Abstract
Transactive response DNA-binding protein 43 (TDP-43) is the pathological signature protein in several neurodegenerative disorders, including the majority of frontotemporal lobar degeneration cases (FTLD-TDP), motor neuron disease, and amyotrophic lateral sclerosis. Pathological TDP-43 is mislocalized from its nuclear location to the cytoplasm, where it accumulates and is proteolytically cleaved to form C-terminal fragments. Although the 25-kDa C-terminal fragment of TDP-43 (TDP-25) accumulates in affected brain regions, its role in the disease pathogenesis remains elusive. To address this problem, we have generated a novel transgenic mouse that selectively expresses TDP-25 in neurons. We show that transgenic mice expressing TDP-25 develop cognitive deficits associated with the build-up of soluble TDP-25. These cognitive deficits are independent of TDP-43–positive inclusions and occur without overt neurodegeneration. Additionally, we show that the expression of TDP-25 is sufficient to alter the processing of endogenous full-length TDP-43. These studies represent the first in vivo demonstration of a pathological role for TDP-25 and strongly suggest that the onset of cognitive deficits in TDP-43 proteinopathies is independent of TDP-43 inclusions. These data provide a framework for understanding the molecular mechanisms underlying the onset of cognitive deficits in FTLD-TDP and other TDP-43 proteinopathies; thus, the TDP-25 transgenic mice represent a unique tool to reach this goal.
Transactive response DNA-binding protein 43 (TDP-43) is a conserved and ubiquitously expressed DNA-RNA binding protein. It is encoded by the TARDBP gene on chromosome 1, which is made of six exons that can be alternatively spliced to yield 11 different isoforms, with the mRNA encoding TDP-43 being the major species.1 Functionally, TDP-43 appears to be involved in exon skipping and alternative splicing.2,3 Structural studies have confirmed the presence of two RNA recognition motifs (RRM1 and RRM2) and a glycine-rich C-terminal tail, which is thought to mediate protein–protein interactions.4 However, TDP-43 function appears not to be confined to the nucleus. Indeed, recent evidence indicates that TDP-43 may play a role in synaptic plasticity by regulating local translation in dendritic spines upon neuronal stimuli.5
Frontotemporal lobar degeneration (FTLD) is a heterogeneous disorder that represents the second most common form of dementia in people under the age of 65 years.6 The majority of FTLD cases are characterized by the accumulation of TDP-43 and are referred to as FTLD-TDP.7 Additionally, mutations in the TARDBP gene are linked to amyotrophic lateral sclerosis (ALS),8 a neurodegenerative disorder with clinical and neuropathological features overlapping with FTLD-TDP.9 Pathological TDP-43 is mislocalized from its nuclear location to the cytoplasm, where it accumulates and is proteolytically cleaved to form C-terminal fragments, mainly of 25 and 35 kDa.7 Notably, the accumulation of a 25-kDa C-terminal fragment of TDP-43 (TDP-25) is an invariable feature of FTLD-TDP. Even though it has been proposed that TDP-25 may play a role in neurodegeneration,7,10–15 little is known about the role of this peptide in disease pathogenesis. Animal models overexpressing TDP-43 recapitulate several key features of FTLD-TDP and ALS16–22; however, these models do not elucidate the role of TDP-25 in TDP-43 proteinopathies. Toward this end, multiple laboratories, including ours, have shown that the expression of TDP-43 C-terminal fragments is toxic and leads to ubiquitinated and abnormally phosphorylated cytoplasmic inclusions in a variety of cell lines.10,11,13–15 However, it remains to be established whether TDP-25 plays a role in the pathogenesis of FTLD-TDP in vivo. To address this critical aspect in FTLD-TDP, we report here the generation of a novel transgenic mouse that selectively expresses TDP-25 in neurons, thereby providing direct in vivo evidence for a role of TDP-25 in the onset of cognitive deficits associated with TDP-43 proteinopathies.
Materials and Methods
Generation of the Transgenic Mice
To clone TDP-25 in the Thy1.2 expression cassette,23 we used a homologous recombination-based approach (InFusion system; BD Biosciences, Franklin Lakes, NJ). TDP-25 was amplified by PCR using full-length human TDP-43 as a template and a proof-checking DNA polymerase with two primers that included 16 bp of homology to the site of insertion within the Thy1.2 multiple cloning site (forward primer 5′-TCTCGACGGCGTCGACCACCATGGATGTGATGGATGTCAAG-3′; reverse primer 5′-GAGGAAGGACCTCGAGCTAGTGATTCATTCCCCAGCCAG-3′). The forward primer also included a Kozak consensus site (CCACC) and an ATG starting codon. To prepare the expression cassette, we linearized the empty Thy1.2 plasmid by restriction digestion using SalI and XhoI enzymes. Both the PCR product and the linearized plasmid were subsequently purified by gel extraction before the recombination reaction. To elicit recombination between the Thy1.2 plasmid and the PCR insert, we combined 100 ng of linearized Thy1.2 plasmid and 50 ng of the PCR product within 10 μL of water; the mix was added to the InFusion Dry-Down Mix (BD Biosciences) and incubated at room temperature for 30 minutes. The InFusion mix contains a proprietary recombinase enzyme that catalyzes the specific recombination of two double-stranded homologous regions of DNA. Given the primer's design, this reaction led to a highly accurate and specific recombination event and insertion of the PCR product within the Thy1.2 expression cassette. We next screened via restriction digestion individual colonies obtained from transforming chemically competent Escherichia coli cells (DH5α) to identify those expressing the Thy1.2-TDP-25 construct. To further confirm the identity of our construct, the entire transgene of two clones was sequenced, and its position within the Thy1.2 plasmid was also confirmed. The Thy1.2-TDP-25 construct was released from the vector via PvuI-EcoRI digestion and purified using a gel purification system. For the generation of transgenic mice, the transgenes were injected into oocytes isolated from pure C57BL/6 mice by the transgenic mouse facility at the University of Texas at Austin. Founders, identified by PCR of DNA extracted from tail biopsies, were backcrossed to C57BL/6 mice to generate F1s.
Real-Time PCR and Determination of Copy Number
Total RNA was extracted using the RT2 qPCR-Grade RNA Isolation Kit (SABioscience, Qiagen, Valencia, CA) according to the manufacturer's protocol and quantified using a Bio Spec-1601 (SHIMADZU, Kyoto, Japan) spectrophotometer. One microgram of total RNA was used to make the cDNA by using RT2 First Strand Kit (SABioscience) in a total volume of 20 μL according to the manufacturer's protocol. After real-time PCR, 1 μL of cDNA was amplified and quantified in 96-well plates using ABI Prism 7000 sequence detection (Applied Biosystems, Foster City, CA). For the determination of copy number, DNA isolated from the B and F lines (n = 5/line) was amplified and quantified in 96-well plates using ABI Prism 7000 sequence detection (Applied Biosystems). The following specific primers were used: 5′-CCCATGTTCTGAGATATTTG-3′ and 5′-GTAATAACCAAAACCAAGGC-3′. Notably, the forward primer was complementary to the promoter region immediately upstream of the TDP-25 sequence. The reverse primer was complementary to the 5′-end region of the TDP-25 region. Using these primers, we amplified a region unique to the transgene, and no endogenous bands were amplified. The amplification conditions consisted of an initial activation step at 95°C for 10 minutes, and 40 cycles at 95°C for 15 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. The fluorescence of the accumulated double-stranded products was monitored in real time. The relative DNA levels were normalized to the reference housekeeping gene presenilin 1 using the following primers: forward, 5′-CACACGCACACTCTGACATGCACAGGC-3′ and reverse, 5′-AGCCAGGAAGATCACGTGTTCCAAGTAC-3′. The copy number in transgenic mice was determined using a serial dilution of mouse genomic DNA containing 0 to 10 copies per cell equivalent of the plasmid DNA used to generate the mice. A standard curve in threshold cycle (Ct) and copy number was generated in Excel (Microsoft, Redmond, WA), and unknowns were determined using the equation generated during the linear regression analysis.
Behavioral Testing
Open-Field Locomotion
The test was conducted in a clear Plexiglas box (40 × 40 cm) and was recorded with a video camera mounted above the testing box. Mice were handled for 5 days prior to the beginning of the test. The test consisted of 10 minutes of free exploration. With the EthoVision XT tracking system (Noldus Information Technology, Leesburg, VA), we recorded the total distance traveled by the mice and the time spent in the center and close to the box walls.
Object Recognition
Mice were left free to explore two objects for 5 minutes in the same arena used for open-field activity. After a 10-minute delay, where the mice were returned to the home cage, mice were retested in the arena where one of the two objects was replaced with a new object. The percentage of time spent exploring the new object was measured with EthoVision.
T-Maze
We previously reported a detailed protocol for the T-maze.24 Briefly, the T-maze consists of a central main arm with two side arms positioned perpendicular to the main arm. The central arm is 65 cm long, and the two side arms are each 30 cm long. The maze width is 13.5 cm. The walls of the maze are made of black acrylic and are 20 cm tall. At the beginning of each test, mice were placed in the main stem while one side arm was blocked by a barrier so that the mice were forced to choose the other arm. Once a mouse entered the side arm, the entrance to that arm was blocked, thereby retaining the mouse within the side arm. Mice were allowed to explore that arm for 120 seconds, at the end of which time period, they were placed back in the main arm of the maze with both side arms open. During this test, mice were free to choose the arm that they had already explored or the new arm. Each animal was tested daily for 7 days, and each day the side arms were alternately blocked. The number of alternations and the latency to make a choice during the free trial were recorded.
Rotarod
Each mouse was trained for three consecutive days (six trials/day) where the speed of the rotor was accelerated from 0 to 15 rpm in 20 seconds and then kept at 15 rpm for 70 additional seconds. Twenty-four hours after the last training session, the mice were tested in a probe trial consisting of six consecutive trials on an accelerating rod (1 rpm/second). The latency to fall was then recorded.
Tissue Processing, Immunohistochemistry, and Western Blot Analysis
After completion of the behavioral tasks, mice were perfused with ice-cold PBS, then their brains were extracted and cut sagitally: one-half of each brain was frozen in dry ice and used for biochemical analyses, and the other half was fixed in 4% ice-cold paraformaldehyde for 48 hours and subsequently sliced for immunohistochemical analysis, as we previously reported.25 Sections were stained with an anti-ubiquitin antibody (Sigma-Aldrich, St. Louis, MO), and with anti–TDP-43 C-terminus 405-414 and anti-phospho TDP-43 pS409/410-2 (CosmoBio, Tokyo, Japan).
Sequential Biochemical Extraction
Frozen hemibrains were homogenized in 1 mL of low-salt solution [10 mmol/L Tris (pH 7.5), 5 mmol/L EDTA, 1 mmol/L DTT, 10% sucrose, protease inhibitors] using power homogenizer, centrifuged at 25,000 × g for 30 minutes at 4°C. The supernatant was saved as a low-salt fraction. The pellet was rehomogenized in 1 mL of high-salt solution (1% Triton X-100, 0.5 mol/L NaCl, protease inhibitor) and centrifuged at 90,000 × g for 1 hour at 4°C. The supernatant was saved as a high-salt fraction. The pellet underwent further sequential extraction in sarkosyl buffer (low-salt + 1% sarkosyl + 0.5 mol/L NaCl) and in urea buffer (7 mol/L urea, 2 M thiourea, 4% CHAPS, 30 mmol/L Tris).
Nuclear and Cytosolic Fractionation
Frozen hemibrains were washed in PBS and placed in a Dounce homogenizer with 2 mL of solution A [10 mmol/L Hepes (pH 7.9), 10 mmol/L KCL, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 1 mmol/L DTT, protease inhibitors]. After five strokes, we added 0.5% vol/vol of NP40, and the tissue was further homogenized with five additional strokes. The solution was then equally divided into four different tubes and centrifuged 1 minute at 12,000 × g. The supernatant was removed and stored at −80°C as the cytosolic fraction. The pellet was resuspended in 250 μL of Solution C [20 mmol/L Hepes (pH 7.9), 400 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L DTT, protease inhibitors], mixed by pipetting and incubated for 15 minutes at 4°C. Subsequently, the tissue was centrifuged 5 minutes at 12,000 × g. and the supernatant was stored at −80°C as the nuclear fraction. Western blot analyses was conducted as previously described.26 The TDP-43 blots were probed with the anti-TDP TARDBP polyclonal antibody (ProteinTech Group, Chicago, IL).
Fluoro-Jade Staining and Quantification
Fluoro-Jade was purchased from Millipore (Billerica, MA) and used as a marker of degenerating neurons as previously described.27 Briefly, sections were mounted on slides and dried overnight at room temperature. Sections were then sequentially immersed in 100% ethanol for 5 minutes, 70% ethanol for 2 minutes, and then rinsed in deionized water for 2 additional minutes. Sections were then immersed in 0.06% KMnO4 while shaking for 10 minutes and then rinsed in water for 2 minutes. At that point, the sections were incubated for 20 minutes with gentle shaking in the Fluoro-Jade working solution (4 mL of 0.01% stock solution of Fluoro-Jade + 96 mL of 0.1% acetic acid vehicle). The final concentration of Fluoro-Jade was 0.0004%. After staining, the sections were rinsed three times in water, dried at 37°C, and then covered with coverslips. To quantify the number of Fluoro-Jade–positive cells, staining was conducted on every 10th section to cover the entire brain. Three pseudo-random pictures for each section were taken at a ×20 magnification, and the number of Fluoro-Jade–positive cells was counted.
Statistical Analyses
We have previously reported detailed statistical analyses.28 Briefly, data were analyzed using one-way and two-way analysis of variance via GraphPad Prism (La Jolla, CA). Post hoc Bonferroni test was then used to determine individual differences among groups. The Student's t-test was used when suitable.
Results
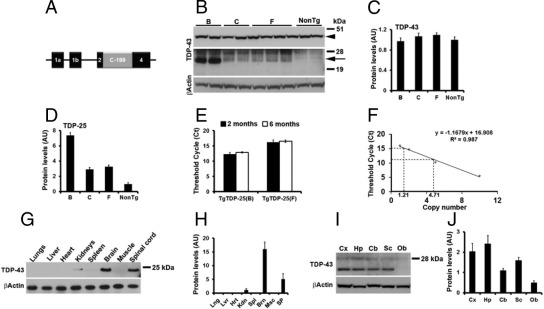
Generation of Transgenic Mice Expressing a 25-kDa Fragment of TDP-43
In FTLD-TDP, pathological TDP-43 is hyperphosphorylated, ubiquitinated, and cleaved in a caspase-dependent manner to generate C-terminal fragments.7,12,29 Interestingly, the accumulation of a 25-kDa C-terminal fragment of TDP-43 (herein referred to as TDP-25) is restricted to affected brain regions,7,12 suggesting that this TDP-43 C-terminal fragment may be directly involved in disease pathogenesis. To assess the role of TDP-25 in the pathogenesis of FTLD-TDP, we cloned the sequence encoding the last 199 amino acids of TDP-43 (Entrez accession number NP_031401) in the Thy1.2 expression cassette (Figure 1A), which includes the Thy1 mRNA polyadenylation sequence.30 An ATG starting codon and a Kozak sequence were also added at the 5′ end of the sequence. For the generation of transgenic mice, the Thy1.2-TDP25 construct was injected into oocytes isolated from pure C57BL/6 mice, and six founders were identified. Initial expression analysis in the brains of 2-month-old transgenic mice using Western blot experiments indicated that three lines expressed the transgene (Figure 1, B and D). Line B and F, herein referred to as TgTDP-25(B) and TgTDP-25(F), were identified as high- and low-expression lines, respectively, and backcrossed to C57BL/6 wild-type mice for further analyses. Notably, the steady-state levels of full-length TDP-43 were similar between transgenic and nontransgenic (NonTg) littermate control mice (Figure 1, B and C). Furthermore, expression analysis by real-time PCR shows that the expression levels of the transgene were similar between 2 and 6 months of age in both transgenic lines (Figure 1E). To estimate the transgene copy number in these two founder lines, we amplified the transgenes by real-time PCR (n = 5/line) and compared the threshold cycle (Ct) of the founder DNA to a standard curve generated from NonTg mouse DNA spiked with 0 to 10 copies per cell equivalent of the transgene (see Materials and Methods). We found that the TgTDP-25(B) and TgTDP-25(F) mice had 4.71 ± 0.09 and 1.21 ± 0.16 copies of the transgene, respectively (Figure 1F).
Figure 1.
Generation of the TgTDP-25 mice. A: Schematic representation of the construct used to generate the TgTDP-25 mice. The entire mouse Thy1.2 genomic sequence is shown with exons depicted as boxes, and noncoding sequences as thin lines. TDP-25 (gray box) was cloned into exon 3 of the murine Thy1.2 gene. The expression cassette includes the Thy1.2 mRNA polyadenylation sequence. B–D: Representative Western blots and quantitative analyses of proteins extracted from the brains of 2-month-old TgTDP-25 mice (lines B, C, and F) and NonTg mice using the low-salt buffer. The arrow points to the transgene, and the arrowhead points to the endogenous, full-length TDP-43. E: Real-time PCR experiments indicated that the mRNA levels of the transgene did not change between 2 and 6 months of age. F: The graph shows a standard curve generated by real-time PCR by measuring the Ct values of known copy numbers of the transgene added to NonTg mouse DNA. The Ct values of the founders DNA was then plotted into the standard curve (dotted lines). G and H: Representative Western blots and quantitative analyses of proteins extracted from the indicated tissues. I and J: Representative Western blots and quantitative analyses of proteins extracted from different brain regions of TgTDP-25(B) mice (n = 4). Brn, brain; Cb, cerebellum; Cx, cortex; Hp, hippocampus; Hrt, heart; Kdn, kidneys; Lng, lungs; Lvr, liver; Msc, muscle; Ob, olfactory bulb, Sc, spinal cord; SP, spinal cord; Spl, spleen. Blots in panels B, G, and I were probed with an anti–TDP-43 polyclonal antibody from ProteinTech. β-Actin was used as a loading control, and quantifications of the Western blots were done by normalizing the protein of interest to β-actin. Data are presented as means ± SEM and analyzed by one-way analysis of variance.
The Thy1.2 promoter is widely used to achieve neuronal-specific expression (eg, Oddo et al23 and Caroni30). Protein extracts in low-salt buffer from multiple tissues of the TgTDP-25(B) line were analyzed by Western blot using an anti–TDP-43 polyclonal antibody. We found that TDP-25 was expressed predominantly, if not exclusively, in the central nervous system (Figure 1, G and H). To determine in which regions of the central nervous system the transgene was expressed, we microdissected and prepared proteins extracted from different brain regions of TgTDP-25(B) and TgTDP-25(F) mice (n = 6/genotype) and measured the transgene levels by Western blot using a polyclonal anti–TDP-43 antibody. We found that in both lines, TDP-25 was mainly expressed in the cortex and hippocampus (Figure 1, I and J). Additionally, the transgene was expressed in cerebellum and spinal cord, with very little expression present in the olfactory bulb (Figure 1, I and J).
TgTDP-25 Mice Develop Cognitive Deficits
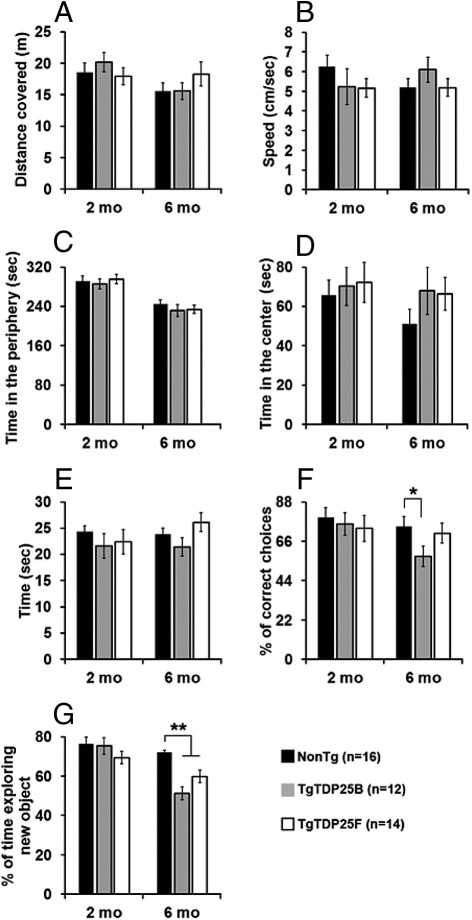
Accumulation of TDP-25 is an invariable feature of FTLD-TDP, and several in vitro studies have shown that the expression of this fragment is toxic.7,10–15 To determine whether expression of TDP-25 is sufficient to cause behavioral alterations, we tested 2- and 6-month-old TgTDP-25 mice from both the B and F lines (n = 14/genotype/time point) in a series of cognitive and noncognitive behavioral tasks. FTLD presents overlapping clinical and neuropathological features with ALS and other motor-neuron disorders.9 We thus used the open-field activity test to measure general motor function. We found that the spontaneous activity and gross motor function of 2- and 6-month-old TgTDP-25(B) and TgTDP-25(F) mice were similar to NonTg mice, as assessed by the distance traveled in the activity chamber and the average speed during the test (Figure 2, A and B, respectively; P > 0.05). We next assessed anxiety and stress by measuring open-field thigmotaxis and the time spent in the center of the arena. As expected, mice spent more time in the periphery compared to the center of the arena. This response was independent of age or genotype as no statistically significant differences were found across the three different genotypes at any of the ages analyzed (Figure 2, C and D). Additionally, we did not find any significant difference between NonTg and TgTDP-25 mice at both ages analyzed; however, a clear trend was observed in 6-month-old mice, where mice of both transgenic lines spent more time in the center of the arena compared to NonTg mice (Figure 2D). To further elucidate motor function in the TgTDP-25 mice, we tested the mice using the rotarod, which is widely used to assess motor coordination. Mice were trained for 90 seconds (six trials/day for 3 days) on a rod at a constant speed of 15 rpm. Six 90-second probe trials were conducted on day 4 on an accelerating rod (1 rpm/second). We found that the TgTDP-25 mice were able to stay on the rod as long as the NonTg mice, and no statistically significant differences were observed at the ages analyzed (Figure 2E; P > 0.05). Taken together, these data indicate that 2- and 6-month-old TgTDP-25 mice do not show any detectable motor dysfunction.
Figure 2.
The TgTDP-25 mice develop cognitive deficits. Behavioral testing was conducted in 2- and 6-month-old NonTg, TgTDP-25(B) and TgTDP-25(F) mice (n = 14/genotype/time point). A–D: The open-field activity test was conducted to measure spontaneous activity and anxiety. No statistically significant differences were found among the three groups (at any of the ages analyzed) in the distance covered during the exploration time (A) or the speed of exploration (B), indicating that gross motor function was intact in both lines of TgTDP-25 mice. Also, no differences among the groups were found when measuring the time spent in the periphery and center of the arena (C and D, respectively), indicating that the TgTDP-25 mice had no detectable anxiety defects. E: To measure motor coordination, we used the accelerating rotarod and found no statistically significant changes among the three groups analyzed. F: T-maze data show that at 2 months of age, working memory was similar among the three groups of mice. In contrast, 6-month-old TgTDP-25(B) mice performed significantly worse compared to NonTg and TgTDP-25(F) mice. G: Novel object recognition tests, a behavioral task highly dependent on the cortex, show that at 2 months of age all three groups of mice performed similarly to each other. At 6 months of age, however, both lines of TDP-25 transgenic mice were significantly impaired compared to NonTg mice as they spent less time exploring the new object compared to the NonTg mice. *P < 0.01, **P < 0.001. Data are presented as means ± SEM, and each time point was independently analyzed by one-way analysis of variance.
Executive function, which is controlled by the frontal cortex, is altered in FTLD patients (eg, Ref. 31). Moreover, cognitive functions controlled by other cortical regions in the temporal lobe are also impaired in FTLD cases.32 These cognitive alterations are consistent with the neuropathological observations showing predominant brain atrophy in frontal and temporal lobes.33 To assess working memory in the TgTDP-25 mice, we used the T-maze, which relies on the tendency of mice to alternate free choices in a T-maze during successive trials.34 This task is dependent on several brain regions, including the basal forebrain, hippocampus, and prefrontal cortex.34 At 2 months of age, the working memory was similar among the three groups of mice (Figure 2F; P > 0.05). In contrast, 6-month-old TgTDP-25(B) mice performed at a chance level, making the correct choice in only 57.5% ± 5.7% of the trials (Figure 2F). A one-way analysis of variance indicated a significant genotype effect for this task (P < 0.05). A Bonferroni post hoc analysis indicated that the TgTDP-25(B) mice were significantly different compared to NonTg and TgTDP-25(F) mice. In contrast, TgTDP-25(F) mice (the low-expressing line) performed as well as NonTg mice (Figure 2F; P > 0.05).
The novel object recognition task was used to measure cortical and hippocampal function by observing spontaneous mouse behavior to explore a novel object.35 During training, mice were exposed to two objects, object A and object B, and were left free to explore for 5 minutes. As expected, mice spent the same amount of time exploring the two objects at all ages analyzed. Indeed, at 2 months of age, the NonTg and TgTDP-25(B) mice spent 47.5% ± 3.2% and 49.5% ± 2.4%, respectively, exploring object A. Similarly, at 6 months of age, the NonTg and TgTDP-25(B) mice spent 51.5% ± 1.8% and 49.4%S ± 1.4%, respectively, exploring object A. During the probe trials, object B was replaced with a new object. At 2 months of age, all three groups showed a clear preference for the novel object, and no statistical differences were detected between transgenic and NonTg mice (Figure 2G; P > 0.05). At 6 months of age, the NonTg mice spent 72.1% ± 1% of their time exploring the new object. In contrast, the TgTDP-25(B) mice did not discriminate between the novel and old object and performed at a chance level, spending 51.3% ± 3.3% of their time exploring the new object (Figure 2G). The TgTDP-25(F) mice performed better than the TgTDP-25(B) mice, but not as well as the NonTg mice, because they spent 59.8% ± 3.1% of their time exploring the new object (Figure 2G). One-way analysis of variance indicated a significant genotype effect for this task (P < 0.0001). A Bonferroni post hoc analysis indicated that the TgTDP-25(B) and TgTDP-25(F) mice were significantly impaired compared to NonTg mice (P < 0.001 and P < 0.01, respectively). The difference between TgTDP-25(B) and TgTDP-25(F) mice did not reach statistical significance (P > 0.05). Taken together, these data indicate that although the TgTDP-25 mice have no gross alterations in motor function, they develop cognitive dysfunctions in two independent tasks. Furthermore, deficits in the object recognition in both transgenic lines clearly indicate that the cognitive impairments in the TgTDP-25 mice are not simply due to an effect of integration of the transgene.
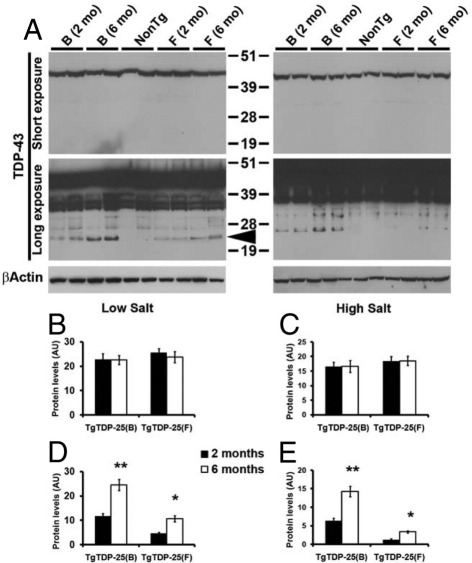
Increased Soluble TDP-25 Levels in 6-Month-Old Transgenic Mice
In TDP-43 proteinopathies, the solubility of TDP-43 and its C-terminal fragments is altered, leading to the formation of insoluble aggregates.7 We next wanted to determine the molecular and biochemical bases of the onset of cognitive deficits in the TgTDP-25 mice. We first extracted proteins from the brains of 2- and 6-month-old TgTDP-25(B) and TgTDP-25(F) and NonTg mice with buffers of increasing strength and analyzed the samples by Western blot. Although the steady-state levels of full-length TDP-43 in the low- and high-salt fractions did not change between 2 and 6 months of age (Figure 3, A-C), we found a significant increase in TDP-25 levels in the low-salt fraction from the brains of the TgTDP-25(B) and TgTDP-25(F) mice (Figure 3, A and D). We also found that the levels of TDP-25 in different fractions change between 2 and 6 months of age. Indeed, we could only detect a faint ∼25-kDa band in the high-salt fraction from the brains of 2-month-old TgTDP-25(B) mice, whereas in the TgTDP-25(F) low-expressing line, TDP-25 was not detectable at this age (Figure 3A). In contrast, as the mice aged, we found a marked and significant increase in TDP-25 levels in both transgenic lines (Figure 3, A and E). TDP-43 C-terminal fragments were below detection levels in the sarkosyl and urea fractions. Together these data suggest that soluble steady-state levels of TDP-25 increase between 2 and 6 months of age, which is consistent with other reports.17
Figure 3.
Increased soluble TDP-25 levels in 6-month-old TgTDP-25 mice. A: Representative Western blots of proteins extracted from the brains of 2- and 6-month-old transgenic and NonTg mice, in low- and high-salt buffers. Blots were probed with an anti–TDP-43 polyclonal antibody from ProteinTech. Two different exposure times are presented to show the less abundant, low molecular weight bands. B: Quantitative analyses of the blots from low-salt extraction show that the levels of full-length TDP-43 were similar between the TgTDP-25(B) and TgTDP-25(F) mice and did not change as a function of age. C: In contrast, we found the steady-state levels of TDP-25 (arrow) significantly increased as a function of age in both the TgTDP-25(B) and TgTDP-25(F) mice. D: Quantitative analyses of the blots from high-salt extracts show that the levels of full-length TDP-43 were similar between the TgTDP-25(B) and TgTDP-25(F) mice and did not change as a function of age. E: TDP-25 levels increased as a function of age in the high-salt fraction in both TgTDP-25 lines, suggesting a change in aggregation of this fragment as a function of age. n = 6/genotype/age. *P < 0.05, **P < 0.01. β-Actin was used as a loading control, and quantifications of the Western blots were done by normalizing the protein of interest to β-actin. Data are presented as means ± SEM and analyzed by two-way analysis of variance, with genotype and age as independent variables.
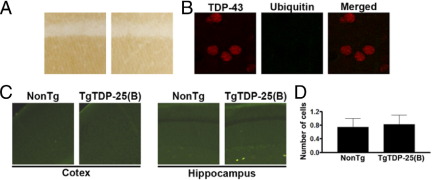
In FTLD-TDP, TDP-43 undergoes several post-translational modifications, including phosphorylation and ubiquitination.7 We thus sought to determine whether the expression of TDP-25 was sufficient to cause phosphorylation and ubiquitination of endogenous TDP-43. Toward this end, sections from 6-month-old TgTDP-25(B) and age-matched NonTg mice (n = 6/genotype) were stained with a TDP-43 antibody that selectively recognizes TDP-43 when phosphorylated at S409/410. Only background staining was found in both groups of mice (Figure 4A). Additionally, we found that at 6 months of age, TgTDP-25(B) mice were devoid of ubiquitin-positive TDP-43 inclusions (Figure 4B), which is consistent with the lack of TDP-43 C-terminal fragments in the sarkosyl and urea fractions. To determine whether the expression of TDP-25 was sufficient to cause neurodegeneration, we stained sections from 6-month-old TgTDP-25(B) and age-matched NonTg mice (n = 6/genotype) with Fluoro-Jade, a dye known to label degenerating neurons.27 The number of Fluoro-Jade–positive cells was very scarce for both groups of mice (Figure 4C), and quantitative analysis showed no statistically significant difference between the two genotypes (Figure 4D).
Figure 4.
Absence of ubiquitin-positive inclusions and cell death in TgTDP-25 mice. A: Representative hippocampal sections from 6-month-old NonTg and TgTDP-25(B) mice stained with a phospho-specific TDP-43 antibody (n = 6/genotype). B: Cortical sections from 6-month-old TgTDP-25(B) mice were double stained with a C-terminal anti-TDP-43 antibody and with an anti-ubiquitin antibody. The overlay shows that at this age, the TgTDP-25(B) mice have no ubiquitin-positive TDP-43 inclusions. C: Representative cortical and hippocampal sections from 6-month-old NonTg and TgTDP-25(B) mice stained with Fluoro-Jade. D: Quantitative analysis of the Fluoro-Jade–positive cells, conducted as described in Materials and Methods, showed no statistically significant difference between the two groups of mice (n = 6/genotype). Data are presented as means ± SEM and analyzed by Student's t-test.
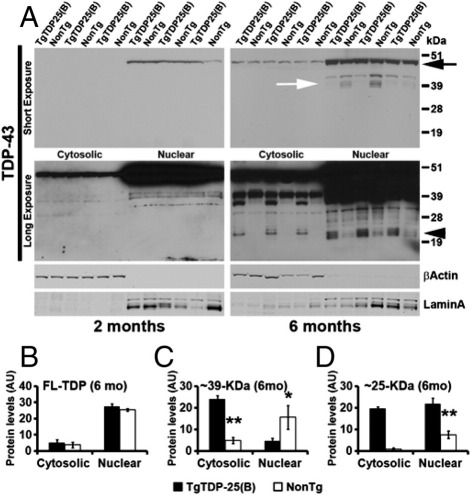
It is well established that in FTLD-TDP, TDP-43 is mislocalized from its nuclear location to the cytosol.7 Furthermore, in vitro studies show that the expression of C-terminal fragments of TDP-43 leads to a mislocalization of endogenous full-length TDP-43, even though the literature does not show general agreement.10,12–15 To biochemically study the intracellular localization of TDP-43 and its C-terminal fragments, we extracted nuclear and cytosolic fractions from 2- and 6-month-old TgTDP-25(B) and NonTg mice (Figure 5A). As expected, most of the full-length TDP-43 was in the nuclear fraction at both ages (Figure 5, A and B); additionally, at 2 and 6 months of age, the levels of full-length TDP-43 were similar between TgTDP-25(B) and NonTg mice (Figure 5, A and B). Notably, in the cytosolic and nuclear fractions of 6-month-old mice, we found a series of TDP-43 fragments in both TgTDP-25(B) and NonTg mice that were not present at 2 months of age (Figure 5A). In the cytosolic fraction, we found an ∼25-kDa band, which was exclusively present in the TgTDP-25(B) mice (Figure 5, A and D) and most likely corresponds to the TDP-25 transgene. In the nuclear fraction, we found a triplet of fragments of similar molecular weight that were significantly higher in the TgTDP-25(B) mice compared to the NonTg mice (Figure 5, A and D). Notably, the higher molecular weight band of this triplet was present only in the TgTDP-25(B) mice and most likely represents the TDP-25 transgene. The other two bands are also present in the NonTg mice, although the levels are significantly lower than those of the TgTDP-25(B) mice, and thus they may represent different fragments of endogenous TDP-43 and not the transgene. Additionally, we found another triplet of bands of ∼35 kDa (Figure 5A). Surprisingly, these fragments were significantly higher in the NonTg mice compared to the TgTDP-25(B) mice (Figure 5, A and C), suggesting that these are derived from a normal processing of TDP-43. Thus, it is tempting to speculate that the expression of TDP-25 is altering the processing of full-length, endogenous TDP-43, leading from a process that mainly generates ∼39-kDa fragments in the NonTg mice to one that mainly forms ∼25-kDa fragments in the TgTDP-25(B) mice. As expected, in the cytosolic fraction, we found that the levels of the TDP-25 were significantly higher in 6-month-old TgTDP-25(B) mice compared to age-matched NonTg mice (Figure 5, A and D). Additionally, in the cytosolic fraction, we found a robust and significant accumulation of a 35-kDa fragment in the TgTDP-25(B) mice (Figure 5, A and C), further suggesting that the expression of the transgene has altered the normal processing of endogenous, full-length TDP-43.
Figure 5.
TDP-25 expression leads to altered processing of full-length endogenous TDP-43. A: Representative Western blots extracted from 2- and 6-month-old TgTDP-25 and NonTg mice. Different exposures are presented to show the less abundant, low molecular TDP-43 fragments. Proteins were extracted from the cytosolic and nuclear fraction, as depicted in the figure. TDP-43 blots were probed with an anti-TDP-43 polyclonal antibody from ProteinTech. β-Actin and LaminA were used as loading controls for the cytosolic and nuclear fractions, respectively. B: As expected, quantitation of the full-length TDP-43 band (panel A, top right blot, black arrow), shows that the levels of TDP-43 are significantly higher in the nucleus compared to the cytoplasm in both transgenic and NonTg mice. Additionally, the levels of TDP-43 were not different in the cytosolic or nuclear fractions between TgTDP-25(B) and NonTg mice. C: Quantitative analysis of the three bands of ∼39-kDa (panel A, top right blot, white arrow). Notably, these bands were not present in 2-month-old mice, irrespective of the genotype. In the nuclear fraction of 6-month-old mice, the levels of these fragments were significantly higher in NonTg mice compared to TgTDP-25(B) mice. In the TgTDP-25(B) mice, however, there was a striking increase of an ∼35-kDa band in the cytosolic fraction compared to NonTg mice, suggesting that in the TgTDP-25(B) mice, there is a possible redistribution of the ∼35-kDa bands from the nucleus (where they are more abundant in NonTg mice) to the cytosol. D: Quantitative analysis of the 25-kDa bands (panel A, bottom right blot, arrowhead) shows that the TDP-25 transgene is also present in the nuclear fraction, despite the lack of putative nuclear localization signals. *P < 0.05, **P < 0.01. β-Actin and LaminA were used as loading controls for cytosolic and nuclear fractions, respectively. Quantifications of the Western blots were done by normalizing the fragment of interest to β-actin of LaminA. Data are presented as means ± SEM and analyzed by two-way analysis of variance, with genotype and age as independent variables.
Discussion
Neuropathologically, TDP-43 has been linked to FTLD-TDP; indeed, TDP-43 has been identified as the major component of ubiquitin-positive inclusions that characterize FTLD-TDP.7 Genetically, mutations in TDP-43 lead to ALS,8 a neurodegenerative disorder with clinical and neuropathological features overlapping with FTLD.9 Pathological TDP-43 is cleaved to form TDP-25, a C-terminal fragment of TDP-43 that consistently has been isolated in human cases of FTLD-TDP and ALS.7 Furthermore, we and others have previously shown that the expression of TDP-25 in vitro is toxic and recapitulates key pathological features of TDP-proteinopathies.10,12–15 Here, we report the significant finding that expression of TDP-25 in vivo is sufficient to cause cognitive deficits in transgenic mice, highlighting new mechanistic insights into the pathogenesis of TDP-43 proteinopathies, including FTLD-TDP and ALS. Indeed, these two disorders may share similar pathogenic mechanisms because the biochemical profile of TDP-43 is consistent between ALS and FTLD-TDP.
Pathological TDP-43 is mislocalized from its physiological nuclear location to the cytoplasm, where it forms ubiquitin-positive inclusions.7 Thus, it is not clear whether the primary culprit in the disease pathogenesis is the accumulation of ubiquitin-positive TDP-43 inclusions (through a gain-of-function mechanism) or the depletion of nuclear TDP-43 (through a loss-of-function mechanism). The data reported here indicate that neither inclusions nor nuclear depletions are necessary for the onset of behavioral alterations. Indeed, we show that the earliest detectable cognitive deficits in the TgTDP-25(B) mice occur at 6 months of age. Notably, at this age, the only overt change in the brain of these mice is the increase in soluble TDP-25 levels. In view of these data, we suggest that the onset of cognitive deficits is due to the build-up of soluble TDP-25 most likely through a toxic gain of function. This is a recurring event in several neurodegenerative diseases. For example, strong evidence shows that in Alzheimer's disease, the accumulation of soluble species of amyloid-β and tau may be the primary event in the disease pathogenesis, whereas the formation of insoluble inclusions could represent a secondary event.36 Analysis of TDP-43 transgenic mice expressing wild-type or mutant full-length TDP-43 indicates the possibility of a relation between gene dosage and the formation of TDP-43 inclusions.17 We cannot exclude the possibility that increasing TDP-25 levels in the TgTDP-25(B) mice (eg, by generating homozygous mice) may lead to TDP-43–positive inclusions. Nevertheless, data clearly indicate that such inclusions are not necessary for the onset of cognitive deficits. However, it should be pointed out that the data presented here are not meant to exclude the possibility that TDP-43 inclusions and the nuclear depletion of TDP-43 are toxic events for overall brain functioning. Indeed, data generated from studies of transgenic mice expressing full-length wild-type and mutant TDP-43 show the toxicity of TDP-43–positive inclusions.17,19–21 Furthermore, the loss of TDP-43 is also toxic as shown by knockout experiments: homozygous TDP-43 knockout mice are not vital; heterozygous mice show muscle weakness.37,38 Consistent with these results, conditional knockout mice die within 9 days following ablation of the TDP-43 gene.39 These data, together with the data presented here, indicate that the onset of behavioral deficits is due to the increase of soluble TDP-25 levels. As the disease progresses, TDP-43 is mislocalized and accumulates in the cytoplasm, and both the toxic gain of function, due to the presence of such cytoplasmic inclusions, and the loss of nuclear TDP-43 function further contribute to the progression of the disease.
The cause of neurodegeneration in TDP-43 proteinopathies remains to be elucidated. Transgenic mice and flies have been generated and recapitulate several key features of the disease, including neurodegeneration. Notably, however, because these models show ubiquitin-positive inclusions, TDP-43 deposits, and accumulation of C-terminal fragments, it has been challenging to identify the primary cause of neurodegeneration in TDP-43 proteinopathies. At the ages analyzed, the TDP-25(B) mice do not show apparent neurodegeneration, indicating that the build-up of soluble TDP-25 is not required for neurodegeneration. These data are consistent with a recent report showing that neurodegeneration in a transgenic mouse model expressing full-length TDP-43 lacking the two nuclear localization signals (NLSs) occurs independently of TDP-43 inclusions and C-terminal fragments.18
Structurally, TDP-43 has two NLSs both localized between amino acids 82 and 98, well outside the 25-kDa C-terminal fragment expressed by the TgTDP-25(B) mice.40 Although in vitro experiments show that removing both NLSs leads to cytosolic expression of TDP-43,40 in vivo TDP-43 sorting appears to be more complex. For example, in mice expressing TDP-43 lacking both NLSs, the transgene is found in both the cytoplasm and the nucleus.18 Here, we show that the levels of TDP-25 are significantly higher in the nucleus than in the cytoplasm, despite the fact that TDP-25 lacks the NLSs. Although it is possible that processing of endogenous TDP-43 may contribute to the high levels of nuclear TDP-25, our data are consistent with previous work reporting that when expressed in vitro, TDP-25 localizes to the nucleus.10,11,13–15 Further supporting changes in TDP-43 processing and trafficking, we reported the striking finding of the mislocalization of ∼35-kDa fragments, which in NonTg mice are mainly present in the nuclear fraction, whereas in TgTDP-25(B) mice, these fragments accumulate in the cytosolic fraction. Although the nature and significance of these fragments remain to be elucidated, these data indicate that the expression of the TDP-25 fragment is sufficient to alter the physiological endogenous TDP-43 processing. Overall, it is evident that more work needs to be done to understand the basic molecular mechanisms underlying TDP-43 processing and its import and export from the nucleus. Understanding such mechanisms may be lead to a better understanding of the disease pathogenesis.
Footnotes
Supported by a Pilot grant from the San Antonio Nathan Shock Center of Excellence in the Biology of Aging (NIA 5P30AG13319) (S.O.).
References
- 1.Wang H.Y., Wang I.F., Bose J., Shen C.K. Structural diversity and functional implications of the eukaryotic TDP gene family. Genomics. 2004;83:130–139. doi: 10.1016/s0888-7543(03)00214-3. [DOI] [PubMed] [Google Scholar]
- 2.Buratti E., Baralle F.E. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J Biol Chem. 2001;276:36337–36343. doi: 10.1074/jbc.M104236200. [DOI] [PubMed] [Google Scholar]
- 3.Buratti E., Dork T., Zuccato E., Pagani F., Romano M., Baralle F.E. Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J. 2001;20:1774–1784. doi: 10.1093/emboj/20.7.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buratti E., Baralle F.E. Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front Biosci. 2008;13:867–878. doi: 10.2741/2727. [DOI] [PubMed] [Google Scholar]
- 5.Wang I.F., Wu L.S., Chang H.Y., Shen C.K. TDP-43, the signature protein of FTLD-U, is a neuronal activity-responsive factor. J Neurochem. 2008;105:797–806. doi: 10.1111/j.1471-4159.2007.05190.x. [DOI] [PubMed] [Google Scholar]
- 6.Harvey R.J., Skelton-Robinson M., Rossor M.N. The prevalence and causes of dementia in people under the age of 65 years. J Neurol Neurosurg Psychiatry. 2003;74:1206–1209. doi: 10.1136/jnnp.74.9.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neumann M., Sampathu D.M., Kwong L.K., Truax A.C., Micsenyi M.C., Chou T.T., Bruce J., Schuck T., Grossman M., Clark C.M., McCluskey L.F., Miller B.L., Masliah E., Mackenzie I.R., Feldman H., Feiden W., Kretzschmar H.A., Trojanowski J.Q., Lee V.M. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 8.Pesiridis G.S., Lee V.M., Trojanowski J.Q. Mutations in TDP-43 link glycine-rich domain functions to amyotrophic lateral sclerosis. Hum Mol Genet. 2009;18:R156–R162. doi: 10.1093/hmg/ddp303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen-Plotkin A.S., Lee V.M., Trojanowski J.Q. TAR DNA-binding protein 43 in neurodegenerative disease. Nat Rev Neurol. 2010;6:211–220. doi: 10.1038/nrneurol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caccamo A., Majumder S., Deng J.J., Bai Y., Thornton F.B., Oddo S. Rapamycin rescues TDP-43 mislocalization and the associated low molecular mass neurofilament instability. J Biol Chem. 2009;284:27416–27424. doi: 10.1074/jbc.M109.031278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igaz L.M., Kwong L.K., Chen-Plotkin A., Winton M.J., Unger T.L., Xu Y., Neumann M., Trojanowski J.Q., Lee V.M. Expression of TDP-43 C-terminal fragments in vitro recapitulates pathological features of TDP-43 proteinopathies. J Biol Chem. 2009;284:8516–8524. doi: 10.1074/jbc.M809462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Igaz L.M., Kwong L.K., Xu Y., Truax A.C., Uryu K., Neumann M., Clark C.M., Elman L.B., Miller B.L., Grossman M., McCluskey L.F., Trojanowski J.Q., Lee V.M. Enrichment of C-terminal fragments in TAR DNA-binding protein-43 cytoplasmic inclusions in brain but not in spinal cord of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Am J Pathol. 2008;173:182–194. doi: 10.2353/ajpath.2008.080003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y.J., Xu Y.F., Cook C., Gendron T.F., Roettges P., Link C.D., Lin W.L., Tong J., Castanedes-Casey M., Ash P., Gass J., Rangachari V., Buratti E., Baralle F., Golde T.E., Dickson D.W., Petrucelli L. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci U S A. 2009;106:7607–7612. doi: 10.1073/pnas.0900688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dormann D., Capell A., Carlson A.M., Shankaran S.S., Rodde R., Neumann M., Kremmer E., Matsuwaki T., Yamanouchi K., Nishihara M., Haass C. Proteolytic processing of TAR DNA binding protein-43 by caspases produces C-terminal fragments with disease defining properties independent of progranulin. J Neurochem. 2009;110:1082–1094. doi: 10.1111/j.1471-4159.2009.06211.x. [DOI] [PubMed] [Google Scholar]
- 15.Nonaka T., Kametani F., Arai T., Akiyama H., Hasegawa M. Truncation and pathogenic mutations facilitate the formation of intracellular aggregates of TDP-43. Hum Mol Genet. 2009;18:3353–3364. doi: 10.1093/hmg/ddp275. [DOI] [PubMed] [Google Scholar]
- 16.Wegorzewska I., Bell S., Cairns N.J., Miller T.M., Baloh R.H. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci U S A. 2009;106:18809–18814. doi: 10.1073/pnas.0908767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wils H., Kleinberger G., Janssens J., Pereson S., Joris G., Cuijt I., Smits V., Ceuterick-de Groote C., Van Broeckhoven C., Kumar-Singh S. T.D.P-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci U S A. 2010;107:3858–3863. doi: 10.1073/pnas.0912417107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Igaz L.M., Kwong L.K., Lee E.B., Chen-Plotkin A., Swanson E., Unger T., Malunda J., Xu Y., Winton M.J., Trojanowski J.Q., Lee V.M. Dysregulation of the ALS-associated gene TDP-43 leads to neuronal death and degeneration in mice. J Clin Invest. 2011;121:726–738. doi: 10.1172/JCI44867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Y.F., Gendron T.F., Zhang Y.J., Lin W.L., D'Alton S., Sheng H., Casey M.C., Tong J., Knight J., Yu X., Rademakers R., Boylan K., Hutton M., McGowan E., Dickson D.W., Lewis J., Petrucelli L. Wild-type human TDP-43 expression causes TDP-43 phosphorylation, mitochondrial aggregation, motor deficits, and early mortality in transgenic mice. J Neurosci. 2010;30:10851–10859. doi: 10.1523/JNEUROSCI.1630-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou H., Huang C., Chen H., Wang D., Landel C.P., Xia P.Y., Bowser R., Liu Y.J., Xia X.G. Transgenic rat model of neurodegeneration caused by mutation in the TDP gene. PLoS Genet. 2010;6:e1000887. doi: 10.1371/journal.pgen.1000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai K.J., Yang C.H., Fang Y.H., Cho K.H., Chien W.L., Wang W.T., Wu T.W., Lin C.P., Fu W.M., Shen C.K. Elevated expression of TDP-43 in the forebrain of mice is sufficient to cause neurological and pathological phenotypes mimicking FTLD-U. J Exp Med. 2010;207:1661–1673. doi: 10.1084/jem.20092164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stallings N.R., Puttaparthi K., Luther C.M., Burns D.K., Elliott J.L. Progressive motor weakness in transgenic mice expressing human TDP-43. Neurobiol Dis. 2010;40:404–414. doi: 10.1016/j.nbd.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Oddo S., Caccamo A., Shepherd J.D., Murphy M.P., Golde T.E., Kayed R., Metherate R., Mattson M.P., Akbari Y., LaFerla F.M. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 24.Oddo S., Vasilevko V., Caccamo A., Kitazawa M., Cribbs D.H., LaFerla F.M. Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem. 2006;281:39413–39423. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- 25.Caccamo A., Majumder S., Richardson A., Strong R., Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caccamo A., Maldonado M.A., Majumder S., Medina D.X., Holbein W., Magri A., Oddo S. Naturally secreted amyloid-{beta} increases mammalian target of rapamycin (mTOR) activity via a PRAS40-mediated mechanism. J Biol Chem. 2011;286:8924–8932. doi: 10.1074/jbc.M110.180638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmued L.C., Hopkins K.J. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- 28.Caccamo A., Maldonado M.A., Bokov A.F., Majumder S., Oddo S. CBP gene transfer increases BDNF levels and ameliorates learning and memory deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2010;107:22687–22692. doi: 10.1073/pnas.1012851108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y.J., Xu Y.F., Dickey C.A., Buratti E., Baralle F., Bailey R., Pickering-Brown S., Dickson D., Petrucelli L. Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J Neurosci. 2007;27:10530–10534. doi: 10.1523/JNEUROSCI.3421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caroni P. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J Neurosci Methods. 1997;71:3–9. doi: 10.1016/s0165-0270(96)00121-5. [DOI] [PubMed] [Google Scholar]
- 31.Johns E.K., Phillips N.A., Belleville S., Goupil D., Babins L., Kelner N., Ska B., Gilbert B., Inglis G., Panisset M., de Boysson C., Chertkow H. Executive functions in frontotemporal dementia and Lewy body dementia. Neuropsychology. 2009;23:765–777. doi: 10.1037/a0016792. [DOI] [PubMed] [Google Scholar]
- 32.Neary D., Snowden J.S., Gustafson L., Passant U., Stuss D., Black S., Freedman M., Kertesz A., Robert P.H., Albert M., Boone K., Miller B.L., Cummings J., Benson D.F. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 33.McKhann G.M., Albert M.S., Grossman M., Miller B., Dickson D., Trojanowski J.Q. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 34.Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- 35.Mumby D.G., Gaskin S., Glenn M.J., Schramek T.E., Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haass C., Selkoe D.J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 37.Wu L.S., Cheng W.C., Hou S.C., Yan Y.T., Jiang S.T., Shen C.K. TDP-43, a neuro-pathosignature factor, is essential for early mouse embryogenesis. Genesis. 2010;48:56–62. doi: 10.1002/dvg.20584. [DOI] [PubMed] [Google Scholar]
- 38.Kraemer B.C., Schuck T., Wheeler J.M., Robinson L.C., Trojanowski J.Q., Lee V.M., Schellenberg G.D. Loss of murine TDP-43 disrupts motor function and plays an essential role in embryogenesis. Acta Neuropathol. 2010;119:409–419. doi: 10.1007/s00401-010-0659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiang P.M., Ling J., Jeong Y.H., Price D.L., Aja S.M., Wong P.C. Deletion of TDP-43 down-regulates Tbc1d1, a gene linked to obesity, and alters body fat metabolism. Proc Natl Acad Sci U S A. 2010;107:16320–16324. doi: 10.1073/pnas.1002176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winton M.J., Igaz L.M., Wong M.M., Kwong L.K., Trojanowski J.Q., Lee V.M. Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation. J Biol Chem. 2008;283:13302–13309. doi: 10.1074/jbc.M800342200. [DOI] [PMC free article] [PubMed] [Google Scholar]