IGPR-1 is a novel adhesion molecule that regulates cell–cell interaction. IGPR-1 associates with several SH3-containing proteins, including SPIN90/WISH, and regulates capillary tube formation of primary endothelial cells.
Abstract
Angiogenesis—the growth of new blood vessels from preexisting vessels—is an important physiological process and is considered to play a key role in tumor growth and metastasis. We identified the immunoglobulin-containing and proline-rich receptor-1 (IGPR-1, also called TMIGD2) gene as a novel cell adhesion receptor that is expressed in various human organs and tissues, mainly in cells with epithelium and endothelium origins. IGPR-1 regulates cellular morphology, homophilic cell aggregation, and cell–cell interaction. IGPR-1 activity also modulates actin stress fiber formation and focal adhesion and reduces cell migration. Silencing of expression of IGPR-1 by small interfering RNA (siRNA) and by ectopic overexpression in endothelial cells showed that IGPR-1 regulates capillary tube formation in vitro, and B16F melanoma cells engineered to express IGPR-1 displayed extensive angiogenesis in the mouse Matrigel angiogenesis model. Moreover, IGPR-1, through its proline-rich cytoplasmic domain, associates with multiple Src homology 3 (SH3)–containing signaling proteins, including SH3 protein interacting with Nck (SPIN90/WISH), bullous pemphigoid antigen-1, and calcium channel β2. Silencing of expression of SPIN90/WISH by siRNA in endothelial cells showed that SPIN90/WISH is required for capillary tube formation. These features of IGPR-1 suggest that IGPR-1 is a novel receptor that plays an important role in cell–cell interaction, cell migration, and angiogenesis.
INTRODUCTION
Angiogenesis is a coordinated cascade of numerous complex cellular processes, which include endothelial cell migration, proliferation, sprouting, and lumen formation, which ultimately leads to the formation of new vessels. These coordinated cellular events are regulated by the function of various cell surface receptors and soluble ligands (Rahimi, 2006; Carmeliet and Jain, 2011a). The ability of endothelial cells to form capillary tubes is prerequisite for the establishment of a continuous vessel lumen that routes the blood flow. Several key receptor tyrosine kinases such as vascular endothelial growth factor (VEGF) receptor-1 (VEGFR-1), VEGFR-2, and VEGFR-2, and cell adhesion molecules (CAMs), including cadherins, integrins, selectins, and immunoglobulin (Ig) superfamily proteins, all are involved in angiogenesis (Bach et al., 1998; Barreiro et al., 2002; Bazzoni, 2003). The roles of proangiogenic molecules, such as VEGF and VEGFRs (i.e., VEGFR-1, VEGFR-2, and VEGFR-3), are well known in regulation of differentiation, survival, proliferation, and migration of endothelial cells (Gory-Faure et al., 1999; Rahimi, 2006). Studies using knockouts or blocking antibodies also demonstrated a key role for integrins in angiogenesis. Vascular endothelial cadherin (VE-cadherin), an endothelium-specific member of the cadherin family of adhesion proteins (Bach et al., 1998), and other CAM proteins, such as PECAM-1, ICAM-1, and JAM-A, are also linked to angiogenesis (Bach et al., 1998; Barclay, 2003; Bazzoni, 2003).
The Ig-containing adhesion molecules are known for their vital role in embryonic development and pathological conditions such as cancer and inflammation by modulating cell–cell adhesion and cell migration (Takai et al., 2008; Yamagata and Sanes, 2008; DeLisser et al., 2010). The Ig domains engage in protein–protein interactions, such as the homophilic (i.e., trans-dimerization) interaction of cell adhesion receptors, and protein–ligand interactions, such growth factor receptors and soluble growth factors (Barclay, 2003; Rahimi, 2006). The Ig-containing cell adhesion molecules, through homophilic and/or heterophilic interactions, selectively contribute to the specificity of cell–cell recognition and cell adhesion (Barclay, 2003; Rahimi, 2006). At the intracellular compartment, they interact with various cytoplasmic signaling proteins, which are often linked to the cytoskeleton (Takai et al., 2008).
In this study we identified a previously uncharacterized receptor—immunoglobulin-containing and proline-rich receptor-1 (IGPR-1)—as a novel adhesion molecule with a broad expression in epithelial and endothelial cells. IGPR-1 regulates cellular morphology, cell–cell interaction, and cell migration. More importantly, IGPR-1 associates with several SH3-containing proteins and regulates angiogenesis in vivo and in vitro.
RESULTS
Identification of IGPR-1 as a novel cell surface receptor
Searching the human genome sequence database for Ig-containing proteins, we identified an uncharacterized protein—transmembrane and immunoglobulin domain–containing protein 2 (TMIGD2)—that has a single Ig domain, a single transmembrane domain, and a stretch of 110–amino acid cytoplasmic region highly rich in proline residues (Figure 1A). The extracellular region of this protein also contains two possible glycosylation sites (Figure 1A). Because of the presence of an immunoglobulin domain in its extracellular region and a proline-rich motif in its cytoplasmic region, we named this protein immunoglobulin and proline-rich receptor-1, or IGPR-1, and refer to it as such throughout this article. The orthologue of human IGPR-1 is found only in eukaryotes, including primates, the guinea pig, canines, felines, dolphins, bovines, the llama, bats, the common shrew, and horses (Figure 1B). Of interest, the IGPR-1 gene is absent in mouse and rat genomes. The immunoglobulin domain of IGPR-1 was predicted to be Ig V (variable) fold and was found to be highly similar to the Ig domain of myelin-associated glycoprotein (MAG; Breithaupt et al., 2003). Using the Ig domain of MAG as a template, we constructed a structural model of IGPR-1. IGPR-1 seems to adapt a typical Ig V-like fold consisting of a sandwich of two antiparallel β-sheets (Figure 1C).
FIGURE 1:
Identification of IGPR-1 as a novel cell surface glycoprotein. (A) The deduced amino acid sequence of human IGPR-1. Amino acids 1–22 are a putative signal sequence (underlined). The immunoglobulin domain of IGPR-1 is shown (red), together with a proline-rich cytoplasmic region. (B) Orthologues of IGPR-1 in various species. (C) The predicted immunoglobulin domain of IGPR-1, which is based on the Ig domain of myelin-associated glycoprotein. IGPR-1 was cloned into retroviral vector (pMSCV), and PAE cells were transduced with viruses of empty vector or IGPR-1. (D) Whole-cell lysates derived from PAE cells were subjected to Western blot analysis using anti-IGPR-1 antibody. (E) Whole-cell lysates derived from PAE cells expressing IGPR-1 were either untreated or treated with PNGase and immunoblotted with anti-IGPR-1 antibody. (F) Immunofluorescence microscopy of PAE cells expressing IGPR-1. (G) PAE cells expressing IGPR-1 were subjected to cell surface biotinylation as described in Materials and Methods, and biotinylated IGPR-1 was detected by blotting with an anti–streptavidin-horseradish peroxidase antibody. (H) The same membrane was reblotted with anti–IGPR-1.
To examine its cellular and biochemical properties, IGPR-1 was cloned into a retroviral expression vector and expressed in porcine aortic endothelial (PAE) cells. Moreover, to detect expression of IGPR-1, we developed a polyclonal anti–IGPR-1 antibody against its cytoplasmic domain, which specifically recognizes IGPR-1 (Figure 1D). The predicted molecular weight of IGPR-1 protein is 31 kDa; however, the apparent molecular weight of IGPR-1 ectopically expressed in PAE cells as detected by Western blot analysis was ∼55 kDa (Figure 1D). We reasoned that the higher molecular weight of IGPR-1 could be associated with its potential glycosylation at its extracellular region. Treatment of cell lysates derived from PAE cells with N-glycosidase F (PNGase), which is known to hydrolyze nearly all types of N-glycan chains from glycoproteins, generated a 31-kDa protein (Figure 1E), suggesting that IGPR-1 is highly glycosylated in vivo and that glycosylation is responsible for its apparent high molecular weight. Of interest, treatment of cells with tunicamycin, a commonly used inhibitor of N-linked glycosylation in vivo, which is known to cause ER stress, also caused a rapid degradation of IGPR-1, suggesting that perhaps glycosylation of IGPR-1 is important for its stability and agents that induce ER stress promote degradation of IGPR-1 (unpublished data). Because IGPR-1 is predicted to be a plasma membrane protein, we also analyzed its membrane localization in PAE cells. The immunofluorescence microscopy assessment of cells expressing IGPR-1 showed that IGPR-1 is localized in the plasma membrane (Figure 1F). Additional analysis, including cell surface biotinylation, further demonstrated cell surface localization of IGPR-1 (Figure 1G). Taken together, the data establish IGPR-1 as a novel immunoglobulin containing membranous glycoprotein.
IGPR-1 is expressed in various human organs and cells
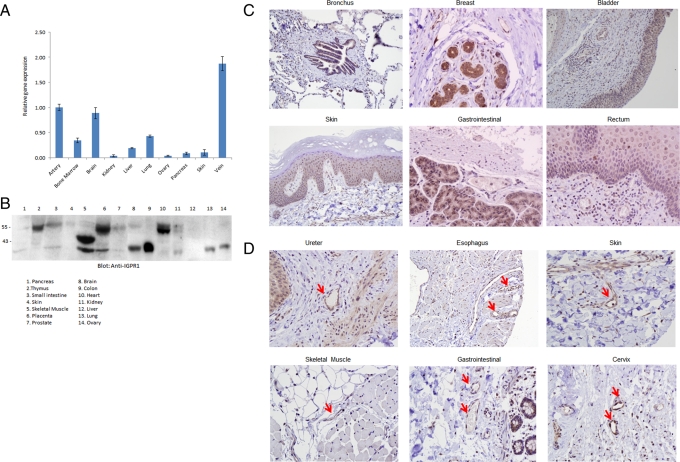
To gain insight into the tissue distribution of IGPR-1, we initially examined its expression in normal human tissues by analyzing the transcript of IGPR-1 by quantitative PCR (qPCR). The qPCR analysis using primers designed for exons 2 and 3 of IGPR-1 showed that IGPR-1 transcript was highly present in artery, vein, and brain. IGPR-1 transcript was also moderately detected in bone marrow, liver, and lung. IGPR-1 transcript, however, was relatively low in kidney, ovary, pancreas, and skin (Figure 2A). Additional analysis using qPCR primers designed for exons 3 and 4 yielded similar results (unpublished data), confirming that IGPR-1 transcript is broadly present in these tissues. To examine the expression of IGPR-1 in human tissues at the protein level, we analyzed cell lysates derived from various human organs/tissues for expression of IGPR-1. IGPR-1 protein was detected in thymus, placenta, heart, small intestine, skin, and kidney with an apparent molecular weight of 55 kDa (Figure 2B). Of interest, cell lysates derived from skeletal muscle, brain, colon, lung, and ovary IGPR-1 were detected with an apparent molecular weight of 35 kDa (Figure 2B), suggesting that perhaps IGPR-1 in these cell lysates is not fully glycosylated, although one could not discount possible proteolytic degradation due to sample preparation and handling.
FIGURE 2:
Expression of IGPR-1 in human organs and tissues. (A) Total RNA derived from various human organs was subjected to qPCR using primers specifically designed to amplify exons 2 and 3 of IGPR-1. The relative levels of IGPR-1 are shown. (B) Cell lysates derived from various human tissues were subjected to Western blot analysis using anti–IGPR-1 antibody. The human tissue microarray of normal human tissues is stained for IGPR-1. The array was viewed under light microscope and pictures were taken at 20× (C) or 40× (D). The red arrow indicates blood vessels.
To further determine the cellular distribution of IGPR-1, we used a normal human tissue microarray consisting of major human organs/tissues and subjected to immunohistochemical analysis using anti–IGPR-1 antibody. Immunohistochemical staining showed that IGPR-1 is mainly expressed by cells of epithelial origin, including bronchial epithelial cells of lung, breast glandular and lobular epithelia cells, urothelium of the bladder, skin epidermis, epithelium of gastrointestinal, and rectum (Figure 2C). Moreover, endometrial glands of the uterus, the ureter, fallopian tube epithelium, colonic epithelium, small bowl epithelium, stomach epithelium, including both chief and parietal cells, trophoblastic epithelium of placenta, and pancreatic acinar cells were all positive for IGPR-1 (unpublished data). Of note, thyroid, cerebellum, cerebral cortex, and thymus were negative for IGPR-1 (unpublished data). Beyond epithelial cells that were positive for IGPR-1 across the tissue microarray staining, endothelial cells present in vein and arteries also consistently were positive for IGPR-1, as shown in tissue sections derived from ureter, esophagus, skin, skeletal muscle, gastrointestinal, and cervix (Figure 2D). As noted, IGPR-1 transcript was also highest in vein and artery (Figure 2A). Taken together, the data demonstrate that IGPR1 is expressed in various organs; however; its expression is mainly in epithelial and endothelial cell types.
IGPR-1 activity regulates angiogenesis in vivo and in vitro
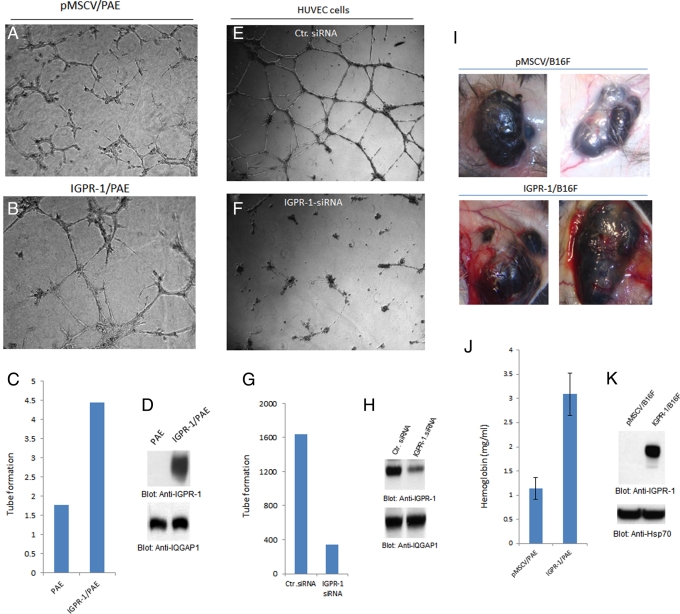
Adhesion molecules are known to regulate capillary tube formation of endothelial cells and angiogenesis (Gory-Faure et al., 1999; DeLisser et al., 2010). IGPR-1 is expressed by endothelial cells (Figure 2D), suggesting a possible role for IGPR-1 in angiogenesis. To examine the role of IGPR-1 in angiogenesis, we subjected PAE cells overexpressing IGPR-1 to an in vitro Matrigel-based angiogenesis assay. Our analysis showed that PAE cells that are engineered to express IGPR-1 undergo increased capillary tube formation (Figure 3A; compare with Figure 3B). Quantification of capillary tube formation of PAE cells and expression of IGPR-1 in PAE cells also are shown (Figure 3, C and D).
FIGURE 3:
IGPR-1 activity regulates angiogenesis. (A, B) PAE cells expressing empty vector (pMSCV) or IGPR-1 were subjected to Matrigel assay, and pictures were taken after 24 h. (C) Quantification of capillary tube formation. (D) Expression of IGPR-1 in PAE cells and protein loading control. (E, F) HUVECs transfected with control (Ctr) siRNA or IGPR-1 siRNA and then subjected to Matrigel assay. (G, H) Quantification of capillary tube formation of HUVECs and expression of IGPR-1. (I) B16F cells expressing empty vector or IGPR-1 were mixed with Matrigel and injected under skin for 8 d, and pictures were taken. (J) Quantification of angiogenesis was determined using Drabkin's reagent, which measures the hemoglobin content in tumor tissue. (K) Expression of IGPR-1 in B16F cells .
To examine the role of IGPR-1 in angiogenesis, in particular the role of endogenous IGPR-1 in capillary tube formation of endothelial cells, we silenced expression of IGPR-1 in human umbilical vein endothelial cells (HUVECs) by small interfering RNA (siRNA). Our analysis showed that IGPR-1 siRNA significantly reduced expression of IGPR-1 in HUVECs (Figure 3H). Knockdown of IGPR-1 by siRNA also resulted in a significant reduction in the capillary tube formation of HUVECs (Figure 3F). To address in vivo potential of IGPR-1 in angiogenesis, we engineered B16F melanoma cells to express IGPR-1 and subjected to in vivo Matrigel plug angiogenesis assay. Expression of IGPR-1 in B16F cells significantly increased the potential of B16F cells to stimulate angiogenesis, as blood vessels surrounding tumor mass are visibly notable in B16F cells expressing IGPR-1 (Figure 3I). Angiogenesis associated with tumor cells was further quantified by measuring the hemoglobin amount in the tumor cells (Figure 3J). Expression of IGPR-1 in B16F cells is shown (Figure 3K). Taken together, the data demonstrate that expression of IGPR-1 in endothelial cells regulates angiogenesis.
IGPR-1 regulates cellular morphology and focal adhesion
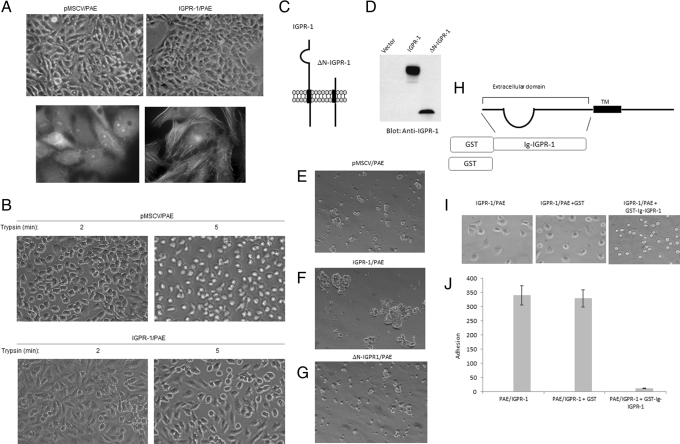
We noted that PAE cells ectopically expressing IGPR-1 display a distinct morphological change. PAE cells expressing IGPR-1 displayed rectangular shape morphology and appeared to be in tight contact, whereas the typical morphology of PAE cells is more elongated (Figure 4A). To demonstrate morphological changes associated with IGPR-1 in PAE cells, we stained these cells for actin filament formation using fluorescein isothiocyanate (FITC)–labeled phalloidin. Expression of IGPR-1 in PAE cells significantly increased actin filament formation (Figure 4A), indicating that IGPR-1 activity induces morphological changes in PAE cells. In general, the adhesive properties of a given cell type determine trypsinization time in cell culture. PAE cells expressing IGPR-1 were also resistant to trypsinization. Within 2–5 min of treatment with tissue culture trypsin/EDTA medium, PAE cells become fully dislodged from the cell culture plate, as they all showed a rounded morphology. However, most PAE cells expressing IGPR-1 after 5 min of treatment with trypsin/EDTA medium were still attached to the plate and mostly retained their adherent morphology (Figure 4B). After 8–10 min, eventually all were detached from the plate. The observation suggests that IGPR-1 is involved in regulation of cell morphology and adhesion.
FIGURE 4:
IGPR-1 regulates cellular morphology and adhesion. Morphology of PAE cells expressing IGPR-1 and PAE cells expressing empty vector. Pictures were taken under light microscopy (top). (A) PAE cells expressing empty vector or IGPR-1 were stained with FITC-labeled phalloidin, and pictures were taken under immunofluorescence microscopy. (B) PAE cells expressing either empty vector or IGPR-1 were trypsinized with 0.05% trypsin/EDTA for indicated times, cells were viewed under the microscope, and pictures were taken with a digital camera. (C) Schematic of IGPR-1 and N-terminus-deleted IGPR-1 ((ΔN-IGPR-1). (D) Expression of IGPR-1 and ΔN-IGPR-1 in PAE cells. (E–G) PAE cells expressing empty vector (pMSCV), IGPR-1, or ΔN-IGPR-1 were subjected to aggregation assay as described in Materials and Methods, and pictures were taken under a light microscope attached to a digital camera. (H) Schematic of generation of recombinant GST-IGPR-1. PAE cells expressing IGPR-1 were incubated either with DMEM medium alone, DMEM plus GST, or DMEM plus GST-N terminus/extracellular domain of IGPR-1. After 15 min of incubation, cells were plated in 24-well plates and allowed to adhere. (I) Pictures of cells were taken after 30 min of incubation in 24-well plates. (J) After 30 min, nonadherent cells were removed, and adherent cells were counted a under microscope (three randomly selected fields were counted in each well) and presented.
Because overexpression of IGPR-1 increases the adhesive phenotype of PAE cells, we examined the possible role of IGPR-1 in cell–cell interaction. To this end, we generated PAE cell lines expressing IGPR-1 and ΔN-IGPR-1 (where the extracellular domain of IGPR-1 was deleted) and subjected them to an aggregation assay. Expression of IGPR-1 and ΔN-IGPR-1 in PAE cells is shown in Figure 4, C and D. PAE cells expressing wild-type IGPR-1 but not ΔN-IGPR-1 or empty vector formed large aggregates of cells (Figure 4, E– G), suggesting that IGPR-1 mediates homophilic cell–cell interaction and that the extracellular domain is necessary for its function to mediate this. To examine role of IGPR-1 in cell adhesion and the role of its Ig-containing extracellular domain in this process, we generated a recombinant glutathione S-transferase (GST)–Ig containing the extracellular domain of IGPR-1 (Figure 4H). PAE cells expressing IGPR-1 and PAE cells expressing empty vector were preincubated with GST protein and both adhered to the plate in a similar manner. However, incubation of PAE cells expressing IGPR-1 with the soluble extracellular domain of IGPR-1 totally inhibited adhesion/spreading of these cells to the plate (Figure 4I). As noted, preincubated cells with soluble IGPR-1 only loosely adhered, with no spreading (Figure 4I), and they come off the tissue plate (unpublished data). Quantification of adherent cells also is shown (Figure 4J). Taken together, the data suggest that IGPR-1 activity regulates cell–cell interaction and adhesion, and its extracellular domain is critically important for its ability to mediate these cellular events.
IGPR-1 activity regulates focal adhesion and cell migration
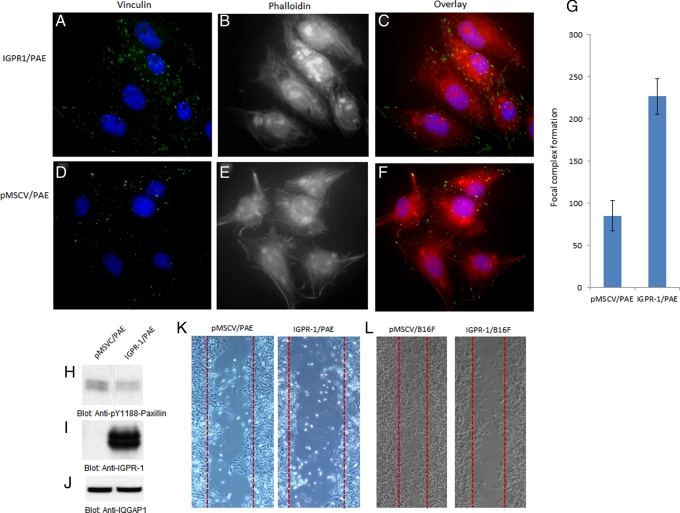
To further investigate biological responses associated with IGPR-1 activity in PAE cells, we analyzed focal adhesion formation in PAE cells. Immunofluorescence staining of PAE cells with vinculin demonstrated that expression of IGPR-1 in PAE cells markedly increases focal adhesion as measured by vinculin localization with respect to focal contact in these cells (Figure 5A). Of note, IGPR-1 expression in PAE cells increased both number and size of focal adhesions (Figure 5A). Of interest, adhesions in PAE cells expressing IGPR-1 were higher at the cell periphery, and consistent with their observed morphology, they were aligned along each other with a tight contact (Figure 5C; compare with Figure 5F). Quantitative analysis based on the number of focal adhesions showed that IGPR-1 expression in PAE cells resulted in an increased number of focal adhesions by 62% (Figure 5G). Staining of cells for actin with FITC-labeled phalloidin also demonstrated that expression of IGPR-1 in PAE cells alters actin stress fibers and cytoskeleton remodeling. PAE cells expressing empty vector form stress fibers primarily at the periphery of cells, with membrane ruffling, which is reflective of actin filament assembly (Figure 5F). PAE cells expressing IGPR-1, however, displayed a distinct actin stress fiber formation mainly in central regions of the cells, with a significantly reduced level of membrane ruffles (Figure 5B; compare with Figure 5E). Taken together, the data suggest that IGPR-1 regulates focal adhesion and actin stress fiber remodeling.
FIGURE 5:
IGPR-1 regulates focal adhesion and inhibits paxillin phosphorylation and cell migration. PAE cells expressing either empty vector or IGPR-1 were grown in 10% fetal bovine serum/DMEM medium and subjected to immunofluorescence staining as described in Materials and Methods. The immunofluorescence staining (green is vinculin staining, purple is nuclear staining, and red is phalloidin staining for F-actin) of PAE cells expressing empty vector vs. IGPR-1 is shown (A vs. D, B vs. E, C vs. F). (G) Quantification of focal adhesion PAE cells expressing empty vector, pMSCV, or IGPR-1. Cell lysates derived from PAE cells expressing empty vector and IGPR-1 were immunoblotted with anti–phospho-Y118 paxillin (H), anti–IGPR-1 (I), and IQGAP1 for protein loading control (J). (K, H) PAE and B16F cells expressing empty vector vs. IGPR-1 were subjected to wounding assay, and migration of cells toward the wound area was documented as described in Materials and Methods.
Because IGPR-1 activity regulates focal adhesion and reduced membrane ruffles, which are linked to cellular migration, we decided to analyze the possible function of IGPR-1 in cell migration. The activity of various proteins, including paxillin, an adaptor protein that localizes to focal adhesion, was implicated in the regulation of different steps of cell migration (Abou Zeid et al., 2006), and so we measured the phosphorylation of paxillin. The data indicate that expression of IGPR-1 in PAE cells reduces the phosphorylation of paxillin (Figure 5H). Phosphorylation of paxillin is known to inhibit cell migration (Abou Zeid et al., 2006; Zaidel-Bar et al., 2007), suggesting that perhaps IGPR-1 activity inhibits cell migration. To address the direct role of IGPR-1 in cell migration, we subjected PAE cells and B16F cells expressing IGPR-1 to wounding assay. Expression of IGPR-1 in two different cell types significantly inhibited cell migration (Figure 5, K and L). Taken together, the data suggest that IGPR-1 stimulates dephosphorylation of paxillin and inhibits cellular migration.
IGPR-1 associates with SH3-containing cytoplasmic signaling proteins
The cytoplasmic region of IGPR-1 is an emblematic proline-rich motif (Figure 1A). The proline-rich motif is known to interact with SH3-containing signaling proteins (Kaneko et al., 2008). To identify signaling proteins that interact with IGPR-1 and potentially mediate its cellular function, we used an SH3 protein array consisting of 34 SH3 domains derived from 34 individual proteins. The blotting of the SH3 array membrane with purified recombinant GST–proline-rich motif of IGPR-1 identified four distinct SH3 domains, including SH3 protein interacting with Nck (SPIN90/WISH; 90 kDa), calcium channel β2 (CACNB2), bullous pemphigoid antigen-1 (BPAG1), and melanoma inhibitory activity (MIA; Figure 6B).
FIGURE 6:
IGPR-1 associates with SH3-containing cytoplasmic signaling proteins. (A) Schematic of detection of IGPR-1–associated SH3 containing proteins. A protein array containing SH3 domains of 34 different proteins was blotted with a recombinant cytoplasmic domain of IGPR1 (rec-cIGPR-1) protein and was detected with anti–IGPR-1 antibody. (B) SH3 domains that interacted with rec-cIGPR-1 protein. (C) The other SH3 domains that were present in array but did not interact with IGPR-1. (D) Schematic of SPIN-90, BPAG1, and CACNB1. (E) The GST-SH3 domain of SPIN-90, CANCB2, and BPAG1 were incubated with cell lysates derived from PAE cells expressing IGPR-1 and subsequently was blotted with anti-IGPR-1 antibody. (E) PAE cells expressing empty vector and IGPR-1 were subjected to immunoprecipitation with anti-IGPR-1 and immunoblotted for SPIN90. (F) The same membrane was reblotted for IGPR-1 (lower panel).
SPIN90 is known to play a key role in cell adhesion and in actin cytoskeleton reorganization (Lim et al., 2001; Takenawa and Suetsugu, 2007). CACNB2 is one of the subunits of L-type voltage-pendent calcium channels. Voltage-dependent calcium channels are oligomeric proteins composed of α1, α2δ, β(1–4), and γ subunits, in which they control calcium entry into cells (Yamagata and Sanes, 2008). The β subunit is a member of membrane-associated guanylate kinase–like proteins with a guanylate kinase domain and an SH3 domain. BPAG1 is a member of plakin family proteins, comprising cytoskeleton-binding proteins (Fuchs and Yang, 1999; Fuchs and Karakesisoglou, 2001; Fuchs et al., 2004). BPAG1 is involved in anchoring keratin intermediate filaments to the cytoplasmic side of hemidesmosomes (Matsumura et al., 1997). MIA is a small, secreted protein that interacts with extracellular matrix proteins (Bosserhoff et al., 1999). To further validate the array data, we created a GST recombinant SH3 domain of SPIN90, BPAG1, and CANCB2 and tested their binding to IGPR-1 in a GST pull-down assay. The MIA binding to IGPR-1 was not considered for further analysis due the fact that it is a secreted protein. The data showed that IGPR-1 strongly binds to SH3 domains of SPIN90, BPAG1, and CANB2 (Figure 6E). The binding of SPIN90 and BPAG1 was stronger than that of CANCB2, suggesting that perhaps these SH3-containing proteins interact with IGPR-1 more readily than with CANCB2. We further validated the in vivo binding of SPIN90 with IGPR-1 by coimmunoprecipitation in PAE cells expressing IGPR-1. Cell lysates derived from PAE cells expressing IGPR-1 immunoprecipitated with anti–IGPR-1 selectively coprecipitated with endogenous SPIN90, as detected with anti-SPIN90 antibody (Figure 6F).
SPIN90 activity is required for angiogenesis
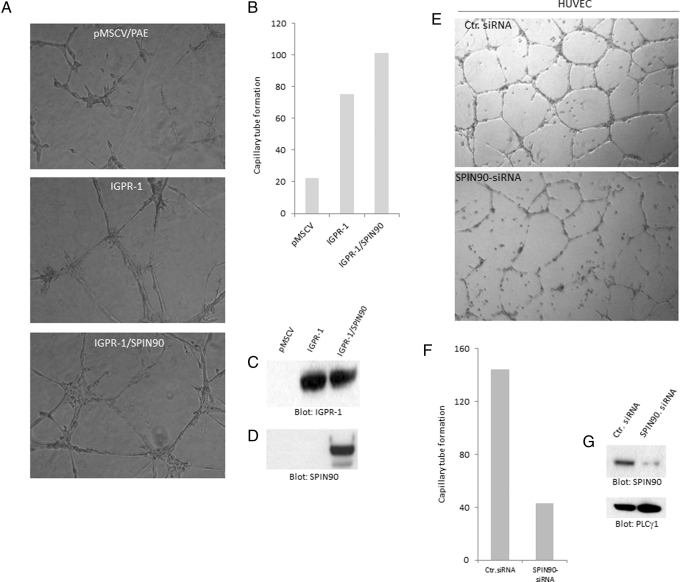
Identification of SPIN90 as an IGPR-1–interacting protein suggests that SPIN90 might play a role in IGPR-1–mediated angiogenesis. To further determine the biological importance of SPIN90 in angiogenesis, we initially overexpressed SPIN90 in PAE cells and examined its potential to stimulate capillary tube formation of PAE cells. Expression of SPIN90 in PAE cells increased capillary tube formation (Figure 7A), indicating that SPIN90 activity is linked to angiogenic events in endothelial cells. Quantification of capillary tube formation and expression of IGPR-1 and SPIN90 in PAE were also found (Figure 7, B and C). To address the biological importance of endogenous SPIN90 in angiogenesis, we silenced expression of SPIN90 by siRNA and examined the biological consequence of depletion of SPIN90 in HUVECs. Depletion of SPIN90 significantly reduced capillary tube formation of HUVECs (Figure 7E). Quantification of capillary tube formation of HUVECs transfected with control siRNA or SPIN90 siRNA is shown in Figure 7F. The effect of SPIN90 siRNA in silencing the expression of SPIN90 in HUVECs is shown in Figure 7G. Taken together, the data indicate that SPIN90 associates with IGPR-1 through its SH3 domain, and its activity regulates angiogenesis.
FIGURE 7:
SPIN90 activity regulates angiogenesis. PAE cells induced to express IGPR-1 alone or coexpress IGPR-1 with SPIN90. (A) Cells were subjected to Matrigel-based angiogenesis assay, and capillary tube formation was documented after 48 h. (B) Quantification of capillary tube formation determined using ImageJ software. (C, D) Whole-cell lysates derived from these cells blotted for IGPR-1 and SPIN90. (E) HUVECs were transfected with control siRNA or SPIN90-siRNA, and after 46 h, cells were subjected to Matrigel-based capillary tube formation, and pictures were taken after 48 h. (F) Quantification of capillary tube formation. (G) Expression of SPIN90 and protein loading control, PLCγ1, in HUVECs.
DISCUSSION
In this study we identified IGPR-1 as a novel cell surface protein expressed in various human organs and tissues, predominantly in cells of epithelial and endothelial origins. On the basis of the observed characteristics of IGPR-1, such as promoting cellular aggregation, cell–cell interaction, morphological change of cells, and increase in focal adhesion, we propose IGPR-1 as a novel CAM. Various classes of CAMs have been described, including, cadherins, mucins, integrins, selectins, and the Ig superfamily (Takai et al., 2008). CAMs mediate homophilic (like-binds-like) adhesion between cells of a single type and heterophilic adhesion between cells of different types, and the cytoplasmic regions of these proteins are usually connected to the cytoskeleton (Takai et al., 2008). Consistent with its observed effect on cell adhesion, transfer of IGPR-1 into PAE cells significantly increases the expression of vinculin. Vinculin is a major constitutive component of focal adhesion and adherens junctions, where it binds to several cytoskeletal proteins, linking microfilaments to CAMs (Pawlak and Helfman, 2001). Consistent with CAM-mediated phenotypic characteristics such as focal adhesion and cellular morphology, introducing IGPR-1 to PAE cells resulted in the acquisition of cubical morphology and elevated focal adhesion. These cells also were resistant to trypsin/EDTA-mediated detachment from cell culture plates and acquired increased cell adhesion properties. The function of IGPR-1 is highly correlated with those of other well-known CAMs. Cadherins are known to stabilize cell–cell contacts to form adherent junctions in epithelial cells, fibroblasts, and endothelial cells (Matsumura et al., 1997; Bach et al., 1998; Gory-Faure et al., 1999).
In agreement with a possible role of IGPR-1 in cell adhesion and cell–cell interaction, expression of IGPR-1 in PAE cells and B16F cells inhibits cell migration. Of interest, the reduced migration of these cells by IGPR-1 also correlates with the inhibition of phosphorylation of paxillin at tyrosine 118 (Y118). Phosphorylation of Y118 of paxillin is linked to inhibition of cell migration (Abou Zeid et al., 2006; Zaidel-Bar et al., 2007), suggesting that IGPR-1 inhibits cellular migration, in part by stimulating dephosphorylation of paxillin either by increasing protein phosphatase activity or preventing its phosphorylation by inhibiting tyrosine kinases such as Src family kinases and focal adhesion kinases, which are involved in the phosphorylation of paxillin. The cytoplasmic domain of IGPR-1 contains a proline-rich motif that recognizes SH3-containing signaling proteins. The data presented in this article demonstrate that the cytoplasmic domain of IGPR-1 interacts with several SH3-containing signaling proteins, including SPIN90. SPIN90 activity is known to regulate cell adhesion through actin cytoskeleton reorganization (Lim et al., 2001; Takenawa and Miki, 2001), suggesting that the proline-rich cytoplasmic domain of IGPR-1, through recruitment of SPIN90, modulates cell adhesion and cellular morphology. Our observation indicates that SPIN90 regulates capillary tube formation of endothelial cells, demonstrating a significant role for SPIN90 in IGPR-1–mediated biological responses, particularly in angiogenesis.
In general, CAMs interact with distinct signaling proteins whose activities are linked to the cytoskeleton. For example, cadherins are known to interact with β-catenin (Harris and Tepass, 2010), nectins interact with the filamentous (F)-actin–binding protein afadin and PAR3 (Takai et al., 2008), ICAM-1 associates with α-actinin and ERM proteins (Barreiro et al., 2002), and JAMs (junctional adhesion molecules) bind to ZO1 (Bazzoni, 2003). Unlike the known CAMs, IGPR-1 distinctively interacts with SH3-containing proteins, including SPIN90, BPAG1, and CANCB2, which might differentiate IGPR-1 from the already known CAMs.
Another interesting and important aspect of this work is that IGPR-1 is mainly expressed in cells of epithelial and endothelial cell origin. For example, epithelium and endothelium of various organs/tissues were positive for IGPR-1, suggesting that IGPR-1 as a novel adhesion molecule could play a significant role in the pathobiology of endothelial and epithelial cells. Angiogenesis—the growth of new blood vessels from preexisting blood vessels—is an important physiological process in the body and is required for normal wound healing and female reproductive activity. Pathological angiogenesis, either excessive or insufficient, is now recognized as a common denominator underlying a number of deadly and debilitating human diseases such as cancer, age-related macular degeneration, diabetic retinopathy, and cardiovascular diseases (Carmeliet and Jain, 2011b). Acquisition of angiogenesis by tumor cells is considered the most critical step in tumor growth and metastasis. To grow beyond 2 mm in diameter, a tumor needs to undergo angiogenesis, which is often established by hypoxia-induced expression of VEGF and other hypoxia-induced growth factors (Carmeliet and Jain, 2011b). The present study suggests an important role for IGPR-1 in angiogenesis. Modulation of expression of IGPR-1 by ectopic expression or silencing in endothelial cells significantly altered the angiogenic phenotype of endothelial cells in culture, and introducing IGPR-1 to tumor cells increased tumor angiogenesis.
In conclusion, we identified IGPR-1 as a novel adhesion molecule involved in cell–cell interaction and regulation of cell migration. IGPR-1 associates with several SH3-containing signaling proteins, including SPIN90, and regulates angiogenesis, suggesting a significant role for IGPR-1 in angiogenesis-associated diseases such as cancer. Further studies should illuminate the mechanisms by which IGPR-1 regulates cell–cell interaction and its biological importance in human diseases, in particular in angiogenesis-associated diseases such as cancer.
MATERIALS AND METHODS
General reagents
BS3 was purchased from Pierce Biotechnology (Rockford, IL). SYBR green qPCR reagents were purchased from Applied Biosystems (Carlsbad, CA). Immunohistochemistry/immunofluorescence microscopy reagents included a Dako primary staining kit, the EnVision System-HRP (DAB) for rabbit primary antibodies (K4010), antibody diluent, background reducer (S3022), citrate buffer (S1966), and Protein Block Serum-Free (X0909), all from Dako (Carpinteria, CA). Hematoxylin (AMH100907) was purchased from Thermo Scientific (Waltham, MA). The proliferation kit was purchased from Promega (Madison, WI). PNGase F was purchased from New England BioLabs (Ipswich, MA). Total human RNA was purchased from BioChain (Newark, CA).
Plasmids, primers, siRNAs, and antibodies
The cDNA corresponding to IGPR-1 (MGC:23244, IMAGE:4811204), which was purchased from Open Biosystems, Thermo Biosystems, Huntsville, AL, was PCR amplified and cloned into retroviral vector pMSCV.puro (Invitrogen, Carlsbad, CA) via XhoI and EcoRI restriction sites. IGPR-1 was cloned in frame of the pcDNA3.1.His.Myc vector (Invitrogen), and the identity of IGPR-1 was confirmed by sequencing. The N-terminus–truncated IGPR-1 (ΔN-IGPR-1) was PCR amplified using IGPR-1 as a template, which results in the deletion of 133 amino acids. Polyclonal rabbit anti–IGPR-1 antibody was developed by injecting a KLH (keyhole limpet hemocyanin)-conjugated peptide corresponding to the cytoplasmic domain of IGPR-1 into rabbits. Anti–IGPR-1 antibody was purified by protein A–agarose column. The specificity of the antibody was further tested by preincubation with the corresponding peptide with antibody, which eliminated its ability to detect IGPR-1. SPIN90 siRNA (sc-76564) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). IGPR-1 siRNAs were custom made and synthesized by Thermo Scientific/Dharmacon (Lafayette, CO): CAGCAAAGGGACUCAGGUAUU and AGGUAACAGCCCAGGAAAUUU. The qPCR primers used for IGPR-1 are 3′CTG AGT TGG AGG AGG CTG AG and CGA TCC GGT TTC TGT TCT GT.
Aggregation assay
Single-cell suspensions were obtained by detaching cells from a plate with 1 mM EDTA in phosphate-buffered saline (PBS) solution. The cells were then washed twice in 10% DMEM medium. The cells were resuspended in DMEM. Approximately 5 × 105 cells per 2.5 ml were incubated in a six-well plate (precoated with 1% bovine serum albumin at 37°C for 2 h) with gentle shaking at 37°C for 1 h, followed by no shaking for 1 h. Cells were viewed under a light microscope, and images were taken.
Cell adhesion assay
For cell adhesion assay, cells expressing IGPR-1 were detached from plates incubated in suspension for 2 h with GST or the GST-Ig domain of IGPR-1 and replated onto tissue culture dishes and allowed to adhere for 2 h at 37°C. Nonadherent cells were removed by washing twice with PBS, after which the adherent cells were fixed for 5 min in methanol and counted under a microscope.
RT-qPCR analysis
RT-qPCR (reverse transcription quantitative PCR) was performed as recommended by the manufacturer (Applied Biosystems) and 18S used as internal controls.
Mouse Matrigel angiogenesis assay
Mice (six animals for each experimental group) were injected with Matrigel (10 mg/ml) plus B16 melanoma cells (1 × 107) that were engineered to express IGPR-1 or empty vector. Before injection, the animals were sedated with Avertin (0.3 ml per 20-g mouse). A 25-gauge needle was used to inject 0.3 ml of Matrigel mixture subdermally into mice. After 8 d, animals were scarified, and tumor-induced angiogenesis and Matrigel plugs were removed for further analysis as described (Meyer et al., 2011a). The tumor plugs were homogenized in 1 ml of deionized H2O on ice and cleared by centrifugation at 10,000 rpm for 5 min at 4°C. The supernatant was collected and used in duplicate to measure hemoglobin content with Drabkin's reagent along with hemoglobin standards as suggested by the manufacturer (Sigma-Aldrich, St. Louis, MO). The absorbance was read at 540 nm.
Endothelial cell capillary tube formation and migration assays
Endothelial cells were seeded on Matrigel with endothelial cell growth medium (Clonetics, San Diego, CA), and capillary tube formation was viewed under microscope and photographed after 24 h as described (Meyer et al., 2008). Quantification of capillary tube formation was established by ImageJ (National Institutes of Health, Bethesda, MD). Migration assay was performed basically by creating a “wound” in a cell monolayer by scratch using the tip of the tissue culture pipette. After 10 h, images were captured under microscope and documented.
Site-directed mutagenesis
All the site-directed mutagenesis was performed using a PCR-based, site-directed mutagenesis strategy (Meyer et al., 2006). The identities of deletions were confirmed by sequencing the plasmids. All the cDNAs were either cloned into pcDNA3.1His.Myc vector or into the retroviral vector pMSCV.puro (Clontech, Mountain View, CA). In some cases the PCR products were cloned into pGEX2T vector (Pharmacia, GE Healthcare, Piscataway, NJ) and used to make GST-fusion protein in Escherichia coli.
Virus production and transient transfection
For virus production pMSCV.puro vector containing IGPR-1 or other cDNA of interest was transfected into 293-GPG cells, and viral supernatants were collected for 5 d as previously described (Rahimi et al., 2000).
Immunoprecipitation, Western blotting, and cell surface biotinylation
Cells were prepared, lysed, and subjected to immunoprecipitation, or whole-cell lysates were subjected to Western blot analysis as described before (Meyer et al., 2011b). Normalized whole-cell lysates were subjected to Western blot analysis using IGPR-1 antibody or with appropriate antibody as indicated in the figure legends. Cell surface biotinylation was performed as follows. Equal numbers of PAE cells expressing IGPR-1 were washed three times with ice-cold PBS. Surface proteins were biotinylated by incubating cells with 0.5 mg/ml of the membrane-impermeable biotin analogue EZ-Link Sulfo-NHS-LC-Biotin (Pierce) in ice-cold PBS (pH 7.4) for 30 min at 4°C. Unreacted biotin was quenched and removed by three washes with ice-cold H/S buffer (25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.4, 150 mM NaCl, and 2 mM Na3VO4) at 4°C. Biotinylated cells were lysed, equal amounts of protein from each lysate were immunoprecipitated using a rabbit polyclonal anti–IGPR-1 antibody, and immunoprecipitated proteins were subjected to Western blot analysis using streptavidin-horseradish peroxidase conjugate and detected by an ECL System (Amersham-Pharmacia Biotech, GE Healthcare Bio-Sciences, Piscataway, NJ).
Immunohistochemical analysis/immunofluorescence microscopy
Normal human tissue microarray (MC0961) was purchased from US BioMax (Rockville, MD). Immunohistochemical staining was performed using rabbit anti–IGPR-1 antibody. After standard formalin fixation and paraffin embedding (FFPE), all FFPE slides were subjected to heat-induced epitope retrieval through the BioCare Medical Decloaking chamber, with citrate buffer at pH 6.0. Antibody detection was preformed with the anti–Rabbit-HRP Envision+ Kit (Dako) with extra blocking with serum-free protein block (Dako) for 1 h. For immunofluorescence microscopy PAE cells expressing IPR-1 were fixed with 4% paraformaldehyde for 30 min and, after washing with PBS (3×), permeabilized with 0.01% Triton X-100 for 1 min. Cells were incubated with 5% bovine serum albumin for 45 min with rocking and then incubated with anti–IPR-1 antibody. After extensive washing and incubation with secondary antibody they were mounted with anti-fade/4′,6-diamidino-2-phenylindole (Vectastain; Vector Laboratories, Burlingame, CA).
Acknowledgments
This work was supported in part through grants from the National Eye Institute, National Institutes of Health, to N.R. This work was also supported by a grant from the Department of Pathology, Boston University, and a Massachusetts Lions Foundation grant to the Department of Ophthalmology. We thank Srimathi Srinivasan for helping with siRNA transfection of HUVECs.
Abbreviations used:
- CAM
cell adhesion molecule
- IGPR-1
immunoglobulin-containing and proline-rich receptor-1
- JAM-A
junctional adhesion molecule-A
- TMIGD2
immunoglobulin domain–containing protein 2
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-11-0934) on March 14, 2012.
REFERENCES
- Abou Zeid N, Valles AM, Boyer B. Serine phosphorylation regulates paxillin turnover during cell migration. Cell Commun Signal. 2006;4:8. doi: 10.1186/1478-811X-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach TL, Barsigian C, Chalupowicz DG, Busler D, Yaen CH, Grant DS, Martinez J. VE-Cadherin mediates endothelial cell capillary tube formation in fibrin and collagen gels. Exp Cell Res. 1998;238:324–334. doi: 10.1006/excr.1997.3844. [DOI] [PubMed] [Google Scholar]
- Barclay AN. Membrane proteins with immunoglobulin-like domains—a master superfamily of interaction molecules. Semin Immunol. 2003;15:215–223. doi: 10.1016/s1044-5323(03)00047-2. [DOI] [PubMed] [Google Scholar]
- Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol. 2002;157:1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni G. The JAM family of junctional adhesion molecules. Curr Opin Cell Biol. 2003;15:525–530. doi: 10.1016/s0955-0674(03)00104-2. [DOI] [PubMed] [Google Scholar]
- Bosserhoff AK, Lederer M, Kaufmann M, Hein R, Stolz W, Apfel R, Bogdahn U, Buettner R. MIA, a novel serum marker for progression of malignant melanoma. Anticancer Res. 1999;19:2691–2693. [PubMed] [Google Scholar]
- Breithaupt C, Schubart A, Zander H, Skerra A, Huber R, Linington C, Jacob U. Structural insights into the antigenicity of myelin oligodendrocyte glycoprotein. Proc Natl Acad Sci USA. 2003;100:9446–9451. doi: 10.1073/pnas.1133443100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011a;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev. Drug Discov. 2011b;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- DeLisser H, et al. Vascular endothelial platelet endothelial cell adhesion molecule 1 (PECAM-1) regulates advanced metastatic progression. Proc Natl Acad Sci USA. 2010;107:18616–18621. doi: 10.1073/pnas.1004654107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Karakesisoglou I. Bridging cytoskeletal intersections. Genes Dev. 2001;15:1–14. doi: 10.1101/gad.861501. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Yang Y. Crossroads on cytoskeletal highways. Cell. 1999;98:547–550. doi: 10.1016/s0092-8674(00)80041-0. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Hutzler P, Handschuh G, Hermannstadter C, Brunner I, Hofler H, Luber B. Dynamics of cell adhesion and motility in living cells is altered by a single amino acid change in E-cadherin fused to enhanced green fluorescent protein. Cell Motil Cytoskeleton. 2004;59:50–61. doi: 10.1002/cm.20019. [DOI] [PubMed] [Google Scholar]
- Gory-Faure S, Prandini MH, Pointu H, Roullot V, Pignot-Paintrand I, Vernet M, Huber P. Role of vascular endothelial-cadherin in vascular morphogenesis. Development. 1999;126:2093–2102. doi: 10.1242/dev.126.10.2093. [DOI] [PubMed] [Google Scholar]
- Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev. Mol Cell Biol. 2010;11:502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Li L, Li SS. The SH3 domain—a family of versatile peptide- and protein-recognition module. Front Biosci. 2008;13:4938–4952. doi: 10.2741/3053. [DOI] [PubMed] [Google Scholar]
- Lim CS, Park ES, Kim DJ, Song YH, Eom SH, Chun JS, Kim JH, Kim JK, Park D, Song WK. SPIN90 (SH3 protein interacting with Nck, 90 kDa), an adaptor protein that is developmentally regulated during cardiac myocyte differentiation. J Biol Chem. 2001;276:12871–12878. doi: 10.1074/jbc.M009411200. [DOI] [PubMed] [Google Scholar]
- Matsumura T, Wolff K, Petzelbauer P. Endothelial cell tube formation depends on cadherin 5 and CD31 interactions with filamentous actin. J Immunol. 1997;158:3408–3416. [PubMed] [Google Scholar]
- Meyer RD, Husain D, Rahimi N. c-Cbl inhibits angiogenesis and tumor growth by suppressing activation of PLCgamma1. Oncogene. 2011a;30:2198–2206. doi: 10.1038/onc.2010.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RD, Qian X, Guo HC, Rahimi N. Leucine motif-dependent tyrosine autophosphorylation of type III receptor tyrosine kinases. J Biol Chem. 2006;281:8620–8627. doi: 10.1074/jbc.M512309200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RD, Sacks DB, Rahimi N. IQGAP1-dependent signaling pathway regulates endothelial cell proliferation and angiogenesis. PloS One. 2008;3:e3848. doi: 10.1371/journal.pone.0003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RD, Srinivasan S, Singh AJ, Mahoney JE, Gharahassanlou KR, Rahimi N. PEST motif serine and tyrosine phosphorylation controls vascular endothelial growth factor receptor 2 stability and downregulation. Mol Cell Biol. 2011b;31:2010–2025. doi: 10.1128/MCB.01006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak G, Helfman DM. Cytoskeletal changes in cell transformation and tumorigenesis. Curr Opin Genet Dev. 2001;11:41–47. doi: 10.1016/s0959-437x(00)00154-4. [DOI] [PubMed] [Google Scholar]
- Rahimi N. VEGFR-1 and VEGFR-2: two non-identical twins with a unique physiognomy. Front Biosci. 2006;11:818–829. doi: 10.2741/1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi N, Dayanir V, Lashkari K. Receptor chimeras indicate that the vascular endothelial growth factor receptor-1 (VEGFR-1) modulates mitogenic activity of VEGFR-2 in endothelial cells. J Biol Chem. 2000;275:16986–16992. doi: 10.1074/jbc.M000528200. [DOI] [PubMed] [Google Scholar]
- Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev. Mol Cell Biol. 2008;9:603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- Takenawa T, Miki H. WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J Cell Sci. 2001;114:1801–1809. doi: 10.1242/jcs.114.10.1801. [DOI] [PubMed] [Google Scholar]
- Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev. Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci. 2007;120:137–148. doi: 10.1242/jcs.03314. [DOI] [PubMed] [Google Scholar]