Abstract
β-Secretase, the rate-limiting enzymatic activity in the production of the amyloid-β (Aβ) peptide, is a major target of Alzheimer's disease (AD) therapeutics. There are two forms of the enzyme: β-site Aβ precursor protein cleaving enzyme (BACE) 1 and BACE2. Although BACE1 increases in late-stage AD, little is known about BACE2. We conducted a detailed examination of BACE2 in patients with preclinical to late-stage AD, including amnestic mild cognitive impairment, and age-matched controls, cases of frontotemporal dementia, and Down's syndrome. BACE2 protein and enzymatic activity increased as early as preclinical AD and were found in neurons and astrocytes. Although the levels of total BACE2 mRNA were unchanged, the mRNA for BACE2 splice form C (missing exon 7) increased in parallel with BACE2 protein and activity. BACE1 and BACE2 were strongly correlated with each other at all levels, suggesting that their regulatory mechanisms may be largely shared. BACE2 was also elevated in frontotemporal dementia but not in Down's syndrome, even in patients with substantial Aβ deposition. Thus, expression of both forms of β-secretase are linked and may play a combined role in human neurologic disease. A better understanding of the normal functions of BACE1 and BACE2, and how these change in different disease states, is essential for the future development of AD therapeutics.
Alzheimer's disease (AD) is the most common age-related neurodegenerative disease. Although progressive memory loss is the best known clinical symptom, patients also exhibit a wide range of other behavioral disturbances, from paranoia and delusions to a gradual decline in language ability. In the affected brain, two forms of neuropathology develop in characteristic patterns: extracellular amyloid deposits, referred to as plaques, and intracellular neurofibrillary tangles (NFTs). The presence of all three—NFTs, plaques, and cognitive impairment—define AD as a clinical entity. The lack of an effective, disease-modifying therapy for AD is one of the greatest unmet medical needs facing modern society.
Despite gaps in our knowledge about the general causes of AD, much is known about the molecular biology underlying several key pathways. A large body of compelling evidence provides broad support for the idea that the amyloid-β (Aβ) peptide is the causative pathologic agent in AD. The Aβ peptide is produced from the larger Aβ precursor protein (APP) through two sequential enzymatic activities, β- and γ-secretase. β-Secretase first cleaves APP to release a large secreted derivative, sAPPβ. A membrane-bound fragment, C-terminal fragment of APP (CTF) β, remains and is, in turn, proteolytically cleaved by γ-secretase to generate Aβ and a cytosolic fragment, which may offer a clue to the physiologic function of APP.1 β-Secretase is the rate-limiting enzyme in the production of Aβ, a limitation that likely arises because it does not often encounter its APP substrate in the cell.2 Consequently, targeting β-secretase is considered a prime candidate strategy for developing an effective AD therapy.
The atypical aspartyl protease β-site APP cleaving enzyme (BACE1) was identified as the major β-secretase enzyme by multiple research groups slightly more than a decade ago.3 Mature BACE1 is a palmitoylated glycoprotein that is processed to an active form by removal of a prodomain by a furin-like convertase activity.4 BACE1 is involved in nervous system myelination,5 and increases in BACE1 after traumatic brain injury may reflect this function.6 The BACE1 cleavage product of APP (sAPPβ), may have distinct physiologic functions that distinguish it from the α-secretase product (sAPPα).7 There are five BACE1 splice variants, although it is unknown whether their variation is related to the development of AD.8
Considerably less is known about the homologous enzyme BACE2. BACE1 and BACE2 compete for substrate and can both cleave APP at the β-site.9,10 Although BACE2 is present in most peripheral tissues,11 there is a moderate amount of BACE2 expression in the human brain.12 In the brain, BACE2 is believed to be mostly astrocytic, whereas BACE1 is largely neuronal.10 BACE2 is located on chromosome 21 in the Down's syndrome (DS) obligate region and may contribute to amyloid abnormalities in these individuals.13,14
Knockout of BACE1 in the mouse leads to the abolishment of Aβ, sAPPβ, and CTFβ production in the brain.15 Crossing BACE1 knockout mice with models of amyloid deposition leads to reduced brain Aβ and improved cognitive function.16 However, BACE1 knockout animals have many subtle phenotypic alterations, including deficits in long-term potentiation, increased seizure susceptibility, and schizophrenic behavior.17,18 BACE1 knockouts exhibit residual β-secretase activity that may be attributable to BACE2.15,19,20 Our understanding of the relationship between BACE1 and BACE2, and how they may be connected in human disease, is incomplete.
The amount of active BACE1 protein is elevated in the brain in late-stage AD,21–25 and higher levels of BACE1 are also observed in the cerebrospinal fluid.26 Individuals with mild cognitive impairment (MCI), presumed to be the earliest detectable clinical phase of AD, also show elevated BACE1 expression in cerebrospinal fluid27 and increased β-secretase activity from platelets.28 These studies have raised the intriguing possibility that changes in β-secretase activity may be directly connected to the development of AD; however, important questions remain unanswered. To be causally involved in AD, the increase in BACE1 should occur in the brain at an early, preclinical stage. This has not been conclusively established. Furthermore, minimal information is available on how specific the changes in BACE1 are to AD compared with other neurologic diseases.26 Finally, the function of BACE2 in the brain, and its possible role in disease, is unresolved. We recently observed unexpectedly high levels of active BACE2 protein in the AD brain,22 raising the question of whether it may have been overlooked in earlier studies. In this study, we attempted to answer these questions by conducting a detailed examination of BACE1 and BACE2 in a broad cohort of patients.
Materials and Methods
Subjects
We selected two series of cases to study two overlapping questions (Table 1). The first case series (see Supplemental Table S1 at http://ajp.amjpathol.org) was chosen to examine the role of β-secretase in age-related neurodegenerative disease. These samples were obtained from the tissue repository of the Alzheimer's Disease Center at the University of Kentucky, Sanders-Brown Center on Aging. Controls (n = 9) were age matched to disease-affected cases [preclinical AD (PCAD): n = 10; amnestic MCI: n = 7; and AD: n = 10]. PCAD cases (often referred to as high-pathology controls) were defined as those that met the National Institute on Aging–Reagan Institute neuropathology criteria for likely AD but exhibited no clinical signs of dementia.29 Amnestic MCI was defined as per the criteria of Petersen et al.30 We included six cases of frontotemporal dementia (FTD) as an additional neurodegenerative disease and specificity control.31 Aβ is not considered to play a significant role in FTD. Details of the recruitment, inclusion criteria, and mental status test battery for the control group have been described previously.32 Details of the tissue collection procedures and consensus diagnosis have also been described (see Supplemental Figure S1 at http://ajp.amjpathol.org).33 The second case series (see Supplemental Table S2 at http://ajp.amjpathol.org) was selected to better elucidate the role of Aβ deposition as a feedback mechanism for changes in β-secretase. Individuals with DS develop Aβ deposition and other AD-like abnormalities with age. DS cases and controls were obtained from the Alzheimer's Disease Center of the University of California at Irvine brain tissue repository and the National Institute of Child Health and Human Development Brain and Tissue Bank for Developmental Disorders (University of Maryland). Control brains had no history of antemortem dementia. An analysis of RNA integrity numbers, controlling for age and postmortem interval, indicated no significant difference in tissue quality between sites (n = 46; F1,42 = 0.9, P < 0.4).
Table 1.
Subject Demographic Data
| Sex, M/F (no.) | Age (years)⁎ | PMI (hours)⁎ | Brain weight (g)⁎ | Braak stage (median) | MMSE score⁎ | |
|---|---|---|---|---|---|---|
| Case series I | ||||||
| Control | 5/4 | 84.3 ± 5.1 | 2.8 ± 0.8 | 1234 ± 163 | I | 28.4 ± 1.5 |
| FTD | 3/3 | 61.0 ± 14.6 | 4.8 ± 1.0 | 941 ± 169 | 0 | 7.8 ± 9.0 |
| Amnestic MCI | 3/4 | 89.0 ± 5.8 | 2.8 ± 0.5 | 1118 ± 132 | IV | 24.8 ± 3.1 |
| Preclinical AD | 1/9 | 85.6 ± 3.7 | 2.6 ± 0.5 | 1173 ± 110 | IV | 29.4 ± 0.7 |
| AD | 4/6 | 83.4 ± 5.7 | 3.1 ± 0.6 | 1074 ± 89 | VI | 9.9 ± 6.0 |
| Case series II | ||||||
| Control | 10/10 | 37.0 ± 21.8 | 9.5 ± 5.5 | NA | NA | NA |
| DS | 18/10 | 34.4 ± 21.2 | 13.5 ± 8.5 | NA | NA | NA |
Series I measurements were made from the SMTG (areas 21 and 22) and the CB; series II measurements were made from the middle frontal gyrus (area 9).
F, female; M, male; MMSE, Mini-Mental State Examination; NA, not applicable or unavailable; PMI, postmortem interval.
Values are given as mean ± SD.
Tissue Collection and Processing
Frozen samples were homogenized using a PowerMax Advanced Homogenizing System 200 (VWR, Batavia, IL) in five volumes (wet w/v) of tissue lysis buffer [10 mmol/L sodium acetate (NaOAc), 3.0 mmol/L NaCl, 0.1% Triton X-100 (Roche Diagnostics GmbH, Mannheim, Germany), 0.32 mol/L sucrose, pH 5.0]. The buffer was supplemented with a complete protease inhibitor cocktail with EDTA (PIC; Amresco, Solon, OH), with 100 nmol/L pepstatin A added (Sigma-Aldrich; St. Louis, MO). Whole tissue homogenate was centrifuged at ∼2000 × g for 15 minutes to pellet insoluble material, followed by an additional spin at 20,000 × g for 30 minutes. Pelleted material was sequentially extracted in an equal volume of radioimmunoprecipitation assay (RIPA) buffer (0.1% SDS, 0.5% deoxycholate, 1.0% Triton X-100, 50 mmol/L Tris base, 150 mmol/L NaCl, pH 8.0, with PIC) or 2% SDS (w/v, with PIC) to determine detergent-soluble Aβ, followed by 70% (v/v) formic acid (FA) to determine insoluble Aβ.34 In each case, the pellet was extracted by brief sonication (10 × 0.5-second microtip pulses at 20% power; Fisher sonic dismembrator, model 500, Fisher Scientific, Pittsburgh, PA) followed by centrifugation to pellet-insoluble material (detergent-soluble fraction: 20,000 × g for 30 minutes at 14°C; FA fraction: 20,000 × g for 1 hour at 4°C). Protein content was determined by bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL).
Immunohistochemical Analysis
For immunofluorescence, sections were labeled for BACE1 (rabbit monoclonal EPR3956; Epitomics Inc., Burlingame, CA), BACE2 (rabbit polyclonal Ab1; EMD Biosciences, San Diego, CA), glial fibrillary acidic protein (for astrocytes; clone GA5, MAB360; Millipore, Billerica, MA), Iba-1 (for microglia; Biocare Medical, Concord, CA), 4G8 (for amyloid deposits; Covance, Denver, PA), or PHF-1 (for pathologic tau; a gift from Dr. Peter Davies, Albert Einstein College of Medicine, Bronx, NY) (see Supplemental Table S3 at http://ajp.amjpathol.org for a detailed list of all the antibodies used in this study). Sections were deparaffinized in SafeClear (Fisher Scientific) and rehydrated before blocking endogenous peroxidase activity with 3% hydrogen peroxide in 10% methanol. All the sections were subjected to antigen retrieval by boiling for 10 minutes in citric acid buffer (10 mmol/L sodium citrate + 0.05% Tween 20, pH 5.0) and blocked (PBS + 2.5% normal goat serum, 2.5% normal donkey serum, and 0.05% Tween 20). Secondary antibodies were anti-rabbit Alexa Fluor 568 (red) or anti-mouse Alexa Fluor 488 (green) (Invitrogen, Carlsbad, CA). Slides were treated with autofluorescence eliminator reagent (Millipore) and coverslipped with Vectashield (Vector Laboratories, Burlingame, CA). Alternatively, we used the Vector avidin-biotin complex kit (Vector Laboratories) with either 3,3′-diaminobenzidine or Vector SG (Vector Laboratories) as substrate.
Enzyme Activity Assays
We recently described and validated the assays for BACE1 and BACE2 for human tissue in considerable detail, including reagent specificity.22 This method is similar to that of Fukumoto et al.35 Determination of BACE1 (MAB931, raised against the BACE1 ectodomain; R&D Systems, Minneapolis, MN) or BACE2 (Ab1, raised against amino acids 496–511 of BACE2; EMD Biosciences) activities was as described.22 In some cases, validation experiments were performed using two different antibodies directed against the opposite ends of BACE1 (C-terminus, EPR3956; Epitomics Inc.) or BACE2 (N-terminus, rabbit polyclonal Ab2; EMD Biosciences). For neprilysin (NEP) activity, tissue samples were homogenized in ∼10 volumes of 50 mmol/L potassium phosphate buffer, pH 7.3. Samples were centrifuged at 500 × g for 15 minutes at 4°C, and the supernatant was used to measure NEP activity.36 Specificity was demonstrated by inhibition with 100 μmol/L phosphoramidon or 2 μmol/L CGS24592.37
ELISA
Detergent- and FA-soluble pools of Aβ were measured in tissue samples using a standard, well-characterized sandwich enzyme-linked immunosorbent assay (ELISA).34,38,39 To determine the total amount of Aβ, capture was performed using monoclonal antibody Ab9 (against the amino-terminus of Aβ), and detection was performed using biotinylated 4G8 against Aβ17-24 (Covance), followed by horseradish peroxidase–conjugated NeutrAvidin (Pierce Biotechnology); Aβ40 was detected with Ab13.1.1, and Aβ42 was detected with 12F4 (Covance).38 Alternatively, Aβ42 was captured using Ab2.1.3 and was detected with Ab9; alternative antibody combinations gave essentially identical results. For the measurement of APP or APP CTFs by ELISA, we used a similar procedure. Antibody 22C11 (Millipore) was used to capture full-length APP from the sample, which was then detected using biotinylated 6E10 against Aβ1–16 (Covance). The cleared sample was then transferred to a second plate, coated with affinity-purified antibody CT20, and raised against the last 20 amino acids of APP (see Supplemental Figure S2 at http://ajp.amjpathol.org). Captured CTFs were detected using either antibody 6E10 (for CTFβ) or 4G8 (for total CTFs). Oligomeric Aβ was measured using the single-site 4G8/4G8 sandwich ELISA.34
Real-Time PCR
For RNA isolation, 100 mg of frozen tissue was homogenized using TRIzol reagent (Invitrogen) followed by phenol/chloroform extraction and ethanol precipitation, as per the manufacturer's instructions. RNeasy cleanup columns (Qiagen, Valencia, CA) were run for each sample. The reverse transcriptase reaction (iScript select cDNA synthesis kit; Bio-Rad Laboratories, Hercules, CA) used 1 μg of RNA, purified RNase H, MMLV reverse transcriptase, and a mixture of random hexamers and oligo dT primer, according to the manufacturer's instructions. Primers were designed with the following forward and reverse sequences, respectively: APP: 5′-AACCAGTGACCATCCAGAAC-3′ and 5′-ACTTGTCAGGAACGAGAAGG-3′; BACE1: 5′-TATCATGGAGGGCTTCTACGTTG-3′ and 5′-GTCCTGAACTCATCGTGCACAT-3′; BACE2: 5′-GCAACCATGAACTCAGCTATTAAGAA-3′ and 5′-AGAAAGCGCCACCATCGA-3′; and BACE2Δ7: 5′-GCCCCAGAAGGTGTTTGAT-3′, 5′-GCTGAATGTAAAGCTGAGGC-3′, and 5′-GGCTGAATGTAAAGCAGAG-3′. Additional primers were designed for housekeeping genes with the following forward and reverse sequences, respectively: tumor protein translationally controlled 1 (TPT1): 5′-GATCGCGGACGGGTTGT-3′ and 5′-TTCAGCGGAGGCATTTCC-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH): 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′; hypoxanthine purine phosphoribosyl-transferase 1 (HPRT): 5′-GACCAGTCAACAGGGGACAT-3′ and 5′-AACACTTCGTGGGGTCCTTTTC-3′; and ribosomal protein L32 (RPL32): 5′-CATCTCCTTCTCGGCATCA-3′ and 5′-AACCCTGTTGTCAATGCCTC-3′.40 Standardization was performed to the geometric mean of at least two housekeeping genes. Quantitative real-time PCR reactions contained ∼20 ng of sample cDNA together with PerfeCTa SYBR green supermix (Quanta BioSciences Inc., Gaithersburg, MD).
SDS-PAGE and Western Blot Analysis
Proteins were separated using a range of SDS-PAGE Criterion gels (Bio-Rad Laboratories) and were electrically transferred to 0.45-μm nitrocellulose or polyvinylidene difluoride membranes. Primary antibodies used were rabbit polyclonal CT20, mouse monoclonal MAB931 (BACE1), rabbit monoclonal EPR3956 (BACE1), rabbit polyclonal Ab1 (BACE2), rabbit polyclonal Ab5670 (BACE2, amino acids 441-457; Abcam Inc., Cambridge, MA), rabbit polyclonal Ab2 (BACE2), rabbit polyclonal anti-GAPDH (Abcam Inc.), or mouse monoclonal AC15 (against β-actin; Sigma-Aldrich). Horseradish peroxidase–conjugated secondary antibodies and enhanced chemiluminescent detection reagents were obtained from Pierce Biotechnology.
Data Analyses
Data were analyzed using SPSS (SPSS Inc., Chicago, IL). Simple group comparisons were made using either Student's t-test or the Mann-Whitney U-test, where appropriate. Group data were analyzed by a general linear model analysis of variance, covarying for age and postmortem interval when necessary, and post hoc comparisons were performed using Dunnett's test. Correlations were determined using either Pearson's r or Spearman's ρ, where appropriate.
Results
BACE1 and BACE2 Are Both Present in Neurons
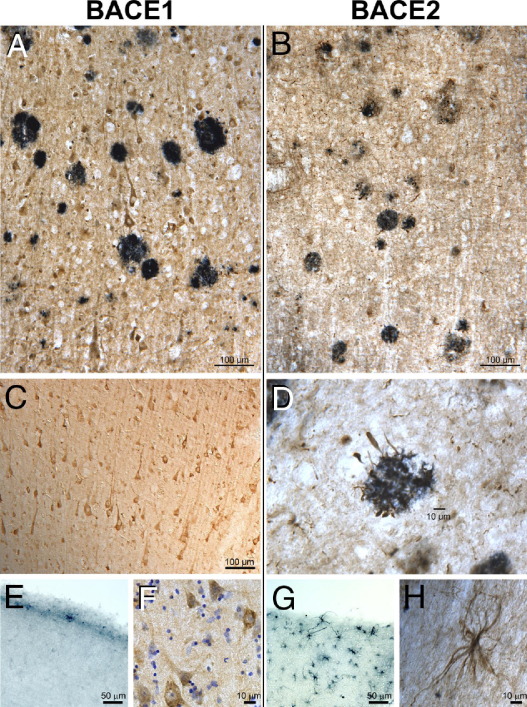
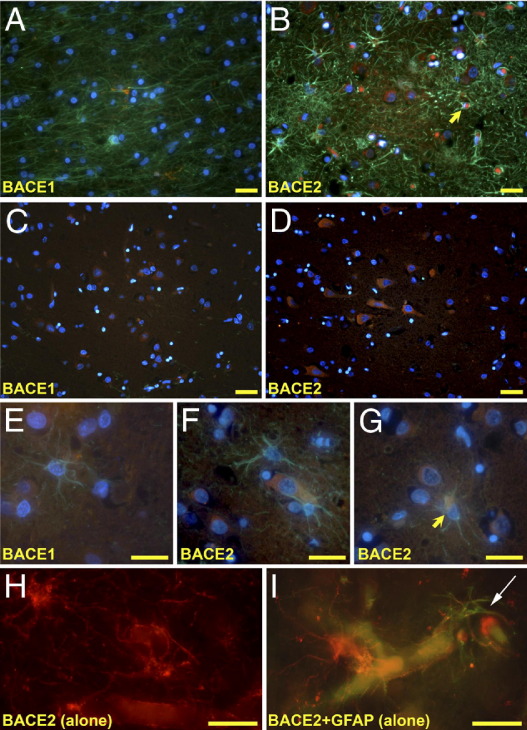
Using well-characterized antibodies,22 we first examined the localization patterns of BACE1 and BACE2 in the human brain (Figure 1). BACE1 and BACE2 immunoreactivity was present in AD and control cases. BACE1-positive cells were abundant throughout the neocortex and were often of distinct pyramidal cell morphology. BACE1 immunoreactivity was mainly present as punctate perinuclear and cytoplasmic staining, consistent with a primary localization to the endosomal/lyosomal compartment. We observed numerous BACE2-positive cells that had the signature stellate appearance of astrocytes, particularly close to the brain surface. BACE2-positive pyramidal neurons were also abundant. These areas frequently contained few BACE1-positive cells. BACE2 immunoreactivity within cells of astrocytic morphology was more widely dispersed, and the processes were frequently labeled extensively. We did not observe a clear relationship between BACE1- or BACE2-positive cells and deposits of the Aβ peptide in either AD cases or controls. However, BACE2-positive processes resembling dystrophic neurites were found closely associated with some plaques. Even in areas where astrocytes were present in large numbers, we did not observe any that were clearly BACE1 positive (Figure 2). Although we did find many examples of BACE2-positive astrocytes, these were uncommon. We observed BACE2 astrocytic processes in association with blood vessels. We did not observe significant immunostaining for either BACE1 or BACE2 in microglia (see Supplemental Figure S3 at http://ajp.amjpathol.org). Hence, although there were some differences in distribution patterns, both BACE1 and BACE2 were found in neurons.
Figure 1.
BACE1 (left panels) and BACE2 (right panels) show distinct patterns of cellular immunoreactivity in the human brain. A and C: BACE1 and BACE2 immunoreactivity was more prevalent in AD brain. Strongly BACE1-positive cells (brown) of distinct neuronal morphology are found throughout the neocortex and are not clearly associated with Aβ deposits (blue); this was true for AD and control cases. B and D: BACE2 immunoreactivity (brown) was also found in neurons, but rare BACE2-positive processes were observed intertwined with plaques (blue). E and G: Many BACE2-positive cells with a distinctive astrocytic morphology are clustered in the outer cortical layer, in areas largely devoid of BACE1-positive cells. F and H: Although some BACE1 is found in cellular processes, most BACE1 immunoreactivity is localized in a punctate perinuclear pattern; in contrast, BACE2 has a broader distribution that may fill the entire cell (counterstain: cresyl violet). Antibodies: BACE1, mouse monoclonal MAB931; BACE2, rabbit polyclonal Ab2; and Aβ, mouse monoclonal 4G8. Substrates: 3,3′-diaminobenzidine (brown) or Vector SG (blue).
Figure 2.
BACE2 was observed in neurons and astrocytes. Most BACE2 immunoreactivity was found in the cortical layer near the surface of the brain and in areas surrounding blood vessels. A: BACE1 was not seen in astrocytes, even in areas where substantial numbers of astrocytes were clearly labeled or in areas of extensive gliosis. B: BACE2-positive astrocytes were rarely found (arrowhead) in areas of extensive neurodegeneration and gliosis. C and D: BACE1 and BACE2 were present in neocortical pyramidal neurons. E and F: BACE1- and BACE2-negative astrocytes in close proximity to positive-labeled cells, some of which are distinctively neuronal in morphology. G: A BACE2-positive astrocyte (yellow arrow) showing strong cytoplasmic labeling. H and I: BACE2-positive astrocytic processes (white arrow) are often found in association with blood vessels. Antibodies: BACE1, rabbit monoclonal EPR3956 (red); BACE2, rabbit polyclonal Ab2 (red); and glial fibrillary acidic protein, mouse monoclonal MAB360 (green). Counterstain: DAPI (blue). Scale bars: 25 μm.
BACE1 and BACE2 Proteins and Activities Increase in Neurodegenerative Disease
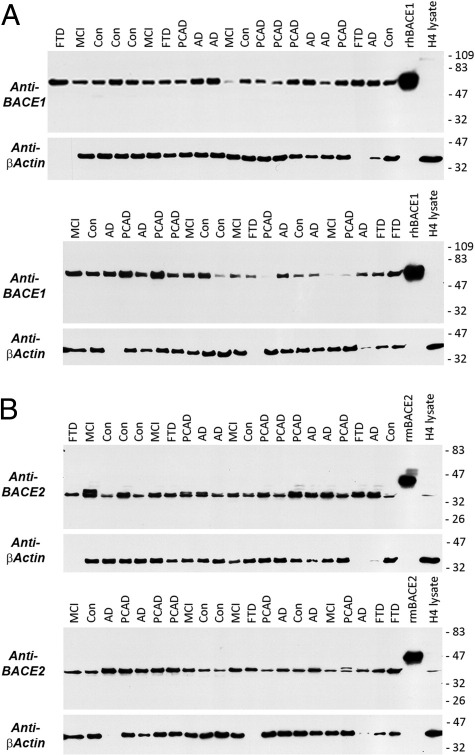
In an earlier study, we noted that BACE2 was nearly as abundant in human brain as was BACE1.22 This was also true in this larger series of cases (Figure 3). We determined the amount of BACE1 and BACE2 proteins using standard curves generated from recombinant standards run on the same Western blot. Although the difference was not large, the mean ± SD amount of BACE1 (4.7 ± 0.73 ng/μg) was higher than that of BACE2 (3.2 ± 0.46 ng/μg; t-test, P < 0.001; five controls and six AD cases). The BACE1 isolated from brain migrates near its expected molecular weight based on the recombinant protein standard. However, BACE2 migrates at a slightly lower-than-expected apparent molecular weight. There are two common splice variants of BACE2 in addition to the full-length (splice form A) version: BACE2 splice form B uses an alternative splice site for exon 8 (resulting in a truncated form with a different C-terminal end), and splice form C omits exon 7 but is otherwise unchanged (BACE2Δ7). The lower observed relative molecular weight coupled with the pattern of immunoreactivity (eg, Ab5670 will not detect BACE2 splice form B) indicates that the major form of BACE2 protein in the human brain is likely splice form C.
Figure 3.
BACE1 (A) and BACE2 (B) are abundant in human brain. Equal amounts of protein from the SMTG (25 μg) were separated by SDS-PAGE under reducing conditions on Criterion 10% to 20% Tris-glycine gels (Bio-Rad Laboratories). Controls included human recombinant BACE1 (100 ng; R&D Systems) or mouse recombinant BACE2 (100 ng; R&D Systems) and cell lysate from H4 neuroglioma cells (25 μg; these cells contain relatively little BACE1 but have readily detectable BACE2). Samples were transferred to 0.45-μmol/L nitrocellulose membranes and blocked overnight. After probing for BACE1 (MAB931) or BACE2 (Ab5670), blots were stripped and reprobed for β-actin. Although β-actin was broadly similar, in some cases the levels were very low. Comparison with recombinant protein standards indicates similar quantities of BACE1 and BACE2 protein, although there is slightly more BACE1 (see Results). Although the BACE1 band migrates close to the recombinant protein standard, BACE2 runs at a slightly lower apparent molecular weight. The lower-than-expected molecular weight of this band and the observed pattern of antibody immunoreactivity together indicate that the active form of BACE2 in the brain is likely BACE2Δ7 (splice form C).
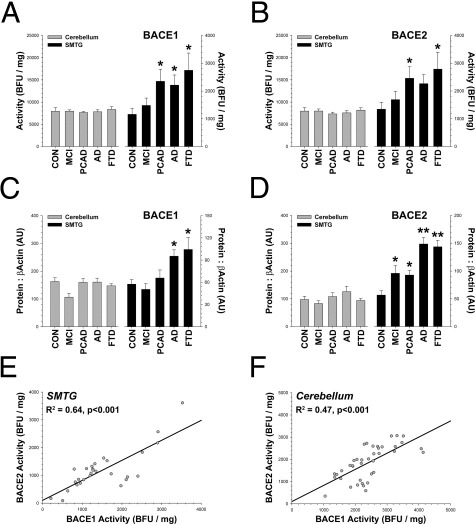
Overall, BACE1 (F4,32 = 2.89, P < 0.04) and BACE2 (F4,32 = 2.87, P < 0.04) activities were increased in neurodegenerative disease (Figure 4). The increase occurred in a disease-affected region [the superior and middle temporal gyri (SMTG), areas 21 and 22] but not in a brain region unaffected by disease [the cerebellum (CB)]. Although enzymatic activity was not increased in the CB with disease, BACE1 (R2 = 0.25, P < 0.001) and BACE2 (R2 = 0.08, P < 0.04) activities in the CB were still significantly correlated with activities in the SMTG. The same increases in a disease-affected region (SMTG) but not in an unaffected region (CB) were seen for BACE1 (F4,32 = 5.87, P < 0.001) and BACE2 (F4,32 = 15.03, P < 0.0001) proteins using Western blot analysis (Figure 3), when examined by densitometry. The results were unchanged when the data were not standardized to β-actin as a loading control. The results were also the same when MCI and PCAD cases were treated as a single, combined group (the amount of pathologic disease in these cases is nearly identical; Supplemental Table S1 and Supplemental Figures S1 and S4 at http://ajp.amjpathol.org). As expected from earlier assay validation studies, BACE1 and BACE2 activities were correlated with their respective protein bands detected by immunoblot analysis (BACE1: R2 = 0.17, P < 0.006; BACE2: R2 = 0.21, P < 0.002). Also, consistent with a general increase in β-secretase activity, the APP CTFβ/CTFα ratio was higher in AD in the SMTG (F4,34 = 6.15, P < 0.001) but not in the CB, as determined by immunoblot analysis (see Supplemental Figure S5 at http://ajp.amjpathol.org). The CTFβ/CTFα ratio was also modestly correlated with BACE1 (R2 = 0.07, P < 0.05) and BACE2 (R2 = 0.07, P < 0.05) activity. The outcome was similar when the SMTG ratio was standardized to the CTFβ/CTFα ratio in the CB, and the overall disease effect was significant (F4,34 = 4.97, P < 0.01). These data indicate that an increase in BACE1 and BACE2 activities and protein levels likely occurs at an early disease stage.
Figure 4.
BACE1 and BACE2 are increased in neurodegenerative disease. A: BACE1 activity (as determined by the MAB931 capture assay) is higher in PCAD, AD, and FTD. B: BACE2 activity (as determined by the Ab1 capture assay) is higher in FTD and PCAD and strongly trends toward an increase in AD (P < 0.07). C: BACE1 protein is higher using Western blot analysis in FTD and AD (Figure 3). D: BACE2 protein is higher using Western blot analysis in MCI, PCAD, AD, and FTD (Figure 3). Immunoblot analysis results were essentially unchanged when not standardized to β-actin, indicating that outlier cases with very low β-actin levels had minimal effect on the analysis. E and F: BACE1 and BACE2 activities are highly correlated, a phenomenon observed with multiple antibodies and assay conditions (shown: BACE1, EPR3956 capture assay; BACE2, Ab2 capture assay). Dunnett's test, *P < 0.05, †P < 0.01. Scale: 1000 base fluorescence units (BFU)/mg = 2.3 nmol · min−1 · mg−1 total protein.
We observed a striking correlation between BACE1 and BACE2 in human brain, a phenomenon observed with multiple antibody combinations and assay conditions. Using a standard assay for BACE1 (MAB931) and BACE2 (Ab1) activities, we found strong correlations between the two enzymatic activities in the SMTG (R2 = 0.9, P < 0.0001) and the CB (R2 = 0.7, P < 0.001), indicating that this was unrelated to disease. To confirm this finding, we repeated the assay using a different method. In the validation test, we reversed the orientation of the assay and used different antibodies, capturing BACE1 at the C-terminus (using EPR3956) and BACE2 at the N-terminus (using Ab2). BACE1 and BACE2 activities remained highly correlated in the SMTG (R2 = 0.64, P < 0.001) and the CB (R2 = 0.47, P < 0.001) (Figure 4). We observed similar disease-related increases in BACE1 and BACE2 using this alternate method (see Supplemental Figure S6 at http://ajp.amjpathol.org). Finally, we examined the BACE1 (using MAB931) and BACE2 (using Ab5670) relationship by immunoblot analysis and detected a similarly strong correlation (R2 = 0.32, P < 0.001). The correlation was significant regardless of whether the data were standardized to β-actin. Hence, we detected strong correlations between BACE1 and BACE2 proteins and activities using different methods.
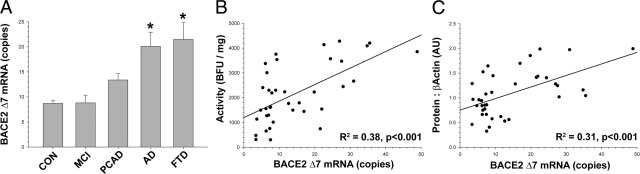
We next wanted to determine whether changes in the β-secretase enzymes occurred only at the level of protein and activity or extended down to the mRNA level. The total amount of BACE1 or BACE2 mRNA did not change with disease state (see Supplemental Figure S7 at http://ajp.amjpathol.org). The amounts of BACE1 and BACE2 mRNA correlated with each other in the SMTG (R2 = 0.48, P < 0.001) and the CB (R2 = 0.72, P < 0.001), but in neither case did the amount of total mRNA correlate with the amount of BACE1 or BACE2 activity or protein. Because immunoblot data indicated that the form of BACE2 in the brain might be missing exon 7, we developed an assay to specifically detect this form. Although the total BACE2 mRNA level did not change with disease state, the BACE2Δ7 mRNA level increased (F4,28 = 3.07, P < 0.04) in a pattern similar to that seen for protein and activity (Figure 5A). Increases in the amount of BACE2Δ7 mRNA were positively correlated with BACE2 enzymatic activity (R2 = 0.38, P < 0.001; Figure 5B) and the amount of BACE2 protein detected by Western blot analysis (R2 = 0.31, P < 0.001; Figure 5C). BACE2 enzymatic activity and BACE2 protein by Western blot analysis were determined using antibodies directed against different regions of the BACE2 protein, both of which do not detect BACE2 splice form B (the other shorter form of BACE2). Together, these data suggest that the principal active isoform of BACE2 in the human brain lacks exon 7 and increases in neurodegenerative disease.
Figure 5.
The mRNA for the splice form of BACE2 missing exon 7 (splice form C, or BACEΔ7) is increased in neurodegenerative disease. A: The BACE2Δ7 mRNA expression increased with disease state (P < 0.04) and was significantly elevated in late-stage AD and FTD; values were standardized to the geometric mean of RPL32, GAPDH, and HPRT (total mRNA did not change). The increase in BACE2Δ7 mRNA was positively correlated (P < 0.001) with enzymatic activity (as determined by Ab1 capture assay) (B) and the amount of BACE2 protein (as determined by Ab5670 immunoblot) (C) (Figure 4). Dunnett's test, *P < 0.05. Scale: 1000 base fluorescence units (BFU)/mg = 2.3 nmol · min−1 · mg−1 total protein.
BACE1 and BACE2 Proteins and Activities Are Unchanged in DS
Because we detected a substantial amount of both forms of β-secretase in the brain, we were interested in how each might be connected to AD-related neuropathology and to the amount of the Aβ peptide. We reasoned that these questions might be examined in detail by studying postmortem brain specimens from individuals with DS, who possess an additional copy of the APP and BACE2 genes. As individuals with DS age, they develop substantial Aβ deposition, along with other aspects of AD pathology.41 This may be related to the overexpression of APP, BACE2, or both because the genes for both proteins reside in the DS obligate region of chromosome 21.
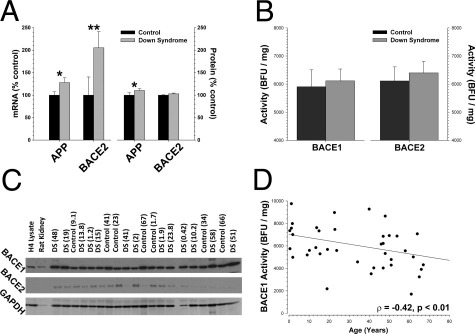
As expected, total mRNA levels for BACE2 (Mann-Whitney U-test, P < 0.01) and APP (P < 0.05) were increased in DS brains versus controls (Figure 6A). The total mRNA level for BACE1 was unchanged in DS cases (data not shown). We detected a small increase in the amount of APP protein (P < 0.05) but not in the amount of BACE2 protein. Expression of full-length APP protein did decline with increasing age (ρ = −0.437, P < 0.002), which may partially account for why the overall APP increase in DS was relatively small across the entire pool of subjects. BACE1 (F1,34 = 0.96, P < 0.34) and BACE2 (F1,34 = 1.14, P < 0.30) enzymatic activities (Figure 6B) and protein levels (Figure 6C; also see Supplemental Figure S8 at http://ajp.amjpathol.org) were not different between DS cases and the series of age-matched control cases. Power calculations (δ = 2.80) indicated that we are able to detect a difference of ∼19% between the two groups with this number of subjects. Because the mRNA for BACE2 is increased approximately twofold in DS, this leads us to conclude that the additional message is not efficiently translated into protein. Similarly, using immunoblot analysis, we did not see differences between CTFβ that might correspond to differences in β-secretase activity in DS. We also examined CTFβ standardized to APP by ELISA and also did not detect any change that might correspond to altered β-secretase activity (see Supplemental Figure S9 at http://ajp.amjpathol.org). BACE1 activity decreased a small amount with age across DS and control cases (Figure 6D; P < 0.01). As with the previous set of neurodegenerative disease–affected cases, the activities of BACE1 and BACE2 were highly correlated (R2 = 0.9, P < 0.0001).
Figure 6.
BACE1 and BACE2 enzymatic activities and protein levels are not increased in DS. A: As expected, total mRNA levels for BACE2 and APP were significantly higher in DS cases; BACE1 mRNA was unchanged (data not shown). DS cases had slightly more APP protein but no additional BACE2 protein. B: BACE1 and BACE2 enzymatic activities were unchanged between DS cases and controls. C: Neither BACE1 (MAB931) nor BACE2 (Ab5670) protein levels differed between DS cases (mean ± SD: BACE1, 89.9 ± 19.7; BACE2, 218.9 ± 10.5) and controls (mean ± SD: BACE1, 89.8 ± 21.4; BACE2, 222.3 ± 6.5) (arbitrary units). D: BACE1 showed a slight age-related decrease in activity. Mann-Whitney U-test, *P < 0.05, †P < 0.01. Scale: 1000 base fluorescence units (BFU)/mg = 2.3 nmol · min−1 · mg−1 total protein.
BACE1 and BACE2 Activities Are Related to Soluble but Not Insoluble Aβ in the Brain
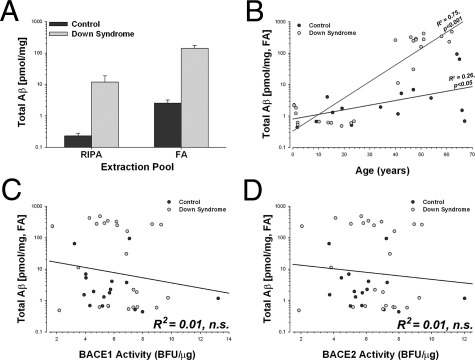
Because individuals with DS have an extra copy of APP and BACE2, they represent a unique population in which to evaluate the interactions between β-secretase expression and the deposition of Aβ in the brain. The amount of Aβ in all fractions analyzed was substantially higher in DS cases (Figure 7A; F4,31 = 16.14, P < 0.001) compared with controls, and the amount of Aβ increased substantially with age (Figure 7B; F4,31 = 4.13, P < 0.01). There was no relationship between the amount of either RIPA (not shown) or FA-soluble Aβ and BACE1 (Figure 7C) or BACE2 (Figure 7D) activities. The results did not change when the analysis was restricted to only Aβ42, to DS cases alone, or to control cases alone. These data were somewhat surprising given recent studies suggesting that a positive feedback mechanism may exist in which Aβ drives the up-regulation of BACE1 expression.42,43
Figure 7.
Aβ increases with age in DS but is not related to β-secretase activity. A: The overall amount of Aβ was higher in DS in all fractions (P < 0.01). Shown: total Aβ extracted in either RIPA buffer (F1,34 = 14.14, P < 0.001) or 70% FA (F1,34 = 40.09, P < 0.0001). B: The amount of Aβ increased with age in DS cases (P < 0.001) and matched controls (P < 0.05). Neither BACE1 (C) nor BACE2 (D) enzymatic activities were related to the amount of either RIPA (not shown) or FA-soluble Aβ in the brain. Scale: 1 base fluorescence unit (BFU)/μg = 2.3 nmol · min−1 · mg−1 total protein.
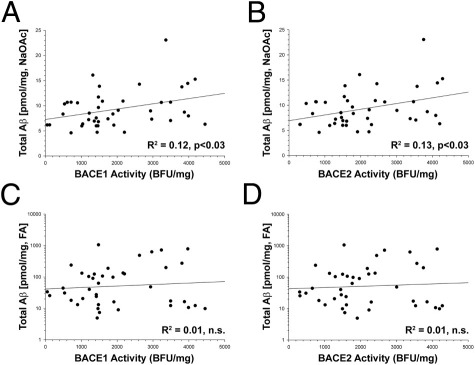
We decided to explore this relationship in greater detail by revisiting the cases from our first group, in which we had access to more detailed neuropathologic data. Neither BACE1 nor BACE2 activity could predict the number of diffuse plaques or neuritic plaques (NPs) (data not shown). BACE1 (R2 = 0.12, P < 0.03) and BACE2 (R2 = 0.13, P < 0.03) activities were significantly positively related to the amount of Aβ in the NaOAc-soluble fraction isolated from the SMTG in this case series. Although these relationships were modest (Figure 8, A and B), we found similar results in the CB in these same cases (BACE1: R2 = 0.10, P < 0.03; BACE2: R2 = 0.09, P < 0.03; data not shown). The NaOAc fraction is the first in the extraction series, contains the most soluble pool of Aβ peptides, and is also where BACE1 activity is localized.22 There was no relationship between either BACE1 or BACE2 enzymatic activities and any form of Aβ (total Aβ, Aβ40, Aβ42, or oligomeric Aβ) extracted in the RIPA, SDS, or FA fractions (Figure 8, C and D). The amount of CTFβ in SMTG was related to the amount of Aβ in multiple fractions (R2 = 0.22 to 0.15, P < 0.05) and was also related to the number of NPs (R2 = 0.22, P < 0.002); this stronger relationship is likely due to CTFβ being the immediate precursor to Aβ, whereas β-secretase activity is a step further removed.
Figure 8.
BACE1 and BACE2 enzymatic activities are related to soluble, but not insoluble, Aβ in the brain. BACE1 (A) and BACE2 (B) enzymatic activities were significantly correlated with the amount of Aβ solubilized in the NaOAc fraction, which contains the most soluble pool of the peptide. Neither BACE1 (C) nor BACE2 (D) enzymatic activities were correlated with less-soluble forms of Aβ, such as the pool soluble in 70% FA. Scale: 1000 base fluorescence units (BFU)/mg = 2.3 nmol · min−1 · mg−1 total protein.
Examining BACE1 and BACE2 using methods other than enzymatic activity produced largely the same results, with some important differences. First, the amount of BACE2 as determined by Western blot analysis was correlated with the number of NPs (R2 = 0.14, P < 0.02) but not with the number of diffuse plaques (R2 = 0.03, P < 0.3). Also, the number of NFTs was positively correlated with BACE2 protein (R2 = 0.16, P < 0.01) and mRNA (R2 = 0.15, P < 0.02). The relationship between BACE2, NFTs, and NPs may be connected to BACE2-positive neurites (Figure 1). However, we did not detect any strong cellular co-localization between BACE2-positive cells and pathologic forms of the microtubule-binding tau protein (see Supplemental Figure S10 at http://ajp.amjpathol.org). This finding suggests that although BACE2 may increase in parallel with tangle pathology, this may be occurring in general in broad areas of neurodegeneration rather than in specific cells. Finally, the amounts of BACE1 (R2 = 0.10, P < 0.05) and BACE2 (R2 = 0.16, P < 0.01) proteins showed a slight positive correlation with the amount of SDS-extractable Aβ. It is possible that this may partially account for discrepancies between the present data and other published results.
Insoluble Aβ in the Brain Is Accounted for by Decreased NEP Activity
The activities of neither BACE1 nor BACE2 significantly accounted for the least-soluble forms of Aβ in the brain. The amount of Aβ in the brain at the time of death, and the number of amyloid deposits, reflects the steady-state level of the peptide. Because β-secretase is the rate-limiting enzymatic activity in the production of Aβ, and because neither form of β-secretase can explain most of the Aβ peptide in the brain at the time of autopsy, we reasoned that the process of Aβ catabolism would likely account for the remaining insoluble peptide. We tested this hypothesis by evaluating the activity of NEP, one of the major Aβ clearance enzymes in the brain.44
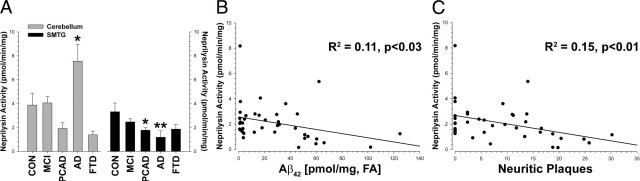
NEP activity decreased in the SMTG in neurodegenerative disease (F4,30 = 2.72, P < 0.05; Figure 9A). This effect was driven primarily by lower NEP activity in AD (Dunnett's test, P < 0.005), although PCAD cases were also significantly reduced (P < 0.04). In the CB, NEP activity was significantly higher in disease (F4,27 = 4.87, P < 0.005), and this increase was prominent in late-stage AD cases (P < 0.05). NEP activity showed a significant negative correlation with the total amount of Aβ in the brain, as determined by two independent replications (R2 = 0.14, P < 0.02; R2 = 0.13, P < 0.03). A stepwise multiple regression analysis revealed that the variance in NEP activity is explained by a model combining SDS- and FA-soluble Aβ (R2 = 0.22; F2,35 = 5.00, P < 0.02), whereas BACE1 (R2 = 0.13; F1,39 = 5.67, P < 0.03) and BACE2 (R2 = 0.14; F1,39 = 6.31, P < 0.02) are significantly related only to the amount of Aβ soluble in NaOAc (Figure 8). The FA-soluble Aβ42 pool accounted for the greatest proportion of the variance in NEP in the SMTG (R2 = 0.11, P < 0.03; Figure 9B). NEP activity was not related to BACE1 or BACE2 enzymatic activity, oligomeric Aβ, or the number of diffuse plaques. However, NEP activity showed a significant negative correlation with the number of NPs (R2 = 0.15, P < 0.01; Figure 9C). Due to constraints on tissue availability, analysis was limited on the DS cases. Using the same method as in the first case series, we again observed that NEP activity was inversely related to the amount of Aβ42 in the least-soluble fraction, FA (R2 = 0.28, P < 0.03; see Supplemental Figure S11 at http://ajp.amjpathol.org). Together, these data are consistent with decreased NEP activity accounting for the increased quantity of insoluble Aβ deposits in the diseased brain.
Figure 9.
NEP enzymatic activity decreases in neurodegenerative disease. A: NEP activity is decreased in a disease-affected region (SMTG; P < 0.05) and increased in an unaffected region (CB; P < 0.01); these effects were mostly driven by large differences in late-stage AD cases. B: NEP activity was inversely correlated (P < 0.05) with the pool of least-soluble Aβ peptide in the brain, FA-extractable Aβ42. C: NEP activity was significantly and negatively correlated (P < 0.01) with the number of NPs in the brain; the number of NPs and the amount of FA-soluble Aβ42 are strongly correlated with each other (R2 = 0.61, P < 0.0001). NEP was not correlated with the number of diffuse plaques (data not shown). Dunnett's test, *P < 0.05, †P < 0.01.
Discussion
The key involvement of β-secretase in AD is now well-established. Until recently, most research in this area focused on furthering our understanding of BACE1. This emphasis has been driven by the results of several studies in genetically modified mice indicating that most of the Aβ peptide in the brain is produced by BACE115,19,20 but also by other studies showing that BACE2 is less prominent in the brain.11 In this study, we demonstrate for the first time that disease-related increases in BACE1 are accompanied by corresponding increases in BACE2, that both increase at an early disease stage, and that these increases are not necessarily specific to AD or neurodegeneration in general. Furthermore, these increases do not necessarily accompany aging or deposition of the Aβ peptide in the brain. Finally, only the more soluble pool of Aβ is related to β-secretase activity, with the quantity of the least-soluble peptide being better accounted for by the activity of catabolic enzymes, such as NEP.
The amount of BACE2 and BACE1 protein and activity increased early in the progression of AD, before the onset of dementia. This finding indicates that increasing β-secretase activity is an early feature of the disease and may play a causal role in the development of neuropathology. Higher levels of β-secretase activity have been reported in disease-affected brain regions in late-stage AD,21,22,24,25 although these studies have focused largely on BACE1. Furthermore, although there have been earlier reports of modest increases in BACE1 protein or β-secretase activity in cerebrospinal fluid 27 and platelets28 from amnestic MCI cases, this is the first report describing a detailed examination in brain tissue from early-stage disease. Two earlier studies attempted to separate BACE2 and BACE1 in the AD brain; one observed no change22 and the other reported a small decrease45 in the amount of BACE2 protein. However, both of these studies used 16 or fewer cases and compared only late-stage AD cases with controls. It is also possible that discrepancies between these two studies and the present study may arise from the study of different tissue brain regions or that these other studies used tissue specimens with longer postmortem intervals. Stockley et al45 evaluated only BACE2 by immunoblot analysis but saw a small decrease in a prominent band at ∼75 kDa. It is possible that this BACE2 band may represent a stable dimer of what we report herein as the active form because BACE1 is thought to exist as a functional dimer.46 It is also possible that disease-related differences may alter the proportion of BACE2 in a monomeric versus dimeric state.
In contrast to the increases in BACE1 and BACE2 in neurologic disease, we did not see an increase in either form of β-secretase in DS. Despite the additional copy of the BACE2 gene, the BACE2 protein is not thought to be overexpressed in DS.47–49 A possible relationship between BACE2 and other AD-like neuropathologic features (such as NFTs) in older DS cases was reported,14 but this has not been extensively studied. Other researchers have reported that BACE1 and BACE2 are comparable in DS brain47 and that overall β-secretase activity in DS brain is similar to that in normal individuals over the same range of ages.50 It would, thus, seem that increases in β-secretase activity, either BACE1 or BACE2, are not a feature of DS. However, individuals with DS have dysfunctions in the endosomal/lysosomal system that depend on APP and BACE1, but not on Aβ, overexpression.51 This finding implies that the CTFβ generated as a consequence of β-secretase activity may be directly involved in DS neuropathology at a different level.
We did not observe disease-related increases in the total amount of mRNA for either BACE2 or BACE1. Although several studies indicate that BACE1 protein and activity increase in the absence of changes in mRNA,24,43,52 the issue has not been completely resolved.21,53 As an important positive control, we detected more total mRNA for BACE2 and APP in the brains of patients with DS but no increase in the total amount of BACE1 mRNA, findings similar to others.48,49,51 Although we saw no change in the total amount of mRNA, we detected an increase in mRNA for BACE2Δ7 (splice form C) that approximately corresponded to BACE2 protein and activity. Although it is beyond the scope of this study, this finding raises the issue that at least some of the discrepancies in BACE1 data between other studies may relate to the primer or probe sets used because only one or more splice forms may actually change in the diseased brain. BACE1 activity, but not mRNA, has been reported to increase in Tg2576 mice with age,54 although there is a tendency toward an increase in the mRNA of a more active splice variant (I-501). The relationship between this splice form of BACE1 and β-secretase activity in the AD brain has not been examined in great detail, other than a report of its regional distribution.8 Disease-related changes may not be uniform between affected brain regions, and additional confusion may derive from the lack of good reference genes that are stable across disease states.53
Although we detected strong, reproducible increases in both forms of β-secretase in early- and late-stage AD, this increase was not necessarily specific to AD. In FTD, we observed increases in BACE1 and BACE2 that were of approximately the same magnitude as what we saw in late-stage AD. This finding indicates that perhaps neurodegeneration in general leads to up-regulation of β-secretase expression. This could correspond to other known functions of BACE1, such as its reported role in myelination,5 because BACE1 might be expected to be up-regulated during repair processes triggered in damaged regions.6 The observed increase in BACE2 could be related to its expression in astrocytes, which express essentially no BACE1 at the protein level.55 However, we did not observe increases in either form of BACE in DS cases, even in older cases with AD-related neuropathology. Older individuals with DS show substantial evidence of white and gray matter degeneration and loss of brain weight.56 It would, thus, seem that the increase in β-secretase cannot be broadly attributed to neurodegeneration in general. A detailed study documenting the differences among AD, FTD, and DS may help elucidate the underlying elements directly responsible for controlling BACE1 and BACE2 expression.
We found a striking relationship between BACE1 and BACE2 in the human brain: both forms of β-secretase were highly and reproducibly correlated with each other. We observed this relationship using multiple methods (RT-PCR, immunoblot analysis, and enzymatic activity assays), with different antibodies directed against different portions of the proteins, in different brain regions (middle frontal gyrus, SMTG, and CB), and in autopsy specimens obtained from different sources comprising brains of varying ages and neurologic statuses. The parsimonious explanation for this finding is that BACE1 and BACE2 must, to a large extent, share their mechanisms of regulation. BACE1 transcription is regulated by STAT3,57 which can be controlled by cyclin-dependent kinase 5 signaling but also by the extracellular signal-regulated kinase 1 or c-Jun N-terminal kinase pathways.58 The BACE1 5′ untranslated region is a strong suppressor of translation59 and is probably a major site of the posttranscriptional control of BACE1 expression.43 However, several microRNA species are involved in the posttranscriptional regulation of BACE1 through its 3′ untranslated region,60,61 and BACE1 expression may be regulated globally by its naturally occurring antisense transcript.62 Considerably less is known about the factors that regulate BACE2 expression. However, the major shared transcription factor between the BACE1 and BACE2 promoters is Sp1.63 This point suggests that at least one component of the relationship we observed may be driven by shared changes in this transcription factor. Changes in Sp1 levels track with the amount of BACE1 mRNA,64 and Sp1 dysregulation occurs in AD65; it is unclear whether this relationship is direct. The BACE1 and BACE2 promoters also share several additional transcription factors, including AP1, AP2, and GATA1,12,63,66 and it is conceivable that these may be more important for the connection between the two. For example, the AP2 site may be important for BACE1 expression in neurons.67 Because BACE1 transcription is likely controlled from a single nexus, whereas BACE2 is controlled from at least two,67 this would also make AP2 a good candidate for a shared regulator. Regardless, because BACE1 is absent from glial cells55 and BACE2 is found in neurons and glia,9 the major point of overlap between the two enzymes is likely in neurons. If there are cell-specific mechanisms that control β-secretase expression at multiple levels, some of these may be either normally absent in neurons or lost during the course of disease.
We detected a significant correlation between the most-soluble pool of Aβ and β-secretase enzymatic activity but not with the least-soluble Aβ pool. It is the least-soluble pool of Aβ that shows the strongest correlations with the number of plaques in the brain, and these were also poorly correlated with β-secretase activity. This finding is consistent with the more-soluble Aβ being the most recently generated, dynamic pool (and, therefore, more likely to correlate with β-secretase activity), whereas the insoluble pool reflects Aβ accumulated over an extended period and, therefore, subject to many other factors. Furthermore, although there were substantial amounts of total Aβ in the brains of older DS cases, there were no associated increases in either BACE1 or BACE2. This finding indicated that age-related Aβ deposition in DS is likely driven more by an overabundance of APP substrate than by increased β-secretase. We also observed large increases in BACE1 and BACE2 protein and activity in the brains of individuals with FTD, although the amount of deposited Aβ was negligible in these cases. The FTD cases were significantly younger than the other cases, and it is possible that had these individuals lived longer they would have developed some amyloid deposition. However, this seems unlikely because Aβ deposition is not considered a feature of FTD neuropathology. These data suggest that it is also unlikely that there is a simple positive feedback mechanism in which Aβ deposition drives β-secretase expression, as has been suggested.21,25,42,43 BACE1 or β-secretase activity has also been shown not to correlate with Aβ-related pathologic findings in the brain or to be a weak association.35 It is possible that under some circumstances the relationship may be less clear, such as with forms of truncated or modified Aβ that may be missed or underestimated by certain extraction methods or immunoassays.68 Zhao et al42 noted that the increase in BACE1 immunoreactivity occurred only around a subset of plaques in the AD brain. We detected a relationship between BACE1 and BACE2, as determined by immunoblot analysis, and the amount of less-soluble Aβ and between BACE2 and NPs. Taken together, although it would seem that there is a relationship between Aβ, BACE1, and BACE2 in the human brain, this is not as robust as other disease-related changes.
Although there is a substantial amount of Aβ in the brain of an individual with AD, the final amount represents the balance between its production, deposition, and clearance. For instance, the overall rate of clearance of Aβ from the brain is impaired in AD, but its production is not.69 In light of the weaker-than-expected relationship between β-secretase activity and Aβ, we investigated the relative relationship between NEP activity and neuropathologic features in these same cases. NEP and insulin-degrading enzyme account for most Aβ degradation in the brain,44,70 and the activity of both decreases in normal aging and in disease-affected regions.71,72 NEP, but not insulin-degrading enzyme, is also negatively correlated with the amount of Aβ in the brains of AD cases, suggesting that it is actually the major enzyme responsible for Aβ catabolism in the brain.73 NEP activity may also decrease in cerebrospinal fluid in the early stages of AD.74 Consistent with these observations, we found that the reduced NEP activity better accounted for the amount of insoluble Aβ in the brain (including the number of NPs) than did either BACE1 or BACE2 activity. In areas not prone to Aβ deposition, including the periphery, NEP expression was much greater than in AD-vulnerable brain regions.52 In fact, we observed a significant increase in NEP activity in the CB of AD cases, possibly explaining the relative lack of neuropathologic findings in this region despite significant β-secretase activity and substantial quantities of Aβ. Note, however, that NEP is largely a synaptic protein, and, therefore, decreases observed in disease-affected regions may reflect significant synaptic loss. In recent studies by Miners et al, NEP activity increased in AD75 and DS76 cases when approximately standardized to neuron number, suggesting that further study is needed in other disease states and brain regions.
Although significant effort has been invested to further our understanding of the role of BACE1 in AD, little attention has been given to the closely related BACE2. This emphasis should be reevaluated for several reasons. First, both forms of β-secretase cleave APP at the normal β-site (M−1-D1) and at internal positions in the Aβ peptide sequence: Y10-E11 for BACE1 and F19-F20-A21 for BACE2. Thus, although the preferences are slightly different, BACE2 can generate cleavage products similar to BACE1, including the full-length Aβ peptide.9,11,20,63 Second, because both forms of BACE compete for the same substrate pool,10 even small changes in BACE2 in the human brain may indirectly contribute to the development of AD simply by altering the available substrate pool for BACE1. Third, although BACE1 knockout substantially reduces the amount of Aβ in the mouse brain,20 there is residual Aβ production (likely glial) that can be attributed to BACE2. Because the population of glia is an order of magnitude greater than the population of neurons in the brain, this could be a significant disease mechanism. Finally, the finding that the overexpression of BACE2 in the mouse brain does not necessarily increase Aβ77 does not rule out a role for this form of the enzyme in the human disease because the overexpression of BACE1 often gives ambiguous results.78,79 The present findings indicate that in human brain, the expressions of both forms of β-secretase are tightly linked and may play a combined role in neurologic disease. A better understanding of the normal function of BACE1 and BACE2, and how this function changes in different disease states, is essential for future therapeutic development.
Acknowledgments
We thank Fred Schmitt, Peter Nelson, Erin Abner, and the late William Markesbery for providing the clinical and neuropathologic data and for assistance with its analysis; Feng Li, Ela Patel, Sonya Anderson, Sarah B. Martin, and Sarita S. Hardas for technical assistance; Harry LeVine for helpful discussions; and Paula Thomason for her expertise in editing the final manuscript. We are very grateful to all the participants in the longitudinal aging study.
Footnotes
Supported by NIH grants NS058382 and AG005119 (M.P.M.) and HD064993 (E.H.). Additional support was provided by NIH training grant HL086341 (R.L.W.), the Coins for Alzheimer's Research Trust, and the Alzheimer's AssociationIIRG-10-172905 (M.P.M. and D.M.N.). Tissue samples were provided by the University of Maryland National Institute of Child Health and Human Development Brain and Tissue Bank for Developmental Disorders (HD90011), the Alzheimer's Disease Center of the University of California at Irvine (AG16573), the University of Kentucky (AG028383), and the Sanders-Brown Center on Aging. The synthesis of the NEP inhibitor CGS24592 was performed by the Chemical Synthesis Core of the Department of Molecular and Cellular Biochemistry at the University of Kentucky (RR020171).
C.J.H. and R.L.W. contributed equally to this work.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.09.034.
Supplementary data
Examples of neuropathology (20× objective) in parietal cortex (Ctx) (Bielschowsky staining) and subiculum (Gallyas staining) from representative cases of PCAD, amnestic MCI, and AD. Although the level of cognitive impairment varies from minimal to demented, all three disease states display the same basic elements of neuropathology, frequently at similar levels. Basic methods: Briefly, brain weights were determined at the time of autopsy, followed by a gross neuropathologic evaluation. At autopsy, tissue samples were dissected and immediately frozen or fixed in 4% paraformaldehyde. For histologic analysis, paraffin-embedded specimens were cut at 8 μm and stained with H&E and the modified Bielschowsky method. Braak staging (Braak and Braak, 1991) was performed using Gallyas-stained sections from ventromedial temporal lobe structures and Bielschowsky-stained neocortical sections. Diffuse plaques (without neurites) and NPs (with neurites) were counted in neocortical sections using Bielschowsky-stained sections. Plaques were counted using a 10× objective (field size: 2.35 mm2) in the five most involved fields in each section and were classified as either diffuse plaques or NPs. NFTs were counted in the ventromedial temporal lobe structures using Gallyas-stained sections with a 20× objective (field size: 0.586 mm2).
CT20-18 (AbCT20) is specific for full-length βAPP (APP) and βAPP CTFs. A: Samples were lysed in Tris-buffered saline + 1% Triton X-100, and approximately 90 μg of protein was loaded per lane of a Criterion XT 4% to 12% gel. After blocking, three separate membranes were probed with either the pre-immune serum, unpurified antiserum, or antiserum absorbed with the peptide immunogen. APP(+) = human H4 neuroglioma cells, overexpressing human APPΔNL695; APP(−) = nontransgenic mouse brain. B: CTFs were immunoprecipitated from APP(+) cell lysate using CT20-18 (AbCT20) and then separated and immunoblotted as in A.
Neither BACE1 (top panel: MAB931, R&D Systems; 3,3′-diaminobenzidine, brown) nor BACE2 (bottom panel: Ab2, EMD Biosciences; 3,3′-diaminobenzidine, brown) were detected in microglia (rabbit anti-Iba1, Biocare Medical; Vector SG, blue) in significant amounts; note the numerous BACE1- and BACE2-negative microglia in both panels. Scale bar = 60 μm.
The mean amount of extractable Aβ was similar between MCI and PCAD cases and minimal in FTD cases. Total Aβ showed a disease-related increase in the SMTG (F4,32 = 16.38, P < 0.001) and the CB (F4,29 = 30.14, P < 0.001), although the overall amount of Aβ was far lower in the CB (however, two data points in the AD group that were ∼20-fold higher than the group mean were dropped from the CB analysis; this did not affect overall significance). **P < 0.01, Dunnett's test. Error bars represent SEM. CON, control.
Mean CTFβ/CTFα levels, as determined by AbCT20 immunoblot analysis, were increased in AD (A) and were modestly correlated with BACE1 and BACE2 activity (B). Results were similar regardless of whether SMTG values were standardized to values from the CB. Error bars represent SEM. *P < 0.05, Dunnett's test. Scale: 1 base fluorescence unit (BFU)/μg = 2.3 nmol · min−1 · mg−1 total protein. AU, arbitrary units; CON, control.
Repeating the BACE1 (A) and BACE2 (B) activity assays using a different combination of antibodies raised against opposite ends of the proteins did not substantially alter the results. Overall, BACE1 (P < 0.01) and BACE2 (P < 0.05) were still higher in the SMTG from disease-affected cases compared with controls (CON). Using this method, the group differences by disease state were essentially unchanged from those of the standard MAB931/Ab1 method (cf. Figure 4). The overall significance is that this second run was reduced due to slightly lower power because this was a subset of cases. PCAD (BACE1, t9 = 3.24, P < 0.01; BACE2, t9 = 2.40, P < 0.03) and AD (BACE1, t11 = 1.86, P < 0.05) were the only groups that showed differences versus the control group. We observed marginally lower BACE2 activity (P < 0.05) in the CB in disease-affected cases with this combination of antibodies; this finding is inconsistent with the first data set and possibly spurious. *P < 0.05, **P < 0.01, Dunnett's test. Error bars represent SEM.
The mean total mRNA levels (ie, all splice forms) for BACE1 (A) and BACE2 (B) did not show consistent alterations across multiple disease states in either the SMTG or CB (all comparisons, P > 0.2). APP mRNA was also unchanged (not shown). Values were standardized to the geometric mean of GAPDH and TPT1. Error bars represent SEM. CON, control.
BACE2 protein levels (as determined by immunoblot analysis) were not elevated in DS cases relative to matched controls (inset) and did not change with age. BACE2 levels were determined using Ab5670 (cf. Figure 6C) and were standardized to GAPDH levels in the same lane. BACE1 protein levels also did not change (data not shown).
Mean CTFβ levels, standardized to full-length APP, were increased in AD and not in DS cases. Results were similar when CTFβ values were standardized to full CTFα levels by Western blot analysis or when values from a disease-affected region (the SMTG) were standardized to an unaffected region (the CB). *P < 0.05, Dunnett's test. Error bars represent SEM. AU, arbitrary units; CON, control.
BACE2 and PHF-1 immunoreactivity increase in AD, but not in the same cells. A: Even in areas near the brain surface with many BACE2-positive cells (green) (yellow arrows), minimal overlap with PHF-1 (red) was observed. B: Fewer BACE2-positive cells (yellow arrows) are located near the brain surface of an age-matched control case with considerably less PHF-1 immunoreactivity. C: Lower-magnification micrograph in AD brain showing numerous BACE2-positive cells in an intensely PHF-1–positive field, showing almost no overlap between individual cells. D: Section from AD brain with no primary antibodies but both secondaries; background fluorescence is minimal (cell nuclei are stained with DAPI in all the panels).
FA-soluble Aβ42 levels in the DS brain (n =17; DS cases only) were inversely related to NEP activity (P < 0.05, using phosphoramidon to demonstrate specificity).
References
- 1.Cao X., Sudhof T.C. A transcriptively active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 2.Capell A., Meyn L., Fluhrer R., Teplow D.B., Walter J., Haass C. Apical sorting of β-secretase limits amyloid β-peptide production. J Biol Chem. 2002;277:5637–5643. doi: 10.1074/jbc.M109119200. [DOI] [PubMed] [Google Scholar]
- 3.Vassar R., Bennett B.D., Babu-Khan S., Kahn S., Mendiaz E.A., Denis P., Teplow D.B., Ross S., Amarante P., Loeloff R., Luo Y., Fisher S., Fuller J., Edenson S., Lile J., Jarosinski M.A., Biere A.L., Curran E., Burgess T., Louis J.C., Collins F., Treanor J., Rogers G., Citron M. β-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 4.Capell A., Steiner H., Willem M., Kaiser H., Meyer C., Walter J., Lammich S., Multhaup G., Haass C. Maturation and pro-peptide cleavage of β-secretase. J Biol Chem. 2000;275:30849–30854. doi: 10.1074/jbc.M003202200. [DOI] [PubMed] [Google Scholar]
- 5.Willem M., Garratt A.N., Novak B., Citron M., Kaufmann S., Rittger A., DeStrooper B., Saftig P., Birchmeier C., Haass C. Control of peripheral nerve myelination by the β-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 6.Loane D.J., Pocivavsek A., Moussa C.E., Thompson R., Matsuoka Y., Faden A.I., Rebeck G.W., Burns M.P. Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat Med. 2009;15:377–379. doi: 10.1038/nm.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H., Wang B., Wang Z., Guo Q., Tabuchi K., Hammer R.E., Sudhof T.C., Zheng H. Soluble amyloid precursor protein (APP) regulates transthyretin and Klotho gene expression without rescuing the essential function of APP. Proc Natl Acad Sci U S A. 2010;107:17362–17367. doi: 10.1073/pnas.1012568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zohar O., Cavallaro S., D'Agata V., Alkon D.L. Quantification and distribution of β-secretase alternative splice variants in the rat and human brain. Brain Res Mol Brain Res. 2003;115:63–68. doi: 10.1016/s0169-328x(03)00182-7. [DOI] [PubMed] [Google Scholar]
- 9.Hussain I., Powell D.J., Howlett D.R., Chapman G.A., Gilmour L., Murdock P.R., Tew D.G., Meek T.D., Chapman C., Schneider K., Ratcliffe S.J., Tattersall D., Testa T.T., Southan C., Ryan D.M., Simmons D.L., Walsh F.S., Dingwall C., Christie G. ASP1 (BACE2) cleaves the amyloid precursor protein at the β-secretase site. Mol Cell Neurosci. 2000;16:609–619. doi: 10.1006/mcne.2000.0884. [DOI] [PubMed] [Google Scholar]
- 10.Basi G., Frigon N., Barbour R., Doan T., Gordon G., McConlogue L., Sinha S., Zeller M. Antagonistic effects of β-site amyloid precursor protein-cleaving enzymes 1 and 2 on β-amyloid peptide production in cells. J Biol Chem. 2003;278:31512–31520. doi: 10.1074/jbc.M300169200. [DOI] [PubMed] [Google Scholar]
- 11.Bennett B.D., Babu-Khan S., Loeloff R., Louis J.C., Curran E., Citron M., Vassar R. Expression analysis of BACE2 in brain and peripheral tissues. J Biol Chem. 2000;275:20647–20651. doi: 10.1074/jbc.M002688200. [DOI] [PubMed] [Google Scholar]
- 12.Maloney B., Ge Y.W., Greig N.H., Lahiri D.K. Characterization of the human β-secretase 2 (BACE2) 5′-flanking region: identification of a 268-bp region as the basal BACE2 promoter. J Mol Neurosci. 2006;29:81–99. doi: 10.1385/JMN:29:1:81. [DOI] [PubMed] [Google Scholar]
- 13.Acquati F., Accarino M., Nucci C., Fumagalli P., Jovine L., Ottolenghi S., Taramelli R. The gene encoding DRAP (BACE2), a glycosylated transmembrane protein of the aspartic protease family, maps to the down critical region. FEBS Lett. 2000;468:59–64. doi: 10.1016/s0014-5793(00)01192-3. [DOI] [PubMed] [Google Scholar]
- 14.Motonaga K., Itoh M., Becker L.E., Goto Y., Takashima S. Elevated expression of β-site amyloid precursor protein cleaving enzyme 2 in brains of patients with Down syndrome. Neurosci Lett. 2002;326:64–66. doi: 10.1016/s0304-3940(02)00287-2. [DOI] [PubMed] [Google Scholar]
- 15.Luo Y., Bolon B., Kahn S., Bennett B.D., Babu-Khan S., Denis P., Fan W., Kha H., Zhang J., Gong Y., Martin L., Louis J.-C., Yan Q., Richards W.G., Citron M., Vassar R. Mice deficient in BACE1, the Alzheimer's β-secretase, have normal phenotype and abolished β-amyloid generation. Nature neuroscience. 2001;4:231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- 16.Ohno M., Cole S.L., Yasvoina M., Zhao J., Citron M., Berry R., Disterhoft J.F., Vassar R. BACE1 gene deletion prevents neuron loss and memory deficits in 5XFAD APP/PS1 transgenic mice. Neurobiol Dis. 2007;26:134–145. doi: 10.1016/j.nbd.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu X., Zhou X., He W., Yang J., Xiong W., Wong P., Wilson C.G., Yan R. BACE1 deficiency causes altered neuronal activity and neurodegeneration. J Neurosci. 2010;30:8819–8829. doi: 10.1523/JNEUROSCI.1334-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savonenko A.V., Melnikova T., Laird F.M., Stewart K.A., Price D.L., Wong P.C. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc Natl Acad Sci U S A. 2008;105:5585–5590. doi: 10.1073/pnas.0710373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai H., Wang Y., McCarthy D., Wen H., Borchelt D.R., Price D.L., Wong P.C. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nature neuroscience. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 20.Dominguez D., Tournoy J., Hartmann D., Huth T., Cryns K., Deforce S., Serneels L., Camacho I.E., Marjaux E., Craessaerts K., Roebroek A.J., Schwake M., D'Hooge R., Bach P., Kalinke U., Moechars D., Alzheimer C., Reiss K., Saftig P., De Strooper B. Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J Biol Chem. 2005;280:30797–30806. doi: 10.1074/jbc.M505249200. [DOI] [PubMed] [Google Scholar]
- 21.Li R., Lindholm K., Yang L.B., Yue X., Citron M., Yan R., Beach T., Sue L., Sabbagh M., Cai H., Wong P., Price D., Shen Y. Amyloid β peptide load is correlated with increased β-secretase activity in sporadic Alzheimer's disease patients. Proc Natl Acad Sci U S A. 2004;101:3632–3637. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed R.R., Holler C.J., Webb R.L., Li F., Beckett T.L., Murphy M.P. BACE1 and BACE2 enzymatic activities in Alzheimer's disease. J Neurochem. 2010;112:1045–1053. doi: 10.1111/j.1471-4159.2009.06528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukumoto H., Cheung B.S., Hyman B.T., Irizarry M.C. β-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002;59:1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 24.Holsinger R.M., McLean C.A., Beyreuther K., Masters C.L., Evin G. Increased expression of the amyloid precursor β-secretase in Alzheimer's disease. Ann Neurol. 2002;51:783–786. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- 25.Yang L.B., Lindholm K., Yan R., Citron M., Xia W., Yang X.L., Beach T., Sue L., Wong P., Price D., Li R., Shen Y. Elevated β-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med. 2003;9:3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- 26.Holsinger R.M., Lee J.S., Boyd A., Masters C.L., Collins S.J. CSF BACE1 activity is increased in CJD and Alzheimer disease versus [corrected] other dementias. Neurology. 2006;67:710–712. doi: 10.1212/01.wnl.0000229925.52203.4c. [DOI] [PubMed] [Google Scholar]
- 27.Zetterberg H., Andreasson U., Hansson O., Wu G., Sankaranarayanan S., Andersson M.E., Buchhave P., Londos E., Umek R.M., Minthon L., Simon A.J., Blennow K. Elevated cerebrospinal fluid BACE1 activity in incipient Alzheimer disease. Arch Neurol. 2008;65:1102–1107. doi: 10.1001/archneur.65.8.1102. [DOI] [PubMed] [Google Scholar]
- 28.Liu W.W., Todd S., Craig D., Passmore A.P., Coulson D.T., Murphy S., Irvine G.B., Johnston J.A. Elevated platelet β-secretase activity in mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;24:464–468. doi: 10.1159/000110739. [DOI] [PubMed] [Google Scholar]
- 29.Price J.L., McKeel D.W., Jr, Buckles V.D., Roe C.M., Xiong C., Grundman M., Hansen L.A., Petersen R.C., Parisi J.E., Dickson D.W., Smith C.D., Davis D.G., Schmitt F.A., Markesbery W.R., Kaye J., Kurlan R., Hulette C., Kurland B.F., Higdon R., Kukull W., Morris J.C. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 31.Cairns N.J., Bigio E.H., Mackenzie I.R., Neumann M., Lee V.M., Hatanpaa K.J., White CL C.L., III, Schneider J.A., Grinberg L.T., Halliday G., Duyckaerts C., Lowe J.S., Holm I.E., Tolnay M., Okamoto K., Yokoo H., Murayama S., Woulfe J., Munoz D.G., Dickson D.W., Ince P.G., Trojanowski J.Q., Mann D.M. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitt F.A., Davis D.G., Wekstein D.R., Smith C.D., Ashford J.W., Markesbery W.R. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- 33.Nelson P.T., Abner E.L., Schmitt F.A., Kryscio R.J., Jicha G.A., Smith C.D., Davis D.G., Poduska J.W., Patel E., Mendiondo M.S., Markesbery W.R. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20:66–79. doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beckett T.L., Niedowicz D.M., Studzinski C.M., Weidner A.M., Webb R.L., Holler C.J., Ahmed R.R., LeVine H., 3rd, Murphy M.P. Effects of nonsteroidal anti-inflammatory drugs on amyloid-β pathology in mouse skeletal muscle. Neurobiol Dis. 2010;39:449–456. doi: 10.1016/j.nbd.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukumoto H., Rosene D.L., Moss M.B., Raju S., Hyman B.T., Irizarry M.C. β-Secretase activity increases with aging in human, monkey, and mouse brain. Am J Pathol. 2004;164:719–725. doi: 10.1016/s0002-9440(10)63159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinall H., Song E.S., Hersh L.B. Susceptibility of amyloid β peptide degrading enzymes to oxidative damage: a potential Alzheimer's disease spiral. Biochemistry. 2005;44:15345–15350. doi: 10.1021/bi050650l. [DOI] [PubMed] [Google Scholar]
- 37.De Lombaert S., Erion M.D., Tan J., Blanchard L., el-Chehabi L., Ghai R.D., Sakane Y., Berry C., Trapani A.J. N-Phosphonomethyl dipeptides and their phosphonate prodrugs, a new generation of neutral endopeptidase (NEP, EC 3.4.24.11) inhibitors. J Med Chem. 1994;37:498–511. doi: 10.1021/jm00030a009. [DOI] [PubMed] [Google Scholar]
- 38.McGowan E., Pickford F., Kim J., Onstead L., Eriksen J., Yu C., Skipper L., Murphy M.P., Beard J., Das P., Jansen K., Delucia M., Lin W.L., Dolios G., Wang R., Eckman C.B., Dickson D.W., Hutton M., Hardy J., Golde T. Aβ42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Head E., Pop V., Sarsoza F., Kayed R., Beckett T.L., Studzinski C.M., Tomic J.L., Glabe C.G., Murphy M.P. Amyloid-β peptide and oligomers in the brain and cerebrospinal fluid of aged canines. J Alzheimers Dis. 2010;20:637–646. doi: 10.3233/JAD-2010-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ling I.F., Estus S. Role of SFRS13A in low-density lipoprotein receptor splicing. Hum Mutat. 2010;31:702–709. doi: 10.1002/humu.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemere C.A., Blusztajn J.K., Yamaguchi H., Wisniewski T., Saido T.C., Selkoe D.J. Sequence of deposition of heterogeneous amyloid β-peptides and APO E in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol Dis. 1996;3:16–32. doi: 10.1006/nbdi.1996.0003. [DOI] [PubMed] [Google Scholar]
- 42.Zhao J., Fu Y., Yasvoina M., Shao P., Hitt B., O'Connor T., Logan S., Maus E., Citron M., Berry R., Binder L., Vassar R. β-Site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques: implications for Alzheimer's disease pathogenesis. J Neurosci. 2007;27:3639–3649. doi: 10.1523/JNEUROSCI.4396-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Connor T., Sadleir K.R., Maus E., Velliquette R.A., Zhao J., Cole S.L., Eimer W.A., Hitt B., Bembinster L.A., Lammich S., Lichtenthaler S.F., Hebert S.S., De Strooper B., Haass C., Bennett D.A., Vassar R. Phosphorylation of the translation initiation factor eIF2α increases BACE1 levels and promotes amyloidogenesis. Neuron. 2008;60:988–1009. doi: 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwata N., Tsubuki S., Takaki Y., Shirotani K., Lu B., Gerard N.P., Gerard C., Hama E., Lee H.J., Saido T.C. Metabolic regulation of brain Aβ by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- 45.Stockley J.H., Ravid R., O'Neill C. Altered β-secretase enzyme kinetics and levels of both BACE1 and BACE2 in the Alzheimer's disease brain. FEBS Lett. 2006;580:6550–6560. doi: 10.1016/j.febslet.2006.10.076. [DOI] [PubMed] [Google Scholar]
- 46.Westmeyer G.G., Willem M., Lichtenthaler S.F., Lurman G., Multhaup G., Assfalg-Machleidt I., Reiss K., Saftig P., Haass C. Dimerization of β-site β-amyloid precursor protein-cleaving enzyme. J Biol Chem. 2004;279:53205–53212. doi: 10.1074/jbc.M410378200. [DOI] [PubMed] [Google Scholar]
- 47.Cheon M.S., Dierssen M., Kim S.H., Lubec G. Protein expression of BACE1: BACE2 and APP in Down syndrome brains. Amino Acids. 2008;35:339–343. doi: 10.1007/s00726-007-0618-9. [DOI] [PubMed] [Google Scholar]
- 48.Barbiero L., Benussi L., Ghidoni R., Alberici A., Russo C., Schettini G., Pagano S.F., Parati E.A., Mazzoli F., Nicosia F., Signorini S., Feudatari E., Binetti G. BACE-2 is overexpressed in Down's syndrome. Exp Neurol. 2003;182:335–345. doi: 10.1016/s0014-4886(03)00049-9. [DOI] [PubMed] [Google Scholar]
- 49.Sun X., He G., Song W. BACE2, as a novel APP θ-secretase, is not responsible for the pathogenesis of Alzheimer's disease in Down syndrome. FASEB J. 2006;20:1369–1376. doi: 10.1096/fj.05-5632com. [DOI] [PubMed] [Google Scholar]
- 50.Nistor M., Don M., Parekh M., Sarsoza F., Goodus M., Lopez G.E., Kawas C., Leverenz J., Doran E., Lott I.T., Hill M., Head E. α- and β-secretase activity as a function of age and β-amyloid in Down syndrome and normal brain. Neurobiol Aging. 2007;28:1493–1506. doi: 10.1016/j.neurobiolaging.2006.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang Y., Mullaney K.A., Peterhoff C.M., Che S., Schmidt S.D., Boyer-Boiteau A., Ginsberg S.D., Cataldo A.M., Mathews P.M., Nixon R.A. Alzheimer's-related endosome dysfunction in Down syndrome is Aβ-independent but requires APP and is reversed by BACE-1 inhibition. Proc Natl Acad Sci U S A. 2010;107:1630–1635. doi: 10.1073/pnas.0908953107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yasojima K., McGeer E.G., McGeer P.L. Relationship between β amyloid peptide generating molecules and neprilysin in Alzheimer disease and normal brain. Brain Res. 2001;919:115–121. doi: 10.1016/s0006-8993(01)03008-6. [DOI] [PubMed] [Google Scholar]
- 53.Coulson D.T., Beyer N., Quinn J.G., Brockbank S., Hellemans J., Irvine G.B., Ravid R., Johnston J.A. BACE1 mRNA expression in Alzheimer's disease postmortem brain tissue. J Alzheimers Dis. 2010;22:1111–1122. doi: 10.3233/JAD-2010-101254. [DOI] [PubMed] [Google Scholar]
- 54.Zohar O., Pick C.G., Cavallaro S., Chapman J., Katzav A., Milman A., Alkon D.L. Age-dependent differential expression of BACE splice variants in brain regions of tg2576 mice. Neurobiol Aging. 2005;26:1167–1175. doi: 10.1016/j.neurobiolaging.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 55.Bettegazzi B., Mihailovich M., Di Cesare A., Consonni A., Macco R., Pelizzoni I., Codazzi F., Grohovaz F., Zacchetti D. β-Secretase activity in rat astrocytes: translational block of BACE1 and modulation of BACE2 expression. Eur J Neurosci. 2011;33:236–243. doi: 10.1111/j.1460-9568.2010.07482.x. [DOI] [PubMed] [Google Scholar]
- 56.Coyle J.T., Oster-Granite M.L., Gearhart J.D. The neurobiologic consequences of Down syndrome. Brain Res Bull. 1986;16:773–787. doi: 10.1016/0361-9230(86)90074-2. [DOI] [PubMed] [Google Scholar]
- 57.Wen Y., Yu W.H., Maloney B., Bailey J., Ma J., Marie I., Maurin T., Wang L., Figueroa H., Herman M., Krishnamurthy P., Liu L., Planel E., Lau L.F., Lahiri D.K., Duff K. Transcriptional regulation of β-secretase by p25/cdk5 leads to enhanced amyloidogenic processing. Neuron. 2008;57:680–690. doi: 10.1016/j.neuron.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamagno E., Guglielmotto M., Giliberto L., Vitali A., Borghi R., Autelli R., Danni O., Tabaton M. JNK and ERK1/2 pathways have a dual opposite effect on the expression of BACE1. Neurobiol Aging. 2009;30:1563–1573. doi: 10.1016/j.neurobiolaging.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 59.Lammich S., Schobel S., Zimmer A.K., Lichtenthaler S.F., Haass C. Expression of the Alzheimer protease BACE1 is suppressed via its 5′-untranslated region. EMBO Rep. 2004;5:620–625. doi: 10.1038/sj.embor.7400166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang W.X., Rajeev B.W., Stromberg A.J., Ren N., Tang G., Huang Q., Rigoutsos I., Nelson P.T. The expression of microRNA miR-107 decreases early in Alzheimer's disease and may accelerate disease progression through regulation of β-site amyloid precursor protein-cleaving enzyme 1. J Neurosci. 2008;28:1213–1223. doi: 10.1523/JNEUROSCI.5065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hebert S.S., Horre K., Nicolai L., Papadopoulou A.S., Mandemakers W., Silahtaroglu A.N., Kauppinen S., Delacourte A., De Strooper B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer's disease correlates with increased BACE1/β-secretase expression. Proc Natl Acad Sci U S A. 2008;105:6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Faghihi M.A., Modarresi F., Khalil A.M., Wood D.E., Sahagan B.G., Morgan T.E., Finch C.E., St Laurent G., III, Kenny P.J., Wahlestedt C. Expression of a noncoding RNA is elevated in Alzheimer's disease and drives rapid feed-forward regulation of β-secretase. Nat Med. 2008;14:723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun X., Wang Y., Qing H., Christensen M.A., Liu Y., Zhou W., Tong Y., Xiao C., Huang Y., Zhang S., Liu X., Song W. Distinct transcriptional regulation and function of the human BACE2 and BACE1 genes. FASEB J. 2005;19:739–749. doi: 10.1096/fj.04-3426com. [DOI] [PubMed] [Google Scholar]
- 64.Dosunmu R., Wu J., Adwan L., Maloney B., Basha M.R., McPherson C.A., Harry G.J., Rice D.C., Zawia N.H., Lahiri D.K. Lifespan profiles of Alzheimer's disease-associated genes and products in monkeys and mice. J Alzheimers Dis. 2009;18:211–230. doi: 10.3233/JAD-2009-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Citron B.A., Dennis J.S., Zeitlin R.S., Echeverria V. Transcription factor Sp1 dysregulation in Alzheimer's disease. J Neurosci Res. 2008;86:2499–2504. doi: 10.1002/jnr.21695. [DOI] [PubMed] [Google Scholar]
- 66.Sambamurti K., Kinsey R., Maloney B., Ge Y.W., Lahiri D.K. Gene structure and organization of the human β-secretase (BACE) promoter. FASEB J. 2004;18:1034–1036. doi: 10.1096/fj.03-1378fje. [DOI] [PubMed] [Google Scholar]
- 67.Lahiri D.K., Maloney B., Ge Y.W. Functional domains of the BACE1 and BACE2 promoters and mechanisms of transcriptional suppression of the BACE2 promoter in normal neuronal cells. J Mol Neurosci. 2006;29:65–80. doi: 10.1385/JMN:29:1:65. [DOI] [PubMed] [Google Scholar]
- 68.Liu K., Solano I., Mann D., Lemere C., Mercken M., Trojanowski J.Q., Lee V.M. Characterization of Aβ11–40/42 peptide deposition in Alzheimer's disease and young Down's syndrome brains: implication of N-terminally truncated Aβ species in the pathogenesis of Alzheimer's disease. Acta Neuropathol. 2006;112:163–174. doi: 10.1007/s00401-006-0077-5. [DOI] [PubMed] [Google Scholar]
- 69.Mawuenyega K.G., Sigurdson W., Ovod V., Munsell L., Kasten T., Morris J.C., Yarasheski K.E., Bateman R.J. Decreased clearance of CNS β-amyloid in Alzheimer's disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang S.M., Mouri A., Kokubo H., Nakajima R., Suemoto T., Higuchi M., Staufenbiel M., Noda Y., Yamaguchi H., Nabeshima T., Saido T.C., Iwata N. Neprilysin-sensitive synapse-associated amyloid-β peptide oligomers impair neuronal plasticity and cognitive function. J Biol Chem. 2006;281:17941–17951. doi: 10.1074/jbc.M601372200. [DOI] [PubMed] [Google Scholar]
- 71.Caccamo A., Oddo S., Sugarman M.C., Akbari Y., LaFerla F.M. Age- and region-dependent alterations in Aβ-degrading enzymes: implications for Aβ-induced disorders. Neurobiol Aging. 2005;26:645–654. doi: 10.1016/j.neurobiolaging.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 72.Miners J.S., Van Helmond Z., Chalmers K., Wilcock G., Love S., Kehoe P.G. Decreased expression and activity of neprilysin in Alzheimer disease are associated with cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2006;65:1012–1021. doi: 10.1097/01.jnen.0000240463.87886.9a. [DOI] [PubMed] [Google Scholar]
- 73.Wang S., Wang R., Chen L., Bennett D.A., Dickson D.W., Wang D.S. Expression and functional profiling of neprilysin, insulin-degrading enzyme, and endothelin-converting enzyme in prospectively studied elderly and Alzheimer's brain. J Neurochem. 2010;115:47–57. doi: 10.1111/j.1471-4159.2010.06899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maruyama M., Higuchi M., Takaki Y., Matsuba Y., Tanji H., Nemoto M., Tomita N., Matsui T., Iwata N., Mizukami H., Muramatsu S., Ozawa K., Saido T.C., Arai H., Sasaki H. Cerebrospinal fluid neprilysin is reduced in prodromal Alzheimer's disease. Ann Neurol. 2005;57:832–842. doi: 10.1002/ana.20494. [DOI] [PubMed] [Google Scholar]
- 75.Miners J.S., van Helmond Z., Kehoe P.G., Love S. Changes with age in the activities of β-secretase and the Aβ-degrading enzymes neprilysin, insulin-degrading enzyme and angiotensin-converting enzyme. Brain Pathol. 2010;20:794–802. doi: 10.1111/j.1750-3639.2010.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miners J.S., Morris S., Love S., Kehoe P.G. Accumulation of insoluble amyloid-β in Down's syndrome is associated with increased BACE-1 and neprilysin activities. J Alzheimers Dis. 2011;23:101–108. doi: 10.3233/JAD-2010-101395. [DOI] [PubMed] [Google Scholar]
- 77.Azkona G., Levannon D., Groner Y., Dierssen M. In vivo effects of APP are not exacerbated by BACE2 co-overexpression: behavioural characterization of a double transgenic mouse model. Amino Acids. 2010;39:1571–1580. doi: 10.1007/s00726-010-0662-8. [DOI] [PubMed] [Google Scholar]
- 78.Lee E.B., Zhang B., Liu K., Greenbaum E.A., Doms R.W., Trojanowski J.Q., Lee V.M. BACE overexpression alters the subcellular processing of APP and inhibits Aβ deposition in vivo. J Cell Biol. 2005;168:291–302. doi: 10.1083/jcb.200407070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rockenstein E., Mante M., Alford M., Adame A., Crews L., Hashimoto M., Esposito L., Mucke L., Masliah E. High β-secretase activity elicits neurodegeneration in transgenic mice despite reductions in amyloid-β levels: implications for the treatment of Alzheimer disease. J Biol Chem. 2005;280:32957–32967. doi: 10.1074/jbc.M507016200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Examples of neuropathology (20× objective) in parietal cortex (Ctx) (Bielschowsky staining) and subiculum (Gallyas staining) from representative cases of PCAD, amnestic MCI, and AD. Although the level of cognitive impairment varies from minimal to demented, all three disease states display the same basic elements of neuropathology, frequently at similar levels. Basic methods: Briefly, brain weights were determined at the time of autopsy, followed by a gross neuropathologic evaluation. At autopsy, tissue samples were dissected and immediately frozen or fixed in 4% paraformaldehyde. For histologic analysis, paraffin-embedded specimens were cut at 8 μm and stained with H&E and the modified Bielschowsky method. Braak staging (Braak and Braak, 1991) was performed using Gallyas-stained sections from ventromedial temporal lobe structures and Bielschowsky-stained neocortical sections. Diffuse plaques (without neurites) and NPs (with neurites) were counted in neocortical sections using Bielschowsky-stained sections. Plaques were counted using a 10× objective (field size: 2.35 mm2) in the five most involved fields in each section and were classified as either diffuse plaques or NPs. NFTs were counted in the ventromedial temporal lobe structures using Gallyas-stained sections with a 20× objective (field size: 0.586 mm2).
CT20-18 (AbCT20) is specific for full-length βAPP (APP) and βAPP CTFs. A: Samples were lysed in Tris-buffered saline + 1% Triton X-100, and approximately 90 μg of protein was loaded per lane of a Criterion XT 4% to 12% gel. After blocking, three separate membranes were probed with either the pre-immune serum, unpurified antiserum, or antiserum absorbed with the peptide immunogen. APP(+) = human H4 neuroglioma cells, overexpressing human APPΔNL695; APP(−) = nontransgenic mouse brain. B: CTFs were immunoprecipitated from APP(+) cell lysate using CT20-18 (AbCT20) and then separated and immunoblotted as in A.
Neither BACE1 (top panel: MAB931, R&D Systems; 3,3′-diaminobenzidine, brown) nor BACE2 (bottom panel: Ab2, EMD Biosciences; 3,3′-diaminobenzidine, brown) were detected in microglia (rabbit anti-Iba1, Biocare Medical; Vector SG, blue) in significant amounts; note the numerous BACE1- and BACE2-negative microglia in both panels. Scale bar = 60 μm.
The mean amount of extractable Aβ was similar between MCI and PCAD cases and minimal in FTD cases. Total Aβ showed a disease-related increase in the SMTG (F4,32 = 16.38, P < 0.001) and the CB (F4,29 = 30.14, P < 0.001), although the overall amount of Aβ was far lower in the CB (however, two data points in the AD group that were ∼20-fold higher than the group mean were dropped from the CB analysis; this did not affect overall significance). **P < 0.01, Dunnett's test. Error bars represent SEM. CON, control.
Mean CTFβ/CTFα levels, as determined by AbCT20 immunoblot analysis, were increased in AD (A) and were modestly correlated with BACE1 and BACE2 activity (B). Results were similar regardless of whether SMTG values were standardized to values from the CB. Error bars represent SEM. *P < 0.05, Dunnett's test. Scale: 1 base fluorescence unit (BFU)/μg = 2.3 nmol · min−1 · mg−1 total protein. AU, arbitrary units; CON, control.
Repeating the BACE1 (A) and BACE2 (B) activity assays using a different combination of antibodies raised against opposite ends of the proteins did not substantially alter the results. Overall, BACE1 (P < 0.01) and BACE2 (P < 0.05) were still higher in the SMTG from disease-affected cases compared with controls (CON). Using this method, the group differences by disease state were essentially unchanged from those of the standard MAB931/Ab1 method (cf. Figure 4). The overall significance is that this second run was reduced due to slightly lower power because this was a subset of cases. PCAD (BACE1, t9 = 3.24, P < 0.01; BACE2, t9 = 2.40, P < 0.03) and AD (BACE1, t11 = 1.86, P < 0.05) were the only groups that showed differences versus the control group. We observed marginally lower BACE2 activity (P < 0.05) in the CB in disease-affected cases with this combination of antibodies; this finding is inconsistent with the first data set and possibly spurious. *P < 0.05, **P < 0.01, Dunnett's test. Error bars represent SEM.
The mean total mRNA levels (ie, all splice forms) for BACE1 (A) and BACE2 (B) did not show consistent alterations across multiple disease states in either the SMTG or CB (all comparisons, P > 0.2). APP mRNA was also unchanged (not shown). Values were standardized to the geometric mean of GAPDH and TPT1. Error bars represent SEM. CON, control.
BACE2 protein levels (as determined by immunoblot analysis) were not elevated in DS cases relative to matched controls (inset) and did not change with age. BACE2 levels were determined using Ab5670 (cf. Figure 6C) and were standardized to GAPDH levels in the same lane. BACE1 protein levels also did not change (data not shown).
Mean CTFβ levels, standardized to full-length APP, were increased in AD and not in DS cases. Results were similar when CTFβ values were standardized to full CTFα levels by Western blot analysis or when values from a disease-affected region (the SMTG) were standardized to an unaffected region (the CB). *P < 0.05, Dunnett's test. Error bars represent SEM. AU, arbitrary units; CON, control.
BACE2 and PHF-1 immunoreactivity increase in AD, but not in the same cells. A: Even in areas near the brain surface with many BACE2-positive cells (green) (yellow arrows), minimal overlap with PHF-1 (red) was observed. B: Fewer BACE2-positive cells (yellow arrows) are located near the brain surface of an age-matched control case with considerably less PHF-1 immunoreactivity. C: Lower-magnification micrograph in AD brain showing numerous BACE2-positive cells in an intensely PHF-1–positive field, showing almost no overlap between individual cells. D: Section from AD brain with no primary antibodies but both secondaries; background fluorescence is minimal (cell nuclei are stained with DAPI in all the panels).
FA-soluble Aβ42 levels in the DS brain (n =17; DS cases only) were inversely related to NEP activity (P < 0.05, using phosphoramidon to demonstrate specificity).