Summary
Background
Children with skeletal dysplasia (SD) often have pulmonary disease, which can be life threatening. In clinical practice, chest wall and formal respiratory function tests are difficult to perform owing to the small size and cooperation. The objective of this study was to demonstrate distinct thoracopulmonary function patterns in children with SD.
Methods
We conducted a retrospective study reviewing pulmonary function tests from 17 patients with the diagnosis of SD. Three subgroups were studied: Morquio syndrome (MS), metatropic-spondylocostal dysplasia (MSD), and unspecified skeletal dysplasias (SDU). Rib cage contribution to tidal volume excursions (%RC), phase angle (Phθ), phase relation during total breath (PhRTB), respiratory resistance (Rrs5-35 Hz), respiratory reactance (Xrs5-35 Hz), resonant frequency and their frequency-dependency were analyzed. Values were age-matched and height-matched to reference values of healthy subjects.
Results
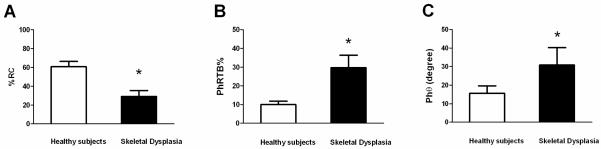
There was a decrease in %RC and an increase in PhRTB (p<0.05) in the SD group. %RC differed between subgroups [MS: 46.4 ± 1.8% SE, MSD: 18.4±2.6% SE, SDU: 27.5±5.2% SE (p< 0.05)]; Phθ was within reference values only in MS, which exhibited a decrease in Xrs at 5 Hz (p<0.05) and an increase in Rrs independent of the frequency. SDU showed a decrease in Xrs at 35 Hz (p<0.05), no differences were found in Rrs. In MS, a correlation was found between RC and Rrs at all frequencies (r=−0.98, p<0.01) and between Xrs5-10 and Phθ (r= −0.93, p<0.05).
Conclusions
Thoracoabdominal dysfunction was associated with altered chest wall reactance at high frequencies in a subgroup of SD patients with abnormal lung reactance and central airway involvement in MS.
Keywords: pulmonary function test, respiratory resistance, Morquio syndrome, young children, respiratory inductance plethysmography
Introduction
The clinical disorders of the thoracic cage affect the thoracic motion and modify the mechanical properties of the lung and chest wall. Restriction of chest wall reduces the total lung capacity and results in an increase in respiratory frequency and decreased tidal volume (VT).1 Children with skeletal dysplasias (SD) frequently have pulmonary disease due to multiple etiologies, including thoracic cage and craniofacial abnormalities predisposing to restrictive disease, airway obstruction, and central apnea.2 In clinical practice, formal respiratory function tests are difficult to achieve owing to the small size and poor patient cooperation. The impulse oscillation system (IOS) is a variant of the forced oscillation technique and a tool to investigate pulmonary disorders. Although this technique was developed in 1956 by Dubois as a forced oscillation technique (FOT), there has been recent commercial development of equipment and software to make these methods available for clinical application. In this regard, this non-invasive pulmonary function test may identify different respiratory patterns in children with SD and their significant pulmonary limitations, as it covers not only children between 2 and 6 years old, but also patients who have difficulties in performing conventional PFT’s (ie, FEV1, etc).3 The impulse oscillation system (IOS) is a variant of the forced oscillation technique and a tool to investigate pulmonary disorders. The method is rapid and non-invasive, allowing the evaluation of total respiratory impedance (Zrs) and providing values of its two components: total respiratory resistance (Rrs) and total respiratory reactance (Xrs).4 Thoracoabdominal motion analysis (TAM) is an analysis of the relative motion and contribution to tidal volume of the thoracic and abdominal compartments.5 In a previous study,6 Allen et al. demonstrated that changes in TAM correlated with changes in resistance and compliance in infants with airflow obstruction.
As far as we are aware, there are no published data about the functional assessment using these techniques in subjects with SD. Recognition of respiratory disease and early intervention improves their survival and quality of life.2 The favorable results that can be achieved with orthopedic and surgical treatment also confirm the need and importance of functional testing over time in order to prevent the development of respiratory insufficiency.
We hypothesized that children with SD would have distinct thoracopulmonary function patterns. Therefore, using non-invasive measurements as reported herein, we believe that this methodology provides valuable information for following the progression of disease stage, evaluation of therapeutic intervention efficacy and (Pre vs. Post) surgical interventions. Three SD subgroups are described herein: Morquio syndrome (MS), metatropic-spondylocostal dysplasia (MSD), and unspecified skeletal dysplasias (SDU).
Methods and Materials
We conducted a retrospective study in children with the diagnosis of SD between 2007 and 2008. The study included 17 patients: 9 females and 8 males with ages ranging from 2 to 20 years in age who had a routine clinical evaluation at the SD clinic and were tested using IOS and/or pneumotachography technique (PNT) and TAM techniques. The diagnosis of the type of SD was made according to orthopedic and genetic criteria. The study was reviewed and approved by the Institutional Review Board (IRB) and the informed consent was waived due to the retrospective nature of the data collection.
The patients were divided into three subgroups according to their type of SD: MS group consisted of patients with Morquio syndrome (n=4). This inherited metabolic disease leads to a reduction in chest wall compliance, reversible and irreversible airway obstruction, chronic obstructive lung disease, and obstructive sleep apnea.7 MSD group consisted of children with metatropic and spondylocostal dysplasias (n=5). These SD are frequently associated with severe restrictive lung disease.2 SDU subgroup included eight children (n=8), three with diastrophic dysplasia (DD), one with kniest displasia (KD), one with cartilage hair hipoplasia, two with spondylometaphyseal dysplasia, and one with epiphyseal dysplasia. Both DD and KD have been associated with restrictive lung disease, tracheomalacia, and bronchomalacia.
Data Collection
The pulmonary function device used for the oscillatory measurements was the Master Screen-IOS device (E. Jaeger; Höchberg, Germany). The method and equipment have been previously described.8,9 Briefly, small mechanical impulses are superimposed on the spontaneous breathing pattern. Through the phase relationship, the Zrs is partitioned into Rrs and Xrs. Rrs includes the airway, lung tissue, and chest wall resistance, whereas Xrs represent the balance of two (an elastic and an inertial) components, or the net effect of two opposite (a compliant and an inertial) components. For each impulse, 32 sample points were analyzed. Daily calibration using a calibration pump (3.0 ± 0.01 L SD, Jaeger; Höchberg, Germany) and a reference impedance of 0.2–2.5 kPa/l/s were performed. After a brief explanation, the children performed some practice trials. When the child was feeling comfortable, the study testing began. The subjects performed the test in sitting position breathing quietly for 15 to 30 seconds using an oval mouthpiece with the head in a neutral position, nose clips in place, and supporting both cheeks with help by the staff. The following parameters were measured: Rrs and Xrs at 5, 10, 15, 20, 25, 35Hz and resonant frequency (Fres) [ie, the frequency at which the reactance crosses zero]. Three replicate oscillatory values were used to calculate the mean and the coefficient of variation (CV) for Rrs5. Coherence value was used as a quality control parameter and the CV for Rrs5 was used as a reliability index. Measurements were discarded if the time-flow and volume pattern showed interruption of the oscillatory signal.
TAM was performed by means of respiratory inductance plethysmography using the SomnoStarPT Unit (Sensormedics, Yoba Linda, CA). The technique and equipment have been previously described by Allen et al.5,6 Briefly, the signals from the inductive bands around the rib cage (RC) and the abdomen (ABD) are treated mathematically as sine waves and the phase angle (Phθ) is then calculated. This and other TAM indices express the degree to which chest and ABD excursion are out of phase call thoracoabdominal asynchrony (TAA). For the recordings, the child was breathing quietly with two flexible self-adherent bands (RespiBands Plus, VIASYS, CA), the chest band was positioned around the rib cage under the arms right above the nipple line, and the abdominal band was placed around the abdomen at the umbilicus level. The size of the bands was chosen to fit comfortably, yet snug enough to obtain adequate recordings, any excess of band material was folded and pressed together using a plastic forceps (Devon, tyco/Healthcare) in each band to avoid any unintentional changed in position. Real-time raw signals and Konno-Mead loops were monitored during the test to ensure proper signal and adequate quality using RespiEvents software 5.2g (Nims Inc). Figure-of-eight loops were discarded. Phθ, phase relation during total breath (PhRTB) and the percentage of rib cage contribution to the tidal volume (%RC) were analyzed. For the %RC, an appropriate sized pneumotachometer was used along with a CO2SMO PFT monitor (CO2SMO, Respironics, Wallingford, CT) to measure actual tidal in milliliters. Before the IOS and TAM measurements were taken, airflow, volume data, flow-volume loops and oximetry signals using pneumotachography technique (PNT) were recorded using a pediatric monitoring system and a software package that interfaced with this system (Analysis Plus, Novametrix). Testing was performed in order to collect at least 10 uniform breaths (RIP and PNT) and the data were stored on a laptop for analysis, the breaths were collected from a long epoch (2–4 minutes). The data were later screened for uniformity and means (± SE) were calculated for each subject. The most significant features for selecting these lung function techniques are their effort-independence and simplicity in young children and patients who are not able to perform forces breathing maneuvers.3,8,10 Measured values of Rrs and Xrs were age-matched and height-matched compared to the reference values or reference equations of healthy subjects.3,8,11–13 Values of TAM were also compared.14–16 Dependent variables were measured and consisted of analysis of means ±SD or SE, normal score and Student t-test. Two-way analysis of variance, Pearson and Spearman correlation coefficients tests were used. The significance level was set at P < 0.05.
Results
RIP and Respiratory Profile
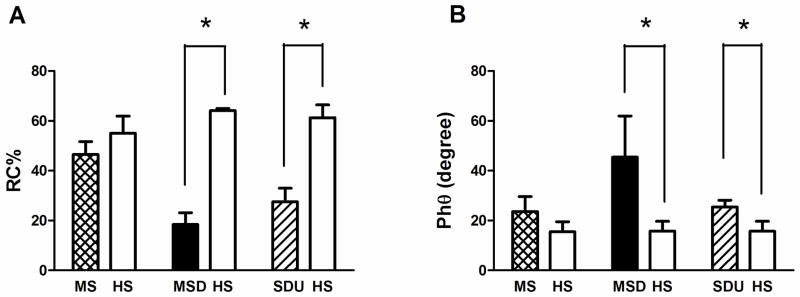
Mean values of basic respiratory profile for the entire SD group and subgroups are presented in Table 1. In addition, RIP parameters including mean (±SE) values are displayed in Table 2 for the entire SD group and subgroups. As an entire group, SD patients’ mean values of basic respiratory parameters were within the normal range except for values of respiratory rate (RR) which were greater than the normal range. Pulse oximetry (SpO2) and end tidal CO2 values were within normal ranges (SpO2 >95% and 35 torr <end tidal CO2 <45 torr). In addition, as an entire group, SD patients’ mean values of %RC were reduced, and mean PhRTB and Phθ values increased significantly (p<0.05) (Figure 1 and table 2). In the subgroups analysis, there were no differences with respect to age, height and weight. RR was higher in MSD subgroup, where values were abnormal in 4 (80%) subjects. Total mean values of minute ventilation (VE) and tidal volume VT were normal in each subgroup (MS, MSD and SDU) with no inter-subgroup differences. %RC differed between subgroups (p< 0.05), MSD demonstrated lower %RC where compared to the SDU subgroup %RC values. MS was the only subgroup where %RC values were within the normal range; Phθ as an index of the degree of thoracoabdominal asynchrony was above the reference values only in MSD and SDU subgroups (Figure 2), with no subgroup differences. No subgroup difference of PhRTB was found; however, all 3 subgroups demonstrated abnormal values.
Table 1.
Mean values ±SD of the basic and respiratory profile in an entire group and subgroups of patients with skeletal dysplasias.
| Parameter | Units | MS subgroup n=4 | MSD subgroup n=5 | SDU subgroup n=8 | SD group n=17 |
|---|---|---|---|---|---|
| Age | (yrs) | 13±6 | 6±4 | 7±4 | 8±5 |
| Sex | (M:F) | 2:2 | 3:2 | 4:4 | 9:8 |
| Height | (cm) | 119±19 | 90±19 | 90±19 | 95±20 |
| Weight | (Kg) | 27±12 | 16±7 | 19±11 | 20±10 |
| SpO2 | (%) | 97±1 | 96±1 | 97±0.9 | 97±1 |
| Respiratory Rate | (br/min) | 20±7 | 41±18 * | 23±6 | 27±14† |
| Tidal Volume | (ml/kg) | 9.7±3 | 7.8±4 | 8.9±4 | 8.7±4 |
| VE | ( L/m) | 4.8±1 | 4.5±2 | 4.7±3 | 4.7±2.5 |
| End Tidal CO2 | (torr) | 36±2 | 36±3 | 37± 3 | 37± 3 |
p<0.05 between subgroups;
values out of normal range.
MS: Morquio syndrome; MSD: Metatropic and spondylocostal dysplasias; SDU: Unspecified skeletal dysplasias. SD: Skeletal dysplasias; VE: Minute ventilation.
Table 2.
Mean values ±SE of the RIP parameters in an entire group and subgroups of patients with skeletal dysplasias.
| RIP Parameters | MS subgroup n=4 | MSD subgroup n=5 | SDU subgroup n=8 | SD group n=17 | |
|---|---|---|---|---|---|
| %RC | (%) | 46.4±1.8† | 18.4±2.6*† | 27.5±5.2* | 30.7±3.2* |
| PhRTB | (%) | 20.5±5* | 35.3±7* | 35.2±5* | 30.3±5.6* |
| Phθ | (degree) | 23.5±6 | 45.4±16* | 25.4± 2* | 31.4± 8* |
Values out of normal range;
p<0.05 differences between subgroups.
MS: Morquio syndrome; MSD: Metatropic and spondylocostal dysplasias; SDU: Unspecified skeletal dysplasias; SD: Skeletal dysplasias. %RC: rib cage contribution to the tidal volume excursions; PhRTB: phase relation during total breath; Phθ: phase angle.
Figure 1.
Rib cage contribution to the tidal volume excursions (A); phase relation during total breath (B); and phase angle (C) in subjects with skeletal dysplasia vs. reference values of healthy subjects. *p<0.05. Data expressed as mean ± SE; %RC= percentage of rib cage contribution to the tidal volume excursions; PhRTB= phase relation during total breath; and Phθ= phase angle.
Figure 2.
Rib cage contribution to the tidal volume excursions (A); phase angle (B); and phase relation during total breath (C) trends between subgroups. MS: Morquio syndrome; MSD: Metatropic and spondylocostal; SDU: Unspecified skeletal dysplasias; HS: Reference values of healthy subjects.*p< 0.05 vs. HS. # p<0.05 differences between subgroups. Data expressed as mean ± SE; %RC= Rib cage contribution to the tidal volume excursions; PhRTB= phase relation during total breath; and Phθ= phase angle.
Impulse Oscillometry
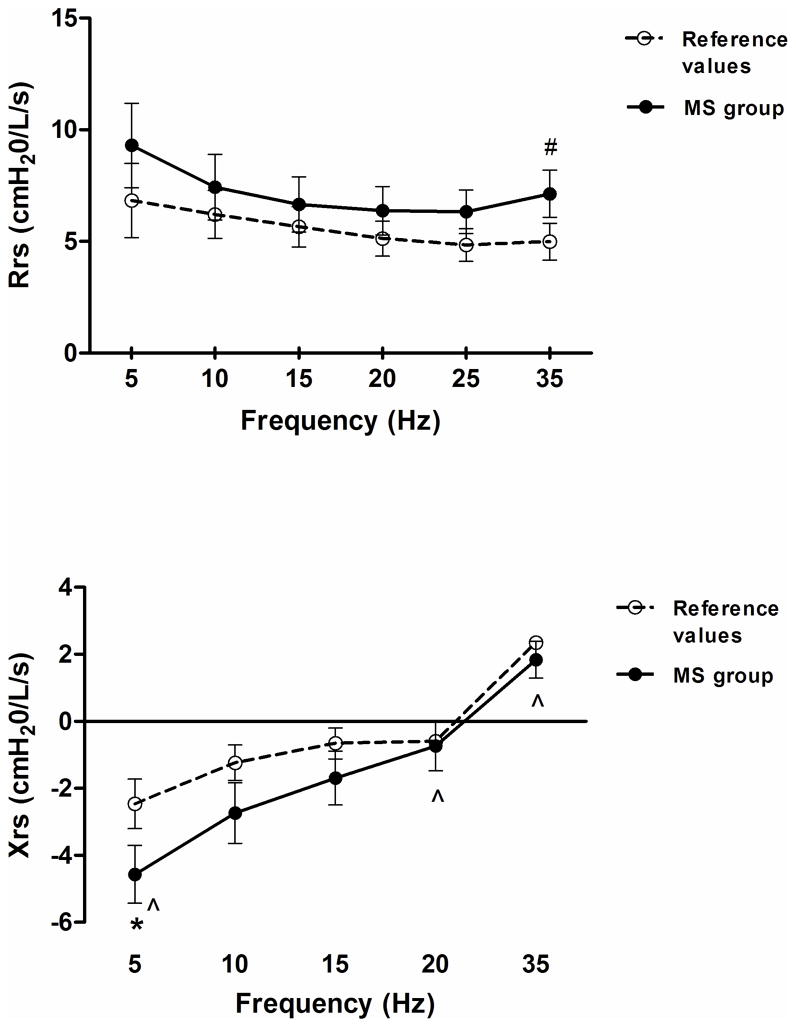
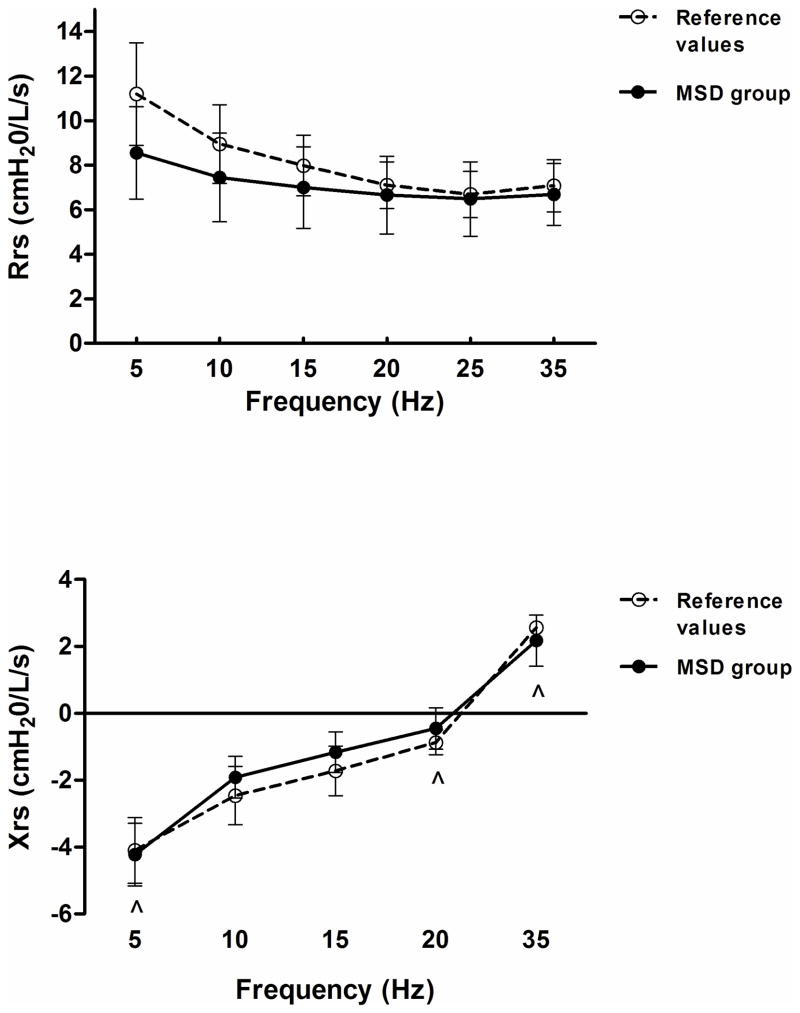
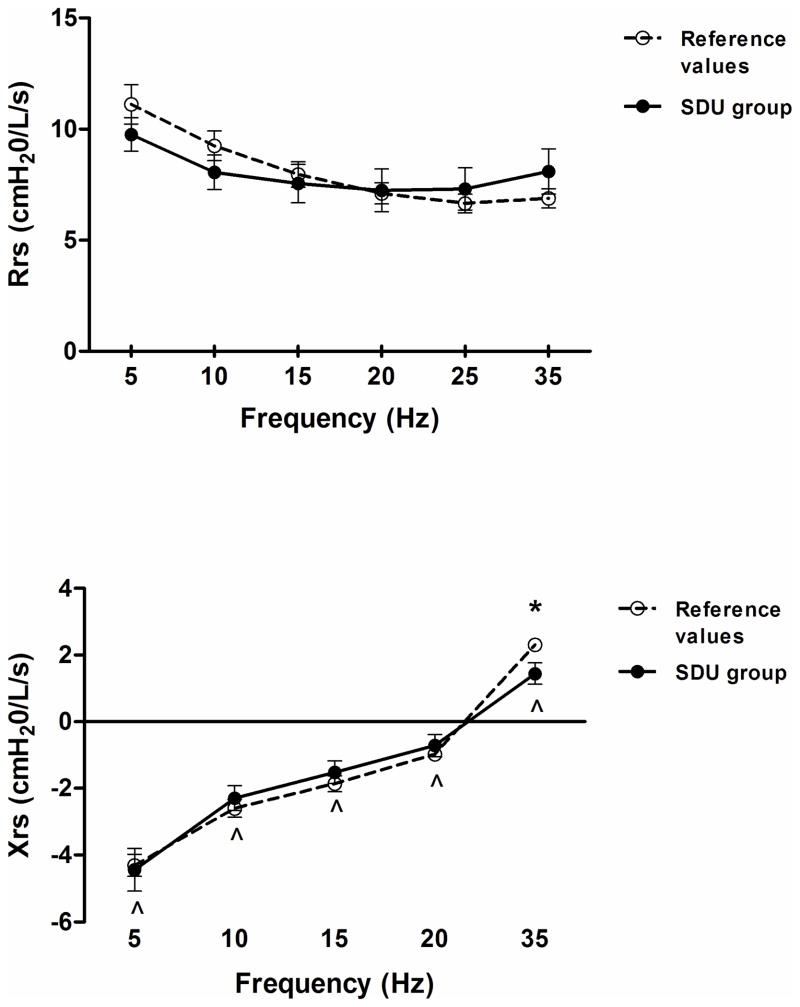
The results demonstrate that the MS group exhibited a decrease in Xrs5 (p<0.05) and an increase in Rrs (p=0.05), independent of the frequency (Figure 3). Also, the MSD group exhibited Xrs values within predicted mean values despite a significant frequency effect (p< 0.05), the Rrs values were not different compared to control data and demonstrated no frequency dependence (Figure 4). The SDU group showed a decrease in Xrs35 (p<0.05), and no differences were found in Rrs as compared to control data (Figure 5). Mean values of Fres were slightly high in the MS group (>10 Hz, p<0.05) and SDU groups (>20Hz) versus MSD (17.9±3.4) based on height with no intergroup differences (p=0.53).
Figure 3.
Total respiratory resistance (Rrs) [top] and Reactance (Xrs) [bottom] trends as a function of oscillatory frequency (5–35 Hz) in MS subgroup. Discontinued line (- - -) represent normal ranges adjusted for age and height. Data expressed as a mean ± SE; *p< 0.05, #p=0.05 vs reference values of healthy subjects; ^p< 0.05 as a function of frequency. MS: Morquio syndrome.
Figure 4.
Total respiratory resistance (Rrs) [top] and Reactance (Xrs) [bottom] trends as a function of oscillatory frequency (5–35 Hz) in MSD subgroup. Discontinued line (- - -) represent normal ranges adjusted for age and height. Data expressed as a mean ± SE; *p< 0.05 vs reference values of healthy subjects; ^p< 0.05 as a function of frequency. MSD: Metatropic and spondylocostal dysplasias.
Figure 5.
Total respiratory resistance (Rrs) [top] and Reactance (Xrs) [bottom] trends as a function of oscillatory frequency (5–35 Hz) in SDU subgroup. Discontinued line (- - -) represent normal ranges adjusted for age and height. Data expressed as a mean ± SE; *p< 0.05 vs reference values of healthy subjects; ^p< 0.05 as a function of frequency. SDU: Unspecified skeletal dysplasias.
Correlation between RIP and IOS
In the MS subgroup, a strong correlation was found between %RC and Rrs at all frequencies (r=−0.98, p<0.01) and between %RC and Xrs20 (r=0.99). PhRTB correlate with Rrs5 (r=0.95, p<0.05) and with Xrs10-15 (r= −0.95/r=−0.96, p<0.05). In addition, there was a significant relationship between Xrs5-10 and Phθ (Table 2). No such correlations were found for the MSD and SDU groups.
Discussion
In this group of children with SD, we have documented three distinct patterns of thoracopulmonary dysfunction.
RIP Findings in MS Subgroup
The patients with MS are the least dysfunctional subgroup in their RC and ABD mechanical interaction. This finding is based on the abnormal percentage of PhRTB (percentage of the total breath duration for which the rib cage and abdomen are moving in opposite directions) only, whereas %RC and Phθ compared to controls are normal in contrast to the other subgroups. Thus, we speculate that PhRTB may be an early indicator of pulmonary dysfunction, since %RC and Phθ were normal. In this regard, Phθ has been associated with an increase in more prominent respiratory muscle load and/or combined airway obstruction which were not demonstrated to a high degree in our patient population.17,18
Based on the literature, chest strapping in healthy subjects can cause an increase of RR and decrease in VT, but VE is not significantly altered.1 However, low VE has been correlated with the severity of the thoracic deformity and its reduction is secondary to a reduction in ventilatory efficiency (maintenance of CO2 removal for a given VE), as demonstrated by Pecorelli.19 In the present study, VE was in the normal range except for one patient; however, mean values of RR and SpO2 remained within the normal range for all subjects.
RIP Findings in MSD Subgroup
In contrast to SD as a whole, the MSD subgroup was the most affected with a severe chest restrictive pattern as expressed by the low %RC and a high degree of TAA (altered Phθ and PhRTB). Mean RR was above reference values and significantly increased when compared to the MS and SDU subgroups; overall VE was within normal range based on 5–6 L/m reference values (p=0.3)20 and are in agreement with previous reports.1,21 The end tidal CO2 normal mean values and the lack of difference with the other subgroups maybe explained by the finding that 3 of 6 patients (60%) in this group had uneven emptying in the CO2 waveforms. The increased RR may be secondary to the chest restrictive pattern of this group as a compensatory mechanism. As mentioned, this group had a stiffer chest as the RC expansion is low relative to ABD excursion; thus, the increased in both Phθ and PhRTB may be secondary to this elevated restrictive respiratory load.
RIP Findings in SDU Subgroup
In the SDU subgroup that includes DD and KD patients, mean values of Phθ and PhRTB were above the reference values (similar to MSD subgroup). These findings are consistent with the literature, in that patients with DD demonstrated normal lung volumes but have abnormally low values of peak expiratory and inspiratory flow associated with decreased movement of the chest wall/or function of the respiratory muscles. Furthermore, it has been reported that KD patients (part of our SDU subgroup) have restrictive disorders.2,22
IOS Findings in MS Subgroup
Differences in average chest and respiratory restriction between groups are reflected in the Rrs and Xrs values as a function of impulse frequency. In MS subgroup, values of Rrs were increased at all frequencies; while clinically important, this difference reached near significance (p= 0.05) only at 35 Hz. Mean values of Xrs at 5Hzdecreased significantly as compared to controls. Xrs5 reflects a reduction of elasticity or elastic recoil in the peripheral lung. “Peripheral” in oscillometry is defined from the functional point of view (peripheral impairment is indicated by changes in Xrs5). Thus, peripheral restriction is an impairment of the peripheral lung represented by Xrs5 in absence of a peripheral obstruction by other means (in our case, by the Rrs IOS pattern and RIP measurements).
The results demonstrate that the MS subgroup exhibited a decrease in Xrs5 (p<0.05) and an increase in Rrs, independent of the frequency. There are some factors which may contribute to the IOS patterns in the MS group. First, in Morquio patients, as in kyphoscoliosis, many properties of airways, lung and chest wall are altered. In patients with Morquio syndrome, a lysosomal storage disease, the metabolic defects lead to a cellular accumulation of mucopolysaccharides causing skeletal, respiratory and neurologic sequelae. The respiratory abnormalities observed and reported in the literature are therefore: 1) Significant restrictive lung disease due to the bony thoracic and vertebral changes, abnormal lung or/and chest wall mechanics, 2) Deposition of mucopolysaccharides throughout the tracheobronchial tree can cause obstructive lung disease or if the deposit is in the lung parenchyma, worsening lung compliance and restrictive lung disease can be seen; and 3) the infiltration of upper airways structures can lead to severe upper airway obstruction.2,23,24 The Rrs pattern is likely to be related to the alteration in central/proximal airway dysfunction; as the site of alteration is inferred from the Rrs values as a function of impulse frequency 25–33
IOS Findings in MSD Subgroup
The MSD subgroup exhibited Xrs values within predicted mean values despite a significant frequency effect (p< 0.05). This result was in contrast to Rrs values which demonstrated no frequency dependence. In this MSD subgroup values of Rrs and Xrs where within the normal range.34 The trend in frequency dependence of Xrs may be explained by pulmonary inhomogeneities and central airway shunt.3
IOS Findings in SDU Subgroup
The SDU subgroup showed a decrease in Xrs35 (p<0.05), and no difference in Rrs as compared to control data. The marked decrease in Xrs at 35 Hz, which may be attributed to the low ratio of chest wall contribution to the inertive properties of the respiratory system. However, we have confirmed low %RC contribution by TAM which is more evident in the high frequency imaginary parts of the impedance spectra.
Finally, Fres was high in the MS subgroup (>10 Hz, p<0.05) and SDU subgroup (>20Hz) versus MSD (17.9±3.4) based on height with no intergroup differences (p=0.53). To our knowledge, there are no reports in the literature on chest wall resistance and Xrs in the types of SD characterized herein.
Correlation of RIP and IOS findings
Correlation analysis showed that PhRTB and %RC are dependent on the degree of chest movement and highly interrelated above 10 Hz in Table 2. Phθ, PhRTB, and %RC are important markers of TAA, which may explain the significant correlation between some of these markers and the Rrs5. Also it should be noted, that %RC correlated with the overall Rrs, but the %RC values in this group, even though low, were within predicted values. Furthermore, although Phθ is not the major determinant in this group with regard to chest/ABD motion status, previous studies have shown that Phθ correlates with changes in compliance.6 In our study, Phθ correlated with Xrs at 5–10 Hz frequencies, and these findings in Xrs at 5 Hz have been associated to peripheral restriction due to a higher percentage of contribution from the lung at this frequency and below. As the Xrs at 5–10 Hz decreased and shifted towards lower values, the mean values of Phθ tended to increase. The parameter Xrs5 reflects a reduction of elasticity or elastic recoil in the peripheral lung. Accordingly, it is likely that changes in lung mechanical properties in Morquio patients play an important role beyond the chest wall and airway component. Perhaps this finding is associated with the reported intraparechymal mucopolysaccharidose deposition which worsens lung compliance and restrictive lung disease in Morquio patients. The lack of correlation between TAM and IOS in MSD and SDU may be due to a no/or small alterations in RIP and IOS parameters respectively in these subgroups.
In conclusion, children with skeletal dysplasias demonstrated distinct thoracopulmonary function patterns using non-invasive tidal breathing techniques. Thoracoabdominal dysfunction is associated with altered chest wall reactance as a function of high impulse frequencies. Patients with Morquio syndrome showed abnormal lung reactance perhaps related to restrictive lung disease. Resistance findings to a lesser degree in this group also support clinically reported airway disease. These non-invasive pulmonary tests may have utility in tracking lung, airway and chest wall properties in skeletal dysplasia children who cannot perform standard pulmonary function tests. Further clinical studies are needed to support the routine clinical use of these diagnostic tests during orthopedic evaluations and for the functional follow-up after orthopedic procedures involving the rib cage and abdomen.
Table 3.
Correlation coefficients between total respiratory resistance, respiratory reactance and thoracoabdominal motion indices of asynchrony in patients with mucopolysaccharidosis type IVA (Morquio syndrome).
| Parameter | %RC (%) | PhRTB (%) | Phθ (o) | |
|---|---|---|---|---|
| Rrs5 | cmH2O/L/s | −0.99 ** | 0.95 * | 0.71 |
| Rrs10 | cmH2O/L/s | −0.96 ** | 0.89 | 0.61 |
| Rrs15 | cmH2O/L/s | −0.99 ** | 0.83 | 0.50 |
| Rrs20 | cmH2O/L/s | −0.99 ** | 0.84 | 0.51 |
| Rrs25 | cmH2O/L/s | −0.99 ** | 0.85 | 0.53 |
| Rrs35 | cmH2O/L/s | −0.99 ** | 0.82 | 0.43 |
| Xrs5 | cmH2O/L/s | 0.80 | −0.89 | −0.93 * |
| Xrs10 | cmH2O/L/s | 0.80 | −0.95 * | −0.93 * |
| Xrs15 | cmH2O/L/s | 0.80 | −0.96 * | −0.82 |
| Xrs20 | cmH2O/L/s | 0.99 ** | −0.92 | −0.73 |
| Xrs35 | cmH2O/L/s | 0.80 | −0.90 | −0.80 |
p<0.01;
p< 0.05.
Rrs: respiratory resistance; Xrs: respiratory reactance; f: frequency; %RC: rib cage contribution to the tidal volume excursions; PhRTB: phase relation during total breath; Phθ: phase angle.
Acknowledgments
The authors thank Barbara Gray, CPM, for her administrative contribution and RuthAnn Deveney for her medical editorial assistance.
This study was supported in part by a grant from Nemours Foundation and NIH COBRE [Grant 1 P20 RR20173] (The Center for Pediatric Research). Contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
This work performed at the Nemours Research Lung Center and Skeletal Dysplasia Clinic, Alfred I. duPont Hospital for Children, Wilmington, DE 19803.
Financial/no financial disclosures: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript. The authors have reported that no significant conflict of interest exist with any companies/organizations whose products or services may be discussed in this article.
This article was presented in part at the 2008 International Conference of American Thoracic Society, in Toronto, Ontario, Canada, during May 16–21, 2008. Part of the work also (abstract supplement) was included as a part of a poster presentation at the 2008 CHEST annual meeting of ACCP on October 29, 2008.
References
- 1.Caro CG, Butler J, Dubois AB. Some effects of restriction of chest cage expansion on pulmonary function in man: an experimental study. J Clin Invest. 1960;39:573–583. doi: 10.1172/JCI104070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mogayzel PJ, Marcus CL. Skeletal dysplasias and their effect on the respiratory system. Paediatr Respir Rev. 2001;2(4):365–371. doi: 10.1053/prrv.2001.0173. [DOI] [PubMed] [Google Scholar]
- 3.Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, Bisgaard H, Davis GM, Ducharme FM, Eigen H, Gappa M, Gaultier C, Gustafsson PM, Hall GL, Hantos Z, Healy MJ, Jones MH, Klug B, Lodrup Carlsen KC, McKenzie SA, Marchal F, Mayer OH, Merkus PJ, Morris MG, Oostveen E, Pillow JJ, Seddon PC, Silverman M, Sly PD, Stocks J, Tepper RS, Vilozni D, Wilson NM. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175(12):1304–1345. doi: 10.1164/rccm.200605-642ST. [DOI] [PubMed] [Google Scholar]
- 4.Nagels J, Landser FJ, van der Linden L, Clement J, Van de Woestijne KP. Mechanical properties of lungs and chest wall during spontaneous breathing. J Appl Physiol. 1980;49(3):408–416. doi: 10.1152/jappl.1980.49.3.408. [DOI] [PubMed] [Google Scholar]
- 5.Allen JL, Wolfson MR, McDowell K, Shaffer TH. Thoracoabdominal asynchrony in infants with airflow obstruction. Am Rev Respir Dis. 1990;141(2):337–342. doi: 10.1164/ajrccm/141.2.337. [DOI] [PubMed] [Google Scholar]
- 6.Allen JL, Greenspan JS, Deoras KS, Keklikian E, Wolfson MR, Shaffer TH. Interaction between chest wall motion and lung mechanics in normal infants and infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 1991;11(1):37–43. doi: 10.1002/ppul.1950110107. [DOI] [PubMed] [Google Scholar]
- 7.Northover H, Cowie RA, Wraith JE. Mucopolysaccharidosis type IVA (Morquio syndrome): a clinical review. J Inherit Metab Dis. 1996;19(3):357–365. doi: 10.1007/BF01799267. [DOI] [PubMed] [Google Scholar]
- 8.Hellinckx J, De Boeck K, Bande-Knops J, van der Poel M, Demedts M. Bronchodilator response in 3–6. 5 years old healthy and stable asthmatic children. Eur Respir J. 1998;12(2):438–443. doi: 10.1183/09031936.98.12020438. [DOI] [PubMed] [Google Scholar]
- 9.Smith HJ, Reinhold P, Goldman MD. Forced oscillation technique and impulse oscillometry. Eur Respir Mon. 2005;31:72–105. [Google Scholar]
- 10.Vink GR, Arets HG, van der Laag J, van der Ent CK. Impulse oscillometry: a measure for airway obstruction. Pediatr Pulmonol. 2003;35(3):214–219. doi: 10.1002/ppul.10235. [DOI] [PubMed] [Google Scholar]
- 11.Frei J, Jutla J, Kramer G, Hatzakis GE, Ducharme FM, Davis GM. Impulse oscillometry: reference values in children 100 to 150 cm in height and 3 to 10 years of age. Chest. 2005;128(3):1266–1273. doi: 10.1378/chest.128.3.1266. [DOI] [PubMed] [Google Scholar]
- 12.Klug B, Bisgaard H. Specific airway resistance, interrupter resistance, and respiratory impedance in healthy children aged 2–7 years. Pediatr Pulmonol. 1998;25(5):322–331. doi: 10.1002/(sici)1099-0496(199805)25:5<322::aid-ppul6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 13.Lebecque P, Desmond K, Swartebroeckx Y, Dubois P, Lulling J, Coates A. Measurement of respiratory system resistance by forced oscillation in normal children: a comparison with spirometric values. Pediatr Pulmonol. 1991;10(2):117–122. doi: 10.1002/ppul.1950100214. [DOI] [PubMed] [Google Scholar]
- 14.Hershenson MB, Colin AA, Wohl ME, Stark AR. Changes in the contribution of the rib cage to tidal breathing during infancy. Am Rev Respir Dis. 1990;141(4 Pt 1):922–925. doi: 10.1164/ajrccm/141.4_Pt_1.922. [DOI] [PubMed] [Google Scholar]
- 15.Mayer OH, Clayton RG, Sr, Jawad AF, McDonough JM, Allen JL. Respiratory inductance plethysmography in healthy 3- to 5-year-old children. Chest. 2003;124(5):1812–1819. doi: 10.1378/chest.124.5.1812. [DOI] [PubMed] [Google Scholar]
- 16.Tabachnik E, Muller N, Toye B, Levison H. Measurement of ventilation in children using the respiratory inductive plethysmograph. J Pediatr. 1981;99(6):895–899. doi: 10.1016/s0022-3476(81)80012-1. [DOI] [PubMed] [Google Scholar]
- 17.Hammer J, Eber E, editors. Paediatric Pulmonary Function Testing. Vol. 33. Switzerland: Karger; 2005. pp. 148–156. [Google Scholar]
- 18.Bloch KE, Li Y, Zhang J, Bingisser R, Kaplan V, Weder W, Russi EW. Effect of surgical lung volume reduction on breathing patterns in severe pulmonary emphysema. Am J Respir Crit Care Med. 1997;156(2 Pt 1):553–560. doi: 10.1164/ajrccm.156.2.9608031. [DOI] [PubMed] [Google Scholar]
- 19.Pecorelli F, Grassi V, Ferrini L, Todisco T. Changes in respiratory function in disorders of the thoracic cage. With special reference to the ventilatory mechanism and the regulation in scoliosis Ital. J Orthop Traumatol. 1983;9(1):75–89. [PubMed] [Google Scholar]
- 20.Jakob M. In: Normal Values. Edition F, editor. CA: Borm Bruckmeier Publishing LLC; 2001. pp. 127–130. [Google Scholar]
- 21.Bergofsky EH. Respiratory failure in disorders of the thoracic cage. Am Rev Respir Dis. 1979;119(4):643–669. doi: 10.1164/arrd.1979.119.4.643. [DOI] [PubMed] [Google Scholar]
- 22.Remes V, Helenius I, Peltonen J, Poussa M, Sovijarvi A. Lung function in diastrophic dysplasia. Pediatr Pulmonol. 2002;33(4):277–282. doi: 10.1002/ppul.10069. [DOI] [PubMed] [Google Scholar]
- 23.Buhain WJ, Rammohan G, Berger HW. Pulmonary function in Morquio’s disease: A study of two siblings. Chest. 1975;68(1):41–45. doi: 10.1378/chest.68.1.41. [DOI] [PubMed] [Google Scholar]
- 24.Steven Sims H, Kempiners JJ. Special airway concerns in patients with mucopolysaccharidoses. Respir Med. 2007;101(8):1779–1782. doi: 10.1016/j.rmed.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Navajas DFR. Oscillation mechanics. Eur Respir Mon. 1999;4(12):112–140. [Google Scholar]
- 26.Lorino AM, Zerah F, Mariette C, Harf A, Lorino H. Respiratory resistive impedance in obstructive patients: linear regression analysis vs viscoelastic modelling. Eur Respir J. 1997;10(1):150–155. doi: 10.1183/09031936.97.10010150. [DOI] [PubMed] [Google Scholar]
- 27.Peslin R, Fredberg J. In: Oscillation mechanics of the respiratory system. Macklem P, Mead J, editors. 1986. [Google Scholar]
- 28.Cuijpers CE, Wesseling G, Swaen GM, Wouters EF. Frequency dependence of oscillatory resistance in healthy primary school children. Respiration. 1993;60(3):149–154. doi: 10.1159/000196191. [DOI] [PubMed] [Google Scholar]
- 29.Pasker HG, Schepers R, Clement J, Van de Woestijne KP. Total respiratory impedance measured by means of the forced oscillation technique in subjects with and without respiratory complaints. Eur Respir J. 1996;9(1):131–139. doi: 10.1183/09031936.96.09010132. [DOI] [PubMed] [Google Scholar]
- 30.Clement J, Landser FJ, Van de Woestijne KP. Total resistance and reactance in patients with respiratory complaints with and without airways obstruction. Chest. 1983;83(2):215–220. doi: 10.1378/chest.83.2.215. [DOI] [PubMed] [Google Scholar]
- 31.Daroczy B, Hantos Z. Generation of optimum pseudorandom signals for respiratory impedance measurements. Int J Biomed Comput. 1990;25(1):21–31. doi: 10.1016/0020-7101(90)90058-3. [DOI] [PubMed] [Google Scholar]
- 32.Clement J, Dumoulin B, Gubbelmans R, Hendriks S, van de Woestijne KP. Reference values of total respiratory resistance and reactance between 4 and 26 Hz in children and adolescents aged 4–20 years. Bull Eur Physiopathol Respir. 1987;23(5):441–448. [PubMed] [Google Scholar]
- 33.Solymar L, Aronsson PH, Bake B, Bjure J. Respiratory resistance and impedance magnitude in healthy children aged 2–18 years. Pediatr Pulmonol. 1985;1(3):134–140. doi: 10.1002/ppul.1950010303. [DOI] [PubMed] [Google Scholar]
- 34.Finucane KE, Dawson SV, Phelan PD, Mead J. Resistance of intrathoracic airways of healthy subjects during periodic flow. J Appl Physiol. 1975;38(3):517–530. doi: 10.1152/jappl.1975.38.3.517. [DOI] [PubMed] [Google Scholar]