Abstract
Type 2 diabetes is a key risk factor for ischemia-dependent pathology; therefore, a significant medical need exists to develop novel therapies that increase the formation of new vessels. We explored the therapeutic potential of epidermal growth factor receptor tyrosine kinase (EGFRtk) and extracellular signal–regulated kinase 1/2 (ERK1/2) inhibition in impaired ischemia-induced neovascularization in type 2 diabetes. Unilateral femoral artery ligation was performed in diabetic (db−/db−) and their control (db−/db+) mice for 4 weeks, followed by treatments with EGFRtk and ERK1/2 inhibitors (AG1478, 10 mg/kg/day and U0126, 400 μg/kg/day, respectively) for 3 weeks. Neovascularization, blood flow recovery, vascular and capillary density, and endothelial nitric oxide synthase activity were significantly impaired and were associated with enhanced EGFRtk and ERK1/2 activity in db−/db− mice. EGFRtk and ERK1/2 inhibitors did not have any effect in control mice, while in db−/db− mice there was a significant increase in neovascularization, blood flow recovery, vascular and capillary density, endothelial nitric oxide synthase activity, and were associated with a decrease in EGFRtk and ERK1/2 activity. Our data demonstrated that the inhibition of EGFRtk and ERK1/2 restored ischemia-induced neovascularization and blood flow recovery in type 2 diabetic mice. Thus, EGFRtk and ERK1/2 could be possible targets to protect from ischemia-induced vascular pathology in type 2 diabetes.
Almost 26 million Americans have diabetes and >650,000 new cases are diagnosed every year.1 Large epidemiological studies reveal that diabetes is linked to metabolic syndrome and vascular disease.2 Diabetes is a powerful risk factor for coronary artery disease, stroke, and peripheral arterial disease.3 Because the formation of new vessels in response to ischemia is compromised, diabetes significantly accelerates lower extremity arterial disease and accounts for 60% of all nontraumatic limb amputations in the Unites States.4 In addition, previous studies reported abnormalities in neovascularization in diabetic patients and animal models with peripheral artery disease.5,6 However, the underlying mechanism responsible for impaired ischemia-induced neovascularization in type 2 diabetes is still unclear.
Loss of a limb produces a permanent disability that can impact a patient's self-image, self-care, and mobility, which negatively affects society. Therefore, there is a significant medical need to develop novel therapies to increase the formation of new vessels, especially in patients with type 2 diabetes. Because well-developed new blood vessels are known to lower ischemia-induced pathology, we speculate that the restoration of tissue blood flow by increasing the formation of new vessels would significantly improve patient outcome.
In a previous study, we demonstrated that enhanced epidermal growth factor receptor tyrosine kinase (EGFRtk) activity is involved in microvascular dysfunction in type 2 diabetes.7 We also observed that the mitogen-activated protein kinase (MAPK) family proteins extracellular signal-regulated kinase 1 and 2 (ERK1/2) are implicated in the homeostasis of microvessels. EGFRtk consists of a 1186-amino acid glycoprotein containing a single trans-membrane domain with intracellular portion containing the tyrosine kinase domain.7 EGFRtk can be activated by different ligands such as EGF and heparin-binding EGF-like factor.8 Although the involvement of EGFRtk is well documented in tumor angiogenesis, the role of EGFRtk and the downstream signaling (ERK1/2) in neovascularization in the ischemic hind limb of type 2 diabetic mice is not known. Thus, the purpose of this study was to determine the potential therapeutic effect of EGFRtk and ERK1/2 inhibition to treat impaired ischemia-induced vascular pathology in type 2 diabetic mice.
Materials and Methods
Animal Model and Surgery
Obese homozygote (db−/db−) type 2 diabetic mice lacking the gene encoding for leptin receptor (Lepr) (diabetic, 8 to 10 weeks old) and their control heterozygote Lepr db−/db+ (db−/db+) nondiabetic (control, 8 to 10 weeks old) adult male mice were obtained from the Jackson Laboratories (Bar Harbor, ME). The hind-limb ischemia procedure was performed in all mice by ligation of the proximal segment of the right femoral artery for 4 weeks, as previously described,6,9 and then 3 weeks of treatment in the following groups: control mice without treatment (n = 10); control mice treated with AG1478 (10 mg/kg/day in mini-osmotic pumps, n = 10); control mice treated with U0126 (400 μg/kg/day in mini-osmotic pumps, n = 10); diabetic mice without treatment (n = 10); diabetic mice treated with AG1478 (LC Laboratories, Woburn, MA; 10 mg/kg/day in mini-osmotic pumps, n = 10); and diabetic mice treated with U0126 (LC Laboratories; 400 μg/kg/day in mini-osmotic pumps, n = 10). These studies are conformed to the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Tulane University Institutional Animal Care and Use Committee.
Blood Glucose
Blood glucose measurements were obtained from tail blood samples using a blood glucose meter (Prestige Smart System HDI; Home Diagnostics, Inc., Fort Lauderdale, FL) in all groups of mice after a 6 hours fast.6
Insulin Resistance
Insulin level was determined at the end of treatment using the Ultrasensitive Mouse Insulin enzyme-linked immunosorbent assay (ELISA) protocol (Mercodia, Uppsala, Sweden), which estimates steady-state insulin resistance.6
Blood Pressure
Systolic blood pressure (SBP) was measured by tail-cuff plethysmography (Softron, BP-98A), before treatment and then once a week as previously described.6,10 All mice were trained for tail cuff plethysmography 1 week before the experiments. SBP was measured in conscious mice using the CODA tail-cuff blood pressure system (Kent Scientific, Torrington, CT). Systolic arterial blood pressure measurements were performed at the same time (between 9 and 11 AM) to avoid the influence of the circadian cycle, and the value for SBP was obtained by estimating the average of eight measurements for a single measurement.
Laser Doppler Measurement of Hind-Limb Blood Flow
Each mouse was warmed to a core temperature of 37°C, and then hind-limb blood flow measurements over the region of interest were performed before surgery, immediately after surgery, and serially over a 7-week period with laser Doppler perfusion imaging (LDPI) (Moor Instruments, Wilmington, DE). The blood flow of right and left hind limbs was assessed by scanning the same lower abdomen region and limbs of mice with a laser Doppler blood flow meter as previously reported.6
X-Ray Quantification of the Hind-Limb Angiogenesis
Vessel density was assessed by microangiography at the end of the treatment period, as previously described.6,11 Briefly, mice were anesthetized and a contrast medium (barium sulfate, 0.5 g/mL) was injected through a catheter introduced into the abdominal aorta. The vessel density quantification was determined using Multi Gauge Fujifilm (Tokyo, Japan).
Colorimetric Determination of cGMP
cGMP levels were measured in hind-limb muscle in all groups of mice. Mice were sacrificed and hind-limb muscles were immediately harvested and frozen in liquid nitrogen. Measurements were performed using a sandwich enzyme-linked immunosorbent assay (cGMP enzyme-linked immunosorbent assay kit; Cayman Chemical, Ann Arbor, MI) according to the manufacturer instructions and as previously described.6,12
Immunohistochemistry
Immunohistochemistry was performed as previously described.6,13 After 7 weeks, mice were anesthetized and perfused with formalin for 45 minutes. Hind-limb muscles were then harvested, embedded in paraffin, and sectioned at 5 μm. Sections were heated with citrate buffer at 95°C for 40 minutes for antigen retrieval. After blocking with 5% BSA in phosphate-buffered saline (PBS), sections were incubated overnight at 4°C with rabbit monoclonal antibody against CD31 (BD Pharmigen, San Jose, CA), α-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). For every section, a negative control without primary antibody was processed simultaneously. After 15 minutes of washing in PBS, a secondary rabbit antibody coupled to Alexa Fluor (Molecular Probes, Carlsbad, CA) was added for 1 hour at room temperature. Cell nuclei were counterstained with DAPI (Molecular Probes). Staining was evaluated using fluorescent a microscope. The capillary density was then determined by counting the number of capillaries in each section of muscle.
RT-PCR Assay
EGFR, vascular endothelial growth factor (VEGF), endothelial nitric oxide synthase (eNOS), and MAPK1 mRNA levels were determined in hind-limb tissues from control and diabetic mice. Total RNA was obtained using the RNeasy Fibrous Tissue Mini Kit (Quiagen, Valencia, CA) according to the manufacturer's recommendations. A total of 1 μg of DNase I-treated RNA was reverse transcribed into cDNA using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) with random hexamers in a 20 μL reaction. PCR was performed in duplicate for each sample using 1 μL of cDNA as a template, 1× of TaqMan Universal PCR Master Mix (Applied Biosystems), and 10× of TaqMan Gene Expression Assays (Applied Biosystems) in a 20 μL reaction. Assays-on-Demand (Applied Biosystems) of TaqMan fluorescent real-time PCR primers and probes were used for EGFR (Mm 00433023_m1), VEGF (Mm01281449_m1), eNOS (Mm00435217_m1), MAPK1 (Mm00442479_m1), and18S rRNA (Hs99999901_s1), which was used as an endogenous control to normalize results. Quantitative reverse transcription-PCR was carried out in an Mx 3000 RT-PCR platform (Agilent Technologies Stratagene, La Jolla, CA) using the following conditions: 1 minute at 50°C, 10 minutes at 95°C followed by 40 cycles of 15 seconds at 95°C, and 1 minute at 60°C. Relative EGFR, VEGF, eNOS, and MAPK1 mRNA levels were determined using the ΔΔCt method. Results are expressed as the relative expression of mRNA in the treated control and db/db mice compared with untreated control mice.
Western Blot Analysis
Western blot analysis was performed as previously described.7,10 We used Western blot analysis to assess the phosphorylation and expression of ERK1/2 (Promega, Madison, WI), EGFR (ECM Biosciences, Versailles, KY), eNOS (Cell Signaling, Boston, MA), expression of VEGF (Cell Signaling), and glyceraldehyde-3-phosphate dehydrogenase (Cell Signaling) using specific antibodies. The quantification of Western blot was determined using Fujifilm-Multi Gauge software.
Statistical Analysis
Results are expressed as mean ± SEM. One-way or two-way analysis of variance was used to compare each parameter when appropriate. Comparisons between groups were performed with t-tests when the analysis of variance test was statistically significant. Values of P < 0.05 were considered significant. Differences between specified groups were analyzed using the Student's t-test (two-tailed) for comparing two groups with P < 0.05 considered statistically significant.
Results
Effect of EGFRtk and ERK1/2 Inhibition on Blood Glucose, Insulin Levels, Body Weight, and SBP
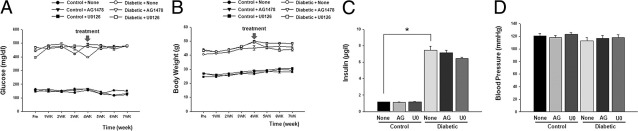
Blood glucose, insulin levels and body weight were increased in diabetic than in control mice; and were not affected by the treatments (Figure 1, A–C). Systolic blood pressure was similar in all groups of mice (Figure 1D).
Figure 1.
Blood glucose, body weight, insulin, and blood pressure in control mice and diabetic mice treated with and without (None) AG1478 (AG) or U0126 (U0). A: Comparison of blood glucose levels between control and diabetic mice with or without AG1478 or U0126, n = 10. B: Comparison of body weight between control and diabetic mice with or without AG1478 or U0126, n = 10. C: Serum insulin levels in all groups, (n = 10); *P < 0.05 for diabetic versus control mice. D: Blood pressure measurements with tail-cuff methods in all groups, n = 10.
Effect of EGFRtk and ERK1/2 MAP Kinase Inhibition on Blood Flow
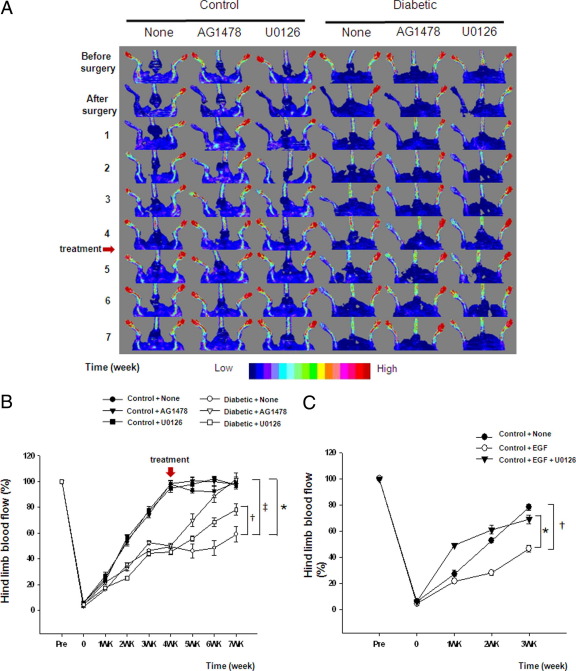
Blood flow was measured using Doppler-flow before, just after surgery, and then once a week for 7 weeks in all groups of mice (Figure 2, A and B). Blood flow was significantly decreased to <5% of control value after femoral artery ligation in all groups of mice. After 4 weeks, blood flow recovery in control mice was 97.62% ± 2.82%. However, blood flow was significantly reduced in the ischemic hind limb from diabetic mice compared with control mice. Four weeks after surgery, control and diabetic mice were then treated with AG1478 or U0126 for 3 weeks. Intriguingly, Figure 2A and B revealed a significant increase in blood flow recovery in the ischemic hind limb from diabetic mice treated with AG1478 or U0126 in comparison with nontreated diabetic mice. The chronic treatment with AG1478 and U0126 did not affect blood flow in control mice (Figure 2, A and B).
Figure 2.
Blood flow analysis in hind limb in diabetic and control mice. A: Blood flow was measured with MoorLDI-Laser in all groups before surgery, just after surgery and once a week for 7 weeks (n = 10). B: Quantitative data of blood flow measurements with MoorLDI-Laser in all groups, (n = 10); *P < 0.05 for diabetic versus control, †P < 0.05 for diabetic treated with U0126 versus diabetic mice, and ‡P < 0.05 statistically significant between diabetic versus diabetic treated with AG1478. Red arrow represents macrophages. C: Quantitative data of blood flow analysis in hind limb of nontreated control mice and control mice injected with EGF or with EGF + U0126, (n = 5); *P < 0.05 indicating a significant difference between control treated with EGF + U0126 versus control treated with EGF, †P < 0.05 for nontreated control versus control treated with EGF.
To determine the direct effect of EGFRtk and ERK1/2 on blood flow recovery regulation, control mice that had femoral artery ligation were locally injected, in the ischemic hind limb, with exogenous EGF (50 ng/mouse) with or without U0126 (400 μg/kg/day) for 3 weeks. Data revealed that increased EGFRtk stimulation significantly reduced blood flow recovery, which was prevented with pretreatment of mice with U0126 (Figure 2C).
Effect of EGFRtk and ERK1/2 Inhibition on Vessel and Capillary Density, and cGMP Content
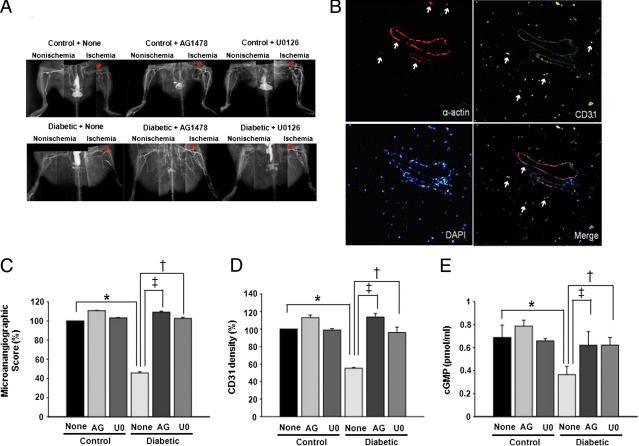
At the end of the experiment, we evaluated vessel density with high-definition microangiography. Contrast media (barium sulfate, 0.5 g/mL) was injected into abdominal aorta. Angiographic measurement of right and left hind limbs was determined using digital X-ray (Figure 3A). Vessel density was quantified with Fujifilm-Multi Gauge software (Figure 3C). Ischemic hind-limb vessel density was similar in control groups of mice with or without treatment. In contrast, vessel density in nontreated diabetic mice was significantly lower compared with control mice. In diabetic mice treated with AG1478 or U0126, vessel density was significantly increased compared with nontreated diabetic mice (Figure 3, A and C).
Figure 3.
A: Microangiography at the end of the treatment period in all groups. Images were assembled to obtain a complete view of the hind limb; each picture is representative of n = 4. Red arrows indicate femoral artery ligation. B: Example of immunostaining with specific antibodies for CD31 (green staining), α-actin (red staining), and nucleus (blue staining) in ischemic hind limbs. White arrows indicate α-actin in top left panel, CD31 in top right panel, and combination in lower right panel. C: Quantitative data (score in percent) showing vessel density in hind limb using Multi Gauge - FUJIFILM by selecting the same area of measurements in all groups, n = 4. D: Quantitative data of CD31 staining in hind limb in all groups. E: cGMP levels in ischemic hind limb in all groups. C–E: *P < 0.05 for diabetic versus control, ‡P < 0.05 indicating a significant difference between diabetic versus diabetic treated with AG1478 (AG), and †P < 0.05 for diabetic versus diabetic treated with U0126 (U0).
Blood flow recovery and microangiographic data were confirmed by capillary density analysis using the CD31 staining (Figure 3B). Capillary density in the ischemic hind limb was similar in control groups of mice with or without treatment. A significant increase in the capillary density was observed in the ischemic hind limb from diabetic mice treated with AG1478 or U0126 compared with nontreated diabetic mice (Figure 3D).
Colorimetric determination of cGMP content in ischemic hind-limb muscle lysates was performed in all groups of mice. The cGMP level was significantly lower in ischemic hind limb in diabetic mice than in control mice. Diabetic mice treated with AG1478 or U0126 displayed a significant increase in cGMP level compared with nontreated diabetic mice (Figure 3E).
Effect of EGFRtk and ERK1/2 Inhibition on mRNA Level of MAPK, EGFRtk, VEGF, and eNOS
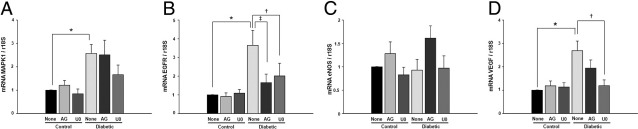
We determined mRNA levels of EGFR, VEGF, MAPK1, and eNOS in ischemic hind limbs from all groups of mice. Figure 4A illustrates that MAPK1 mRNA level was elevated in diabetic mice compared with control mice. The treatment with AG1478 and U0126 did not affect the mRNA level of MAPK1 in control and diabetic mice (Figure 4A). EGFRtk mRNA level was higher in diabetic mice compared with control mice (Figure 4B). Treatment with AG1478 and U0126 did not affect the mRNA level of EGFRtk in control mice but was significantly reduced in diabetic mice (Figure 4B). The mRNA level for eNOS in all groups was not changed (Figure 4C). VEGF mRNA level was increased in diabetic mice compared with control mice with and without treatment (Figure 4D). The treatment with AG1478 and U0126 reduced the mRNA level for VEGF in diabetic mice (Figure 4D).
Figure 4.
Quantitative reverse transcription-PCR assessment of (A) MAPK1 (n = 5), (B) EGFR (n = 5), (C) eNOS (n = 5), and (D) VEGF (n = 5) mRNA levels in ischemic hind-limb muscles in control and diabetic mice with or without AG1478 or U0126. The mRNA level was normalized to 18S rRNA as endogenous control. A–D: Results are expressed as the relative expression of mRNA compared to nontreated control mice; *P < 0.05 for diabetic versus control, †P < 0.05 for diabetic versus diabetic treated with U0126 (U0), and ‡P < 0.05 indicating a significant difference between diabetic versus diabetic treated with AG1478 (AG).
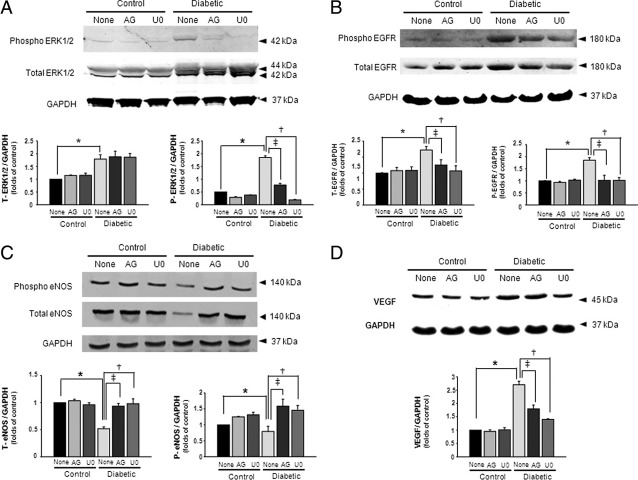
Effect of EGFRtk and ERK1/2 Inhibition on the Expression and Phosphorylation Level of ERK1/2, EGFRtk, eNOS, and VEGF
Figure 5A illustrates an increase in ERK1/2 expression and phosphorylation in diabetic mice compared with control mice. The treatment with AG1478 and U0126 did not affect ERK1/2 expression but significantly reduced phosphorylation levels in diabetic mice (Figure 5A). In control mice, the treatment did not affect ERK1/2 expression and phosphorylation (Figure 5A). We also measured the expression and phosphorylation of EGFRtk in all groups of mice. The data revealed an increase in EGFRtk expression and phosphorylation in diabetic mice, which was significantly reduced by AG1478 and U0126 treatment (Figure 5B). In control mice, AG1478 and U0126 had no effect on EGFRtk expression and phosphorylation (Figure 5B). The expression and phosphorylation of eNOS were significantly reduced in diabetic mice compared with control mice (Figure 5C). Chronic treatment with AG1478 and U0126 increased eNOS expression and phosphorylation in diabetic mice but no effect was observed in control mice (Figure 5C). The VEGF expression was significantly higher in diabetic mice compared treated diabetic mice and all control groups of mice (Figure 5D).
Figure 5.
Western blot analysis of (A) total and phosphorylated ERK1/2 (n = 5), (B) total and phosphorylated EGFR (n = 5), (C) total and phosphorylated eNOS (n = 5), and (D) VEGF (n = 5) protein levels in ischemic hind-limb muscles in control and diabetic mice with or without AG1478 (AG) or U0126 (U0). A–D: Results are normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression; *P < 0.05 for diabetic versus control, †P < 0.05 indicating a significant difference between diabetic versus diabetic treated with U0126, and ‡P < 0.05 for diabetic versus diabetic treated with AG1478.
Discussion
Impaired ischemia-induced neovascularization is a major risk factor for amputation, stroke, and heart attack in type 2 diabetic patients, which represents a major public health issue in the United States and worldwide. The ability to elicit a neovascularization response varies considerably between tissues, species, and even individual patients with similar degrees of ischemia burden. Understanding the key regulatory processes is critical for developing a new strategy for a potential therapy. In the present study, we demonstrated for the first time the involvement of EGFRtk and ERK1/2 in the impaired ischemia-induced neovascularization in type 2 diabetic mice. Importantly, chronic inhibition of EGFRtk and ERK1/2 restored ischemia-induced neovascularization and subsequently blood flow recovery in type 2 diabetic mice.
The induction of neovascularization is thought to be dependent on variety of factors that include cell therapy (stem cells), intermediate signaling (nitric oxide-cGMP), and growth factors (VEGF and EGF).6,14–16 Signaling through EGFR is generally believed to be angiogenic17 in cancer and its inhibition suppresses angiogenesis. The EGFR signaling is completely dysregulated in tumors due to different somatic mutations in the EGF receptor, as it has been previously reported.18,19 In our previous studies, we demonstrated that the enhanced EGFRtk phosphorylation level in type 2 diabetic mice is responsible for microvascular dysfunction. Interestingly the inhibition of EGFRtk reduced EGFR phosphorylation and improved microvascular function in db−/db− mice.7 In the present study, the inhibition of EGFRtk increases ischemia-induced neovascularization. Therefore, it is important to mention that cancer and vascular complications in type 2 diabetes are two different diseases in terms of etiology and mechanisms.
We also showed that ERK1/2, downstream signaling of the EGFRtk, is an important key element in the homeostasis of microvessels.7 Thus, the present study was carried out to chronically inhibit EGFRtk and ERK1/2 and restore ischemia-induced neovascularization in type 2 diabetic mice. Our protocol was first to induce ischemia in hind limb of diabetic and control mice for 4 weeks and then treat the mice with EGFRtk and ERK1/2 inhibitors for 3 weeks.
Glucose, insulin levels and body weight were elevated in diabetic mice compared with control mice. The chronic treatment did not affect these values indicating that EGFRtk and ERK1/2 are not involved in the etiology but rather the consequence of type 2 diabetes. The systolic blood pressure was normal in all groups of mice suggesting that db−/db− mice are normotensive20 and the treatment did not affect arterial blood pressure.
When control and diabetic mice hind limbs were first subjected to ischemia for 4 weeks, blood flow recovery was 100% in control mice compared with 49% in diabetic mice. These data clearly indicate that blood flow recovery in type 2 diabetes is compromised and suggests that neovascularization in response to chronic ischemia is impaired. These data are in agreement with previous studies showing that neovascularization is altered in type 2 diabetic patients and animal models.6,21,22 Importantly, the treatment of diabetic mice with EGFRtk or ERK1/2 inhibitors, started 4 weeks after femoral artery ligation, restored blood flow recovery reaching 100%. These novel findings demonstrate that EGFRtk and ERK1/2 are important factors in the impaired ischemia-induced neovascularization response and are independent of glucose, insulin and obesity regulation; and could be downstream to the effects of hyperglycemia and insulin resistance.
Using an alternative strategy, we evaluated vessel and capillary density in the ischemic hind limb of all groups of mice. We observed that vessel and capillary density were significantly reduced in diabetic mice compared with control mice. Importantly, vessel and capillary density in diabetic mice was reversed by AG1478 or U0126 treatments. These data strengthen our previous findings indicating23 that EGFRtk and ERK1/2 are critical in this process. These data are in agreement with our previous data showing an induction of structural wall remodeling of resistance arteries, which was reduced by EGFRtk inhibition.24
The induction of neovascularization results from the balance between pro-angiogenic and anti-angiogenic factors.25 In this study, we determined the mechanism by which the chronic inhibition of EGFRtk and ERK1/2 enhanced ischemia-induced neovascularization and blood flow recovery in type 2 diabetic mice. It is well established that the NO-cGMP pathway, as an intermediate signaling pathway, regulates VEGF-dependent neovascularization.26–28 In the present study we demonstrated that eNOS phosphorylation and expression, and cGMP levels were significantly reduced in diabetic mice compared with control mice, which suggests that the eNOS pathway is compromised and participates in impaired neovascularization. Importantly, the chronic inhibition of EGFRtk and ERK1/2 enhanced eNOS-cGMP pathway activity and ischemia-induced neovascularization, which suggests that the eNOS pathway is regulated by EGFRtk and ERK1/2 activity. Further studies are needed to delineate the mechanism linking EGFRtk and ERK1/2 to eNOS activity and the downstream signaling molecule cGMP. It is well established that VEGF is critical for neovascularization.29 It is surprising that VEGF level is augmented in diabetic mice indicating that the binding and signaling could be compromised. These data are in agreement with previous studies showing an increase in VEGF levels with reduced VEGF signaling in type 2 diabetes.30–33 The chronic inhibition of EGFRtk and ERK1/2 reduced VEGF levels and was associated with improved ischemia-induced neovascularization indicating an improvement in VEGF binding and signaling. We suggest that EGFRtk and ERK1/2 inhibition improves VEGFR binding and signaling, and this could explain the reduction in VEGF levels associated with increased neovascularization.
There is an association between ERK1/2 and angiogenesis.34 In this study, we found an increase in ERK1/2 expression and phosphorylation in diabetic mice. The chronic inhibition of ERK1/2 significantly reduced ERK1/2 phosphorylation, which was associated with enhanced ischemia-induced neovascularization in diabetic mice. These data suggest that ERK1/2 is an anti-angiogenic factor in type 2 diabetic mice. An effect on ischemia-induced neovascularization was not seen in control mice treated with ERK1/2, indicating that ERK1/2 is not an important factor in neovascularization. These data are not in agreement with a previous study showing that GRb2 is important in angiogenesis signaling through Akt and ERK1/2.34 We previously reported that nitric oxide inhibits ERK1/2 phosphorylation.28 Our current findings are in agreement with our previous study.35,36 Diabetic mice have less nitric oxide, which leads to an increase in ERK1/2 phosphorylation and causes impaired ischemia-induced neovascularization. ERK1/2 is a downstream signal for EGFRtk; therefore, it is more likely that inhibiting EGFRtk would have a readout similar to that of the inhibition of ERK1/2. In addition, a previous study reported that ERK1/2 activation was suppressed in the presence of AG1478, while the phosphorylation of JNK and p38 were not affected.37 It was also reported that EGF increases ERK1/2 activation.38 Thus, the chronic treatment of diabetic mice with ERK1/2 inhibitor significantly enhanced eNOS expression and phosphorylation, associated with augmented ischemia-induced neovascularization. However, eNOS mRNA levels were similar all groups of mice, which indicate a posttranscriptional regulation event39–42 and possible eNOS mRNA degradation in type 2 diabetes. Our data indicate a potential interaction between eNOS pathway, ERK1/2, and ischemia-induced neovascularization in diabetic mice. Further studies are needed to elucidate the mechanism of the interaction between nitric oxide and ERK1/2 in respect to neovascularization in type 2 diabetes.
In conclusion, we observed that in type 2 diabetic mice, chronic inhibition of EGFRtk and ERK1/2 re-established ischemia-induced neovascularization and hind-limb blood flow recovery. Therefore, EGFRtk and ERK1/2 should be potential targets for therapeutic strategy to protect against impaired ischemia-induced vascular pathology in type 2 diabetes.
Footnotes
Supported by NIH grants 1R01HL095566 (K.M.) and 5R01HL097111 (M.T.).
Contributor Information
Souad Belmadani, Email: sbelma@lsuhsc.edu.
Khalid Matrougui, Email: kmatroug@tulane.edu.
References
- 1.Akbari C.M., LoGerfo F.W. Diabetes and peripheral vascular disease. J Vasc Surg. 1999;30:373–384. doi: 10.1016/s0741-5214(99)70154-0. [DOI] [PubMed] [Google Scholar]
- 2.Saydah S.H., Fradkin J., Cowie C.C. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–342. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 3.Entabi F., Albadawi H., Stone D.H., Sroufe R., Conrad M.F., Watkins M.T. Hind limb ischemia-reperfusion in the leptin receptor deficient (db/db) mouse. J Surg Res. 2007;139:97–105. doi: 10.1016/j.jss.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Nathan D.M. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328:1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 5.Rivard A., Silver M., Chen D., Kearney M., Magner M., Annex B., Peters K., Isner J.M. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999;154:355–363. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amin AH, Abd Elmageed ZY, Nair D, Partyka MI, Kadowitz PJ, Belmadani S, Matrougui K: Modified multipotent stromal cells with epidermal growth factor restore vasculogenesis and blood flow in ischemic hind-limb of type II diabetic mice. Lab Invest 90:985–996 [DOI] [PMC free article] [PubMed]
- 7.Belmadani S., Palen D.I., Gonzalez-Villalobos R.A., Boulares H.A., Matrougui K. Elevated epidermal growth factor receptor phosphorylation induces resistance artery dysfunction in diabetic db/db mice. Diabetes. 2008;57:1629–1637. doi: 10.2337/db07-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matrougui K: Diabetes and microvascular pathophysiology: role of epidermal growth factor receptor tyrosine kinase. Diabetes Metab Res Rev 26:13–16 [DOI] [PMC free article] [PubMed]
- 9.Senthilkumar A., Smith R.D., Khitha J., Arora N., Veerareddy S., Langston W., Chidlow J.H., Barlow S.C., Teng X., Patel R.P., Lefer D.J., Kevil C.G. Sildenafil promotes ischemia-induced angiogenesis through a PKG-dependent pathway. Arterioscler Thromb Vasc Biol. 2007;27:1947–1954. doi: 10.1161/ATVBAHA.107.147421. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Villalobos R.A., Satou R., Seth D.M., Semprun-Prieto L.C., Katsurada A., Kobori H., Navar L.G. Angiotensin-converting enzyme-derived angiotensin II formation during angiotensin II-induced hypertension. Hypertension. 2009;53:351–355. doi: 10.1161/HYPERTENSIONAHA.108.124511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobi J., Tam B.Y.Y., Wu G., Hoffman J., Cooke J.P., Kuo C.J. Adenoviral gene transfer with soluble vascular endothelial growth factor receptors impairs angiogenesis and perfusion in a murine model of hind limb ischemia. Circulation. 2004;110:2424–2429. doi: 10.1161/01.CIR.0000145142.85645.EA. [DOI] [PubMed] [Google Scholar]
- 12.Crawford J.H., Chacko B.K., Pruitt H.M., Piknova B., Hogg N., Patel R.P. Transduction of NO-bioactivity by the red blood cell in sepsis: novel mechanisms of vasodilation during acute inflammatory disease. Blood. 2004;104:1375–1382. doi: 10.1182/blood-2004-03-0880. [DOI] [PubMed] [Google Scholar]
- 13.Belmadani S., Matrougui K., Kolz C., Pung Y.F., Palen D., Prockop D.J., Chilian W.M. Amplification of coronary arteriogenic capacity of multipotent stromal cells by epidermal growth factor. Arterioscler Thromb Vasc Biol. 2009;29:802–808. doi: 10.1161/ATVBAHA.109.186189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooke J.P. NO and angiogenesis. Atheroscler Suppl. 2003;4:53–60. doi: 10.1016/s1567-5688(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 15.Bernardini G., Ribatti D., Spinetti G., Morbidelli L., Ziche M., Santoni A., Capogrossi M.C., Napolitano M. Analysis of the role of chemokines in angiogenesis. J Immunol Methods. 2003;273:83–101. doi: 10.1016/s0022-1759(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 16.Morbidelli L., Donnini S., Ziche M. Role of nitric oxide in the modulation of angiogenesis. Curr Pharm Des. 2003;9:521–530. doi: 10.2174/1381612033391405. [DOI] [PubMed] [Google Scholar]
- 17.De Luca A., Carotenuto A., Rachiglio A., Gallo M., Maiello M.R., Aldinucci D., Pinto A., Normanno N. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol. 2008;214:559–567. doi: 10.1002/jcp.21260. [DOI] [PubMed] [Google Scholar]
- 18.Greulich H., Chen T.H., Feng W., Janne P.A., Alvarez J.V., Zappaterra M., Bulmer S.E., Frank D.A., Hahn W.C., Sellers W.R., Meyerson M. Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS Med. 2005;2:e313. doi: 10.1371/journal.pmed.0020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J.C., Vivanco I., Beroukhim R., Huang J.H., Feng W.L., DeBiasi R.M., Yoshimoto K., King J.C., Nghiemphu P., Yuza Y., Xu Q., Greulich H., Thomas R.K., Paez J.G., Peck T.C., Linhart D.J., Glatt K.A., Getz G., Onofrio R., Ziaugra L., Levine R.L., Gabriel S., Kawaguchi T., O'Neill K., Khan H., Liau L.M., Nelson S.F., Rao P.N., Mischel P., Pieper R.O., Cloughesy T., Leahy D.J., Sellers W.R., Sawyers C.L., Meyerson M., Mellinghoff I.K. Epidermal growth factor receptor activation in glioblastoma through novel missense mutations in the extracellular domain. PLoS Med. 2006;3:e485. doi: 10.1371/journal.pmed.0030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senador D., Kanakamedala K., Irigoyen M.C., Morris M., Elased K.M. Cardiovascular and autonomic phenotype of db/db diabetic mice. Exp Physiol. 2009;94:648–658. doi: 10.1113/expphysiol.2008.046474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J.-X., Stinnett A. Disruption of Ang-1/Tie-2 signaling contributes to the impaired myocardial vascular maturation and angiogenesis in type II diabetic mice. Arterioscler Thromb Vasc Biol. 2008;28:1606–1613. doi: 10.1161/ATVBAHA.108.169235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chalothorn D., Moore S.M., Zhang H., Sunnarborg S.W., Lee D.C., Faber J.E. Heparin-binding epidermal growth factor-like growth factor, collateral vessel development, and angiogenesis in skeletal muscle ischemia. Arterioscler Thromb Vasc Biol. 2005;25:1884–1890. doi: 10.1161/01.ATV.0000175761.59602.16. [DOI] [PubMed] [Google Scholar]
- 23.Fukumura D., Gohongi T., Kadambi A., Izumi Y., Ang J., Yun C.O., Buerk D.G., Huang P.L., Jain R.K. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palen D.I., Matrougui K. Role of elevated EGFR phosphorylation in the induction of structural remodelling and altered mechanical properties of resistance artery from type 2 diabetic mice. Diabetes Metab Res Rev. 2008;24:651–656. doi: 10.1002/dmrr.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isner J.M., Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103:1231–1236. doi: 10.1172/JCI6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundberg J.O., Weitzberg E. NO generation from nitrite and its role in vascular control. Arterioscler Thromb Vasc Biol. 2005;25:915–922. doi: 10.1161/01.ATV.0000161048.72004.c2. [DOI] [PubMed] [Google Scholar]
- 27.Eginton M.T., Mays B.W., Kelley H., Hower C.D., Dassow M., Cambria R.A., Towne J.B., Seabrook G.R., Freischlag J.A. cGMP is decreased after acute ischemia in chronically ischemic canine limbs. J Surg Res. 1999;86:167–170. doi: 10.1006/jsre.1999.5712. [DOI] [PubMed] [Google Scholar]
- 28.Zhang R., Wang L., Zhang L., Chen J., Zhu Z., Zhang Z., Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92:308–313. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- 29.von Degenfeld G., Banfi A., Springer M.L., Wagner R.A., Jacobi J., Ozawa C.R., Merchant M.J., Cooke J.P., Blau H.M. Microenvironmental VEGF distribution is critical for stable and functional vessel growth in ischemia. FASEB J. 2006;20:2657–2659. doi: 10.1096/fj.06-6568fje. [DOI] [PubMed] [Google Scholar]
- 30.Li Y., Hazarika S., Xie D., Pippen A.M., Kontos C.D., Annex B.H. In mice with type 2 diabetes, a vascular endothelial growth factor (VEGF)-activating transcription factor modulates VEGF signaling and induces therapeutic angiogenesis after hind limb ischemia. Diabetes. 2007;56:656–665. doi: 10.2337/db06-0999. [DOI] [PubMed] [Google Scholar]
- 31.Sasso F.C., Torella D., Carbonara O., Ellison G.M., Torella M., Scardone M., Marra C., Nasti R., Marfella R., Cozzolino D., Indolfi C., Cotrufo M., Torella R., Salvatore T. Increased vascular endothelial growth factor expression but impaired vascular endothelial growth factor receptor signaling in the myocardium of type 2 diabetic patients with chronic coronary heart disease. J Am Coll Cardiol. 2005;46:827–834. doi: 10.1016/j.jacc.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Hazarika S., Dokun A.O., Li Y., Popel A.S., Kontos C.D., Annex B.H. Impaired angiogenesis after hind limb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res. 2007;101:948–956. doi: 10.1161/CIRCRESAHA.107.160630. [DOI] [PubMed] [Google Scholar]
- 33.Amin A.H., Abd Elmageed Z.Y., Nair D., Partyka M.I., Kadowitz P.J., Belmadani S., Matrougui K. Modified multipotent stromal cells with epidermal growth factor restore vasculogenesis and blood flow in ischemic hind-limb of type II diabetic mice. Lab Invest. 2010;90:985–996. doi: 10.1038/labinvest.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao J, Wang W, Ha CH, Kim JY, Wong C, Redmond EM, Hamik A, Jain MK, Feng G-S, Jin ZG: Endothelial grb2-associated binder 1 is crucial for postnatal angiogenesis. Arterioscler Thromb Vasc Biol 31:1016–1023 [DOI] [PMC free article] [PubMed]
- 35.Palen D.I., Belmadani S., Lucchesi P.A., Matrougui K. Role of SHP-1: Kv 12, and cGMP in nitric oxide-induced ERK1/2 MAP kinase dephosphorylation in rat vascular smooth muscle cells. Cardiovasc Res. 2005;68:268–277. doi: 10.1016/j.cardiores.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 36.Jones M.K., Tsugawa K., Tarnawski A.S., Baatar D. Dual actions of nitric oxide on angiogenesis: possible roles of PKC: ERK, and AP-1. Biochem Biophys Res Commun. 2004;318:520–528. doi: 10.1016/j.bbrc.2004.04.055. [DOI] [PubMed] [Google Scholar]
- 37.Nishinaka T., Yabe-Nishimura C. EGF receptor-ERK pathway is the major signaling pathway that mediates upregulation of aldose reductase expression under oxidative stress. Free Radic Biol Med. 2001;31:205–216. doi: 10.1016/s0891-5849(01)00571-8. [DOI] [PubMed] [Google Scholar]
- 38.Desai A.K., Joshi V.K., Rao C.B. Cyclic neutropenia in a cleft lip and palate patient – a case report. Cleft Palate Craniofac J. 2011 Feb 10 doi: 10.1597/09-251. K G. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Laufs U., Liao J.K. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 40.Laufs U., La Fata V., Plutzky J., Liao J.K. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 41.Yoshizumi M., Perrella M.A., Burnett J.C., Jr, Lee M.E. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ Res. 1993;73:205–209. doi: 10.1161/01.res.73.1.205. [DOI] [PubMed] [Google Scholar]
- 42.Alonso J., Sanchez de Miguel L., Monton M., Casado S., Lopez-Farre A. Endothelial cytosolic proteins bind to the 3′ untranslated region of endothelial nitric oxide synthase mRNA: regulation by tumor necrosis factor alpha. Mol Cell Biol. 1997;17:5719–5726. doi: 10.1128/mcb.17.10.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]