also induce additional antiapoptotic and proapoptotic pathways that affect cell function and survival. Finally, ASK1 not only activates the intrinsic apoptotic pathway but may also directly participate in an inflammatory response.
Renal failure is a well-recognized accompaniment of multiple myeloma. Kyle et al showed that renal dysfunction, as determined by serum creatinine concentration ≥1.3 mg/dL, occurred in nearly half of patients with newly diagnosed myeloma.44 Between 19% to 22% had serum creatinine concentrations above 2 mg/dL.44,45 The majority of patients in this setting have tubulointerstitial renal disease, and usually monoclonal FLCs deposition is the culprit.6,7,36,40 The most common pathology is cast nephropathy, or myeloma kidney, although a smaller percentage of patients have isolated proximal tubular injury attributed to the monoclonal FLCs.7,8,35,46 Consistent with clinical findings, preclinical studies that used monoclonal FLCs demonstrated varying propensity for cast formation and acute tubular injury.6,36,47 In addition, prominent inflammatory and fibrotic changes in the interstitium are also typical of cast nephropathy. Although cast formation per se is a critical determinant of clinically recognized renal failure, the combined findings suggest a facilitating role for alterations in proximal tubule function during the renal metabolism of monoclonal FLCs by promoting apoptosis and stimulating the renal production of chemokines and cytokines. Batuman's laboratory demonstrated that in vivo administration of human monoclonal FLCs increased apoptosis of proximal tubular cells in mice, as detected by a TUNEL-based fluorescence assay.40 Clinically, tubulointerstitial fibrosis can occur rapidly48; only 8% of patients with severe renal failure (serum creatinine ≥4.0 mg/dL) at presentation have reversible renal failure.45 Present clinical evidence supports rapid reduction in circulating levels of monoclonal FLCs,49 but additional therapeutic strategies are needed to slow the progression to end-stage kidney failure in these patients. By demonstrating an important role for activation of ASK1 by monoclonal FLCs, the present study provides another potential target for consideration in the treatment of the tubulointerstitial renal disease associated with myeloma.
Footnotes
Supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, and NIH (R01 DK46199) and the George M. O'Brien Kidney and Urological Research Centers Program (P30 DK079337).
References
- 1.Batuman V., Dreisbach A.W., Cyran J. Light-chain binding sites on renal brush-border membranes. Am J Physiol. 1990;258:F1259–F1265. doi: 10.1152/ajprenal.1990.258.5.F1259. [DOI] [PubMed] [Google Scholar]
- 2.Batuman V., Guan S. Receptor-mediated endocytosis of immunoglobulin light chains by renal proximal tubule cells. Am J Physiol. 1997;272:F521–F530. doi: 10.1152/ajprenal.1997.272.4.F521. [DOI] [PubMed] [Google Scholar]
- 3.Batuman V., Verroust P.J., Navar G.L., Kaysen J.H., Goda F.O., Campbell W.C., Simon E., Pontillon F., Lyles M., Bruno J., Hammond T.G. Myeloma light chains are ligands for cubilin (gp280) Am J Physiol. 1998;275:F246–F254. doi: 10.1152/ajprenal.1998.275.2.F246. [DOI] [PubMed] [Google Scholar]
- 4.Klassen R.B., Allen P.L., Batuman V., Crenshaw K., Hammond T.G. Light chains are a ligand for megalin. J Appl Physiol. 2005;98:257–263. doi: 10.1152/japplphysiol.01090.2003. [DOI] [PubMed] [Google Scholar]
- 5.Holland M.D., Galla J.H., Sanders P.W., Luke R.G. Effect of urinary pH and diatrizoate on Bence Jones protein nephrotoxicity in the rat. Kidney Int. 1985;27:46–50. doi: 10.1038/ki.1985.8. [DOI] [PubMed] [Google Scholar]
- 6.Sanders P.W., Herrera G.A., Chen A., Booker B.B., Galla J.H. Differential nephrotoxicity of low molecular weight proteins including Bence Jones proteins in the perfused rat nephron in vivo. J Clin Invest. 1988;82:2086–2096. doi: 10.1172/JCI113830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanders P.W., Herrera G.A., Galla J.H. Human Bence Jones protein toxicity in rat proximal tubule epithelium in vivo. Kidney Int. 1987;32:851–861. doi: 10.1038/ki.1987.286. [DOI] [PubMed] [Google Scholar]
- 8.Sanders P.W., Herrera G.A., Lott R.L., Galla J.H. Morphologic alterations of the proximal tubules in light chain–related renal disease. Kidney Int. 1988;33:881–889. doi: 10.1038/ki.1988.80. [DOI] [PubMed] [Google Scholar]
- 9.Arimura A., Li M., Batuman V. Potential protective action of pituitary adenylate cyclase–activating polypeptide (PACAP38) on in vitro and in vivo models of myeloma kidney injury. Blood. 2006;107:661–668. doi: 10.1182/blood-2005-03-1186. [DOI] [PubMed] [Google Scholar]
- 10.Pote A., Zwizinski C., Simon E.E., Meleg-Smith S., Batuman V. Cytotoxicity of myeloma light chains in cultured human kidney proximal tubule cells. Am J Kidney Dis. 2000;36:735–744. doi: 10.1053/ajkd.2000.17620. [DOI] [PubMed] [Google Scholar]
- 11.Sengul S., Zwizinski C., Batuman V. Role of MAPK pathways in light chain–induced cytokine production in human proximal tubule cells. Am J Physiol Renal Physiol. 2003;284:F1245–F1254. doi: 10.1152/ajprenal.00350.2002. [DOI] [PubMed] [Google Scholar]
- 12.Li M., Balamuthusamy S., Simon E.E., Batuman V. Silencing megalin and cubilin genes inhibits myeloma light chain endocytosis and ameliorates toxicity in human renal proximal tubule epithelial cells. Am J Physiol Renal Physiol. 2008;295:F82–F90. doi: 10.1152/ajprenal.00091.2008. [DOI] [PubMed] [Google Scholar]
- 13.Wang X.S., Diener K., Jannuzzi D., Trollinger D., Tan T.H., Lichenstein H., Zukowski M., Yao Z. Molecular cloning and characterization of a novel protein kinase with a catalytic domain homologous to mitogen-activated protein kinase kinase kinase. J Biol Chem. 1996;271:31607–31611. doi: 10.1074/jbc.271.49.31607. [DOI] [PubMed] [Google Scholar]
- 14.Ichijo H., Nishida E., Irie K., ten Dijke P., Saitoh M., Moriguchi T., Takagi M., Matsumoto K., Miyazono K., Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 15.Van Laethem A., Nys K., Van Kelst S., Claerhout S., Ichijo H., Vandenheede J.R., Garmyn M., Agostinis P. Apoptosis signal regulating kinase-1 connects reactive oxygen species to p38 MAPK-induced mitochondrial apoptosis in UVB-irradiated human keratinocytes. Free Radic Biol Med. 2006;41:1361–1371. doi: 10.1016/j.freeradbiomed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Hatai T., Matsuzawa A., Inoshita S., Mochida Y., Kuroda T., Sakamaki K., Kuida K., Yonehara S., Ichijo H., Takeda K. Execution of apoptosis signal-regulating kinase 1 (ASK1)-induced apoptosis by the mitochondria-dependent caspase activation. J Biol Chem. 2000;275:26576–26581. doi: 10.1074/jbc.M003412200. [DOI] [PubMed] [Google Scholar]
- 17.Saitoh M., Nishitoh H., Fujii M., Takeda K., Tobiume K., Sawada Y., Kawabata M., Miyazono K., Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R., Al-Lamki R., Bai L., Streb J.W., Miano J.M., Bradley J., Min W. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ Res. 2004;94:1483–1491. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]
- 19.Matsuzawa A., Ichijo H. Redox control of cell fate by MAP kinase: physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim Biophys Acta. 2008;1780:1325–1336. doi: 10.1016/j.bbagen.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Matsuzawa A., Nishitoh H., Tobiume K., Takeda K., Ichijo H. Physiological roles of ASK1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: advanced findings from ASK1 knockout mice. Antioxid Redox Signal. 2002;4:415–425. doi: 10.1089/15230860260196218. [DOI] [PubMed] [Google Scholar]
- 21.Tobiume K., Matsuzawa A., Takahashi T., Nishitoh H., Morita K., Takeda K., Minowa O., Miyazono K., Noda T., Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tobiume K., Saitoh M., Ichijo H. Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J Cell Physiol. 2002;191:95–104. doi: 10.1002/jcp.10080. [DOI] [PubMed] [Google Scholar]
- 23.Izumi Y., Kim-Mitsuyama S., Yoshiyama M., Omura T., Shiota M., Matsuzawa A., Yukimura T., Murohara T., Takeya M., Ichijo H., Yoshikawa J., Iwao H. Important role of apoptosis signal-regulating kinase 1 in ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2005;25:1877–1883. doi: 10.1161/01.ATV.0000174801.76234.bd. [DOI] [PubMed] [Google Scholar]
- 24.Terada Y., Inoshita S., Kuwana H., Kobayashi T., Okado T., Ichijo H., Sasaki S. Important role of apoptosis signal-regulating kinase 1 in ischemic acute kidney injury. Biochem Biophys Res Commun. 2007;364:1043–1049. doi: 10.1016/j.bbrc.2007.10.122. [DOI] [PubMed] [Google Scholar]
- 25.Kim A.H., Khursigara G., Sun X., Franke T.F., Chao M.V. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol. 2001;21:893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee S.G. Cell signaling: H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 27.Wang P.X., Sanders P.W. Immunoglobulin light chains generate hydrogen peroxide. J Am Soc Nephrol. 2007;18:1239–1245. doi: 10.1681/ASN.2006111299. [DOI] [PubMed] [Google Scholar]
- 28.Basnayake K., Ying W.Z., Wang P.X., Sanders P.W. Immunoglobulin light chains activate tubular epithelial cells through redox signaling. J Am Soc Nephrol. 2010;21:1165–1173. doi: 10.1681/ASN.2009101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ying W.Z., Wang P.X., Aaron K.J., Basnayake K., Sanders P.W. Immunoglobulin light chains activate NF-κB in renal epithelial cells through a Src-dependent mechanism. Blood. 2011;117:1301–1307. doi: 10.1182/blood-2010-08-302505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan M.J., Johnson G., Kirk J., Fuerstenberg S.M., Zager R.A., Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994;45:48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- 31.Mead G.P., Carr-Smith H.D., Drayson M.T., Morgan G.J., Child J.A., Bradwell A.R. Serum free light chains for monitoring multiple myeloma. Br J Haematol. 2004;126:348–354. doi: 10.1111/j.1365-2141.2004.05045.x. [DOI] [PubMed] [Google Scholar]
- 32.Hanke J.H., Gardner J.P., Dow R.L., Changelian P.S., Brissette W.H., Weringer E.J., Pollok B.A., Connelly P.A. Discovery of a novel, potent, and Src family–selective tyrosine kinase inhibitor: Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 33.Ouyang M., Shen X. Critical role of ASK1 in the 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J Neurochem. 2006;97:234–244. doi: 10.1111/j.1471-4159.2006.03730.x. [DOI] [PubMed] [Google Scholar]
- 34.Berggård I., Peterson P.A. Polymeric forms of free normal k and λ chains of human immunoglobulin. J Biol Chem. 1969;244:4299–4307. [PubMed] [Google Scholar]
- 35.Sanders P.W., Herrera G.A., Kirk K.A., Old C.W., Galla J.H. Spectrum of glomerular and tubulointerstitial renal lesions associated with monotypical immunoglobulin light chain deposition. Lab Invest. 1991;64:527–537. [PubMed] [Google Scholar]
- 36.Sanders P.W., Booker B.B., Bishop J.B., Cheung H.C. Mechanisms of intranephronal proteinaceous cast formation by low molecular weight proteins. J Clin Invest. 1990;85:570–576. doi: 10.1172/JCI114474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kapur U., Barton K., Fresco R., Leehey D.J., Picken M.M. Expanding the pathologic spectrum of immunoglobulin light chain proximal tubulopathy. Arch Pathol Lab Med. 2007;131:1368–1372. doi: 10.5858/2007-131-1368-ETPSOI. [DOI] [PubMed] [Google Scholar]
- 38.Herrera G.A., Sanders P.W., Reddy B.V., Hasbargen J.A., Hammond W.S., Brooke J.D. Ultrastructural immunolabeling: a unique diagnostic tool in monoclonal light chain–related renal diseases. Ultrastruct Pathol. 1994;18:401–416. doi: 10.3109/01913129409023211. [DOI] [PubMed] [Google Scholar]
- 39.Li X., Pabla N., Wei Q., Dong G., Messing R.O., Wang C.Y., Dong Z. PKC-delta promotes renal tubular cell apoptosis associated with proteinuria. J Am Soc Nephrol. 2010;21:1115–1124. doi: 10.1681/ASN.2009070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan A.M., Li M., Balamuthusamy S., Maderdrut J.L., Simon E.E., Batuman V. Myeloma light chain–induced renal injury in mice. Nephron Exp Nephrol. 2010;116:e32–e41. doi: 10.1159/000317129. [DOI] [PubMed] [Google Scholar]
- 41.Izumi Y., Kim S., Yoshiyama M., Izumiya Y., Yoshida K., Matsuzawa A., Koyama H., Nishizawa Y., Ichijo H., Yoshikawa J., Iwao H. Activation of apoptosis signal-regulating kinase 1 in injured artery and its critical role in neointimal hyperplasia. Circulation. 2003;108:2812–2818. doi: 10.1161/01.CIR.0000096486.01652.FC. [DOI] [PubMed] [Google Scholar]
- 42.Roy S., Khanna S., Azad A., Schnitt R., He G., Weigert C., Ichijo H., Sen C.K. Fra-2 mediates oxygen-sensitive induction of transforming growth factor beta in cardiac fibroblasts. Cardiovasc Res. 2010;87:647–655. doi: 10.1093/cvr/cvq123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ying W.Z., Aaron K., Sanders P.W. Dietary salt activates an endothelial proline-rich tyrosine kinase 2/c-Src/phosphatidylinositol 3-kinase complex to promote endothelial nitric oxide synthase phosphorylation. Hypertension. 2008;52:1134–1141. doi: 10.1161/HYPERTENSIONAHA.108.121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kyle R.A., Gertz M.A., Witzig T.E., Lust J.A., Lacy M.Q., Dispenzieri A., Fonseca R., Rajkumar S.V., Offord J.R., Larson D.R., Plevak M.E., Therneau T.M., Greipp P.R. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 45.Blade J., Fernandez-Llama P., Bosch F., Montoliu J., Lens X.M., Montoto S., Cases A., Darnell A., Rozman C., Montserrat E. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med. 1998;158:1889–1893. doi: 10.1001/archinte.158.17.1889. [DOI] [PubMed] [Google Scholar]
- 46.Herrera G.A., Joseph L., Gu X., Hough A., Barlogie B. Renal pathologic spectrum in an autopsy series of patients with plasma cell dyscrasia. Arch Pathol Lab Med. 2004;128:875–879. doi: 10.5858/2004-128-875-RPSIAA. [DOI] [PubMed] [Google Scholar]
- 47.Sanders P.W., Booker B.B. Pathobiology of cast nephropathy from human Bence Jones proteins. J Clin Invest. 1992;89:630–639. doi: 10.1172/JCI115629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basnayake K., Cheung C.K., Sheaff M., Fuggle W., Kamel D., Nakoinz S., Hutchison C.A., Cook M., Stoves J., Bradwell A.R., Cockwell P. Differential progression of renal scarring and determinants of late renal recovery in sustained dialysis dependent acute kidney injury secondary to myeloma kidney. J Clin Pathol. 2010;63:884–887. doi: 10.1136/jcp.2010.079236. [DOI] [PubMed] [Google Scholar]
- 49.Hutchison C.A., Cockwell P., Stringer S., Bradwell A., Cook M., Gertz M.A., Dispenzieri A., Winters J.L., Kumar S., Rajkumar S.V., Kyle R.A., Leung N. Early reduction of serum-free light chains associates with renal recovery in myeloma kidney. J Am Soc Nephrol. 2011;22:1129–1136. doi: 10.1681/ASN.2010080857. [DOI] [PMC free article] [PubMed] [Google Scholar]