Abstract
T2D is a growing health problem world-wide, but the currently available strategies for therapy and prevention are insufficient. Recent observations indicate that bile acid homeostasis is altered in T2D. Bile acids are metabolic regulators that act as signaling molecules through receptor-dependent and -independent pathways. The most prominent signaling molecules mediating bile acid signaling are the nuclear receptor FXR and the membrane receptor TGR5. Both are implicated in the regulation of lipid, glucose and energy metabolism. Dysregulation of these pathways might contribute to the development of T2D and associated metabolic complications. Interestingly, data from studies with bile acids or bile acid sequestrants indicate that the manipulation of bile acid homeostasis might be an attractive approach for T2D therapy. In this review, we summarize the mechanisms of bile-acid-mediated metabolic control that might be of relevance in the pathogenesis of T2D.
Keywords: Animals; Bile Acids and Salts; metabolism; Diabetes Mellitus, Type 2; metabolism; physiopathology; Dyslipidemias; metabolism; physiopathology; Humans; Obesity; metabolism; physiopathology
Keywords: Bile acids, T2D, FXR, TGR5, bile acid sequestrants, obesity, dyslipidemia, NAFL
Introduction
Type 2 diabetes (T2D) is characterized by relative insulin deficiency due to the resistance of target tissues towards insulin action and the concomitant decline of insulin secretion from the pancreas. T2D pathogenesis includes genetic as well as environmental factors, but despite extensive research in this field the detailed mechanisms are yet incompletely known. The development of T2D is commonly associated with obesity and often with hypertension and dyslipidemia. These latter complications promote the development of cardiovascular disease which presents the most common mortality linked to T2D [1].
In the past 15 years, a growing body of evidence has shown that bile acid metabolism is altered in T2D patients and, conversely, that manipulation of the bile acid pool can improve glycemic control in such patients. Bile acids are increasingly recognized for their function as metabolic regulators. Via the activation of different signaling pathways, they participate in the control of bile acid, lipid and glucose metabolism. In this review, we summarize the mechanisms through which bile acids exert metabolic control and discuss their possible implication in the pathogenesis of T2D.
Bile acid metabolism
The synthesis and metabolism of bile acids is complex and highly regulated. Bile acids are synthesized from cholesterol in the liver. Specific transporters located in the basolateral membrane of the hepatocyte actively secrete bile acids as well as cholesterol and phospholipids into the bile. The ingestion of a meal triggers the release of bile from the gallbladder into the intestinal lumen, where bile acids form micelles with dietary lipids and lipid-soluble vitamins, thereby facilitating their absorption. Specific transporters expressed in the distal ileum assure the re-absorption of ~95% of bile acids conveying them back to the liver, while the remaining 5% are excreted in the feces. In humans, the bile acid pool undergoes this enterohepatic cycle around 12x/day. Even though the bile acid pool size is maintained constant, the flux of bile acids varies during the day. Promoted by the ingestion of a meal, bile acid flux and plasma bile acid concentrations are highest postprandially [2].
Bile acids consist of a 24-carbon steroid core and a side chain carrying a carboxyl group. The nature of the bile acid species is determined by the number and position of hydroxyl groups on the steroid core, forming a hydrophobic and a hydrophilic side of the molecule. This amphiphatic property is essential for the bile acid’s biological function, i.e. facilitating the excretion of hydrophobic molecules into the bile and mediating the absorption of dietary lipids from the intestinal lumen. However, it also turns bile acids into powerful detergents which at high concentrations may be cytotoxic (toxicity increases with hydrophobicity). For this reason, the size and composition of the bile acid pool is strictly controlled. Since the biological activity of a bile acid depends on its chemical structure, the pool composition (and thus hydrophobicity) may further determine to what extent bile acid signaling pathways are activated [3].
Bile acids are synthesized in a multiple step process [4]. The liver produces the primary bile acids cholic acid (CA) and chenodeoxycholic acid (CDCA) in humans, and CA and muricholic acid (MCA) in mice. The majority of bile acids is formed via the so-called classical pathway and only a small quantity (in humans estimated at ~6%) via the alternative pathway. Cholesterol-7α-hydroxylase (CYP7A1) catalyzes the first step of the classical pathway and is submitted to a negative feedback regulation mediated by bile acids themselves. CYP7A1 is considered to be the key enzyme of bile acid synthesis, CYP7A1-deficient mice die from liver failure within three weeks after birth [4]. The key enzymes of the alternative pathway are sterol 27-hydroxylase (CYP27A1) and 25-hydroxycholesterol-7α-hydroxylase (CYP7B1). Even though mice deficient for CYP7B1 are able to maintain a normal bile acid pool, a case of a human newborn has been reported where a CYP7B1 mutation led to severe liver dysfunction, demonstrating the importance of the alternative pathway in humans [2]. While CYP7A1 defines the size of the bile acid pool, its composition strongly depends on the activity of the sterol-12α-hydroxylase (CYP8B1). This enzyme catalyzes the formation of CA by diverting intermediates from CDCA synthesis (or MCA synthesis in mice), thus defining the CA/CDCA ratio in the pool. As CYP7A1, CYP8B1 is negatively regulated by bile acids [4]. The synthesized primary bile acids are conjugated to glycine or taurine before secretion into the bile. Once they have reached the intestine, they may undergo deconjugation and dehydroxylation by bacteria of the gut microflora. The resulting secondary bile acids in humans are mainly deoxycholic acid (DCA) and lithocholic acid (LCA). During the second passage through the liver, a small part of the secondary bile acids is again modified by sulfonation or glucuronidation giving rise to tertiary bile acids.
Bile acids exert negative feedback on their own synthesis as mentioned above, but also on the expression of transport molecules assuring the enterohepatic cycle. Thus, bile acids stimulate their basolateral efflux into the bile (via the bile salt export pump BSEP), inhibit their ileal absorption (via the apical sodium-dependent bile acid transporter ASBT), stimulate their efflux from the enterocyte into the blood (via the organic solute transporter OST) and inhibit their apical uptake into the liver (via the sodium taurocholate-cotransporting polypeptide NTCP). The details of bile acid synthesis and transport regulation have been reviewed in detail elsewhere [2,3].
Bile acid homeostasis in T2D
Due to their long-known role in the digestion of dietary fat, bile acids have classically been associated with lipid metabolism. Over the past decades, a limited number of studies has described alterations of the bile acid pool in T2D patients and animal models. Even though the available data are not fully consistent, they present a link of bile acid and glucose homeostasis.
An early study in patients with uncontrolled T2D reported an increase in the bile acid pool size and fecal bile acid excretion which decreased upon insulin treatment [5]. Consistently, insulin was found to inhibit the expression of CYP7A1 and CYP27A1 in rat hepatocytes, the key enzymes of both bile acid synthesis pathways [2]. However, a second study observed no differences in bile acid synthesis between uncontrolled and insulin-treated diabetic patients [6]. A recent investigation evaluated bile acid kinetics more systematically in T2D patients [7]. Even though no difference was found in the size of the total bile acid pool, the contribution of specific bile acid species to the pool was altered in T2D. Thus, the pool of the secondary bile acid DCA was elevated as was the DCA input rate, whereas the CDCA pool decreased [8]. The CA pool was unchanged, even though the CA synthesis rate was elevated in diabetes. Another study used a metabolomic approach to show lower CA and elevated DCA levels in plasma of self-reported, controlled diabetic patients compared to controls [9]. Hence, CA might be increasingly converted into DCA in the gut of patients with T2D. Interestingly, changes in the gut microflora have lately been associated with the development of metabolic diseases. This area of research might provide further insights into associated changes of bile acid metabolism. Despite the increasing interest in the link between bile acid metabolism and diabetes, the evidence from animal models is still very scarce. In insulin resistant ob/ob mice, leptin administration diminished the size and further decreased the hydrophobicity of the bile acid pool, resulting in an enhanced efflux of cholesterol into the bile [10]. Unfortunately, possible consequences of these alterations on glucose homeostasis were not analyzed. Herrema et al. investigated bile acid kinetics in more detail in diabetic db/db mice and observed an elevation of total bile acid pool size and synthesis, due to an increase in the fractional pool size and synthesis rate of CA [11].
Even though not devoid of discrepancies, the above presented data clearly suggest that changes in bile acid homeostasis are either a cause or a consequence of the metabolic disturbances observed during T2D. This raises the question whether the manipulation of bile acid homeostasis may improve metabolic complications linked to T2D. In this regard, an interesting study looked at bile acid metabolism in high-fat diet-fed rats that were submitted to ileal interposition surgery. This operation moves upwards a distal part of the ileum which is repositioned in the proximal jejunum. The authors found that plasma bile acid levels increased in operated rats, with a higher ratio of primary to secondary bile acids, and that bile acid excretion decreased, indicating an early reabsorption which short-circuits the enterohepatic cycle. Most interestingly, obesity, glucose tolerance and high cholesterol levels improved subsequent to the operation even though high-fat feeding continued [12]. Similar observations were made in gastric bypass patients who, compared to weight-matched controls, had increased plasma bile acid levels which inversely correlated with fasting plasma lipids and postprandial blood glucose [13]. Another recent report confirmed that the increase of the bile acid pool size, in this case by overexpression of CYP7A1 in mice, protected from Western diet-induced obesity, insulin resistance and hepatic steatosis [14].
More evidence for a beneficial effect of the modulation of bile acid metabolism comes from trials in which bile acid sequestrants were administered to T2D patients. These non-absorbable polymers complex bile acids in the intestinal lumen and thus divert them from the enterohepatic cycle. Consequently, the bile acid pool composition is modulated [7]. Successfully applied to lower elevated LDL-cholesterol and prevent cardiovascular disease since a few decades, bile acid sequestrants were found to improve glycemic control in T2D patients. In patients not adequately controlled by common anti-diabetic therapeutics such as insulin, sulfonylurea or metformin, the administration of a bile acid sequestrant decreased plasma glucose and HbA1c concentrations [15]. A pilot study in T2D patients [16] and a report from diabetic mice [17] suggest that an increase in whole-body insulin sensitivity might underlie the improvement in glucose homeostasis upon treatment with bile acid sequestrants. Two other recent investigations found an increase in GLP1 (glucagon-like peptide 1) secretion from the intestine of sequestrant-treated diabetic rats, which might mediate the increased responsiveness towards insulin [18,19].
Bile acids as signaling molecules
Over the past decade, several pathways have been identified, which mediate the regulatory effects of bile acids. The membrane receptor FPR (formyl peptide receptor) was reported to be activated by CDCA and DCA with an immunosuppressive outcome. The nuclear receptors PXR (pregnane X receptor) and VDR (vitamin D receptor) were shown to bind the toxic bile acid LCA and mediate its elimination from the liver and the intestine, providing protection from cholestasis and colon cancer. The most prominent candidates, however, are the nuclear receptor FXR (farnesoid X receptor) and the G-protein-coupled membrane receptor TGR5 [2].
FXR
The nuclear receptor FXR is highly expressed in liver and intestine, the main sites of bile acid metabolism, but also in adipose tissue, pancreas and adrenals [2]. CDCA is most effective in activating FXR, while LCA, DCA and CA bind with lower affinity and specificity. Upon ligand-binding, FXR acts either as a monomer or forms a heterodimer with RXR (retinoid X receptor) to subsequently transactivate or repress the expression of genes that contain a specific FXR response element in their promoter [20]. In addition to the direct regulation of target gene expression, numerous FXR functions are mediated by the induction of SHP (short heterodimer protein) which in turn represses target genes in bile acid, lipid and glucose metabolism [2]. In the intestine, FXR further induces the expression and subsequent secretion of FGF (fibroblast growth factor) 19 (corresponding to FGF15 in mice), which as a hormone can transmit the BA-induced signal to specific target tissues. By binding to the FGF receptor 4 (FGFR4) in the liver, FGF15 inhibits bile acid synthesis via a c-Jun N terminal kinase (JNK) – mediated pathway [21].
TGR5
The identification of the G protein coupled receptor TGR5 (also Gpbar1, M-Bar, BG37) as a bile acid receptor [22,23] has opened new insights in the mechanisms of bile acid signaling. Mainly expressed in the gall bladder, ileum, colon, brown and white adipose tissue, and to a lesser extent in skeletal muscle, liver and immune cells, TGR5 is activated by nanomolar concentrations of LCA and TLCA and micromolar concentrations of CA, DCA and CDCA [15]. After ligand-binding to plasma membrane TGR5, the receptor is internalized, the GαS subunit released and adenylate cyclase activated. The consecutively formed cAMP can activate PKA (protein kinase A) or the transcription factor CREB (cAMP response element binding protein), mediating bile acid functions in immunosuppression, energy and glucose homeostasis [3].
Receptor-independent pathways
Cell signaling networks, e.g. the MAPK pathway, can also be activated by bile acids independent of the mentioned receptors (reviewed in [24]), but their implication in the control of metabolic homeostasis still needs to be determined.
Metabolic regulation by bile acids
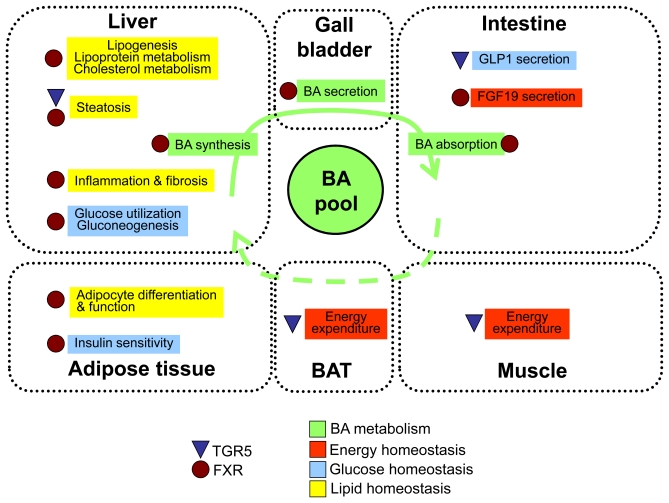
Based on the variety of bile acid-targeted receptors and signaling pathways, it is obvious that bile acids exert multiple regulatory functions. Here, we will focus on the role of bile acids in the regulation of pathways that are affected in T2D and associated metabolic complications (Figure 1).
Figure 1.
Schematic overview of the functions of bile acids (BA) in the regulation of BA, energy, glucose and lipid metabolism via FXR- and TGR5-mediated signaling pathways. For details please see text. GLP1, glucagon-like peptide 1; FGF, fibroblast growth factor; BAT, brown adipose tissue.
Glucose metabolism and insulin sensitivity
T2D is commonly preceded by a state of insulin resistance. In peripheral tissues, insulin resistance leads to a decreased uptake of glucose from the blood, whereas hepatic insulin resistance results in enhanced de novo glucose production. Both phenomena contribute to elevated plasma glucose levels, first in the postprandial and later also in the fasted state. Bile acids are involved in the regulation of hepatic glucose metabolism by FXR-mediated pathways. The expression of FXR itself is decreased in rat models of type 1 and T2D, an effect that could be reversed by the administration of insulin [25]. The evaluation of the fasting-refeeding transition in FXR-deficient mice revealed the importance of functional FXR for the maintenance of stable blood glucose concentrations. In the absence of FXR, the induction of glycolytic (liver pyruvate kinase) and lipogenic genes (fatty acid synthase, acetyl CoA carboxylase) was accelerated, associated with a transient hypoglycaemia [26]. In contrast, the induction of glucose-responsive genes was lower upon FXR activation. Via FXR, bile acids further regulate hepatic glucose production by gluconeogenesis. In one set of studies, FXR activation by CA decreased the expression of the gluconeogenic genes PEPCK (phosphoenol pyruvate kinase), G6Pase (glucose-6-phosphatase) and FBP1 (fructose-1,6-bisphosphatase) [27,28]. In contrast, FXR activation by the synthetic agonist GW4064 was found to induce the expression of PEPCK [29] in another investigation. In line with this finding, PEPCK and G6Pase expression were repressed in FXR-deficient mice [26,28]. Despite the controversy of the available data, bile acids clearly impact on the regulation of hepatic glucose metabolism.
Intact insulin sensitivity is a prerequisite for the maintenance of glucose homeostasis. Several groups have reported that FXR-deficiency leads to peripheral insulin resistance in mice with a reduction of glucose disposal and decreased adipose tissue and skeletal muscle insulin signaling, however they presented conflicting data on the status of the liver [28,30,31]. By contrast, the activation of FXR by GW4064 in insulin-resistant ob/ob mice reduced hyperinsulinemia and improved glucose tolerance [31]. Another study applying the semi-synthetic FXR agonist 6-ethyl-CDCA to diabetic fa/fa rats confirmed the beneficial effect of FXR activation on insulin resistance in liver and skeletal muscle [32]. In addition to this key role of FXR in the control of insulin sensitivity, FXR expression has recently been reported to play a role in the pancreas, where it regulates glucose-induced insulin secretion [33,34] and protects against lipotoxicity [33], a crucial feature in the development of T2D.
Modulation of the bile acid pool by intestinal sequestration has been shown to improve insulin resistance in rat models of diabetes by promoting the secretion of GLP1 from intestinal L-cells [18,19]. The incretin GLP1 is released in response to the ingestion of a meal and, in addition to controlling appetite and gastrointestinal function, promotes insulin secretion from the pancreas. This is why GLP1 based strategies are currently being used for the therapy of T2D. Both studies [18,19] exclude that the observed effect is FXR-dependent. Most interestingly, recent reports provide evidence that bile acids induce GLP1 secretion by TGR5-mediated pathways. LCA and DCA TGR5-dependently induced intracellular cAMP concentrations and GLP1 secretion from intestinal L-cells in vitro [35]. In a second study, the TGR5 agonist oleanolic acid attenuated obesity and improved insulin resistance in mice upon high-fat diet-feeding [36]. The most detailed investigation has been provided by Thomas et al. [37]. The authors show that glucose tolerance reduced by TGR5-deficiency and improved by TGR5 overexpression in high-fat diet-fed mice via increased GLP1 and insulin secretion. Pharmacological activation of TGR5 by the semi-synthetic agonist INT777 also ameliorated insulin sensitivity and raised GLP1 secretion in obese mice. The authors proposed that TGR5 activation raises the intracellular ATP/ADP ratio and enhances calcium influx which promotes GLP1 secretion. Thus, the TGR5-mediated triggering of GLP1 secretion presents a powerful mean for bile acids to control glucose homeostasis.
Very few data have demonstrated that receptor-independent pathways account for the beneficial action of bile acids on glucose homeostasis. The administration of taurine-conjugated ursodeoxycholic acid (TUDCA) to genetically obese mice improved insulin resistance by attenuating endoplasmatic reticulum (ER) stress [38]. A very recent study in human obese subjects found an increase of insulin sensitivity in liver and muscle, but not adipose tissue upon TUDCA administration [39]. However, no effect on markers of ER stress was observed.
Energy metabolism
As mentioned above, visceral obesity is often associated with T2D, which is characterized by a dysregulation of energy metabolism. It is still under discussion to which extent and by which mechanisms obesity is causal for the development of insulin resistance and T2D, but the epidemiological link is clearly established [1].
Even though adipose tissue is not a tissue classically involved in bile acid metabolism, it expresses FXR [31,40]. In FXR-deficient mice, adipose tissue mass and adipocyte size are reduced [31,41], indicating a role for FXR in adipocyte biology. Indeed, FXR controls adipocyte differentiation and function by promoting PPARγ activity and inhibiting the Wnt/β-catenin pathway [40,41]. In the absence of FXR, basal lipolysis is increased whereas lipogenesis is reduced [41]. Further, FXR activation was shown to increase the insulin-mediated uptake of glucose [40].
Activation of FXR induces the expression and secretion of FGF19 in the intestine. Fu et al. have shown that the administration of FGF19 to high-fat diet-fed mice increased energy expenditure, reversed weight gain and as a consequence improves insulin resistance [42]. Changes of the amount or nature of bile acids passing through the intestine can thus have strong systemic effects on metabolic regulation by modulating FXR-mediated FGF19 secretion.
The discovery of TGR5 as a bile acid receptor identified another pathway for the bile acid-mediated regulation of energy metabolism. The supplementation of a high-fat diet with CA increased energy expenditure in brown adipose tissue of mice with a beneficial outcome for obesity and insulin resistance [43]. The authors suggest a TGR5-cAMP-triggered increase in triiodothyronine due to the activation of deiodinase in brown adipose tissue, resulting in increased uncoupling. Even though an attractive concept, several facts put the proposed mechanism into question. Neither the overexpression nor the absence of TGR5 in mice leads to a difference in body weight and the susceptibility to diet-induced obesity is gender-specific [44]. Further, thyroid hormones have recently been shown to exert a central rather than peripheral effect on energy metabolism of brown adipose tissue [45].
Lipid metabolism and NAFL/NASH
Even though the dysregulation of lipid and lipoprotein metabolism is not considered as a direct cause for T2D, it is strongly associated with obesity, insulin resistance and T2D itself. Dyslipidemia increases the risk for cardiovascular incidences in T2D. The implication of bile acids in the regulation of triglyceride and cholesterol metabolism via hepatic FXR became manifest through the observation of dyslipidemia in FXR-deficient mice [46].
CDCA administration was shown to decrease plasma lipids in hypertriglyceridemic patients, long before FXR was identified [47]. Watanabe et al. delineated a FXR-SHP-mediated mechanism which repressed the induction of SREBP1c by LXR and thus reduced lipogenesis and VLDL secretion [48]. Recently, the administration of bile acid sequestrants was reported to induce lipogenesis dependent of FXR and LXR [11]. The reduction of plasma triglycerides by FXR activation was further attributed to an increased clearance from the bloodstream by lipoprotein lipase (LPL). LPL activity is enhanced by the induction of its activator apolipoprotein CII and the repression of its inhibitor apolipoprotein CIII in the liver upon FXR activation [2].
Given that bile acids are synthesized from cholesterol, the direct link of these two metabolic pathways is obvious. Bile acids exert additional control in cholesterol metabolism by regulating numerous FXR target genes [2]. In vitro, FXR activation induced the expression of the LDL receptor and repressed its inhibitor PCSK9. However, this mechanism did not seem to apply in vivo since CDCA administration to patients increased LDL cholesterol levels [20]. The key structural component of HDL, apolipoprotein AI, was reported to be a negative FXR target gene [2]. Data from mice further proposed that FXR stimulates the uptake of HDL cholesterol esters via hepatic SRBI induction [46]. Consequently, FXR activation in patients lowered HDL cholesterol levels [47].
The occurrence of non-alcoholic liver (NAFL) and its progression towards non-alcoholic steatohepatitis (NASH) in association with insulin resistance and T2D development has been re-evaluated in the past years. Several studies suggest that NAFLD might be one of the causal factors for the manifestation of T2D in obese subjects [49]. TUDCA administration was shown to decrease NAFLD in genetically obese mice [38] and TGR5 activation in diet-induced obesity [37], but no directly-underlying mechanisms were proposed. CDCA and CA further prevented fructose-induced hepatic steatosis by protecting against the entry of bacterial endotoxins from the intestine [50]. Several studies have further linked bile acids to NASH via FXR. FXR-deficient mice displayed marked inflammatory cell infiltration and increased hepatic collagen content, whereas FXR activation decreased inflammatory cell infiltration and fibrosis in mice by repression of the fibrotic genes TGF1β, α-SMA and TIMP-1 [20].
Conclusions
A growing body of evidence acknowledges bile acids as metabolic regulators. By binding to the nuclear receptor FXR or the membrane receptor TGR5, they participate in the control of glucose metabolism, insulin sensitivity, energy homeostasis as well as lipid metabolism. During the pathogenesis of T2D, the composition of the circulating bile acid pool is altered, probably contributing to the dysregulation of metabolic homeostasis in T2D. The manipulation of the bile acid pool itself by bile acid substitution or intestinal sequestration, or bile acid receptors such as FXR and TGR5 present promising strategies to develop therapeutics for T2D. However, given the diversity of the metabolic pathways controlled by bile acid signaling, more mechanistic and clinical studies are needed to precipitate the optimal approach.
Acknowledgments
The authors are supported by the EU Grant HEPADIP (N° 018734) and received funding from Daiichi Sankyo.
Footnotes
Author Disclosure Statement
The authors disclose no conflict of interest.
References
- 1.IDF Diabetes Atlas. [Accessed January 2011]. Available at http://www.diabetesatlas.org/
- 2.Lefebvre P, Cariou B, Lien F, et al. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 3.Thomas C, Pellicciari R, Pruzanski M, et al. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 4.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 5.Bennion LJ, Grundy SM. Effects of diabetes mellitus on cholesterol metabolism in man. N Engl J Med. 1977;296:1365–1371. doi: 10.1056/NEJM197706162962401. [DOI] [PubMed] [Google Scholar]
- 6.Abrams JJ, Ginsberg H, Grundy SM. Metabolism of cholesterol and plasma triglycerides in nonketotic diabetes mellitus. Diabetes. 1982;31:903–910. doi: 10.2337/diab.31.10.903. [DOI] [PubMed] [Google Scholar]
- 7.Brufau G, Stellaard F, Prado K, et al. Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology. 2010;52:1455–1464. doi: 10.1002/hep.23831. [DOI] [PubMed] [Google Scholar]
- 8.Brufau G, Bahr MJ, Staels B, et al. Plasma bile acids are not associated with energy metabolism in humans. Nutr Metab. 2010;7:73. doi: 10.1186/1743-7075-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suhre K, Meisinger C, Döring A, et al. Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS One. 2010;5:e13953. doi: 10.1371/journal.pone.0013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyogo H, Roy S, Paigen B, et al. Leptin promotes biliary cholesterol elimination during weight loss in ob/ob mice by regulating the enterohepatic circulation of bile salts. J Biol Chem. 2002;277:34117–34124. doi: 10.1074/jbc.M203912200. [DOI] [PubMed] [Google Scholar]
- 11.Herrema H, Meissner M, van Dijk TH, et al. Bile salt sequestration induces hepatic de novo lipogenesis through farnesoid X receptor- and liver X receptoralpha-controlled metabolic pathways in mice. Hepatology. 2010;51:806–816. doi: 10.1002/hep.23408. This study is the first to give detailed insight into bile acid kinetics after sequestration in diabetic animal models. [DOI] [PubMed] [Google Scholar]
- 12.Kohli R, Kirby M, Setchell KDR, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol. 2010;299:G652–60. doi: 10.1152/ajpgi.00221.2010. This paper elegantly shows that short-circuiting of bile acid cycling might contribute to the metabolic improvements observed after bariatric surgery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patti M, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li T, Owsley E, Matozel M, et al. Transgenic expression of cholesterol 7alpha-hydroxylase in the liver prevents high-fat diet-induced obesity and insulin resistance in mice. Hepatology. 2010;52:678–690. doi: 10.1002/hep.23721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prawitt J, Staels B. Bile acid sequestrants: glucose-lowering mechanisms. Metab Syndr Relat Disord. 2010;8 (Suppl 1):S3–8. doi: 10.1089/met.2010.0096. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz SL, Lai Y, Xu J, et al. The effect of colesevelam hydrochloride on insulin sensitivity and secretion in patients with type 2 diabetes: a pilot study. Metab Syndr Relat Disord. 2010;8:179–188. doi: 10.1089/met.2009.0049. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi M, Ikegami H, Fujisawa T, et al. Prevention and treatment of obesity, insulin resistance, and diabetes by bile acid-binding resin. Diabetes. 2007;56:239–247. doi: 10.2337/db06-0353. [DOI] [PubMed] [Google Scholar]
- 18.Shang Q, Saumoy M, Holst JJ, et al. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am J Physiol Gastrointest Liver Physiol. 2010;298:G419–24. doi: 10.1152/ajpgi.00362.2009. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, McNulty J, Anderson D, et al. Cholestyramine reverses hyperglycemia and enhances GLP-1 release in Zucker Diabetic Fatty rats. J Pharmacol Exp Ther. 2010;334:164–170. doi: 10.1124/jpet.110.166892. [DOI] [PubMed] [Google Scholar]
- 20.Prawitt J, Caron S, Staels B. How to modulate FXR activity to treat the Metabolic Syndrome. Drug Discov Today Dis Mech. 2009;6:e55–e64. [Google Scholar]
- 21.Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 23.Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen A, Bouscarel B. Bile acids and signal transduction: role in glucose homeostasis. Cell Signal. 2008;20:2180–2197. doi: 10.1016/j.cellsig.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Duran-Sandoval D, Mautino G, Martin G, et al. Glucose regulates the expression of the farnesoid X receptor in liver. Diabetes. 2004;53:890–898. doi: 10.2337/diabetes.53.4.890. [DOI] [PubMed] [Google Scholar]
- 26.Duran-Sandoval D, Cariou B, Percevault F, et al. The farnesoid X receptor modulates hepatic carbohydrate metabolism during the fasting-refeeding transition. J Biol Chem. 2005;280:29971–29979. doi: 10.1074/jbc.M501931200. [DOI] [PubMed] [Google Scholar]
- 27.Yamagata K, Daitoku H, Shimamoto Y, et al. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J Biol Chem. 2004;279:23158–23165. doi: 10.1074/jbc.M314322200. [DOI] [PubMed] [Google Scholar]
- 28.Ma K, Saha PK, Chan L, et al. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116:1102–1109. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stayrook KR, Bramlett KS, Savkur RS, et al. Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology. 2005;146:984–991. doi: 10.1210/en.2004-0965. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Lee FY, Barrera G, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cariou B, van Harmelen K, Duran-Sandoval D, et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem. 2006;281:11039–11049. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- 32.Cipriani S, Mencarelli A, Palladino G, et al. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51:771–784. doi: 10.1194/jlr.M001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popescu IR, Helleboid-Chapman A, Lucas A, et al. The nuclear receptor FXR is expressed in pancreatic beta-cells and protects human islets from lipotoxicity. FEBS Lett. 2010;584:2845–2851. doi: 10.1016/j.febslet.2010.04.068. [DOI] [PubMed] [Google Scholar]
- 34.Renga B, Mencarelli A, Vavassori P, et al. The bile acid sensor FXR regulates insulin transcription and secretion. Biochim Biophys Acta. 2010;1802:363–372. doi: 10.1016/j.bbadis.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Katsuma SEA. Bile acids promote glucagon-like peptide-1 secretion through tgr5 in a murine enteroendocrine cell line stc-1. Biochem Biophys Res Commun. 2005;329:386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 36.Sato H, Genet C, Strehle A, et al. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem Biophys Res Commun. 2007;362:793–798. doi: 10.1016/j.bbrc.2007.06.130. [DOI] [PubMed] [Google Scholar]
- 37.Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. This elaborate study is the first to propose a mechanism by which bile acids might improve glucose homeostasis via TGR5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kars M, Yang L, Gregor MF, et al. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59:1899–1905. doi: 10.2337/db10-0308. One of the rare investigations of receptor-independent effects of bile acids on insulin sensitivity in human patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rizzo G, Disante M, Mencarelli A, et al. The farnesoid X receptor promotes adipocyte differentiation and regulates adipose cell function in vivo. Mol Pharmacol. 2006;70:1164–1173. doi: 10.1124/mol.106.023820. [DOI] [PubMed] [Google Scholar]
- 41.Abdelkarim M, Caron S, Duhem C, et al. The Farnesoid X Receptor Regulates Adipocyte Differentiation and Function by Promoting Peroxisome Proliferator-activated Receptor-{gamma} and Interfering with the Wnt/{beta}-Catenin Pathways. J Biol Chem. 2010;285:36759–36767. doi: 10.1074/jbc.M110.166231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu L, John LM, Adams SH, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594–2603. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 44.Maruyama T, Tanaka K, Suzuki J, et al. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endocrinol. 2006;191:197–205. doi: 10.1677/joe.1.06546. [DOI] [PubMed] [Google Scholar]
- 45.López M, Varela L, Vázquez MJ, et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med. 2010;16:1001–1008. doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambert G, Amar MJA, Guo G, et al. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem. 2003;278:2563–2570. doi: 10.1074/jbc.M209525200. [DOI] [PubMed] [Google Scholar]
- 47.Bateson MC, Maclean D, Evans JR, et al. Chenodeoxycholic acid therapy for hypertriglyceridaemia in men. Br J Clin Pharmacol. 1978;5:249–254. doi: 10.1111/j.1365-2125.1978.tb01632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe M, Houten SM, Wang L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27–38. doi: 10.1161/ATVBAHA.107.147538. [DOI] [PubMed] [Google Scholar]
- 50.Volynets V, Spruss A, Kanuri G, et al. Protective effect of bile acids on the onset of fructose-induced hepatic steatosis in mice. J Lipid Res. 2010;51:3414–3424. doi: 10.1194/jlr.M007179. [DOI] [PMC free article] [PubMed] [Google Scholar]