Abstract
Transcriptionally active nuclear extracts from human breast carcinoma cells (T47D) were used to compare the action of progestins and several antiprogestins of the 11 beta-aryl substituted steroid series on the DNA-binding properties and the trans-activating potential of progesterone receptor (PR) in vitro. Using the gel-shift assay we identified a novel type of antiprogestin (ZK98299, type I), which in contrast to type II antiprogestins, including RU486, does not induce binding of PR to progesterone response elements (PREs). In competition experiments excess of type I antiprogestin inhibits induction of DNA binding of PR by progestins and type II antiprogestins suggesting that its binding to PR interferes with the formation of stable receptor dimers. Moreover, we demonstrate that the antagonistic action of ZK98299 can be fully mimicked in vitro by using cell-free nuclear extracts from T47D cells and a 'simple' test promoter. In contrast, type II antiprogestins known to induce certain promoters in vivo exert strong agonistic effects on in vitro transcription of the test template used.
Full text
PDF

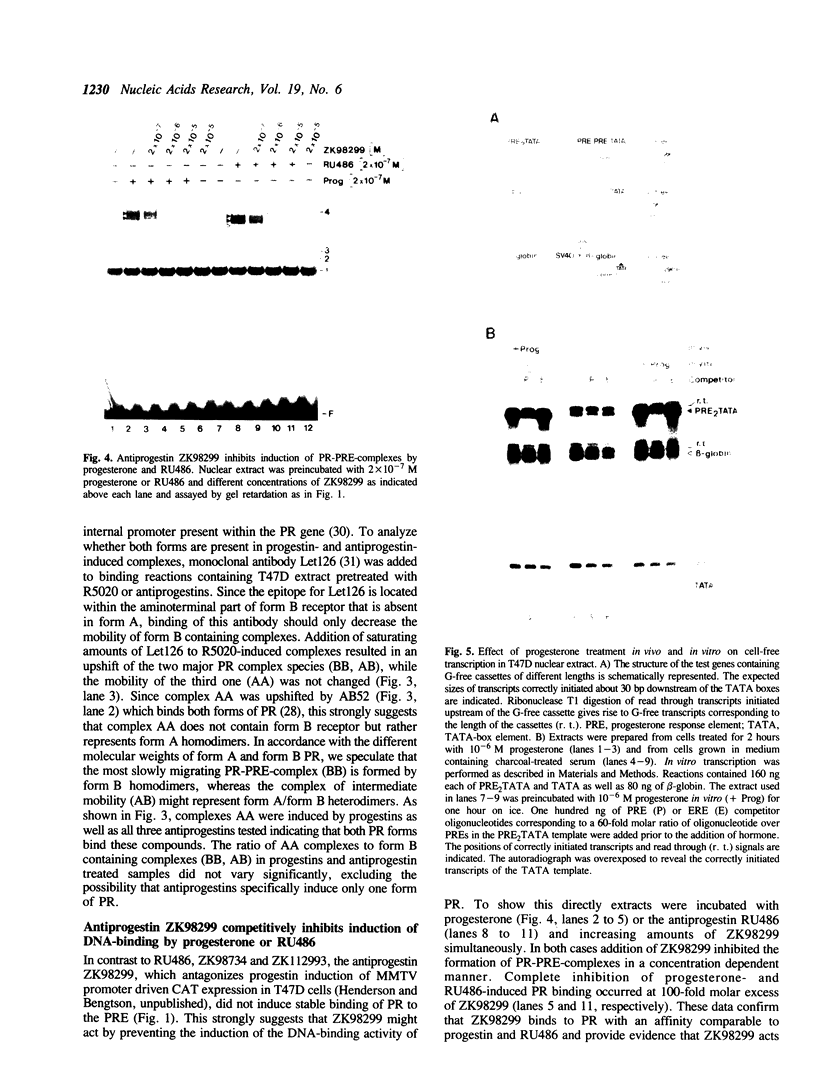
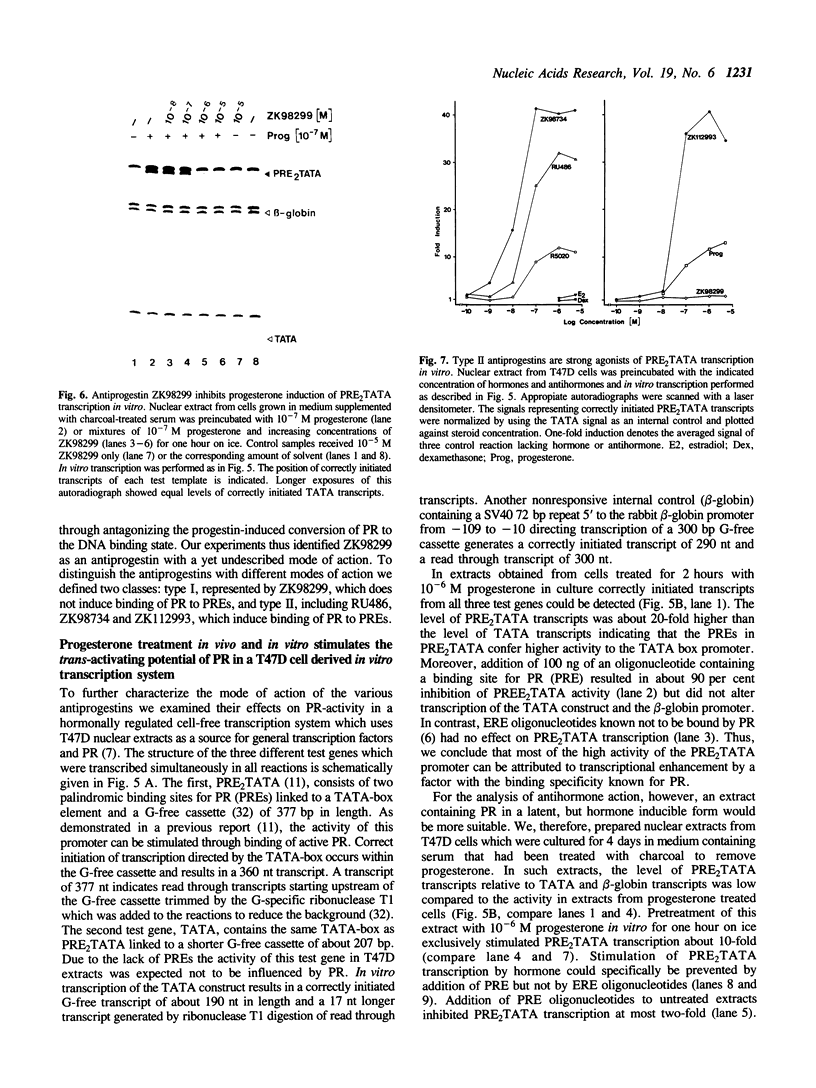



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchi M. K., Elliston J. F., Tsai S. Y., Edwards D. P., Tsai M. J., O'Malley B. W. Steroid hormone-dependent interaction of human progesterone receptor with its target enhancer element. Mol Endocrinol. 1988 Dec;2(12):1221–1229. doi: 10.1210/mend-2-12-1221. [DOI] [PubMed] [Google Scholar]
- Bagchi M. K., Tsai S. Y., Tsai M. J., O'Malley B. W. Identification of a functional intermediate in receptor activation in progesterone-dependent cell-free transcription. Nature. 1990 Jun 7;345(6275):547–550. doi: 10.1038/345547a0. [DOI] [PubMed] [Google Scholar]
- Baulieu E. E. Contragestion and other clinical applications of RU 486, an antiprogesterone at the receptor. Science. 1989 Sep 22;245(4924):1351–1357. doi: 10.1126/science.2781282. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Cato A. C., Henderson D., Ponta H. The hormone response element of the mouse mammary tumour virus DNA mediates the progestin and androgen induction of transcription in the proviral long terminal repeat region. EMBO J. 1987 Feb;6(2):363–368. doi: 10.1002/j.1460-2075.1987.tb04763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato A. C., Miksicek R., Schütz G., Arnemann J., Beato M. The hormone regulatory element of mouse mammary tumour virus mediates progesterone induction. EMBO J. 1986 Sep;5(9):2237–2240. doi: 10.1002/j.1460-2075.1986.tb04490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalepakis G., Arnemann J., Slater E., Brüller H. J., Gross B., Beato M. Differential gene activation by glucocorticoids and progestins through the hormone regulatory element of mouse mammary tumor virus. Cell. 1988 May 6;53(3):371–382. doi: 10.1016/0092-8674(88)90157-2. [DOI] [PubMed] [Google Scholar]
- Elger W., Beier S., Chwalisz K., Fähnrich M., Hasan S. H., Henderson D., Neef G., Rohde R. Studies on the mechanisms of action of progesterone antagonists. J Steroid Biochem. 1986 Nov;25(5B):835–845. doi: 10.1016/0022-4731(86)90314-6. [DOI] [PubMed] [Google Scholar]
- Estes P. A., Suba E. J., Lawler-Heavner J., Elashry-Stowers D., Wei L. L., Toft D. O., Sullivan W. P., Horwitz K. B., Edwards D. P. Immunologic analysis of human breast cancer progesterone receptors. 1. Immunoaffinity purification of transformed receptors and production of monoclonal antibodies. Biochemistry. 1987 Sep 22;26(19):6250–6262. doi: 10.1021/bi00393a045. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell S. E., White R., Hoare S., Sydenham M., Page M., Parker M. G. Inhibition of estrogen receptor-DNA binding by the "pure" antiestrogen ICI 164,384 appears to be mediated by impaired receptor dimerization. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6883–6887. doi: 10.1073/pnas.87.17.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Chambon P. A superfamily of potentially oncogenic hormone receptors. Nature. 1986 Dec 18;324(6098):615–617. doi: 10.1038/324615a0. [DOI] [PubMed] [Google Scholar]
- Guiochon-Mantel A., Loosfelt H., Lescop P., Sar S., Atger M., Perrot-Applanat M., Milgrom E. Mechanisms of nuclear localization of the progesterone receptor: evidence for interaction between monomers. Cell. 1989 Jun 30;57(7):1147–1154. doi: 10.1016/0092-8674(89)90052-4. [DOI] [PubMed] [Google Scholar]
- Guiochon-Mantel A., Loosfelt H., Ragot T., Bailly A., Atger M., Misrahi M., Perricaudet M., Milgrom E. Receptors bound to antiprogestin from abortive complexes with hormone responsive elements. Nature. 1988 Dec 15;336(6200):695–698. doi: 10.1038/336695a0. [DOI] [PubMed] [Google Scholar]
- Ham J., Parker M. G. Regulation of gene expression by nuclear hormone receptors. Curr Opin Cell Biol. 1989 Jun;1(3):503–511. doi: 10.1016/0955-0674(89)90012-4. [DOI] [PubMed] [Google Scholar]
- Herrmann W., Wyss R., Riondel A., Philibert D., Teutsch G., Sakiz E., Baulieu E. E. Effet d'un stéroide anti-progestérone chez la femme: interruption du cycle menstruel et de la grossesse au début. C R Seances Acad Sci III. 1982 May 17;294(18):933–938. [PubMed] [Google Scholar]
- Horwitz K. B., Alexander P. S. In situ photolinked nuclear progesterone receptors of human breast cancer cells: subunit molecular weights after transformation and translocation. Endocrinology. 1983 Dec;113(6):2195–2201. doi: 10.1210/endo-113-6-2195. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B. The antiprogestin RU38 486: receptor-mediated progestin versus antiprogestin actions screened in estrogen-insensitive T47Dco human breast cancer cells. Endocrinology. 1985 Jun;116(6):2236–2245. doi: 10.1210/endo-116-6-2236. [DOI] [PubMed] [Google Scholar]
- Kalff M., Gross B., Beato M. Progesterone receptor stimulates transcription of mouse mammary tumour virus in a cell-free system. Nature. 1990 Mar 22;344(6264):360–362. doi: 10.1038/344360a0. [DOI] [PubMed] [Google Scholar]
- Kastner P., Krust A., Turcotte B., Stropp U., Tora L., Gronemeyer H., Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990 May;9(5):1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Hitpass L., Tsai S. Y., Greene G. L., Clark J. H., Tsai M. J., O'Malley B. W. Specific binding of estrogen receptor to the estrogen response element. Mol Cell Biol. 1989 Jan;9(1):43–49. doi: 10.1128/mcb.9.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Hitpass L., Tsai S. Y., Weigel N. L., Allan G. F., Riley D., Rodriguez R., Schrader W. T., Tsai M. J., O'Malley B. W. The progesterone receptor stimulates cell-free transcription by enhancing the formation of a stable preinitiation complex. Cell. 1990 Jan 26;60(2):247–257. doi: 10.1016/0092-8674(90)90740-6. [DOI] [PubMed] [Google Scholar]
- Klijn J. G., de Jong F. H., Bakker G. H., Lamberts S. W., Rodenburg C. J., Alexieva-Figusch J. Antiprogestins, a new form of endocrine therapy for human breast cancer. Cancer Res. 1989 Jun 1;49(11):2851–2856. [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Lorenzo F., Jolivet A., Loosfelt H., Thu vu Hai M., Brailly S., Perrot-Applanat M., Milgrom E. A rapid method of epitope mapping. Application to the study of immunogenic domains and to the characterization of various forms of rabbit progesterone receptor. Eur J Biochem. 1988 Sep 1;176(1):53–60. doi: 10.1111/j.1432-1033.1988.tb14250.x. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Gronemeyer H., Turcotte B., Bocquel M. T., Tasset D., Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989 May 5;57(3):433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Pornon A., Ji J. W., Bocquel M. T., Chambon P., Gronemeyer H. Agonistic and antagonistic activities of RU486 on the functions of the human progesterone receptor. EMBO J. 1990 Dec;9(12):3923–3932. doi: 10.1002/j.1460-2075.1990.tb07613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michna H., Schneider M. R., Nishino Y., el Etreby M. F. Antitumor activity of the antiprogestins ZK 98.299 and RU 38.486 in hormone dependent rat and mouse mammary tumors: mechanistic studies. Breast Cancer Res Treat. 1989 Dec;14(3):275–288. doi: 10.1007/BF01806299. [DOI] [PubMed] [Google Scholar]
- Michna H., Schneider M. R., Nishino Y., el Etreby M. F. The antitumor mechanism of progesterone antagonists is a receptor mediated antiproliferative effect by induction of terminal cell death. J Steroid Biochem. 1989;34(1-6):447–453. doi: 10.1016/0022-4731(89)90126-x. [DOI] [PubMed] [Google Scholar]
- Neef G., Beier S., Elger W., Henderson D., Wiechert R. New steroids with antiprogestational and antiglucocorticoid activities. Steroids. 1984 Oct;44(4):349–372. doi: 10.1016/s0039-128x(84)80027-6. [DOI] [PubMed] [Google Scholar]
- Picard D., Khursheed B., Garabedian M. J., Fortin M. G., Lindquist S., Yamamoto K. R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990 Nov 8;348(6297):166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Ryffel G. U., Kugler W., Wagner U., Kaling M. Liver cell specific gene transcription in vitro: the promoter elements HP1 and TATA box are necessary and sufficient to generate a liver-specific promoter. Nucleic Acids Res. 1989 Feb 11;17(3):939–953. doi: 10.1093/nar/17.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P., Duboule D., Chambon P. Viral enhancer activity in teratocarcinoma cells. Cold Spring Harb Symp Quant Biol. 1985;50:747–752. doi: 10.1101/sqb.1985.050.01.092. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Factors involved in specific transcription by human RNA polymerase II: analysis by a rapid and quantitative in vitro assay. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4394–4398. doi: 10.1073/pnas.82.13.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. R., Michna H., Nishino Y., el Etreby M. F. Antitumor activity of the progesterone antagonists ZK 98.299 and RU 38.486 in the hormone-dependent MXT mammary tumor model of the mouse and the DMBA- and the MNU-induced mammary tumor models of the rat. Eur J Cancer Clin Oncol. 1989 Apr;25(4):691–701. doi: 10.1016/0277-5379(89)90206-x. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Sharp P. A., Wahli W. W., Keller M. J. A high-efficiency HeLa cell nuclear transcription extract. DNA. 1988 Jan-Feb;7(1):47–55. doi: 10.1089/dna.1988.7.47. [DOI] [PubMed] [Google Scholar]
- Tsai S. Y., Carlstedt-Duke J., Weigel N. L., Dahlman K., Gustafsson J. A., Tsai M. J., O'Malley B. W. Molecular interactions of steroid hormone receptor with its enhancer element: evidence for receptor dimer formation. Cell. 1988 Oct 21;55(2):361–369. doi: 10.1016/0092-8674(88)90059-1. [DOI] [PubMed] [Google Scholar]
- Tsai S. Y., Tsai M. J., O'Malley B. W. Cooperative binding of steroid hormone receptors contributes to transcriptional synergism at target enhancer elements. Cell. 1989 May 5;57(3):443–448. doi: 10.1016/0092-8674(89)90919-7. [DOI] [PubMed] [Google Scholar]
- Turcotte B., Meyer M. E., Bocquel M. T., Bélanger L., Chambon P. Repression of the alpha-fetoprotein gene promoter by progesterone and chimeric receptors in the presence of hormones and antihormones. Mol Cell Biol. 1990 Sep;10(9):5002–5006. doi: 10.1128/mcb.10.9.5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherill P. J., Wilson A. P., Nicholson R. I., Davies P., Wakeling A. E. Interaction of the antioestrogen ICI 164,384 with the oestrogen receptor. J Steroid Biochem. 1988;30(1-6):263–266. doi: 10.1016/0022-4731(88)90103-3. [DOI] [PubMed] [Google Scholar]
- Westley B., Rochefort H. A secreted glycoprotein induced by estrogen in human breast cancer cell lines. Cell. 1980 Jun;20(2):353–362. doi: 10.1016/0092-8674(80)90621-2. [DOI] [PubMed] [Google Scholar]
- von der Ahe D., Janich S., Scheidereit C., Renkawitz R., Schütz G., Beato M. Glucocorticoid and progesterone receptors bind to the same sites in two hormonally regulated promoters. Nature. 1985 Feb 21;313(6004):706–709. doi: 10.1038/313706a0. [DOI] [PubMed] [Google Scholar]