Depending on timing and venue, new blood vessel growth can be either beneficial or pathological. Vigorous vessel growth is absolutely necessary for tissue, organ, and limb development as was graphically demonstrated a few decades ago with thalidomide, now known to be a compound with antiangiogenic properties. As one ages though, the need for angiogenesis decreases or stops entirely except for specialized cases such as wound repair. In fact, neovascularization in the adult is usually associated with some form of pathology—tumor growth, for example, or diabetes. New work by Bouck and coworkers (1), as reported in PNAS, now gives evidence that a natural protein, pigment epithelium-derived factor (PEDF), is a potent inhibitor of abnormal blood vessel growth in a murine model of ocular neovascularization. This, together with previous data from the same group (2), gives compelling evidence that PEDF may be pivotal in controlling both normal and abnormal blood vessel growth. Although this gives hope for ultimately controlling neovascularization, it also raises interesting questions about PEDF's biological activities.
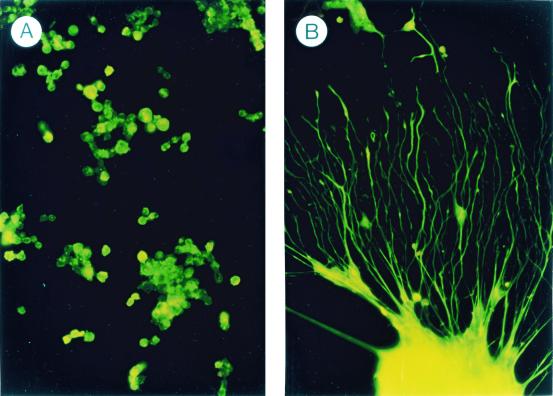
In 1989, a novel neurotrophic activity was first described by Tombran-Tink and Johnson (3) in conditioned medium from cultured fetal retinal pigment epithelial (RPE) cells. In a test system using cultured, primitive retinoblastoma cells, extensive neuronal-like processes were induced after exposure to the RPE conditioned medium (Fig. 1). This factor, PEDF, has now been purified (4) and cloned from both human (5) and mouse (6). The gene is active at 17 weeks in human fetal RPE cells (7), making it a candidate as a factor involved in early neuronal development.
Figure 1.
Effect of PEDF on retinoblastoma morphology. (A) Proliferating, undifferentiated Y-79 retinoblastoma cells in culture. (B) Nonproliferating cells treated with PEDF demonstrating neuronal-like morphology. [Reproduced with permission from ref. 27 (Copyright 1994, Academic Press).]
Subsequent work has demonstrated that PEDF is mainly a secreted glycoprotein of about 50 kDa that is not uniquely synthesized by fetal RPE cells. PEDF mRNA is found in most tissues, normal cell types (neuronal and nonneuronal), and tumors, as surveyed by Western and Northern blot analysis (8). DNA sequence analysis shows that PEDF belongs to the serine protease inhibitor (SERPIN) gene family (5, 8) which includes a large number of proteins of widely divergent function, such as ovalbumin, alpha 1–antitrypsin, angiotensinogen, and GDN/PN-1, another serpin with neurotrophic properties. Interestingly, PEDF does not seem to exhibit antiprotease activity with the neurotrophic active site residing at the other end of the peptide chain from the antiprotease active center (9, 10) seen in the classical serpins. The noninhibitory nature of the PEDF molecule has been well studied by Becerra and coworkers (11).
During the time when the PEDF protein and gene were being isolated, Cristofalo and coworkers (12) described a protein in WI-38 fibroblast cells, a prime model system for studying aging and senescence, whose expression was (i) restricted to the Go stage of the cell cycle in young cells and (ii) not expressed in older, senescent cells. This protein, called EPC-1, was found to be identical to PEDF. Subsequent work (13, 14) reinforces the notion that PEDF is intimately associated with both the cell cycle and senescence. The expression of PEDF mRNA and the secreted protein are much more highly (100-fold) expressed in Go (growth arrest) of early-passaged cells than in rapidly proliferating young cells or in later-passaged (senescent) cells. Palmieri and coworkers (15) have found similar results in PEDF down-regulation in proliferating and in senescent human endometrial stromal fibroblasts.
With the finding that PEDF was neurotrophic in cultured retinoblastoma tumor cells, it was of interest to determine whether PEDF had an effect on normal neurons. For this purpose, the effect(s) of both native and recombinant PEDF on normal cerebellar granule cells (CGCs) in primary culture was examined. Tanawaki and coworkers (16) found that PEDF did not induce a more neuronal phenotype in the CGCs, but did have a marked effect on CGC survival. Usually, most of the cells are dead by 2–3 weeks in culture. However, with PEDF added to the medium, the cells live much longer. It was also established that PEDF did not enhance mitotic activity of the CGCs and thus appeared to have an effect on increased survival instead of increased replication. In more detailed experiments, Araki and coworkers (17) found that cell death of the CGCs was by “natural apoptosis” and that, at least in immature CGCs, PEDF was a potent inhibitor of natural and induced apoptosis. The effects of PEDF are not limited to brain neurons. Houenou and coworkers (18), demonstrated that PEDF effectively promotes both the differentiation and survival of developing spinal motor neurons. Similarly, Bilek and coworkers (19) found that, in an organotypic culture model system for motor neuron degeneration, PEDF spared the spinal chord's gross morphological appearance and total number of neurons. Along with chronic, long-term neurotrophic effect, PEDF is also quite effective in protecting neurons against rapid neurotoxic effects such as glutamate toxicity (20). The protective effect is seen with only a very short preincubation with PEDF (30 min) and at a very low dose (0.47 nM). The level of protection is inversely correlated with the intracellular calcium level after glutamate poisoning. DeCoster and coworkers (21) noted a protective effect of PEDF on hippocampal neurons, albeit of a smaller nature, whereas Cao and coworkers (22) found that rat retinal neurons were protected against hydrogen peroxide-induced apoptosis. It is thus clear that PEDF and its recombinant peptides can protect neurons in both long-term and more acute situations. Protection is seen in both the central nervous system (CNS) and the spine making PEDF of potential use against diseases of the CNS (brain, retina) and of peripheral neurons as well.
If these characteristics were not sufficiently interesting for a protein, Sugita and coworkers (23) noted another striking property of PEDF. CGC cultures contain contaminating glial elements that, as the neurons die, proliferate and ultimately dominate the culture dish. In the presence of PEDF, however, the growth of astroglia is considerably slowed. Microglia change morphologically and become more metabolically active in the presence of PEDF. PEDF also completely blocks GMCSF-stimulated cell division of microglia. This “gliastatic” effect of PEDF, along with the neuron-survival character, could be of benefit in the overall management of a number of diseases with CNS involvement.
Diabetes is certainly a disease with CNS involvement and with neovascular pathology as well. Nowhere is this more “visible” than in the eye, where the neural retina is normally protected by an antiangiogenic shield or environment but, with breaching of the shield, is devastated by neovascular growth as in diabetic retinopathy or retinopathy of prematurity. The article by Bouck and coworkers (1) now gives important information about aberrant blood vessel proliferation in the eye and the possible role of PEDF in controlling this process. By using a well known murine model of ischemia-induced retinopathy (24), they demonstrate that the neovascular process in the eye can be controlled by administering relatively low doses of PEDF. Interestingly, treatment with PEDF is i.p. as opposed to intraocular. The latter is the more usual route used in getting agents to the retina, which is normally protected behind a blood-retina barrier much as in the brain. Retinal neovascularization with tufts extending into the vitreous body were seen in all ischemia-induced animals (Fig. 2 a–d; originally appeared in ref. 1), but new vessel growth was markedly reduced or eliminated in PEDF-treated animals (Fig. 2 e–h). To corroborate these findings, endothelial cell nuclei extending beyond the internal limiting membrane (ILM) of the retina into the vitreal surface were counted. As expected, PEDF was found to fully inhibit extrusion of the endothelial cells into the vitreous cavity. The low dose seen to be effective in these studies is within the same low dose range (i.e., nM) of PEDF needed to effect CGC neuroprotection as cited above. The case for PEDF as a (if not the) major antiangiogenic factor in the eye is greatly strengthened if one considers previous work from Bouck's group. Dawson and coworkers (2) demonstrated that PEDF is probably responsible for the normal avascularity of several of the ocular components. Also, loss of PEDF plays a “permissive role” in ischemia-induced neovascular growth.
Figure 2.
In situ angiogenesis in PEDF-treated retinas. Low-power (Left) and high-power (Right) views of retinas of mice exposed to hyperoxia and subsequently treated with vehicle (a–d), or with PEDF at 11.2 (e and f) or 22.4 (g and h) μg/day.
How does PEDF prevent the retinal endothelial cells from responding to ischemic signals that induce neovascularization? Bouck and coworkers (1) suggest that it may be through an apoptotic mechanism. Experimentally, cultured human endothelial cells were subjected to mild stress and the numbers of TUNEL-positive cells were monitored. Addition of PEDF to the cultures markedly increased the number of positive cells. Similarly, apoptotic endothelial cells were more common in retinas of hyperoxic animals treated with PEDF than in those not receiving PEDF. This raises interesting questions as to the action(s) of PEDF in either promoting apoptosis as seen in the present case or inhibiting it as in the neuron-survival activities cited above. Does PEDF have a single, unified action/function or rather does it have different activities in different cell types or under different conditions? Its differentiating–promoting and gliastatic activities also need to be taken into account as well as the ramifications of PEDF loss at senescence. Could loss of PEDF in older individuals eliminate a critical negative regulator of angiogenesis, contributing to the well-known age-related increase in cancer prevalence?
Finally, do different parts of the PEDF molecule have different intrinsic activities? It is known that antithrombin, a serpin that functions as an inhibitor of thrombin, acquires marked antiangiogenic properties after typical serpin proteolytic cleavage induces a small conformational change in the molecule (25). It seems clear, however, that PEDF functions as a noninhibitory serpin (11) with at least the site responsible for its neuron-survival activity residing closer to the N terminus than the classical serpin active site near the C terminus. However, PEDF, like antithrombin and PAI-1, has the ability to interact with and bind to components of the extracellular matrix (ECM) (26). Perhaps these interactions induce different PEDF conformations and thus different levels or states of activity. Interaction with different ECM components of different cell types could also allow for different PEDF conformations and resultant activities.
Whatever the mechanism(s) of action of PEDF, it is obvious that it exhibits a number of interesting biological activities, most notably as a potent antineovascular agent as elucidated by the outstanding work of Bouck and coworkers (1). In the eye, PEDF's characteristics make it an appealing agent for trying to control diabetic retinopathy, retinopathy of prematurity, and the devastating effects of the wet form of age-related macular degeneration. PEDF could also be useful in controlling other situations, such as tumor growth, where neovascularization is a major factor in disease progression. Finally, in neuronal tissues, the neuron-survival and gliastatic activities of PEDF could perhaps be induced to act together with its antiangiogenic properties in synergistically combating a wide range of primary disease processes and their sequelae.
Footnotes
See companion article on page 2593.
References
- 1.Stellmach V, Crawford S, Zhou W, Bouck N. Proc Natl Acad Sci USA. 2001;98:2593–2597. doi: 10.1073/pnas.031252398. . (First Published January 23, 2001; 10.1073/pnas.031252398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawson D, Volpert O, Gillis P, Crawford S, Xu H-J, Benedict W, Bouck N. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 3.Tombran-Tink J, Johnson L. Invest Ophthalmol Visual Sci. 1989;30:1700–1709. [PubMed] [Google Scholar]
- 4.Tombran-Tink J, Chader G, Johnson L. Exp Eye Res. 1991;53:411–414. doi: 10.1016/0014-4835(91)90248-d. [DOI] [PubMed] [Google Scholar]
- 5.Steele F, Chader G, Johnson L, Tombran-Tink J. Proc Natl Acad Sci USA. 1992;90:1526–1530. doi: 10.1073/pnas.90.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh V, Chader G, Rodriguez I. Mol Vis. 1998;4:7–12. [PubMed] [Google Scholar]
- 7.Tombran-Tink J, Shivaram S, Chader G, Johnson L, Bok D. J Neurosci. 1995;15:4992–5003. doi: 10.1523/JNEUROSCI.15-07-04992.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tombran-Tink J, Mazurek K, Rodriguez I, Chung D, Linker T, Englander E, Chader G. Mol Vis. 1996;2:11–19. [PubMed] [Google Scholar]
- 9.Becerra S, Palmer I, Kumar A, Steele F, Shiloach J, Notario V, Chader G. J Biol Chem. 1993;268:23148–23156. [PubMed] [Google Scholar]
- 10.Becerra S, Sagasti A, Spinella P, Notario V. J Biol Chem. 1995;270:25992–25999. doi: 10.1074/jbc.270.43.25992. [DOI] [PubMed] [Google Scholar]
- 11.Becerra S. In: Chemistry and Biology of Serpins. Church F C, Cunningham D D, Ginsburg D, Hoffman M R, Stone S R, Tollefsen D M, editors. New York: Plenum; 1997. pp. 223–237. [Google Scholar]
- 12.Pignolo R, Cristofalo V, Rotenberg M. J Biol Chem. 1993;268:8949–8957. [PubMed] [Google Scholar]
- 13.Pignolo R, Rotenberg M, Cristofalo V. J Cell Physiol. 1995;162:110–118. doi: 10.1002/jcp.1041620113. [DOI] [PubMed] [Google Scholar]
- 14.Tresini M, Pignolo R, Allen R, Cristofalo V. J Cell Physiol. 1999;179:11–17. doi: 10.1002/(SICI)1097-4652(199904)179:1<11::AID-JCP2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Palmieri D, Watson J, Rinehart C. Exp Cell Res. 1999;247:142–147. doi: 10.1006/excr.1998.4341. [DOI] [PubMed] [Google Scholar]
- 16.Taniwki T, Harashima N, Chader G, Schwartz J. J Neurochem. 1995;64:2509–2517. doi: 10.1046/j.1471-4159.1995.64062509.x. [DOI] [PubMed] [Google Scholar]
- 17.Araki T, Tanawaki T, Becerra S, Chader G, Schwartz J. J Neurosci Res. 1998;53:7–15. doi: 10.1002/(SICI)1097-4547(19980701)53:1<7::AID-JNR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 18.Houenou L, D'Costa A, Turgeon V, Enyadike C, Alberti E, Becerra S. J Comp Neurol. 1999;412:506–514. [PubMed] [Google Scholar]
- 19.Bilak M, Corse A, Lehar M, Tombran-Tink J, Kunel R. J Neuropath Exp Neurol. 1999;58:719–729. doi: 10.1097/00005072-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Tanawaki T, Harashima N, Becerra S, Chader G, Etcheberrigary R, Schwartz J. J Neurochem. 1997;68:26–32. doi: 10.1046/j.1471-4159.1997.68010026.x. [DOI] [PubMed] [Google Scholar]
- 21.DeCoster M, Schabelman E, Tombran-Tink J, Bazan N. J Neurosci Res. 1999;56:604–610. doi: 10.1002/(SICI)1097-4547(19990615)56:6<604::AID-JNR6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 22.Cao W, Tombran-Tink J, Chen W, McGinnis J. Invest Ophthalmol Visual Sci. 1999;40:s160. [Google Scholar]
- 23.Sugita Y, Becerra S, Chafer G, Schwartz J. J Neurosci Res. 1997;49:710–718. doi: 10.1002/(SICI)1097-4547(19970915)49:6<710::AID-JNR5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 24.Smith L, Wesolowski E, McLennan A, Kostyk S, D'Amato R, Sullivan R, D'Amore P. Invest Ophthalmol Visual Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 25.O'Reilly M, Pirie-Shepherd S, Lane W, Folkman J. Science. 1999;285:1926–1928. doi: 10.1126/science.285.5435.1926. [DOI] [PubMed] [Google Scholar]
- 26.Alberdi E, Hyde C, Becerra S. Biochemistry. 1998;30:10642–10652. doi: 10.1021/bi9802317. [DOI] [PubMed] [Google Scholar]
- 27.Tombran-Tink J, Pawar H, Swaroop A, Rodriguez I, Chader G J. Genomics. 1994;19:266–272. doi: 10.1006/geno.1994.1057. [DOI] [PubMed] [Google Scholar]