Abstract
During bacterial and viral infections, unmethylated CpG-DNA released by proliferating and dying microbes is recognized by toll-like receptor (TLR) 9 in host cells, initiating innate immune responses. Many corneal infections occur secondary to epithelial breaches and represent a major cause of vision impairment and blindness globally. To mimic this clinical situation, we investigated mechanisms of TLR9 ligand–induced corneal inflammation in mice after epithelial debridement. Application of CpG oligodeoxynucleotides (ODNs) resulted in neutrophil and macrophage infiltration to the cornea and loss of transparency. By 6 hours after CpG-ODN administration, TLR9 mRNA was increased in the cornea and retina. In vivo clinical examination at 24 hours revealed inflammatory infiltrates in the vitreous and retina, which were confirmed ex vivo to be neutrophils and macrophages, along with activated resident microglia. CpG-ODN–induced intraocular inflammation was abrogated in TLR9−/− and macrophage-depleted mice. Bone marrow reconstitution of irradiated TLR9−/− mice with TLR9+/+ bone marrow led to restored corneal inflammatory responses to CpG-ODN. Fluorescein isothiocyanate–CpG-ODN rapidly penetrated the cornea and ocular media to reach the retina, where it was present within CD68+ retinal macrophages and microglia. These data show that topically applied CpG-ODN induces intraocular inflammation owing to TLR9 activation of monocyte-lineage cells. These novel findings indicate that microbial CpG-DNA released during bacterial and/or viral keratitis can cause widespread inflammation within the eye, including the retina.
Activation of toll-like receptors (TLRs) that recognize distinct pathogen-associated molecular patterns unique to bacteria, viruses, fungi, parasites, and some endogenous ligands1 is well recognized as an initiation step in the inflammatory cascade that follows corneal infection.2–5 Scarring and opacification of the cornea, secondary to various forms of infection, are significant causes of visual impairment globally.6 A complete picture of the distribution and phenotype of resident macrophages and dendritic cells (DCs) in the mouse cornea has emerged in recent years,7–9 and we have previously shown that they play a pivotal role in recognition of the TLR4 ligand lipopolysaccharide (LPS) and the initiation of local innate immune responses when the corneal epithelium is breached.10 In addition, corneal macrophages play a critical role in corneal allograft rejection11 and in the recognition and clearance of Pseudomonas aeruginosa bacteria.12,13
TLR9 recognizes unmethylated CpG-rich motifs that are found in high abundance in bacterial and viral DNA.14 Recognition of the TLR9 ligand, bacterial DNA, and its synthetic homologue [unmethylated CpG oligodeoxynucleotide (ODN)] occurs intracellularly within endosomal compartments.15 Based on their capacity to activate different subsets of myeloid cells, CpG-ODNs are classified as type A, B, or C (C being a combination of types A and B).16 Type A CpG-ODN strongly activates plasmacytoid DCs to produce interferon (IFN)-α/β, whereas type B CpG-ODNs are poor inducers of IFN-α/β but strongly activate B cells and induce transcriptional activation of NF-κβ in monocytes, macrophages, and DCs, resulting in tumor necrosis factor-α production.17 The mouse corneal stroma normally lacks B cells but contains rich networks of resident CD11b+ F4/80+ macrophages and CD11c+ CD11b+ myeloid DCs, along with a small population of CD11c−B220+ plasmacytoid DCs.7,8,18,19
Previous studies have demonstrated that TLR9 signaling plays an important role in the host defense against infectious diseases of the cornea, including Pseudomonas keratitis3 and herpes simplex virus (HSV)-1 keratitis.20 Modulation of the corneal inflammatory response to P. aeruginosa, Staphylococcus aureus, and HSV-1 keratitis has been demonstrated by TLR silencing and the use of TLR knockout mouse models.3–5,20 After exposure of CpG-ODN to the debrided corneal epithelium, we know that TLR9 signaling leads to the production of the chemokines KC and macrophage inflammatory protein-2, which recruit neutrophils to the cornea.21,22 Huang et al23 also revealed that, after subconjunctival injection of CpG-ODN, there was significant up-regulation of IL-1β, macrophage inflammatory protein-2, IL-12, and IFN-γ mRNA in the cornea. It is unclear whether nonhematopoietic corneal cells (epithelial or stromal keratocytes) and/or resident myeloid cells mediate the innate immune responses to topically applied TLR9 ligand5,21 or whether the inflammatory response is restricted to the cornea.
In the present study, we demonstrate, for the first time to our knowledge, that application of CpG-ODN to the abraded cornea leads to inflammation in the anterior uveal tract (iris/ciliary body), vitreous, and retina because of rapid movement of CpG-ODN through the ocular media to the posterior segment of the eye. We reveal that this widespread intraocular inflammation is TLR9 dependent and is abrogated in macrophage-depleted Macrophage Fas ligand-Induced Apoptosis (Mafia) mice. These novel data demonstrate a mechanism by which release of small quantities of bacterial/viral DNA, arising from ocular surface infections, may quickly diffuse through the aqueous ocular environment to initiate inflammation of the retina. This phenomenon is also likely to be relevant in innate immune responses at other sites, such as the meninges and brain parenchyma, the homologues of the cornea and retina, respectively.
Materials and Methods
Animals
C57BL/6 and BALB/c mice, aged 6 to 12 weeks, were sourced from the Animal Resources Centre (Murdoch, WA, Australia) and Monash Animal Services (Monash University, Clayton, VIC, Australia). Six-week-old Mafia mice were obtained from Sandra Burnett (Brigham Young University, Provo, UT). TLR9−/− mice, provided by Dr. S. Akira (Osaka University, Osaka, Japan), were fully backcrossed onto a C57BL/6 background. All animal procedures were performed in accordance with the guidelines of local Animal Ethics Committees at the University of Western Australia (Perth, WA), Monash University, and Case Western Reserve University (Cleveland, OH).
Mouse Model of Corneal Inflammation
Mice were anesthetized by i.p. injection of either ketamine-xylazine or 2,2,2-tribomoethanol (1.2%; Sigma-Aldrich, St. Louis, MO), and the epithelium of the central cornea was debrided using an Algerbrush II corneal rust ring remover with a 0.5-mm burr (Alger Equipment Co, Lago Vista, TX), as previously described.21 Immediately after epithelial debridement, 20 μg of phosphorothioate CpG 1826 (type B) oligonucleotide (5′-TCCATGACGTTCCTGACGTT-3′), control oligonucleotide 1826 (control ODN′; 5′-TCCATGAGCTTCCTGAGCTT-3′), CpG 1585 (type A; 5′-GGGGTCAACGTTGAGGGGG-3′), or 20 μg of Ultra Pure Escherichia coli LPS (strain K12; Invitrogen, San Diego, CA) was applied to each eye. For analysis of ocular inflammation, animals were euthanized at 30 minutes; 2, 6, 24, and 72 hours; and 1 week. To determine whether exposure to CpG-ODN in one eye would be sufficient to induce inflammation in the contralateral eye, some experiments were performed whereby the contralateral eye was debrided and treated with control ODN. Furthermore, to examine whether the intraocular inflammation could be reproduced by systemic exposure to CpG-ODN, mice received an i.p. or i.v. injection of 40 μg of CpG-ODN and one eye was debrided and treated with control ODN while the contralateral eye was left untreated (naïve). To localize the movement of CpG-ODN in the eye, 10 μg of CpG-ODN 1826 fluorescein isothiocyanate (FITC; Invitrogen) was applied to the debrided cornea, and animals were sacrificed at 4 hours, 24 hours, or 1 week after treatment. The lower dosage of 10 µg was chosen as the level of fluorescent staining of the cornea using 20 μg of CpG-FITC made examination of the fundus difficult.
Spectral Domain Optical Coherence Tomography, Scanning Laser Ophthalmoscopy, and Fluorescein Angiography
Immediately before euthanasia, mice were anesthetized using ketamine-xylazine and their pupils were dilated with tropicamide eye drops (Mydriacyl, 0.5%; Alcon Australia, Frenchs Forest, Australia). The fundi of eyes of representative animals were imaged using the combined scanning-laser ophthalmoscope and spectral domain optical coherence tomography (OCT) device (Heidelberg Spectralis-OCT; Heidelberg Engineering, Dossenheim, Germany). Scanning laser ophthalmoscopy fundus images of the central mouse retina and vitreous were acquired using a 55-degree lens. For analysis of retinal vasculature, mice received 50 μL of 10% sodium fluorescein (Alcon Laboratories, Frenchs Forest, NSW, Australia) by i.p. injection, and images were captured using the fluorescein angiography mode. To acquire OCT images of the mouse retina/vitreous, a 30-degree wide-angle lens was used. All data were processed using Eye Explorer Software (Heidelberg Engineering).
Immunohistochemistry
Eyes were enucleated and snap frozen in embedding medium (optical cutting temperature compound, Tissue-Tek; Proscitech, Thuringowa, QLD, Australia) using dry ice. Cryosections (6 μm thick) were fixed in 4% paraformaldehyde or acetone (for F4/80 staining) and immunostained with rat anti-mouse monoclonal antibody NIMP-R14 (Abcam, Cambridge, UK), which specifically stains neutrophils, or with the pan-macrophage marker F4/80 (Serotec, Oxford, UK). Sections were incubated for 45 minutes with Strep Alexa Fluor 594–conjugated anti-rat IgG (1:200; Molecular Probes, Carlsbad, CA) and for 5 minutes with DAPI (Roche Diagnostics, Mannheim, Germany). Cells were counted in each section by a blinded observer (SM; from limbus to limbus; original magnification, ×400) by fluorescence microscopy (Olympus BX60; Olympus, Tokyo, Japan).
Whole-Mount Immunofluorescence
Corneas and retinas were dissected from eyes that had been fixed in 4% paraformaldehyde and processed as whole mounts, as previously described for immunostaining.24 A range of anti-leukocyte monoclonal antibodies were used, including anti-ionized calcium binding adaptor molecule 1 (Iba-1; Wako Pure Chemical Industries, Osaka, Japan), anti-major histocompatibility complex (MHC) class II (BD Pharmingen, San Diego, CA), and anti-neutrophil and biotinylated Griffonia simplicifolia B4 isolectin (IB4; Vector Laboratories, Burlingame, CA). After overnight incubation, tissues were washed with PBS and then incubated with appropriate secondary and tertiary antibodies [namely, goat anti-rabbit biotin (Iba-1), goat anti-rat biotin (MHC II and NIMP), or streptavidin Cy3; Jackson ImmunoResearch, West Grove, PA]. To visualize nuclei, tissues were incubated in DAPI (Roche Applied Science, Mannheim, Germany) for 10 minutes at room temperature. For negative controls, either isotype IgG primary antibodies were used or primary antibodies were omitted altogether. To calculate the mean number of NIMP+ and MHC II+ cells, three random images were taken from each retinal whole mount using a ×20 objective, and immunopositive cells were counted in the three images and the density was averaged.
Real-Time RT-PCR
Eyes were enucleated at 6 or 24 hours after topical application of either control ODN or CpG-ODN, immediately placed into RNAlater (Qiagen GmbH, Hilden, Germany), and stored at 4°C overnight. Total RNA was extracted from corneal and retinal tissue using the RNeasy Mini Kit (Qiagen), following the manufacturer's instructions. cDNA was prepared from 800 ng of total RNA for experimental samples (retina and cornea at 6 and 24 hours) and 2 μg of total RNA for standard curve samples (spleen tissue, High Capacity cDNA-to-RNA Kit; Applied Biosystems Inc., Foster City, CA). SYBR Green–based real-time PCRs were run in triplicate (Fast SYBR Green Master Mix; Applied Biosystems) on the Rotor-Gene 3000 (Corbett Research/Qiagen). Ribosomal protein L32 (forward, 5′-TTAAGCGAAACTGGCGGAAAC-3′; reverse, 5′-TTGTTGCTCCCATAACCGATG-3′) was used as the housekeeping gene. Expression levels of TLR9 (forward, 5′-AGGCTGTCAATGGCTCTCAGTT-3′; reverse, 5′-TGAACGATTTCCAGTGGTACAAGT-3′) and IFN-γ (forward, 5′-AGCAACAGCAAGGCGAAAA-3′; reverse, 5′-CTGGACCTGTGGGTTGTTGA-3′) were normalized against ribosomal protein L32. Fold changes in expression of TLR9 and IFN-γ at 6 and 24 hours after CpG-ODN administration, compared with controls, were determined using 2−ΔΔCT.25
Depletion of CSF-1 Receptor–Expressing Cells Using Mafia Mice
Burnett and colleagues26 generated transgenic mice designed for inducible macrophage and DC depletion by Fas-ligand–induced apoptosis. The transgene is under control of the c-fms promoter that regulates expression of the colony stimulating factor-1 (CSF-1) receptor expressed on macrophages and DCs. These mice express enhanced green fluorescent protein (eGFP) and a membrane-bound suicide protein comprising the human low-affinity nerve growth factor receptor, the FK506 binding protein, and the cytoplasmic domain of Fas. AP20187 is a covalently linked dimer (Ariad Pharmaceuticals, Cambridge, MA) that cross-links the FK506 binding protein region of the suicide protein and induces caspase 8–dependent apoptosis.26 Mafia mice are on a C57BL/6 background and have a normal phenotype in the absence of the dimerizer. To deplete macrophages and DCs, AP20187 was diluted to 0.55 mg/mL in 4% ethanol, 10% polyethylene glycol-400, and 1.7% Tween 80 in sterile water; Mafia mice were injected i.p. daily for 5 days with 40 μg of the AP20187 dimerizer per injection (total, 200 μg) before corneal application of CpG-ODN. Control Mafia mice received injections of sterile saline.
Generation of Bone Marrow Chimeric Mice
Recipient TLR9−/− and wild-type (WT) C57BL/6 mice, aged 6 to 8 weeks, were irradiated with two doses of 5.5 Gy 3 hours apart. The femur and tibia bones were harvested from donor mice (Cx3cr1gfp/+), and the shafts were centrifuged at 4500 × g for 30 seconds at 4°C, as previously described.27 The pellet was resuspended in RPMI 1640 medium (Sigma Chemical Co, St Louis, MO) and centrifuged at 250 × g for 5 minutes at room temperature, and live cells were counted by trypan blue exclusion. Recipient mice received an i.v. injection of 5 × 106 bone marrow cells (in 150 μL of RPMI 1640 medium) via the lateral tail vein. Antibiotic cover was provided in drinking water to recipient mice [800 μL/L enrofloxacin (Baytril 25); Bayer Health Care, Pymble, NSW, Australia] for 10 days after irradiation. Chimeric mice were treated at 12 weeks after reconstitution (n = 4 to 6 per treatment group).
In Vivo Examination of Stromal Haze and eGFP+ Monocyte Infiltration into the Cornea
Immediately after euthanasia, corneas were examined by in vivo confocal microscopy (Confoscan 4; Nidek Technologies, New Orleans, LA). Images were captured every 2 μm throughout the thickness of the cornea, and light intensity (reflectivity) values for each image were captured using accompanying software (NAVIS; Lucent Technologies, Murry Hill, NJ) and exported as an Excel file. Reflectivity values for the corneal stroma were plotted in relation to distance from the corneal endothelium to the basal epithelium to generate a curve, and the area under the curve was calculated using GraphPad Prism software (GraphPad Software, San Diego, CA). This measurement was termed stromal haze and is described in arbitrary units of corneal clarity, as in our previous studies.21,28 Measurements were completed within a few minutes after euthanasia, and control animals were treated identically; thus, postmortem corneal edema was minimal. To visualize eGFP+ monocytic cell infiltration in Mafia mice, corneas were imaged and analyzed using a high-resolution stereofluorescence MZFLIII microscope (Leica Microsystems, Inc., Bannockburn, IL) and SpotCam software (RT Slider KE; Diagnostics Instruments, Sterling Heights, MI).27
In Vivo Examination of CpG-FITC in the Posterior Segment
For qualitative analysis of CpG-FITC dissemination in the posterior segment, mouse retinas were imaged using the Micron III retinal imaging microscope (Phoenix Research Laboratories, Inc., San Ramon, CA). At 4 hours, 24 hours, or 1 week after corneal CpG-FITC treatment, anesthetized mice (ketamine-xylazine) received topical Mydriacyl eye drops (0.5%; Alcon Australia, Frenchs Forest, Australia) to dilate pupils. A drop of eye gel (Viscotears; Novartis Pharmaceuticals Australia Pty Limited, Australia) was placed on the objective that comes into contact with the mouse cornea. By using the bright-field and 488 fluorescence filters, images of the mouse retina were captured with a 3 chip color-device camera.
Statistical Analysis
Mean values for each experiment were compared between groups using a one-way analysis of variance and Tukey's post hoc comparison, with P < 0.05 considered statistically significant.
Results
Type B CpG-ODN Induces Acute Neutrophilic Infiltration to the Corneal Stroma and a Delayed Macrophage Response
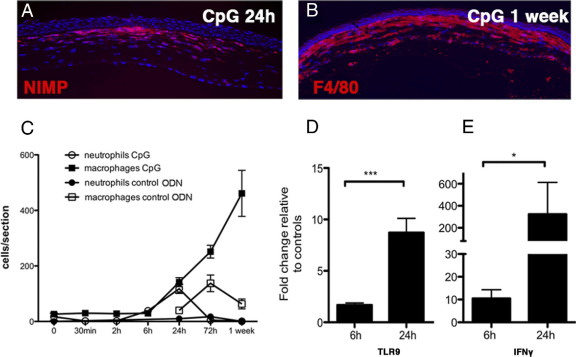
Our laboratory and others5,21 have shown that activation of the mouse cornea by the TLR9 ligand CpG-ODN (type B) causes corneal haze, inflammatory cell infiltration, and production of the IFN-γ–inducible protein CXCL10. The number of neutrophils infiltrating the corneal stroma peaked at 24 hours, whereas macrophage numbers continued to increase 1 week after treatment (Figure 1, A–C). In contrast, application of a control ODN (CpG-ODN), which has no TLR9 binding activity, induced attenuated recruitment of macrophages in the absence of a neutrophil response, elicited by the debridement procedure (Figure 1C). Type A CpG-ODN failed to produce corneal inflammation; thus, in all subsequent experiments, type B CpG-ODN was used.
Figure 1.
Inflammatory cell recruitment to the cornea after topically applied CpG-ODN. Immunohistochemical staining of NIMP+ neutrophils in the central cornea at 24 hours (A) and F4/80+ macrophages at 1 week after treatment (DAPI+ nuclei are blue) (B). The kinetics of macrophage and neutrophil recruitment to the cornea after topical application of CpG-ODN or control ODN is shown (C). Up-regulation of TLR9 mRNA (D) and IFN-γ mRNA (E) in isolated corneas from CpG-ODN–treated eyes at 6 and 24 hours (n = 4 to 10 mice per group). Data are given as mean ± SEM.
At 6 hours after treatment, when neutrophil and macrophage numbers were low (Figure 1C), TLR9 mRNA expression was elevated in the CpG-ODN–treated corneas compared with control ODN-treated corneas and was further elevated at 24 hours (Figure 1D). IFN-γ mRNA was elevated 10-fold in the cornea by 6 hours and >200-fold after 24 hours (Figure 1E). These data demonstrate that the TLR9 ligand, type B CpG-ODN, is a potent inducer of acute corneal inflammation when applied to the debrided corneal surface.
TLR9-Dependent CpG-ODN–Induced Keratitis Is Mediated by Cells of Myeloid Origin
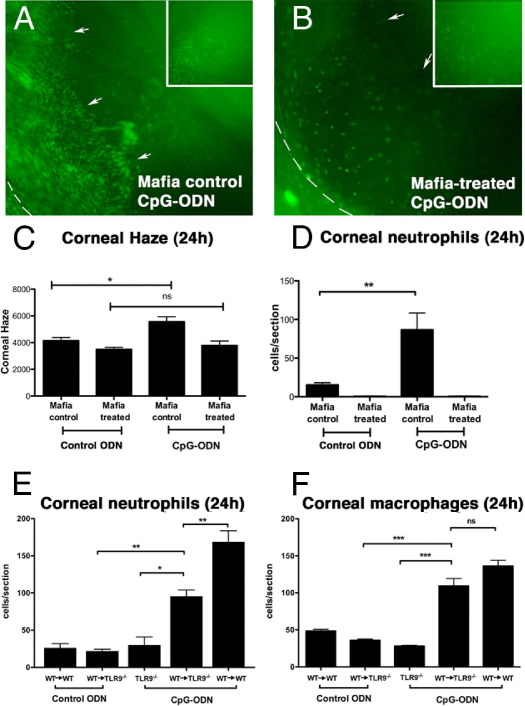
To determine whether resident macrophages and DCs mediate the initial recognition of TLR9 ligands in the cornea, we used Mafia mice, in which macrophages and DCs express eGFP constitutively and undergo cell lineage–specific apoptosis after cross-linking the FK506 binding domain of the membrane-bound suicide protein using the FK506 dimerizer AP20187.26 After depletion of eGFP+ macrophages/DCs, AP20187-treated Mafia mice are unable to respond to LPS10 and Aspergillus fumigatus.29 In vivo imaging of nondimerized Mafia mice 24 hours after corneal application of CpG-ODN demonstrated many eGFP+ cells infiltrating the corneal stroma and iris (Figure 2A). In comparison, dimerizer-treated Mafia mice retained few eGFP+ cells in the corneal stroma (Figure 2B) and displayed reduced corneal haze and neutrophil infiltration after CpG-ODN treatment (Figure 2, C and D). These data clearly demonstrate that monocytic-lineage cells play a critical role in the innate immune response triggered by corneal exposure to TLR9 ligand.
Figure 2.
Inflammatory cell infiltrate and corneal haze in Mafia control (nondimerized; no macrophage depletion) and Mafia-treated (dimerized; macrophage depletion) mice 24 hours after corneal CpG-ODN. In vivo epifluorescence image of a Mafia control mouse eye containing extensive eGFP+ inflammatory macrophages in the central cornea (inset) and iris/anterior chamber (A) compared with a Mafia-treated eye that has fewer eGFP+ inflammatory cells (B). The dashed line demarcates the limbus (cornea-scleral junction), and arrows outline the pupil margin of the underlying iris. CpG-ODN increased corneal haze (C) in Mafia control corneas compared with Mafia-treated corneas. An abrogated neutrophil response in Mafia-treated corneas compared with Mafia control corneas 24 hours after CpG-ODN treatment is shown (D). CpG-ODN induced neutrophil and macrophage infiltration into the corneas of bone marrow chimeric mice (E and F) (n = 3 to 6 mice per group). Data are given as mean ± SEM. ns, not significant. *P < 0.05, **P < 0.01, and ***P < 0.001.
To further investigate the contribution of resident corneal macrophages in the generation of inflammatory responses to topically applied CpG-ODN, TLR9−/− mice were lethally irradiated and given bone marrow from TLR9+/+Cx3cr1gfp/+ mice (WT→TLR9−/−). Previous studies30,31 have demonstrated an almost complete replenishment of resident corneal bone-marrow–derived cells by 12 weeks after transplantation. Thus, at 12 weeks after transplantation, when resident monocyte-derived cells are the only TLR9+/+ cells in the cornea, mice were challenged with topical CpG-ODN or control ODN; 24 hours later, neutrophil and macrophage infiltration was quantified (Figure 2, E and F). In TLR9−/− mice, the number of neutrophils and macrophages was equivalent to control ODN-treated corneas. In WT→TLR9−/− mouse corneas, there was a statistically significant increase in the number of neutrophils recruited to the cornea compared with CpG-ODN–treated TLR9−/− corneas and control ODN-treated corneas. However, the inflammatory response did not reach the levels of inflammation observed in WT→WT chimeric mice (Figure 2E). Quantitation of macrophage infiltration to the cornea revealed a full reconstitution of the CpG-ODN response in the corneas of WT→TLR9−/− mice, which was indistinguishable from WT→WT mice (Figure 2F).
CpG-ODN–Induced Keratitis Is Accompanied by Widespread Intraocular Inflammation
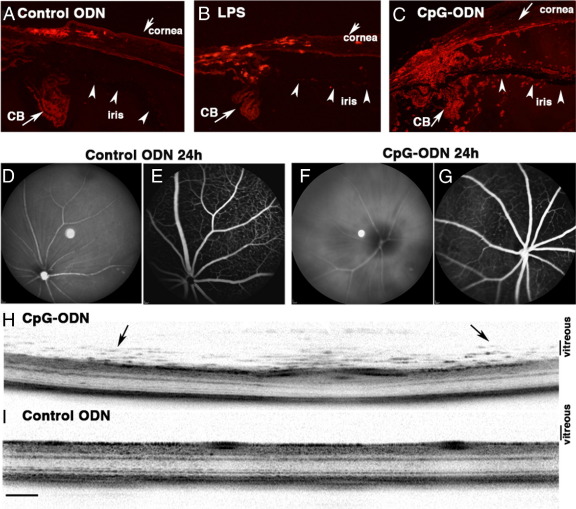
In a similar murine model of LPS-induced corneal inflammation, a mild degree of neutrophilic infiltration is observed in the anterior segment compared with control ODN-treated eyes at 24 hours (Figure 3, A and B). By contrast, CpG-ODN application to the debrided corneal surface resulted in a much more widespread and striking degree of neutrophil and macrophage infiltration, which involves the entire anterior uveal tract (iris/ciliary body), anterior chamber, and posterior chamber (Figure 3C). An examination of the retina and vitreous using in vivo fundus scanning laser ophthalmoscopy, fluorescein angiography, and OCT revealed normal fundus and retinal vasculature in control ODN-treated eyes; however, vitreous haze and inflammatory infiltrates in the vitreous and retina were detected in CpG-ODN–treated eyes (Figure 3, D–I). There did not appear to be any compromise of the blood-retinal barrier because no leakage was detected via fluorescein angiography (Figure 3, E and G).
Figure 3.
Evidence of widespread intraocular inflammation 24 hours after topical application of CpG-ODN to the debrided cornea. Moderate neutrophil infiltration (red) in the limbus and anterior segment of control ODN and LPS-treated eyes (A and B). In CpG-ODN–treated eyes (C), many neutrophils were present in the anterior chamber, iris, and ciliary body (CB). In vivo scanning laser ophthalmoscopy and fluorescein angiography of control ODN-treated eyes demonstrating clear retinal vasculature (D and E) compared with the hazy appearance of the fundus from CpG-ODN–treated eyes (F and G). In vivo optical coherence tomography image demonstrating the presence of inflammatory infiltrates (arrows) in the vitreous/retina of the CpG-ODN–treated eye (H) compared with normal appearance of the central retina from a control ODN-treated eye (I). Scale bar = 200 μm (I).
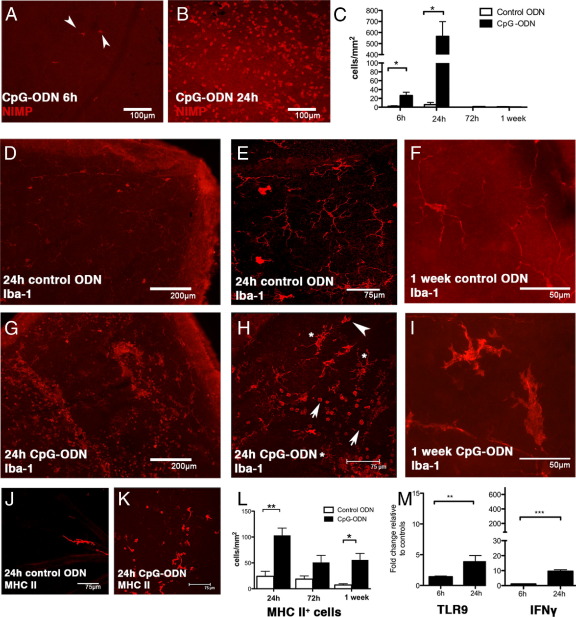
Retinal whole mounts were immunostained to examine the phenotype and kinetics of inflammatory cell infiltration after topically applied CpG-ODN. Adherent intravascular neutrophils appeared in retinal vessels as early as 6 hours after exposure to the corneal surface with CpG-ODN (Figure 4A), with peak infiltration at 24 hours (Figure 4B) and resolution by 72 hours (Figure 4C). In the normal retina, CD45low CD11blow CD68low CX3CR1+ Iba-1+ and MHC class II− monocyte-derived ramified microglia are the predominant tissue resident leukocyte population (Figure 4, D–F).32,33 Hallmarks of microglial activation include up-regulation of Iba-1 and MHC class II and changes in morphological appearance, whereby the distinct fine ramifications are lost and the soma becomes enlarged, with fewer stouter processes.34 At 24 hours after corneal CpG-ODN treatment, microglia in the ganglion cell layer exhibited enlarged soma and there was a large influx of Iba-1+ round cells (Figure 4, G and H). There was evidence of continued activation up to 1 week after treatment (Figure 4I). Rare MHC class II+ DCs are localized to the juxtapapillary margin, optic disc region, and perivascular sites of the normal mouse retina35 (Figure 4J). After CpG-ODN treatment, many round MHC class II+ cells were detected in the inner retina within 24 hours (Figure 4, K and L). By 72 hours, the frequency of these cells had declined, but they remained present in significant numbers for at least 1 week after treatment compared with control eyes (Figure 4L). The IFN-γ mRNA level was elevated at 24 hours (Figure 4M), and at 6 hours after treatment, TLR9 mRNA expression was elevated (1.4-fold) in the retinas of CpG-ODN–treated eyes compared with controls and was further increased (fourfold) at 24 hours (Figure 4M). In summary, these data demonstrate that topical application of a TLR9 ligand to the debrided corneal surface induces infiltration of neutrophils and macrophages into the retina, activation of resident microglia, and local production of IFN-γ.
Figure 4.
Characterization of retinal inflammation after topical application of CpG-ODN onto the debrided cornea. Adherent intravascular neutrophils already present in the retina 6 hours after CpG-ODN treatment (A, arrowheads). Many extravascular neutrophils are evident by 24 hours (B). Quantitation of the time course of neutrophil infiltration on retinal whole mounts revealed a peak at 24 hours (C). Healthy, ramified, microglial morphological characteristics in retinas from control ODN-treated eyes at 24 hours (D: low power; E: higher magnification) or 1 week (F). The fewer bright red dendriform cells (E) are normal vitreous hyalocytes. After CpG-ODN treatment, numerous Iba-1+ inflammatory macrophages (G–I) were observed in the retina. Note the altered morphological characteristics of Iba-1+ microglia (H, asterisks) alongside rounded Iba-1+ activated macrophages (H, arrowheads). At 1 week after corneal CpG-ODN, large Iba-1+ microglia with stout processes (I) are clearly evident, compared with microglia in control retinas (F). Typical rare perivascular MHC class II+ DCs in the control retina (J) can be compared with the increased number of MHC class II+ cells in the retina 24 hours after corneal CpG-ODN treatment (K). The density of MHC class II+ cells in retinal whole mounts from control ODN or CpG-ODN–treated corneas (L). Real-time RT-PCR revealed a significant fold increase (compared with control ODN-treated corneas) in TLR9 mRNA in the retina as early as 6 hours (M), further increasing by 24 hours, whereas IFN-γ up-regulation was only apparent at 24 hours (n = 5 to 10 mice per group). Data are given as mean ± SEM. Scale bars are as shown. *P < 0.05, **P < 0.01, and ***P < 0.001.
Intraocular Inflammation in Response to Corneal CpG-ODN Is TLR9 Dependent
The infiltration of neutrophil and Iba-1+ monocyte-derived cells in the retina after topical exposure to CpG-ODN was absent in TLR9−/− mice (see Supplemental Figure S1, A and B, at http://ajp.amjpathol.org). In dimerizer-treated Mafia mice, there was no retinal inflammation, providing strong evidence for the role of c-fms–expressing myeloid cells in TLR9-dependent responses in the posterior portion of the eye after corneal exposure to CpG-ODN.
Acute Retinal Inflammation in Response to Corneal CpG-ODN Is Selective for TLR9 Ligands and Does Not Occur after Exposure to TLR4 Ligand, LPS
Although there have been extensive studies of the effects of the TLR4 ligand, LPS, on the eye after either direct injection into the anterior chamber or vitreous36 (modeling infections within the eye) or systemic exposure37 (modeling anterior uveitis that often accompanies systemic exposure to LPS), to our knowledge, there have been no reports of retinal inflammation as a sequel to the topical application of TLR4 ligands to the surface of the eye (mimicking models of keratitis). Thus, we treated mice topically with 20 μg of LPS in an identical manner to CpG-ODN and as previously reported.10,21 These experiments revealed no evidence of vitritis or retinal inflammation (see Supplemental Figure S1 at http://ajp.amjpathol.org).
Acute Retinal Inflammation Does Not Occur after Systemic Exposure to CpG-ODN
To examine the possibility that CpG-ODN may be mediating the observed effect on the retina, secondary to its absorption via the nasolacrimal system or conjunctival lymphatics, we performed a further experiment in which a group of animals were given a single i.p. or i.v. injection of 40 μg of CpG-ODN and the cornea of one eye was debrided to mimic a local injury. No corneal or retinal inflammation was noted in this experiment (see Supplemental Figure S1C at http://ajp.amjpathol.org). To determine whether CpG-ODN–induced intraocular inflammation could cause an inflammatory response in the untreated contralateral eye, animals received 20 μg of CpG-ODN in the right eye and either debridement only (to mimic injury) or no treatment in the left eye. No inflammation was observed in the retina of the left eye in either experiment (see Supplemental Figure S1, D and E, at http://ajp.amjpathol.org), confirming that retinal inflammation was localized to the eye topically exposed to CpG-ODN. In summary, systemic exposure to 40 μg of CpG-ODN failed to elicit an acute intraocular response, even in the presence of corneal injury.
Retinal Inflammation Follows Subconjunctival Injection of CpG-ODN
To determine whether the response previously described is specific to corneal application or whether CpG-ODN can access the retina and vitreous from other extrabulbar routes to cause retinal inflammation, 20 μg of CpG-ODN or control ODN was injected into the subconjunctival space. Retinas were examined at 24 hours for evidence of inflammation. Contralateral control-ODN–injected mice displayed no signs of inflammatory cell infiltration (see Supplemental Figure S2A at http://ajp.amjpathol.org). In CpG-ODN–injected eyes, infiltrating IB4+Iba-1+CD68+ macrophages and NIMP+ neutrophils were observed in the retina (see Supplemental Figure S2, B and C, at http://ajp.amjpathol.org), thus confirming that CpG-ODN injected subconjunctivally can result in inflammation in the posterior compartment of the mouse eye.
CpG-ODN Applied to the Debrided Corneal Surface Quickly Reaches the Retina
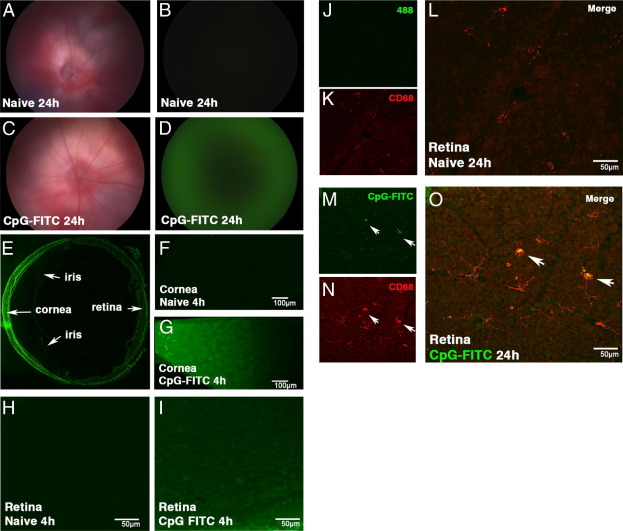
We hypothesized that the retinal inflammation observed secondary to topical application of CpG-ODN was the result of diffusion of this small–mol. wt. molecule through the corneal stroma, aqueous humor, and vitreous humor to reach the posterior segment of the eye, where it directly activates retinal myeloid-derived cells. To test this notion, we tracked the fate of FITC-labeled CpG-ODN (mol. wt., 6.6 kDa) applied to the debrided cornea. The results of a fundus examination at 24 hours were normal in naïve contralateral eyes, and no fluorescence was detected (Figure 5, A and B). In FITC CpG-ODN–treated eyes, retinal vessels were also normal (Figure 5C); however, a fluorescent signal was present throughout the posterior segment at 24 hours (Figure 5D). At 1 week, fluorescence was still detectable in FITC CpG-ODN–treated eyes compared with naïve contralateral eyes (data not shown).
Figure 5.
Distribution of FITC-CpG-ODN in the mouse eye after topical application to the injured cornea. In vivo images of the mouse fundus in BALB/c mice (A–D), showing normal retinal vasculature in bright-field mode (A and C). No detectable fluorescent signal (B) is evident in the naïve contralateral eye; however, a green signal is clearly evident in the FITC-CpG-ODN–treated cornea at 24 hours (D). Cryosection of a C57BL/6 eye 24 hours after FITC-CpG-ODN, showing an intense green signal in the cornea (E). Confocal image of corneal whole mounts of a naïve contralateral eye (F) and an FITC-CpG-ODN–treated eye (G) at 4 hours. Confocal images of retinal whole mounts from a naïve contralateral eye (H) and an FITC-CpG-ODN–treated eye (I). CD68+ FITC− microglia in a naïve contralateral eye at 24 hours (J–L) compared with CD68+ FITC+ macrophages (arrows) in the retina of an FITC-CpG-ODN–treated eye (M–O).
Cryosections of FITC CpG-ODN–treated eyes at 24 hours revealed a gradient in fluorescence, indicative of diffusion from the cornea, through to the posterior segment (Figure 5E). No fluorescence signal was detected in corneal whole mounts of either naïve or debrided contralateral eyes (Figure 5F) compared with intense green signal in the FITC CpG-ODN–treated corneas (Figure 5G). Confocal analysis revealed FITC CpG-ODN in the extracellular space and within keratocytes, macrophages, and DCs in the cornea, with occasional uptake by epithelial cells also noted. Microscopy of retinal whole mounts at 4 hours revealed a lack of FITC signal in the contralateral retina (Figure 5H) compared with obvious fluorescence in the ganglion cell layer of the retina 4 hours after FITC CpG-ODN treatment (Figure 5I). Naïve retinal whole mounts at 24 hours failed to demonstrate colocalization of FITC CpG-ODN (Figure 5J) and CD68+ cells (Figure 5, K and L), contrasting with the obvious uptake of labeled CpG-ODN by large round Iba-1+ IB4+ macrophages and occasional microglia in the inner retina of CpG-ODN–treated animals, where the signal colocalized with CD68+ endosomal compartments of these cells (Figure 5, M–O; see also Supplemental Figure S3, D–F, at http://ajp.amjpathol.org). Additional CpG-FITC localization studies, performed in TLR9−/− mice, confirm the presence of CpG-FITC in the retina 24 hours after topical application; however, there were no infiltrating inflammatory cells present (see Supplemental Figure S3, A–C, at http://ajp.amjpathol.org).
Discussion
In light of the global burden of vision-threatening keratitis secondary to bacterial, viral, fungal, and protozoan infections, the mechanisms of inflammation in the transparent cornea have been extensively investigated. We have used a sterile model of keratitis to investigate the role of macrophages and DCs in the induction of corneal inflammation after application of TLR ligands.4,10,13,21,28 In this sterile model and in infectious microbial keratitis, we21 and others have previously demonstrated the presence and activation of TLR9 in the mouse cornea and characterized the CXC chemokines that recruit neutrophils and macrophages into this avascular tissue.5,22,23 In the present study, we confirmed that CpG-ODN, a synthetic mimic of bacterial and viral DNA, topically applied to the injured corneal epithelium of WT, but not TLR9−/−, mice results in an acute neutrophilic and delayed macrophage/monocytic response in the cornea. We also demonstrate, for the first time to our knowledge, widespread intraocular inflammation involving the anterior uveal tract, vitreous, and retina. The amelioration of the corneal and intraocular response in Mafia mice strongly implicates a role for monocyte-derived macrophages in the recognition of CpG-ODN, which accords with our previous demonstration of such a mechanism for A. fumigatus keratitis29 and TLR4-mediated responses to LPS in the mouse cornea.10 In addition, data from bone marrow chimeras, in which Cx3cr1gfp/+ bone marrow was transplanted into lethally irradiated TLR9−/− mice, support a role for resident corneal monocyte-derived cells in the recruitment of neutrophils and macrophages to the cornea after topical application of TLR9-specific CpG-ODN.
Based on their capacity to activate different subsets of myeloid cells, synthetic TLR9 ligand homologues, unmethylated CpG-ODNs, are classified as type A, B, or C.16 Type A CpG-ODNs activate plasmacytoid DCs to produce IFN-α/β, whereas type B CpG-ODNs are poor inducers of IFN-α/β but strongly activate B cells and induce transcriptional activation of NF-κβ in monocytes, macrophages, and DCs, resulting in tumor necrosis factor-α production.17 We found a lack of inflammation after corneal exposure to type A CpG-ODN, which may reflect the rarity of resident plasmacytoid DCs and natural killer cells in the noninflamed mouse cornea.19 By comparison, the response to type B CpG-ODN was predictable because macrophages are widely distributed throughout the corneal stroma.7,9,18
The unexpected retinal inflammation that accompanied exposure of the injured corneal surface to CpG-ODN appears to be unique to TLR9 responses because previous studies have not described such inflammatory changes after exposure to ultrapure LPS, Pam3Cys, and poly(I:C) (TLR4, TLR2, and TLR3 ligands, respectively). In addition, we did not observe posterior segment inflammation in the present investigation after identical LPS treatment. There are several possible explanations for the retinal inflammation that accompanies corneal CpG-ODN treatment that are worthy of consideration. First, cytokines and chemokines produced in the cornea may diffuse to the anterior uveal tract (iris and ciliary body) and the posterior segment of the eye (vitreous and retina) and, thereby, exert their effects. We believe this unlikely on the grounds that TLR4 or TLR2 ligands induce a more intense corneal neutrophilic infiltration and cause the production of a similar cascade of cytokines and chemokine signals,21,38 yet fail to cause posterior segment inflammation. A second mechanism considered was secondary exposure to CpG-ODN via a systemic route, perhaps as a consequence of the ligand entering the tears or limbal vasculature (the rich plexus of vessels, including lymphatics, encircling the avascular cornea) during debridement and topical application. However, the lack of intraocular inflammation at 24 hours in naïve contralateral eyes, injury-only contralateral eyes, or the eyes of animals that had received a corneal injury in conjunction with an i.v. or i.p. injection of CpG-ODN makes this pathway unlikely. It does not preclude such a mechanism operating at higher doses or during active systemic viral disease, both of which are under investigation in our laboratories.
The detection of FITC-conjugated CpG-ODN in the retina only hours after administration to the injured cornea, and its localization within retinal macrophages led us to propose that CpG-ODN, because of its small size (approximately 6 kDa), readily diffuses through the cornea and aqueous environment of the eye to directly activate TLR9+ myeloid cells in the retina. A similar transit time of hours has been shown during transcorneal iontophoresis of fluorescent ODN (21mer) in rats.39 These data suggest a mechanism by which the unmethylated CpG-ODN motifs released during bacterial or viral infections, perhaps even secondary to bacteriolysis after antibiotic therapy, may directly lead to activation of intraocular myeloid cells. Previous reports have demonstrated that corneal inoculation with P. aeruginosa leads to up-regulation of IL-6 and KC mRNA in the retina,40 and during corneal HSV infection, a few retinal cells in the outer nuclear layer express HSV antigen and undergo apoptosis.41 Both these studies suggest that inoculation of the mouse cornea with HSV or P. aeruginosa results in minor retinal inflammatory changes; however, the mechanism of these posterior segment changes was not investigated.
Evidence that brain microglia are activated by CpG-ODN42,43 would make it extremely likely that retinal microglia would recognize this TLR9 ligand. In a study with parallels to our own, Deng et al44 noted that intracisternal injection (homologous to direct injection into the vitreous or anterior chamber) of CpG-ODN in mice and rats caused meningitis, which could be abrogated by macrophage depletion and anti-sense targeting of NF-κβ, that leads to down-regulation of pro-inflammatory cytokine production.
The findings of the present study lead us to postulate that activation of retinal microglia and other ocular macrophage populations,45 secondary to corneal microbial infection, may have subtle effects on the progression of posterior segment diseases, including age-related macular degeneration.46 Recent evidence47 has shown that, after co-culture with LPS-activated retinal microglia, retinal pigment epithelial cells produced the chemotactic factors chemokine ligand 2, chemokine ligand 5, stromal-cell derived factor-1 and the pro-angiogenic molecule vascular endothelial growth factor. The latter growth factor has a direct role in the wet neovascular form of age-related macular degeneration. Thus, we hypothesize that activation of retinal microglia, occurring as secondary sequelae to innate immune responses to viral and bacterial DNA at the ocular surface, may have a previously unsuspected role in the progression of retinal diseases.
Finally, data from this study demonstrating that corneal CpG-ODN can cause TLR9-mediated inflammation in the retina highlight the need for consideration of contaminating CpG motifs in antisense ODN or plasmids used in ocular gene therapy. Specific depletion of unmethylated CpG motifs from plasmid DNA has been shown in other models to improve the safety of gene therapy treatments by reducing unwanted inflammation.48 In addition, the rapid dissemination of immunostimulatory ODN to the posterior segment of the eye after application to a compromised corneal epithelium or via subconjunctival injection could offer promise of a novel mode of therapeutic delivery to treat intraocular inflammation using inhibitory ODN.49
Acknowledgments
We thank Dr. Matt Wikstrom (Lions Eye Institute, Nedlands, Australia), Manpreet Sidhu (Monash University, Clayton, Australia), and Catherine Doller and Scott Howell (Case Western Reserve University, Cleveland, OH) for excellent technical assistance; Dr. Shaun Summers (Monash Medical Centre, Monash University, Australia) for kindly donating breeding pairs of TLR9−/− mice; the facilities and scientific and technical assistance of the Australian Microscopy and Microanalysis Research Facility at the Center for Microscopy, Characterization and Analysis, University of Western Australia (a facility funded by the University, State, and Commonwealth Governments); and the staff at Monash Micro Imaging for their excellent technical assistance with confocal microscopy.
Footnotes
Supported by grants from the National Health and Medical Research Council Project (572709), the Elvina King Fund, the Lions Eye Institute, the Lions Save-Sight Foundation of Western Australia, Inc., through the Brian King Postdoctoral Research Fellowship (N.B.), and a grant from the NIH (R01 EY14362 to Y.S. and E.P.).
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.09.041.
Supplementary data
CpG-ODN–induced intraocular inflammation in WT, but not TLR9−/−, mice. Retinas from eyes whose corneas were treated with either CpG-ODN or control ODN revealed an absence of inflammation in TLR9−/− mice compared with WT mice at 24 hours (A). At 24 hours after corneal CpG-ODN, numerous round Iba-1+ inflammatory macrophages and neutrophils were present in the retina of WT, but not TLR9−/−, mice (B). Neutrophil infiltration was observed in the retina after CpG-ODN application to the debrided cornea but was absent after i.v. injection of CpG-ODN or control CpG-ODN or topical application of LPS to the cornea (C). In vivo OCT images of the CpG-ODN–treated eye (D) showing inflammatory cells in the vitreous compared with the healthy appearance of the retina from the naive contralateral eye (E). Data are given as mean ± SEM (n = 3 to 6 mice per group). Scale bars are as shown.
Retinal inflammation occurred after CpG-ODN was injected into the subconjunctival space. IB4-stained vasculature in retinal whole mounts 24 hours after control ODN injection (A) or CpG-ODN (B) is shown. Note the presence of extravascular IB4+ inflammatory cells in the retina of CpG-ODN–injected eyes. The density of IB4+ and NIMP+ inflammatory cells was increased in the retina of CpG-ODN–injected eyes (C) (n = 3 to 4 mice per group).
Retinal inflammation does not occur in TLR9−/− mice 24 hours after topically applied CpG-FITC. Despite the presence of CpG-FITC in CD68+ cells in the retina of TLR9−/− mice (A–C), no inflammatory response was observed (D–F). CpG-FITC was mostly localized to infiltrating CD68+ inflammatory cells in WT retina (arrowheads) and occasional resident microglia (arrow) (n = 3 to 6 mice per group).
References
- 1.Medzhitov R., Preston-Hurlburt P., Janeway C.A., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 2.Gillette-Ferguson I., Daehnel K., Hise A.G., Sun Y., Carlson E., Diaconu E., McGarry H.F., Taylor M.J., Pearlman E. Toll-like receptor 2 regulates CXC chemokine production and neutrophil recruitment to the cornea in Onchocerca volvulus/Wolbachia-induced keratitis. Infect Immun. 2007;75:5908–5915. doi: 10.1128/IAI.00991-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang X., Du W., McClellan S.A., Barrett R.P., Hazlett L.D. TLR4 is required for host resistance in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2006;47:4910–4916. doi: 10.1167/iovs.06-0537. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y., Hise A.G., Kalsow C.M., Pearlman E. Staphylococcus aureus-induced corneal inflammation is dependent on Toll-like receptor 2 and myeloid differentiation factor 88. Infect Immun. 2006;74:5325–5332. doi: 10.1128/IAI.00645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wuest T., Austin B.A., Uematsu S., Thapa M., Akira S., Carr D.J. Intact TRL 9 and type I interferon signaling pathways are required to augment HSV-1 induced corneal CXCL9 and CXCL10. J Neuroimmunol. 2006;179:46–52. doi: 10.1016/j.jneuroim.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitcher J.P., Srinivasan M., Upadhyay M.P. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 7.Brissette-Storkus C.S., Reynolds S.M., Lepisto A.J., Hendricks R.L. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci. 2002;43:2264–2271. [PMC free article] [PubMed] [Google Scholar]
- 8.Hamrah P., Huq S.O., Liu Y., Zhang Q., Dana M.R. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukoc Biol. 2003;74:172–178. doi: 10.1189/jlb.1102544. [DOI] [PubMed] [Google Scholar]
- 9.Knickelbein J.E., Watkins S.C., McMenamin P.G., Hendricks R.L. Stratification of antigen-presenting cells within the normal cornea. Ophthalmol Eye Dis. 2009;1:45–54. doi: 10.4137/oed.s2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chinnery H.R., Carlson E.C., Sun Y., Lin M., Burnett S.H., Perez V.L., McMenamin P.G., Pearlman E. Bone marrow chimeras and c-fms conditional ablation (Mafia) mice reveal an essential role for resident myeloid cells in lipopolysaccharide/TLR4-induced corneal inflammation. J Immunol. 2009;182:2738–2744. doi: 10.4049/jimmunol.0803505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slegers T.P., Broersma L., van Rooijen N., Hooymans J.M., van Rij G., van der Gaag R. Macrophages play a role in the early phase of corneal allograft rejection in rats. Transplantation. 2004;77:1641–1646. doi: 10.1097/01.tp.0000129410.89410.f2. [DOI] [PubMed] [Google Scholar]
- 12.McClellan S.A., Huang X., Barrett R.P., van Rooijen N., Hazlett L.D. Macrophages restrict Pseudomonas aeruginosa growth, regulate polymorphonuclear neutrophil influx, and balance pro- and anti-inflammatory cytokines in BALB/c mice. J Immunol. 2003;170:5219–5227. doi: 10.4049/jimmunol.170.10.5219. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y., Karmakar M., Roy S., Ramadan R.T., Williams S.R., Howell S., Shive C.L., Han Y., Stopford C.M., Rietsch A., Pearlman E. TLR4 and TLR5 on corneal macrophages regulate Pseudomonas aeruginosa keratitis by signaling through MyD88-dependent and -independent pathways. J Immunol. 2010;85:4272–4283. doi: 10.4049/jimmunol.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemmi H., Takeuchi O., Kawai T., Kaisho T., Sato S., Sanjo H., Matsumoto M., Hoshino K., Wagner H., Takeda K., Akira S. A toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 15.Latz E., Schoenemeyer A., Visintin A., Fitzgerald K.A., Monks B.G., Knetter C.F., Lien E., Nilsen N.J., Espevik T., Golenbock D.T. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 16.Krug A., Rothenfusser S., Hornung V., Jahrsdorfer B., Blackwell S., Ballas Z.K., Endres S., Krieg A.M., Hartmann G. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 17.Hartmann G., Battiany J., Poeck H., Wagner M., Kerkmann M., Lubenow N., Rothenfusser S., Endres S. Rational design of new CpG oligonucleotides that combine B cell activation with high IFN-alpha induction in plasmacytoid dendritic cells. Eur J Immunol. 2003;33:1633–1641. doi: 10.1002/eji.200323813. [DOI] [PubMed] [Google Scholar]
- 18.Chinnery H.R., Ruitenberg M.J., Plant G.W., Pearlman E., Jung S., McMenamin P.G. The chemokine receptor CX3CR1 mediates homing of MHC class II-positive cells to the normal mouse corneal epithelium. Invest Ophthalmol Vis Sci. 2007;48:1568–1574. doi: 10.1167/iovs.06-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sosnova M., Bradl M., Forrester J.V. CD34+ corneal stromal cells are bone marrow-derived and express hemopoietic stem cell markers. Stem Cells. 2005;23:507–515. doi: 10.1634/stemcells.2004-0291. [DOI] [PubMed] [Google Scholar]
- 20.Sarangi P.P., Kim B., Kurt-Jones E., Rouse B.T. Innate recognition network driving herpes simplex virus-induced corneal immunopathology: role of the toll pathway in early inflammatory events in stromal keratitis. J Virol. 2007;81:11128–11138. doi: 10.1128/JVI.01008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson A.C., Heinzel F.P., Diaconu E., Sun Y., Hise A.G., Golenbock D., Lass J.H., Pearlman E. Activation of toll-like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation. Invest Ophthalmol Vis Sci. 2005;46:589–595. doi: 10.1167/iovs.04-1077. [DOI] [PubMed] [Google Scholar]
- 22.Zheng M., Klinman D.M., Gierynska M., Rouse B.T. DNA containing CpG motifs induces angiogenesis. Proc Natl Acad Sci U S A. 2002;99:8944–8949. doi: 10.1073/pnas.132605599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang X., Barrett R.P., McClellan S.A., Hazlett L.D. Silencing Toll-like receptor-9 in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2005;46:4209–4216. doi: 10.1167/iovs.05-0185. [DOI] [PubMed] [Google Scholar]
- 24.McMenamin P.G. Optimal methods for preparation and immunostaining of iris, ciliary body, and choroidal whole mounts. Invest Ophthalmol Vis Sci. 2000;41:3043–3048. [PubMed] [Google Scholar]
- 25.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Burnett S.H., Kershen E.J., Zhang J., Zeng L., Straley S.C., Kaplan A.M., Cohen D.A. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J Leukoc Biol. 2004;75:612–623. doi: 10.1189/jlb.0903442. [DOI] [PubMed] [Google Scholar]
- 27.Carlson E.C., Drazba J., Yang X., Perez V.L. Visualization and characterization of inflammatory cell recruitment and migration through the corneal stroma in endotoxin-induced keratitis. Invest Ophthalmol Vis Sci. 2006;47:241–248. doi: 10.1167/iovs.04-0741. [DOI] [PubMed] [Google Scholar]
- 28.Khatri S., Lass J.H., Heinzel F.P., Petroll W.M., Gomez J., Diaconu E., Kalsow C.M., Pearlman E. Regulation of endotoxin-induced keratitis by PECAM-1, MIP-2, and toll-like receptor 4. Invest Ophthalmol Vis Sci. 2002;43:2278–2284. [PubMed] [Google Scholar]
- 29.Leal S.M., Jr, Cowden S., Hsia Y.C., Ghannoum M.A., Momany M., Pearlman E. Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS Pathog. 2010;6:e1000976. doi: 10.1371/journal.ppat.1000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chinnery H.R., Humphries T., Clare A., Dixon A.E., Howes K., Moran C.B., Scott D., Zakrzewski M., Pearlman E., McMenamin P.G. Turnover of bone marrow-derived cells in the irradiated mouse cornea. Immunology. 2008;125:541–548. doi: 10.1111/j.1365-2567.2008.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura T., Ishikawa F., Sonoda K.H., Hisatomi T., Qiao H., Yamada J., Fukata M., Ishibashi T., Harada M., Kinoshita S. Characterization and distribution of bone marrow-derived cells in mouse cornea. Invest Ophthalmol Vis Sci. 2005;46:497–503. doi: 10.1167/iovs.04-1154. [DOI] [PubMed] [Google Scholar]
- 32.Dick A.D., Ford A.L., Forrester J.V., Sedgwick J.D. Flow cytometric identification of a minority population of MHC class II positive cells in the normal rat retina distinct from CD45lowCD11b/c+CD4low parenchymal microglia. Br J Ophthalmol. 1995;79:834–840. doi: 10.1136/bjo.79.9.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kezic J., Xu H., Chinnery H.R., Murphy C.C., McMenamin P.G. Retinal microglia and uveal tract dendritic cells and macrophages are not CX3CR1 dependent in their recruitment and distribution in the young mouse eye. Invest Ophthalmol Vis Sci. 2008;49:1599–1608. doi: 10.1167/iovs.07-0953. [DOI] [PubMed] [Google Scholar]
- 34.Ransohoff R.M., Perry V.H. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 35.Xu H., Dawson R., Forrester J.V., Liversidge J. Identification of novel dendritic cell populations in normal mouse retina. Invest Ophthalmol Vis Sci. 2007;48:1701–1710. doi: 10.1167/iovs.06-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forrester J.V., Worgul B.V., Merriam G.R., Jr Endotoxin-induced uveitis in the rat. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1980;213:221–233. doi: 10.1007/BF00417543. [DOI] [PubMed] [Google Scholar]
- 37.Rosenbaum J.T., McDevitt H.O., Guss R.B., Egbert P.R. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286:611–613. doi: 10.1038/286611a0. [DOI] [PubMed] [Google Scholar]
- 38.Sun Y., Pearlman E. Inhibition of corneal inflammation by the TLR4 antagonist Eritoran tetrasodium (E5564) Invest Ophthalmol Vis Sci. 2009;50:1247–1254. doi: 10.1167/iovs.08-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voigt M., de Kozak Y., Halhal M., Courtois Y., Behar-Cohen F. Down-regulation of NOSII gene expression by iontophoresis of anti-sense oligonucleotide in endotoxin-induced uveitis. Biochem Biophys Res Commun. 2002;295:336–341. doi: 10.1016/s0006-291x(02)00656-3. [DOI] [PubMed] [Google Scholar]
- 40.Cole N., Hume E., Khan S., Madigan M., Husband A.J., Garthwaite L., Willcox M. Contribution of the cornea to cytokine levels in the whole eye induced during the early phase of Pseudomonas aeruginosa challenge. Immunol Cell Biol. 2005;83:301–306. doi: 10.1111/j.1440-1711.2005.01324.x. [DOI] [PubMed] [Google Scholar]
- 41.Carr D.J., Chodosh J., Ash J., Lane T.E. Effect of anti-CXCL10 monoclonal antibody on herpes simplex virus type 1 keratitis and retinal infection. J Virol. 2003;77:10037–10046. doi: 10.1128/JVI.77.18.10037-10046.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iliev A.I., Stringaris A.K., Nau R., Neumann H. Neuronal injury mediated via stimulation of microglial toll-like receptor-9 (TLR9) FASEB J. 2004;18:412–414. doi: 10.1096/fj.03-0670fje. [DOI] [PubMed] [Google Scholar]
- 43.Dalpke A.H., Schafer M.K., Frey M., Zimmermann S., Tebbe J., Weihe E., Heeg K. Immunostimulatory CpG-DNA activates murine microglia. J Immunol. 2002;168:4854–4863. doi: 10.4049/jimmunol.168.10.4854. [DOI] [PubMed] [Google Scholar]
- 44.Deng G.M., Liu Z.Q., Tarkowski A. Intracisternally localized bacterial DNA containing CpG motifs induces meningitis. J Immunol. 2001;167:4616–4626. doi: 10.4049/jimmunol.167.8.4616. [DOI] [PubMed] [Google Scholar]
- 45.Forrester J.V., Xu H., Kuffova L., Dick A.D., McMenamin P.G. Dendritic cell physiology and function in the eye. Immunol Rev. 2010;234:282–304. doi: 10.1111/j.0105-2896.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- 46.Ramkumar H.L., Zhang J., Chan C.C. Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD) Prog Retin Eye Res. 2010;29:169–190. doi: 10.1016/j.preteyeres.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma W., Zhao L., Fontainhas A.M., Fariss R.N., Wong W.T. Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: a potential cellular interaction relevant to AMD. PLoS One. 2009;4:e7945. doi: 10.1371/journal.pone.0007945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hughes T.S., Langer S.J., Virtanen S.I., Chavez R.A., Watkins L.R., Milligan E.D., Leinwand L.A. Immunogenicity of intrathecal plasmid gene delivery: cytokine release and effects on transgene expression. J Gene Med. 2009;11:782–790. doi: 10.1002/jgm.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duramad O., Fearon K.L., Chang B., Chan J.H., Gregorio J., Coffman R.L., Barrat F.J. Inhibitors of TLR-9 act on multiple cell subsets in mouse and man in vitro and prevent death in vivo from systemic inflammation. J Immunol. 2005;174:5193–5200. doi: 10.4049/jimmunol.174.9.5193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CpG-ODN–induced intraocular inflammation in WT, but not TLR9−/−, mice. Retinas from eyes whose corneas were treated with either CpG-ODN or control ODN revealed an absence of inflammation in TLR9−/− mice compared with WT mice at 24 hours (A). At 24 hours after corneal CpG-ODN, numerous round Iba-1+ inflammatory macrophages and neutrophils were present in the retina of WT, but not TLR9−/−, mice (B). Neutrophil infiltration was observed in the retina after CpG-ODN application to the debrided cornea but was absent after i.v. injection of CpG-ODN or control CpG-ODN or topical application of LPS to the cornea (C). In vivo OCT images of the CpG-ODN–treated eye (D) showing inflammatory cells in the vitreous compared with the healthy appearance of the retina from the naive contralateral eye (E). Data are given as mean ± SEM (n = 3 to 6 mice per group). Scale bars are as shown.
Retinal inflammation occurred after CpG-ODN was injected into the subconjunctival space. IB4-stained vasculature in retinal whole mounts 24 hours after control ODN injection (A) or CpG-ODN (B) is shown. Note the presence of extravascular IB4+ inflammatory cells in the retina of CpG-ODN–injected eyes. The density of IB4+ and NIMP+ inflammatory cells was increased in the retina of CpG-ODN–injected eyes (C) (n = 3 to 4 mice per group).
Retinal inflammation does not occur in TLR9−/− mice 24 hours after topically applied CpG-FITC. Despite the presence of CpG-FITC in CD68+ cells in the retina of TLR9−/− mice (A–C), no inflammatory response was observed (D–F). CpG-FITC was mostly localized to infiltrating CD68+ inflammatory cells in WT retina (arrowheads) and occasional resident microglia (arrow) (n = 3 to 6 mice per group).