Abstract
Systemic inflammation remains a major cause of morbidity and mortality in the United States, across many disease processes. One classic murine model to study this syndrome is lipopolysaccharide (LPS)–induced Toll-like receptor 4 (TLR4)–dependent systemic inflammation. Although most studies have focused on inflammatory cell TLR4 responses, parenchymal cells also express TLR4. Our objective was to define the in vivo role of parenchymal- versus marrow-derived cell activation via TLR4 during LPS-induced inflammation. Mice bearing TLR4 on parenchymal cells only, marrow-derived cells only, both, or neither were generated using bone marrow transplantation. Mortality occurred only in mice that had TLR4 expression on their parenchymal cells. Before onset, virtually all major plasma cytokines and blood neutrophil responses were related to marrow-derived cell activation via TLR4. The only cytokine predictive of oncoming systemic inflammation was the chemokine monocyte chemoattractant protein-1. Late blood neutrophil responses were related to the presence of TLR4 on either parenchymal or marrow cells, whereas plasma cytokine elevations late in LPS-induced systemic inflammation were dependent on mice having TLR4 in both cell compartments. Parenchymal cell activation via TLR4 is a key component of LPS-induced systemic inflammation and mortality, although most plasma cytokine levels and blood neutrophil responses were not key components. Given its unique role, future studies into monocyte chemoattractant protein-1's exact role during systemic inflammation are warranted.
Systemic inflammation remains a major cause of morbidity and mortality in the United States because it is involved in a host of different infectious and noninfectious diseases.1–3 One classic animal model for this process is triggered by lipopolysaccharide (LPS), a Gram-negative bacterial toxin recognized solely by Toll-like receptor 4 (TLR4).4 The role of TLR4 as the much-sought LPS receptor was made over a decade ago through the serendipitous discovery of mouse substrains completely resistant to this toxin.5,6 Since then, TLR4 mutant or knockout mice have been used to study global TLR4 dependence in a host of other systemic inflammatory diseases.7–9
Investigations into the host systemic inflammatory response have focused largely on the actions of inflammatory cells, in part because these cells are easily accessible for study. Responses of these cells to LPS have been well documented, along with their roles in acute and chronic tissue injury.10 It is known, however, that TLR4 is also widely expressed by many different types of parenchymal cells.11 It remains unclear what role parenchymal cell activation via such receptors plays in the pathophysiological characteristics of systemic inflammation. Previous studies12–15 into the roles of these cells in response to LPS have focused on one particular organ or tissue, as opposed to the global host response. The objective of these studies was to determine whether and to what extent parenchymal cell activation by TLR4 contributes to LPS-induced systemic inflammation in terms of both early events (onset and neutropenia) and late events (neutrophilia, organ neutrophil infiltration, and mortality).4 Determining the kinetics of these changes is key to this inquiry because some of the most prominent changes could be the consequence, rather than the cause. Hence, within the context of these studies, we also tested various cytokines and chemokines early and late in the disease process to determine which (if any) are crucial to systemic inflammation. Our hypothesis is that systemic inflammation is actually driven by parenchymal cell activation via TLR4 while plasma cytokine levels are driven instead by marrow-derived cell activation via TLR4.
Materials and Methods
TLR4 Transplantation Mouse Generation
All studies were approved by Mayo Clinic's institutional animal care and use committee. Bone marrow transplantations were conducted between C57BL TLR4 wild-type and/or knockout mice (Jackson Laboratories, Bar Harbor, ME) using previously published methods.16 In brief, recipient mice were administered neomycin (4 mg/mL in drinking water) for 24 hours and then irradiated twice using 5-Gy doses 3 hours apart in a Cs137 irradiator (J.L. Shepard & Associates, San Fernando, CA). Donor mice were euthanized, and bone marrow was sterilely extracted from excised femurs and tibia. Eight million bone marrow cells, suspended in sterile PBS, were introduced into each recipient mouse via jugular vein injection under general anesthesia. Recipient mice continued to receive 4 mg/mL neomycin for 2 weeks, followed by an additional 6- to 8-week recovery period, to reconstitute immunity with donor bone marrow. Throughout the process, mice were given standard chow and water ad libitum. In this manner, two different types of chimeric TLR4 mice, marrow TLR4−/− (transplant of TLR4−/− bone marrow cells into a TLR4+/+ mouse) and parenchymal TLR4−/− (transplant of TLR4+/+ bone marrow cells into a TLR4−/− mouse), along with two types of transplant control mice, TLR4+/+ (transplant of TLR4+/+ bone marrow cells into a TLR4+/+ mouse) and TLR4−/− (transplant of TLR4−/− bone marrow cells into a TLR4−/− mouse), were generated for all experiments. By using this procedure, it was previously demonstrated that 99% of leukocytes and all tissue macrophages are replaced from the donor bone marrow.12,13,16 We confirmed engraftment of donor bone marrow at 7 weeks after transplantation using the presence or absence of peripheral blood mononuclear cell production of tumor necrosis factor (TNF)-α in response to LPS ex vivo (data not shown).
LPS-Induced Systemic Inflammation Model
To induce systemic inflammation, ultra-pure Escherichia coli LPS (Invitrogen, San Diego, CA) was dissolved in PBS and injected i.p. To adjust for variations in LPS biological activity between lots, each lot's LPS activity was quantitated using a chromogenic Limulus amoebocyte lysate kit (GenScript, Piscataway, NJ). A dose of approximately 1.5 × 1015 endotoxin units per kg was selected based on pilot studies demonstrating that this dose achieved systemic inflammation with high mortality in TLR4+/+ mice. A matching volume of PBS injected i.p. was used as a vehicle control.
Survival Curves
Eighteen mice that underwent transplantation (four TLR4+/+, five marrow TLR4−/−, four parenchymal TLR4−/−, and five TLR4−/−) were injected with i.p. LPS for this experiment. These mice were then monitored for onset of systemic inflammation and mortality. Murine systemic inflammation induced by LPS was defined as previously described in a comprehensive review4 of LPS-induced systemic inflammation in small animals. In brief, murine LPS-induced systemic inflammation first appears when mice become: i) less active, ii) disinclined to feed, and iii) ataxic and weak. At this time, severe neutropenia also develops. Later in the process, mice become: i) ataxic and unable to stand; ii) hypothermic; iii) tachypneic, with labored respirations; and iv) immobilized and unresponsive to stimuli. Mice also develop profuse, fluid diarrhea and peripheral blood neutrophilia at this point. Mice were observed by an expert veterinary technician (B.E.K.) who was blinded to the identity of the groups for up to 10 days for development of the initial signs of murine LPS-induced systemic inflammation.
Plasma Cytokines, Blood Neutrophil Content, and Tissue MPO Content
A total of 65 mice that underwent TLR4 transplantation (15 TLR4+/+, 16 marrow TLR4−/−, 18 parenchymal TLR4−/−, and 16 TLR4−/−) were used for this experiment. Two thirds of each group received LPS, and one third received vehicle control, via i.p. injection. For each of these mice, blood was harvested at 1 hour after injection for plasma cytokine and blood neutrophil levels. Both blood and organs (lung, liver, and kidney) were then harvested at 18 hours, near the time of death, based on the results of the previous experiment. Signs of murine systemic inflammation were assessed at 18 hours before sacrifice using all of the previously defined criteria. For a subset of these mice, hypothermia was confirmed by measuring core temperatures via rectal thermometer just before anesthesia induction for sacrifice. Weights were recorded before LPS/saline injection and again before anesthesia induction for sacrifice.
All blood was harvested in 7.5% EDTA. Plasma was isolated from each blood sample and stored along with organs at −80°C for analysis. The remaining packed blood cells were stained using neutrophil marker Gr1-PerCP at 4°C for 45 minutes (eBiosciences, San Diego, CA), the red blood cells were lysed, and the remaining cells were washed and fixed using a whole blood erythrocyte lysing kit (R&D Systems, Minneapolis, MN). Flow cytometry of the fixed cells was conducted using a BD FACS Calibur cytometer (BD Biosciences, San Jose, CA). White blood cells were separated from cellular fragments using standard forward and side scatter gating. Neutrophils were then separated based on forward and side scatter from the lymphocyte/monocyte population and then confirmed using the Gr1-PerCP neutrophil marker. The percentage of neutrophils was calculated using number of neutrophils/total number of gated white blood cells.
Lung, liver, and kidney myeloperoxidase (MPO) content was measured using a commercially available murine MPO enzyme-linked immunosorbent assay kit, as instructed (HyCult Biotech, Plymouth Meeting, PA). Plasma cytokines were measured using the Luminex mouse cytokine 20-plex panel on a Luminex 100 machine, as instructed (Invitrogen, Carlsbad, CA). IL-13 and monokine induced by gamma interferon were not considered for these experiments, per Invitrogen's assay validation information, because EDTA plasma was used.
Statistical Analysis
Kaplan-Meier survival curves were constructed for time to LPS-induced mortality. Comparisons of curves between the four TLR4 transplantation groups were conducted using log-rank testing. LPS-induced plasma cytokine expression, blood neutrophil levels, and tissue MPO content were compared across all four TLR4 transplantation groups initially using one-way analysis of variance and then the Tukey-Kramer honestly significant differences test for all subsequent pairwise testing to account for multiple comparisons. The prediction of oncoming murine systemic inflammation using 1-hour plasma cytokine data was assessed using sensitivity, specificity, and area under the receiver operating characteristic curve. The 95% CIs for sensitivity and specificity were calculated using the exact binomial method. P < 0.05 was considered statistically significant.
Results
Onset and Mortality
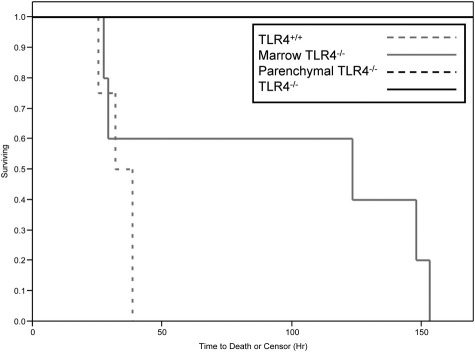
To investigate whether parenchymal- or marrow-derived cell activation via TLR4 was important to the pathophysiological features of systemic inflammation, we first injected LPS into the i.p. space of 18 mice that underwent transplantation (four TLR4+/+, five marrow TLR4−/−, four parenchymal TLR4−/−, and five TLR4−/−). Remarkably, mortality was entirely dependent on whether TLR4 was expressed on parenchymal cells (P = 0.0004, log-rank test; Figure 1). TLR4+/+ mice trended toward having earlier mortality than marrow TLR4−/− mice, but this difference was not significant. In this experiment, murine systemic inflammation started to manifest itself between 2 and 8 hours after injection in the TLR4+/+ and marrow TLR4−/− mice. No signs of murine LPS-induced systemic inflammation appeared in the parenchymal TLR4−/− and TLR4−/− mice, even up to 10 days after injection (data not shown).
Figure 1.
Kaplan-Meier curves for mortality for four groups of mice that underwent TLR4 transplantation and were given LPS. Four TLR4+/+, five marrow TLR4−/−, four parenchymal TLR4−/−, and five TLR4−/− mice were used for this experiment. The Kaplan-Meier curves for parenchymal TLR4−/− and TLR4−/− groups overlap in this figure.
Blood Neutrophil Levels and Distal MPO Content
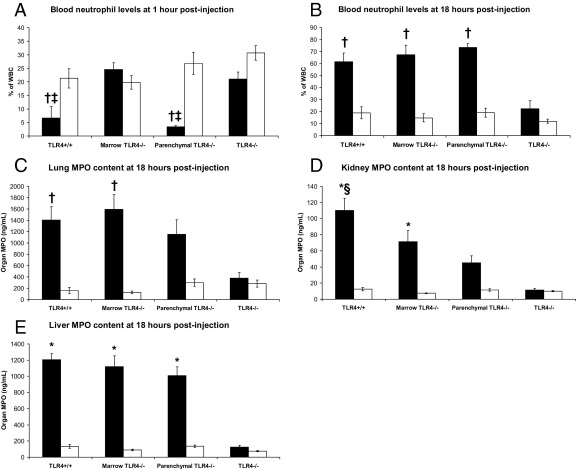
To investigate cell compartment roles on neutrophil responses (neutropenia early and neutrophilia late), we injected mice that underwent TLR4 transplantation with LPS or saline vehicle control and measured blood neutrophil levels.4 We examined these responses both early in the process (1 hour after injection), before LPS-induced systemic inflammation had manifested itself, and again late in the process (18 hours after injection), when TLR4+/+ and marrow TLR4−/− mice showed signs of systemic inflammation, but before they died (Figure 1). We also measured lung, kidney, and liver neutrophilic activity seen late in murine LPS-induced systemic inflammation by assessing organ MPO content to see the role such cellular activity has in this process.
In this experiment, 88% of TLR4+/+, 82% of marrow TLR4−/−, 8% of parenchymal TLR4−/−, and 0% of TLR4−/− mice given LPS developed manifestations of murine systemic inflammation by 18 hours (Table 1). There was also significantly greater weight loss at 18 hours in TLR4+/+, marrow TLR4−/−, and parenchymal TLR4−/− mice given LPS compared with TLR4−/− mice given LPS (P = 0.001, one-way analysis of variance; Table 1).
Table 1.
Responses of 65 Mice that Underwent TLR4 Transplantation and Were Given LPS or Saline Vehicle Control at 18 Hours after Injection
| TLR4+/+ mice |
Marrow TLR4−/− mice |
Parenchymal TLR4−/− mice |
TLR4−/− mice |
|||||
|---|---|---|---|---|---|---|---|---|
| Variable | LPS | Saline | LPS | Saline | LPS | Saline | LPS | Saline |
| Total | 8 | 7 | 11 | 5 | 12 | 6 | 10 | 6 |
| LPS-induced systemic inflammation⁎ | 7 | 0 | 9 | 0 | 1 | 0 | 0 | 0 |
| <32°C (no./total assessed)† | 3/4 | 0/4 | 7/9 | 0/3 | 1/5 | 0/5 | 0/2 | 0/2 |
| Weight loss (g)‡ | 2.0 ± 0.1 | 0.9 ± 0.3 | 1.7 ± 0.2 | 0.0 ± 0.3 | 2.0 ± 0.2 | 0.0 ± 0.2 | 0.8 ± 0.3 | 0.6 ± 0.3 |
Assessed by blinded animal technician based on published description of murine LPS-induced systemic inflammation.4
Rectal temperature assessed before anesthesia administration and completed for the last subsets of mice in this experiment.
Expressed as mean ± SE.
TLR4+/+ and parenchymal TLR4−/− mice experienced significant neutropenia soon after LPS injection compared with marrow TLR4−/− and TLR4−/− mice (P < 0.0001, one-way analysis of variance; Figure 2A). Late in the disease process, there was significant neutrophilia in all mice given LPS, except the TLR4−/− mice (P = 0.001, one-way analysis of variance; Figure 2B). Liver MPO content was significantly elevated at 18 hours after LPS administration in TLR4+/+, marrow TLR4−/−, and parenchymal TLR4−/− mice compared with TLR4−/− mice (P < 0.0001, one-way analysis of variance; Figure 2E). Moreover, lung and kidney MPO content at 18 hours after injection with LPS was significantly elevated in TLR4+/+ and marrow TLR4−/− mice compared with TLR4−/− mice, whereas parenchymal TLR4−/− mice had intermediate levels (P = 0.004 and P < 0.0001, respectively; one-way analysis of variance; Figure 2, C and D).
Figure 2.
Blood neutrophil responses at 1 hour (A) and at 18 hours (B) after injection, along with several organ MPO contents at 18 hours after injection (C–E) for four groups of mice that underwent TLR4 transplantation and were given LPS (black) or saline (white). Fifteen TLR4+/+, 16 marrow TLR4−/−, 18 parenchymal TLR4−/−, and 16 TLR4−/− mice were used for this experiment. Data are represented as the mean ± SE. *P < 0.005 versus TLR4−/− mice given LPS; †P < 0.05 versus TLR4−/− mice given LPS; ‡P < 0.005 versus marrow TLR4−/− mice given LPS; §P = 0.001 versus parenchymal TLR4−/− mice given LPS.
Early and Late Plasma Cytokine Levels
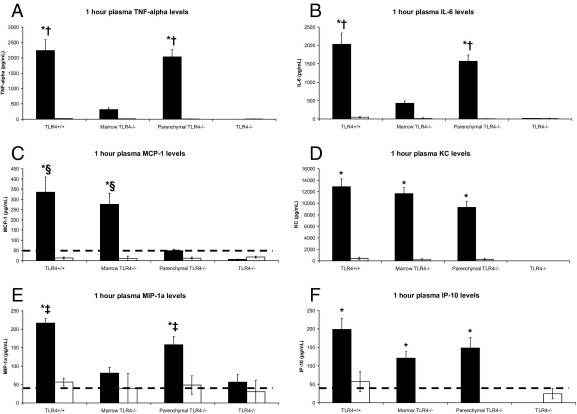
To further define the role of marrow-derived versus parenchymal cell in the development and progression of systemic inflammation, we examined early and late plasma cytokine levels to determine which cytokines, if any, corresponded to LPS-induced systemic inflammation (Figure 3 and Figure 4). In terms of early (1 hour after injection) plasma cytokine levels before manifestation, interferon-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-17, granulocyte-macrophage colony-stimulating factor, and vascular endothelial growth factor were all lower than the detection threshold of the assay (data not shown). There were no significant differences in early plasma levels of IL-10 or IL-12 among any of the groups (data not shown). Both TNF-α and IL-6 elevations corresponded to marrow TLR4 expression but were not associated with oncoming development of LPS-induced systemic inflammation (P < 0.0001, one-way analysis of variance; Figure 3, A and B). Early chemokine levels had a mixture of different expression patterns (Figure 3, C–F). Macrophage inflammatory protein (MIP)-1α followed the expression pattern seen in TNF-α and IL-6, whereas keratinocyte chemoattractant (KC) and interferon gamma-induced protein 10 (IP-10) plasma levels were elevated in TLR4+/+, marrow TLR4−/−, and parenchymal TLR4−/− mice given LPS compared with TLR4−/− mice given LPS (P < 0.0001, one-way analysis of variance; Figure 3, D–F). Of the cytokines and chemokines present, monocyte chemoattractant protein (MCP)-1 followed an expression pattern that best mirrored oncoming disease (P < 0.0001, one-way analysis of variance; Figure 3C). In fact, 1-hour MCP-1 plasma levels predicted murine systemic inflammation at 18 hours (Table 1), regardless of their transplant phenotype, with a sensitivity of 100% (95% CI, 84% to 100%), a specificity of 96% (95% CI, 77% to 100%), and an area under the receiver operating characteristic curve of 0.995 (cutoff, 90 pg/mL).
Figure 3.
Plasma levels at 1 hour after injection of TNF-α (A), IL-6 (B), MCP-1 (C), KC (D), MIP-1α (E), and IP-10 (F) for four groups of mice that underwent TLR4 transplantation and were given LPS (black) or saline (white). Fifteen TLR4+/+, 16 marrow TLR4−/−, 18 parenchymal TLR4−/−, and 16 TLR4−/− mice were used for this experiment. Data are represented as the mean ± SE. The dotted line indicates the minimal-detectable threshold for the assay. *P < 0.005 versus TLR4−/− mice given LPS; †P ≤ 0.001 versus marrow TLR4−/− mice given LPS; ‡P < 0.05 versus marrow TLR4−/− mice given LPS; §P < 0.005 versus parenchymal TLR4−/− mice given LPS.
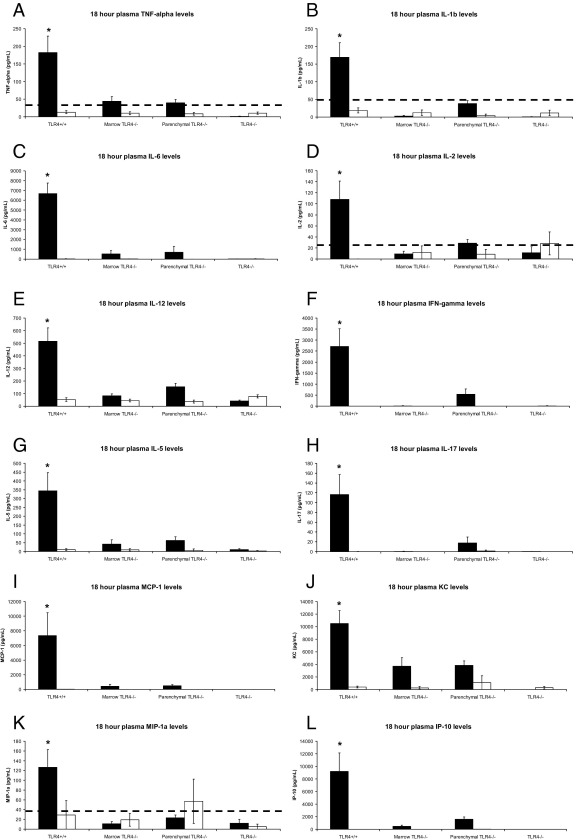
Figure 4.
Plasma levels at 18 hours after injection of TNF-α (A), IL-1β (B), IL-6 (C), IL-2 (D), IL-12 (E), interferon (IFN)-γ (F), IL-5 (G), IL-17 (H), MCP-1 (I), KC (J), MIP-1α (K), and IP-10 (L) for four groups of mice that underwent TLR4 transplantation and were given LPS (black) or saline (white). Fifteen TLR4+/+, 16 marrow TLR4−/−, 18 parenchymal TLR4−/−, and 16 TLR4−/− mice were used for this experiment. Data are represented as the mean ± SE. The dotted line indicates the minimal-detectable threshold for the assay. *P ≤ 0.005 versus marrow TLR4−/−, parenchymal TLR4−/−, and TLR4−/− mice given LPS.
Late (18 hours after injection) plasma cytokine and chemokine levels followed a consistent expression pattern (Figure 4). IL-1α, IL-4, and granulocyte-macrophage colony-stimulating factor plasma levels remained lower than the detection threshold of the assay (data not shown). There was no significant difference in IL-10 plasma levels among any of the groups (data not shown). TNF-α, IL-1β, IL-6, IL-2, IL-12, interferon-γ, IL-5, IL-17, and vascular endothelial growth factor all demonstrated the highest plasma levels in TLR4+/+ mice, with lower or nonexistent levels in the remaining mice that underwent transplantation and were given LPS (P < 0.0005, one-way analysis of variance; Figure 4, A–H; vascular endothelial growth factor data not shown). Plasma chemokine levels (MCP-1, KC, MIP-1α, and IP-10) also reflected this expression pattern among the groups given LPS (P < 0.001, one-way analysis of variance; Figure 4, I–L).
Discussion
These data suggest that parenchymal cell activation via TLR4 drives mortality from murine LPS-induced systemic inflammation (Table 2). At most, marrow-derived cell activation via TLR4 may play a role in accelerating LPS-induced systemic inflammation. These overall findings seem consistent with previous observations of isolated organ systems using chimeric TLR4 mice, in that parenchymal TLR4 drives both neutrophilic sequestering into the lungs during LPS-induced inflammation and leukocyte recruitment to the brain after intracranial LPS injection.12,15 Interestingly, parenchymal TLR4 expression did not cause mortality in a Gram-negative infection model (E. coli peritonitis). This could be because of the multifaceted host responses that compose an effective systemic response to an active infection compared with toxin-induced systemic inflammation.17
Table 2.
Summary of Experimental Results for LPS-Induced Systemic Inflammation in Mice that Underwent TLR4 Transplantation
| Variable | TLR4+/+ mice | Marrow TLR4−/− mice | Parenchymal TLR4−/− mice | TLR4−/− mice |
|---|---|---|---|---|
| Systemic inflammation and mortality | X | X | ||
| Early cytokines | ||||
| TNF-α, IL-6, MIP-1α | X | X | ||
| KC, IP-10 | X | X | X | |
| MCP-1 | X | X | ||
| Early neutropenia | X | X | ||
| Weight loss | X | X | X | |
| Late cytokines | X | |||
| Late neutrophilia | X | X | X | |
| Organ MPO | ||||
| Lung, kidney | X | X | ||
| Liver | X | X | X |
The blank spaces in this table (e.g., under TLR4-/- mice column) indicate no difference compared with saline control mice.
X, differences compared with saline control mice.
Circulating and tissue-bound neutrophils are believed to be significant contributors to tissue damage during the systemic inflammatory response. We found that early neutropenia is related to marrow-derived cell TLR4 expression, suggesting that TLR4 activation of these cells instead induces early neutrophilic margination and extravasation (Figure 2A). Later in the disease process, TLR4 activation of parenchymal and/or marrow-derived cells leads to increased bone marrow release of neutrophils (Figure 2B). In terms of end-organ neutrophilic activity (as assessed by MPO content), lung and kidney neutrophilic activity levels were highest in the TLR4+/+ and marrow TLR4−/− mice given LPS and had lower elevations in the parenchymal TLR4−/− mice given LPS (Figure 2, C and D). These findings appear more consistent with the pattern of LPS-induced murine systemic inflammation observed in the TLR4+/+ and marrow TLR4−/− mice. This pattern in lung and kidney MPO is consistent with previous research12,15 involving chimeric TLR4 mice, which suggested that neutrophil rolling and margination in the brain and lung in response to LPS were solely driven by parenchymal TLR4. However, the differences between TLR4+/+, marrow TLR4−/−, and parenchymal TLR4−/− organ MPO content are not as striking as the differences in LPS-induced murine systemic inflammation, mortality, and blood neutrophil responses (Table 2 and Figures 1 and 2). Based on these considerations, we suggest that it is likely that activation of either cellular compartment via TLR4 helps drive the entry and/or activation of neutrophils in these organs, but neutrophil activity's relationship to the actual manifestations of systemic inflammation may be far more complicated. This pattern of MPO content and TLR4 expression in any cellular compartment is seen more robustly in the liver. This starker effect may be because of intrinsic differences in liver architecture, where capillary endothelial structure is fenestrated, allowing easier organ access from the vasculature. Neutrophilic activation could then be driven by blood neutrophil responses (late neutrophilia) as much as end-organ TLR4 activation.
Given the known functions of cytokines and chemokines in the systemic inflammatory response, we next explored whether these mediators were connected to the pattern of LPS-induced systemic inflammation and subsequent mortality. The nearly complete disconnect we observed between early plasma cytokine levels and signs of LPS-induced systemic inflammation is surprising. These data indicate that most early cytokine production is marrow-derived cell in origin. This is consistent with previously described sources of these mediators. However, classic pro-inflammatory acute-phase cytokines (TNF-α and IL-6) drive hepatic acute-phase protein synthesis, endothelial cell activation, leukocyte extravasation, and shock, consistent with Koch's postulates.18 Yet, we observed high plasma levels of these mediators in one group of chimeric TLR4 mice (parenchymal TLR4−/−) with intact cytokine signaling pathways that did not develop signs of systemic inflammation and much lower plasma levels of these mediators in another group of chimeric TLR4 mice (marrow TLR4−/−) that did develop signs of systemic inflammation (Table 1 and Figure 3). Given the initial interest in these pro-inflammatory cytokines based on models such as LPS-induced systemic inflammation, our results may help to explain their failure as diagnostic and therapeutic targets in the clinic because their role in a classic model of systemic inflammation may not be as crucial as once thought.19–21 Further studies are needed to define mechanisms by which TLR4 signaling within parenchymal cells drives the actual manifestations of systemic inflammation.
The one early plasma cytokine found to have clear prognostic value was MCP-1 (chemokine ligand 2). MCP-1 is a chemokine that attracts monocytes, mast cells, and basophils to the sites of inflammation and can be produced by both immune and nonimmune cells.22–24 Plasma MCP-1 has been elevated in response to LPS in mice, baboons, and humans, although the nature of its role in this systemic inflammatory response is unclear.25,26 Previous reports27,28 of MCP-1 blockade in murine LPS-induced systemic inflammation have demonstrated both protective and detrimental effects, leading to no definitive conclusion. Although the studies presented herein cannot yet address MCP-1's exact role (neutral, beneficial, or harmful), it is clear that its role in the development of LPS-induced systemic inflammation and subsequent mortality may be unique compared with other inflammatory mediators and should be further investigated. It is well established that MCP-1 cannot initiate a systemic inflammatory response when introduced into mice, suggesting a more indirect role in this disease process.29–32 Given the morbidity and mortality associated with systemic inflammation in many disease processes, the value of a plasma molecule predictive of oncoming systemic inflammation clinically would be immense, especially in those situations in which the development of systemic inflammation occurs only in a subset of patients, regardless of its exact function in the process.10,33,34
Even late in LPS-induced systemic inflammation, plasma cytokine profiles continue to be disassociated with present status (Table 1 and Figure 3). Globally, plasma cytokines in the TLR4+/+ mice seem to show an additive or synergistic relationship between marrow-derived cell and parenchymal cell activation during LPS-induced systemic inflammation. However, late plasma cytokine levels (TNF-α, IL-1β, IL-6, IL-5, IL-10, MCP-1, KC, and MIP-1α) remained indistinguishable (or undetectable) in the two chimeric TLR4 mouse groups (marrow TLR4−/− and parenchymal TLR4−/−), even though these two groups have fundamentally different responses to LPS. What this may suggest is that late in the systemic inflammatory disease process, plasma cytokines and chemokines may drive a more rapid course for systemic inflammation, as suggested in Figure 1.
In summary, our findings indicate that expression of TLR4 by parenchymal cells is primarily responsible for the development of LPS-induced systemic inflammation and subsequent mortality. In this model, both early and late blood neutrophil responses and plasma cytokine levels do not predict the development of systemic inflammation, even though exogenous administration of cytokines, such as TNF-α in wild-type mice (capable of signaling via TLR4 in both parenchymal and bone marrow–derived cells), can produce systemic inflammation via NF-κB activation.35
Our study has several limitations that need to be addressed in future studies. First, our definition of systemic inflammation depends primarily on clinical observation. Although the onset of systemic inflammation was associated with mortality, the timing of systemic inflammation onset could be more precise through regular assessment of heart rate, respiratory rate, and temperature. Although TLR4 signaling in parenchymal cells drives the development of LPS-induced systemic inflammation, our data suggest that marrow-derived cell activation via TLR4 may accelerate or exacerbate an already initiated systemic inflammatory process. MCP-1 was unique in its expression pattern early and was the only chemokine to predict the onset of LPS-induced systemic inflammation with high accuracy. Given that exogenous administration of MCP-1 does not produce systemic inflammation in experimental animals, the mechanistic role of MCP-1 in this process awaits further clarification.29–32 The parenchymal cell source of MCP-1 has not yet been defined. These studies are important because existing studies of the role of MCP-1 in preventing or exacerbating host systemic inflammatory responses to LPS are contradictory. Finally, our experimental model, which segregates TLR4 activation in parenchymal cells and bone marrow–derived cells, may be regarded as artificial, in that TLR4 signaling in both compartments is intact during human systemic inflammation. Nevertheless, this model may shed mechanistic insights related to TLR4 signaling in systemic inflammation; further studies with this model may help us understand why plasma chemokine levels often fail to predict outcome during systemic inflammation processes.
Acknowledgments
We thank Cherish Grabau for secretarial assistance and Marshall Behrens for technical assistance with the Luminex plasma cytokine assays.
Footnotes
Supported by NIH grants F30 DK084671, UL1 RR024150, P01 HL85307, P01 AI53733, and R01 HL55552.
Disclosure: The content herein is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Bhatia M. Acute pancreatitis as a model of SIRS. Front Biosci. 2009;14:2042–2050. doi: 10.2741/3362. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia M., He M., Zhang H., Moochhala S. Sepsis as a model of SIRS. Front Biosci. 2009;14:4703–4711. doi: 10.2741/3561. [DOI] [PubMed] [Google Scholar]
- 3.Dahiya P. Burns as a model of SIRS. Front Biosci. 2009;14:4962–4967. doi: 10.2741/3580. [DOI] [PubMed] [Google Scholar]
- 4.Thomas L. The physiological disturbances produced by endotoxins. Annu Rev Physiol. 1954;16:467–490. doi: 10.1146/annurev.ph.16.030154.002343. [DOI] [PubMed] [Google Scholar]
- 5.Poltorak A., He X., Smirnova I., Liu M.Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., Freudenberg M., Ricciardi-Castagnoli P., Layton B., Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi S.T., Lariviere L., Leveque G., Clermont S., Moore K.J., Gros P., Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barsness K.A., Arcaroli J., Harken A.H., Abraham E., Banerjee A., Reznikov L., McIntyre R.C. Hemorrhage-induced acute lung injury is TLR-4 dependent. Am J Physiol Regul Integr Comp Physiol. 2004;287:R592–R599. doi: 10.1152/ajpregu.00412.2003. [DOI] [PubMed] [Google Scholar]
- 8.Levy R.M., Prince J.M., Yang R., Mollen K.P., Liao H., Watson G.A., Fink M.P., Vodovotz Y., Billiar T.R. Systemic inflammation and remote organ damage following bilateral femur fracture requires Toll-like receptor 4. Am J Physiol Regul Integr Comp Physiol. 2006;291:R970–R976. doi: 10.1152/ajpregu.00793.2005. [DOI] [PubMed] [Google Scholar]
- 9.Breslin J.W., Wu M.H., Guo M., Reynoso R., Yuan S.Y. Toll-like receptor 4 contributes to microvascular inflammation and barrier dysfunction in thermal injury. Shock. 2008;29:349–355. doi: 10.1097/shk.0b013e3181454975. [DOI] [PubMed] [Google Scholar]
- 10.Beutler B., Rietschel E.T. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol. 2003;3:169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- 11.Zarember K.A., Godowski P.J. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 12.Andonegui G., Bonder C.S., Green F., Mullaly S.C., Zbytnuik L., Raharjo E., Kubes P. Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs. J Clin Invest. 2003;111:1011–1020. doi: 10.1172/JCI16510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tavener S.A., Long E.M., Robbins S.M., McRae K.M., Van Remmen H., Kubes P. Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circ Res. 2004;95:700–707. doi: 10.1161/01.RES.0000144175.70140.8c. [DOI] [PubMed] [Google Scholar]
- 14.Zhou H., Andonegui G., Wong C.H., Kubes P. Role of endothelial TLR4 for neutrophil recruitment into central nervous system microvessels in systemic inflammation. J Immunol. 2009;183:5244–5250. doi: 10.4049/jimmunol.0901309. [DOI] [PubMed] [Google Scholar]
- 15.Zhou H., Lapointe B.M., Clark S.R., Zbytnuik L., Kubes P. A requirement for microglial TLR4 in leukocyte recruitment into brain in response to lipopolysaccharide. J Immunol. 2006;177:8103–8110. doi: 10.4049/jimmunol.177.11.8103. [DOI] [PubMed] [Google Scholar]
- 16.Spangrude G.J. Assessment of lymphocyte development in radiation bone marrow chimeras. Curr Protoc Immunol. 2008 doi: 10.1002/0471142735.im0406s81. Chap 4:Unit 4.6. [DOI] [PubMed] [Google Scholar]
- 17.Andonegui G., Zhou H., Bullard D., Kelly M.M., Mullaly S.C., McDonald B., Long E.M., Robbins S.M., Kubes P. Mice that exclusively express TLR4 on endothelial cells can efficiently clear a lethal systemic Gram-negative bacterial infection. J Clin Invest. 2009;119:1921–1930. doi: 10.1172/JCI36411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golsby R.A., Kindt T.J., Osborne B.A., Kuby J. Freeman & Co; New York, WH: 2003. Cytokines; pp. 276–298. [Google Scholar]
- 19.Pierrakos C., Vincent J.L. Sepsis biomarkers: a review. Crit Care. 2010;14:R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abraham E., Anzueto A., Gutierrez G., Tessler S., San Pedro G., Wunderink R., Dal Nogare A., Nasraway S., Berman S., Cooney R., Levy H., Baughman R., Rumbak M., Light R.B., Poole L., Allred R., Constant J., Pennington J., Porter S., NORASEPT II Study Group Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. Lancet. 1998;351:929–933. [PubMed] [Google Scholar]
- 21.Remick D.G., Ward P.A. Evaluation of endotoxin models for the study of sepsis. Shock. 2005;24(Suppl 1):7–11. doi: 10.1097/01.shk.0000191384.34066.85. [DOI] [PubMed] [Google Scholar]
- 22.Leonard E.J., Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1) Immunol Today. 1990;11:97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- 23.Bossink A.W., Paemen L., Jansen P.M., Hack C.E., Thijs L.G., Van Damme J. Plasma levels of the chemokines monocyte chemotactic proteins-1 and -2 are elevated in human sepsis. Blood. 1995;86:3841–3847. [PubMed] [Google Scholar]
- 24.Diaz Encarnacion M.M., Warner G.M., Cheng J., Gray C.E., Nath K.A., Grande J.P. n-3 Fatty acids block TNF-α-stimulated MCP-1 expression in rat mesangial cells. Am J Physiol Renal Physiol. 2011;300:F1142–F1151. doi: 10.1152/ajprenal.00064.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansen P.M., van Damme J., Put W., de Jong I.W., Taylor F.B., Jr, Hack C.E. Monocyte chemotactic protein 1 is released during lethal and sublethal bacteremia in baboons. J Infect Dis. 1995;171:1640–1642. doi: 10.1093/infdis/171.6.1640. [DOI] [PubMed] [Google Scholar]
- 26.Sylvester I., Suffredini A.F., Boujoukos A.J., Martich G.D., Danner R.L., Yoshimura T., Leonard E.J. Neutrophil attractant protein-1 and monocyte chemoattractant protein-1 in human serum: effects of intravenous lipopolysaccharide on free attractants, specific IgG autoantibodies and immune complexes. J Immunol. 1993;151:3292–3298. [PubMed] [Google Scholar]
- 27.Zisman D.A., Kunkel S.L., Strieter R.M., Tsai W.C., Bucknell K., Wilkowski J., Standiford T.J. MCP-1 protects mice in lethal endotoxemia. J Clin Invest. 1997;99:2832–2836. doi: 10.1172/JCI119475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramnath R.D., Ng S.W., Guglielmotti A., Bhatia M. Role of MCP-1 in endotoxemia and sepsis. Int Immunopharmacol. 2008;8:810–818. doi: 10.1016/j.intimp.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 29.Fuentes M.E., Durham S.K., Swerdel M.R., Lewin A.C., Barton D.S., Megill J.R., Bravo R., Lira S.A. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J Immunol. 1995;155:5769–5776. [PubMed] [Google Scholar]
- 30.Grewal I.S., Rutledge B.J., Fiorillo J.A., Gu L., Gladue R.P., Flavell R.A., Rollins B.J. Transgenic monocyte chemoattractant protein-1 (MCP-1) in pancreatic islets produces monocyte-rich insulitis without diabetes: abrogation by a second transgene expressing systemic MCP-1. J Immunol. 1997;159:401–408. [PubMed] [Google Scholar]
- 31.Gunn M.D., Nelken N.A., Liao X., Williams L.T. Monocyte chemoattractant protein-1 is sufficient for the chemotaxis of monocytes and lymphocytes in transgenic mice but requires an additional stimulus for inflammatory activation. J Immunol. 1997;158:376–383. [PubMed] [Google Scholar]
- 32.Nakamura K., Williams I.R., Kupper T.S. Keratinocyte-derived monocyte chemoattractant protein 1 (MCP-1): analysis in a transgenic model demonstrates MCP-1 can recruit dendritic and Langerhans cells to skin. J Invest Dermatol. 1995;105:635–643. doi: 10.1111/1523-1747.ep12324061. [DOI] [PubMed] [Google Scholar]
- 33.Seifert J.K., Stewart G.J., Hewitt P.M., Bolton E.J., Junginger T., Morris D.L. Interleukin-6 and tumor necrosis factor-alpha levels following hepatic cryotherapy: association with volume and duration of freezing. World J Surg. 1999;23:1019–1026. doi: 10.1007/s002689900617. [DOI] [PubMed] [Google Scholar]
- 34.Hill G.E. The inflammatory response to cardiopulmonary bypass. Int Anesthesiol Clin. 1996;34:95–108. doi: 10.1097/00004311-199603420-00009. [DOI] [PubMed] [Google Scholar]
- 35.Tracey K.J., Beutler B., Lowry S.F., Merryweather J., Wolpe S., Milsark I.W., Hariri R.J., Fahey T.J., 3rd, Zentella A., Albert J.D., Shires G.T., Cerami A. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]