Abstract
Low extracellular pH (pHe), that is characteristic of many tumours, tends to reduce the uptake of weakly basic drugs, such as doxorubicin, thereby conferring a degree of physiological resistance to chemotherapy. It has been assumed, from pH-partition theory, that the effect of intracellular pH (pHi) is symmetrically opposite, although this has not been tested experimentally. Doxorubicin uptake into colon HCT116 cells was measured using the drug's intrinsic fluorescence under conditions that alter pHi and pHe or pHi alone. Acutely, doxorubicin influx across the cell-membrane correlates with the trans-membrane pH-gradient (facilitated at alkaline pHe and acidic pHi). However, the protonated molecule is not completely membrane-impermeant and, therefore, overall drug uptake is less pHe-sensitive than expected from pH-partitioning. Once inside cells, doxorubicin associates with slowly-releasing nuclear binding sites. The occupancy of these sites increases with pHi, such that steady-state drug uptake can be greater with alkaline cytoplasm, in contradiction to pH-partition theory. Measurements of cell proliferation demonstrate that doxorubicin efficacy is enhanced at alkaline pHi and that pH-partition theory is inadequate to account for this. The limitations in the predictive power of pH-partition theory arise because it only accounts for the pHi/pHe-sensitivity of drug entry into cells but not the drug's subsequent interactions that, independently, show pHi-dependence. In summary, doxorubicin uptake into cells is favoured by high pHe and high pHi. This modified formalism should be taken into account when designing manoeuvres aimed at increasing doxorubicin efficacy.
Introduction
The entry of weakly basic or weakly acidic molecules into cells can show pH-dependence arising from differences in the membrane permeability of their protonated and unprotonated forms [1], [2], [3]. This so-called pH-partitioning is relevant to chemotherapy because many anti-cancer drugs are protonatable and tumours typically have substantially lower extracellular pH (pHe) than normal tissue, whilst maintaining a modestly alkaline intracellular pH (pHi) [3], [4], [5], [6]. pH-partition theory has been tested experimentally by correlating drug uptake or efficacy with the trans-membrane pH gradient (ΔpH = pHe−pHi). Cellular accumulation of the weakly-basic drug doxorubicin [7] has been shown to decrease at low pHe, in accordance with pH-partition theory [1], [2], [8], [9]. The acidic extracellular milieu of tumours is therefore predicted to confer a degree of physiological resistance to weakly-basic drugs [9], [10], and manoeuvres that raise pHe have been proposed to improve doxorubicin efficacy [1], [8]. Doxorubicin resistance may also arise from sequestration of the drug into acidic vesicles [11]. pH-partition theory has been applied to explain this phenomenon, and manoeuvres that raise intra-vesicular pH (pHv) have been shown to enhance doxorubicin efficacy [12], [13].
Many studies of the pH-sensitivity of doxorubicin uptake, or its cytotoxicity, have measured responses to changes in extracellular pH [1], [2], [8], [9]. Such manoeuvres will also change intracellular pH because of the pHe-sensitivity of transporters that regulate the cell's acid-base balance [14]. The effect of pHi – independently of pHe – on doxorubicin uptake has not been tested robustly. It cannot, therefore, be excluded that higher drug uptake at alkaline pHe arises from higher pHi, in an apparent contradiction to pH-partition theory. Recent in vitro work has shown that doxorubicin forms an adduct with DNA [15] in a pH-sensitive manner [16], decreasing as pH is reduced below 7. This process may introduce physiologically-relevant pHi-sensitivity of doxorubicin uptake into cells, independently of pH-partitioning across the membrane. Attempts have been made to disentangle the effects of pHi and pHe on drug uptake by comparing cells with different pHe−pHi relationships, for example, by comparing low-pH adapted CHO cells with control cells [2]. Comparison between phenotypically-distinct sub-populations can introduce additional variables that may affect drug uptake. For instance, at pHe = 7.4, when both normal and low-pH adapted cells have similar pHi, doxorubicin uptake was much higher in the former. Correlations between ΔpH and drug uptake, deemed as evidence for the pH-partition theory, have typically been made over a range of negative ΔpH (i.e. pHe<pHi), but not extended to symmetrically positive ΔpH. In these studies, changes to pHi have typically been much smaller than the associated changes to pHe, making it difficult to characterise the importance of pHi in drug uptake. Similarly, doxorubicin uptake into vesicles has been investigated in the context of changing pHv at near-constant pHi [12], [13]. Further testing of pH-partition theory would require studies performed under conditions where pHi is manipulated to a greater degree than pHe or pHv.
In this study, we use doxorubicin's intrinsic fluorescence to study the drug's uptake and accumulation over a range of pHi and pHe values, manipulated by changing bathing medium pH (to alter pHi and pHe in parallel), applying weak-acids at constant pHe (to alter pHi) or inhibiting membrane-bound acid-base transporters (to alter pHi at constant pHe). We then test the inferences made from isolated cells on multi-cellular 3-D spheroids [17], [18]. Finally, the effects of changing pHi with pHe or pHi alone on doxorubicin efficacy were tested using an index of proliferation. P53 wild-type human colon HCT116 cells were used in the study, as these have relatively low expression levels of the drug resistance-conferring P-glycoprotein [19], [20] and the proposed doxorubicin carrier SLC22A16 [21]. In addition, these cells have well-characterised pH regulation, little acid-extrusion by H+ ATPase pumps (i.e. Na+- and HCO3 −-independent flux) and can form multi-cellular spheroids [22]. Experimental data were analysed with a mathematical model to dissect the relationships between pHi, pHe and drug uptake.
Our results indicate that pH-partition theory alone is inadequate to fully account for the pH-sensitivity of doxorubicin uptake. Firstly, we find that the protonated form of doxorubicin can cross membranes, albeit slower than the unprotonated form. Secondly, once inside the cell, the drug associates with nuclear binding sites in a pH-dependent manner. We conclude that the behaviour of free doxorubicin can be approximated by the pH-partition theory, but its intracellular accumulation requires the introduction of a pHi-sensitive drug-binding process. We propose that doxorubicin accumulation is favoured by a combination of high pHe and high pHi.
Methods
Solutions
All solutions were based on Hepes/Mes-buffered normal Tyrode (125 mM NaCl, 4.5 mM KCl, 20 mM Hepes, 20 mM Mes, 1 mM CaCl2, 1 mM MgCl2, pH adjusted to 7.4). To change pHe (and, secondarily, pHi), solution pH was titrated to 6.4 or 6.8 or 7.8 (whilst maintaining osmolarity by adjusting NaCl). To change pHi at constant pHe: (i) NaCl was iso-osmotically replaced with 40 mM or 80 mM NaAcetate (reduces pHi), (ii) Na+ was replaced with N-methyl-D-glucamine (inactivates NHE and allows CHE to acid-load cell), (iii) 5-(N,N-dimethyl) amiloride (DMA) was added (inhibits NHE and allows CHE to acid-load cell), (iv) Cl− was replaced with gluconate (inactivates CHE and allows NHE to base-load cells), or (v) Hepes/Mes was replaced with CO2/HCO3 − (alters the steady-state pHe−pHi relationship by activating additional pHi regulating transporters).
Imaging isolated cells under superfusion
Colon cancer HCT116 cells (provided by Cancer Research UK; [17], [23]) were grown in Dulbecco's Modified Eagle's Medium (DMEM) containing NaHCO3, in an atmosphere of 5% CO2 for 48–72 hours until confluency. Prior to experiments, cells were re-suspended in Hepes-buffered DMEM (for no more than 3 hours). A 200 µl-aliquot of cell-suspension was applied to a poly-L-lysine pre-treated coverslip, mounted at the base of a Perspex superfusion chamber. After 10 minutes to allow for cell-adhesion (and fluorescent dye-loading, where applicable), cells were superfused with solution heated to 37°C at 2 ml/min [22]. By switching between two solution lines, it was possible to change the superfusate with a time-constant of <8 sec. pHi was measured in cells loaded with carboxy-SNARF-1 (excitation 514 nm, fluorescence emission collected at 580 nm and 640 nm). The emission ratio was calibrated in units of pH by the ‘nigericin’ technique [24]. In separate experiments, doxorubicin (50 µM) uptake was measured using its intrinsic fluorescence (excitation 488 nm, emission 580 nm). This signal was not significantly pH-sensitive (Fig S1A), i.e. the protonated and unprotonated forms are equally fluorescent. By confocally imaging an optical slice, it is possible to measure fluorescence within cells and in the extracellular solution simultaneously.
Measurements with flow cytometry
Cells were pre-equilibrated for 1 hour with a desired Hepes/Mes-buffered solution to attain steady-state pHi. Cells were then treated with doxorubicin (50 µM) for a further 2 hours. Drug accumulation in cells was quantified with a Beckman Coulter Quanta SC flow cytometer (excitation 488 nm; emission 580 nm) in drug-free medium. Measurements were limited to 5 minutes and back-extrapolated to account for slow release of doxorubicin in drug-free bathing media. To measure pHi, cells were subjected to the same procedure (with the exception of doxorubicin treatment) and AM-loaded with carboxy-SNARF-1 for 10 minutes before flow-cytometry (excitation 488 nm; emission 580 nm and 640 nm).
Culturing and imaging spheroids
HCT116 cells were cultured in McCoy's 5A medium. Aggregation into spheroids was initiated by plating 4×106 cells in 250 mL spinner flasks (Techne MCS, UK) spun at 40 rpm for 2–9 days. Spheroids were superfused with buffer containing doxorubicin (50 µM) for 2 hours. To correct for the depth-dependent decrease in light-emission, doxorubicin fluorescence was imaged alternately with the pH-insensitive, UV-excitable and spectrally-resolvable dye, 7-amino-4-methyl coumarin (AMC; 30 µM). Extracellular pH within the spheroid mass was measured with fluorescein sulphonic acid (30 µM) [17].
Nuclear vs cytoplasmic doxorubicin fluorescence
HCT116 cells were grown as confluent monolayers and treated with doxorubicin (50 µM) and the nuclear stain Hoechst 33342 (10 µg/ml) for 2 hours. Fluorescence from doxorubicin (>580 nm) and from Hoechst (<480 nm) were excited at 488 nm and 405 nm, respectively. Doxorubicin fluorescence was summed in nuclear regions (identified by Hoechst fluorescence) and non-nuclear regions (with sub-threshold Hoechst fluorescence). See Figure S2 for details of the image-processing method.
Proliferation assay for determining doxorubicin efficacy
1.0×105 cells were seeded per well in 96-well plates and incubated for 48 hours. Confluent cells at the base of the wells were washed with PBS twice and then bathed in Hepes/Mes-buffered normal Tyrode solution at pH = 7.4 (the control) or one of five solutions that alter pHi: (i–ii) normal Tyrode titrated to pH 6.4 or 7.8 to alter pHe and pHi, (iii–v) normal Tyrode at pH 7.4 containing DMA or lacking Na+ salts or Cl− salts to alter pHi at constant pHe (NB: these manoeuvres change the balance of trans-membrane acid/base fluxes, which alters pHi but not pHe because the bulk extracellular volume is much larger than intracellular volume). After allowing 45 minutes for cells to re-equilibrate their trans-membrane pH gradient, solutions were replaced with ones containing doxorubicin (1 to 300 µM). After 2 hours of drug treatment, cell proliferation was assessed using the CellTiter Blue assay (Promega, UK). Proliferation was calculated as the ratio of absorbance at 562 nm to 595 nm, normalized to that ratio in the absence of doxorubicin (this will take into account doxorubicin-independent differences in cell-survival in the six bathing media used). At >550 nm, absorbance due to doxorubicin is negligible.
Mathematical model of doxorubicin uptake
The model assumes that doxorubicin fluorescence is (i) proportional to its concentration and (ii) a linear combination of free and bound doxorubicin. The drug's pK was taken as 8.2 [1], [2], [8], [9]. Best-fitting was used to estimate the permeability constant of the protonated and unprotonated forms (PHDox, PDox), the maximal binding capacity of the ‘receptor’ (Bmax) and its association and dissociation constants (kon, koff). It was assumed that both forms of drug bind equally to the ‘receptor’. The pH-sensitivity of drug binding was modelled by scaling Bmax (i.e. non-competitive; acid-dissociation constant KH) or by scaling kon (i.e. competitive; acid-dissociation constant QH). The equations describing the concentration of free doxorubicin ([DOX]) and bound doxorubicin ([DOX*]) inside cells are:
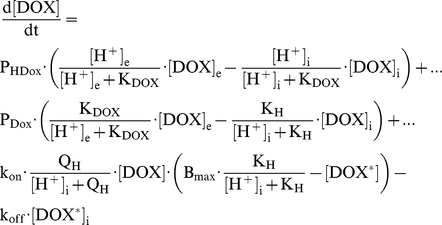 |
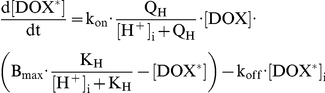 |
Results
Intracellular doxorubicin associates with a slowly-releasing binding site
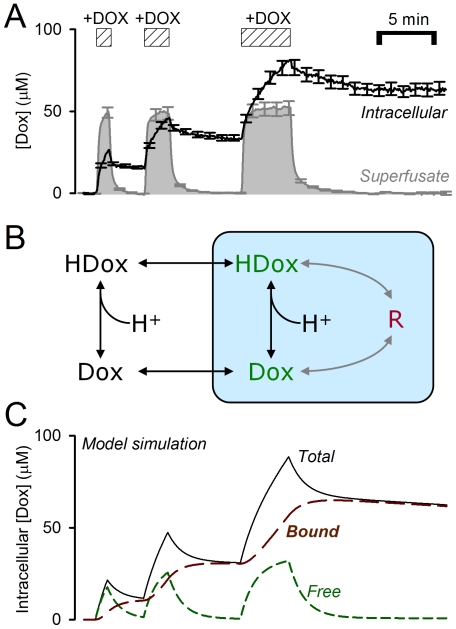
Colon HCT116 cells (resting pHi = 7.2) were exposed transiently to solution (pHe = 7.4) containing 50 µM doxorubicin for 1, 2 and 5 minutes (Fig. 1A). This dose is adequately fluorescent, potently cytotoxic over a broad pHe range [9] and can produce significant binding to DNA for a more accurate characterisation of drug accumulation in cells [16], [25]. Doxorubicin wash-out was considerably slower than its uptake. This asymmetry cannot be explained in terms of the trans-membrane pH gradient (ΔpH of only +0.2). The rise of cell-averaged fluorescence above the extracellular background suggests that the drug concentrates inside cells [11], [13], [15], [26]. The experimental time-courses can be simulated by accounting for doxorubicin-binding to an intracellular ‘receptor’ (Fig. 1B) of fast association (kon) and slow dissociation (koff) kinetics (Fig. 1C). Best-fitting values for the receptor's maximal binding capacity (Bmax), ‘on’ and ‘off’ rate constants (kon and koff) were 506 µM, 32.5 s−1×M−1 and 2.345×10−4 s−1, respectively, giving a doxorubicin binding constant of 7.2 µM. Total intracellular doxorubicin concentration is therefore given by the sum of ‘free’ and ‘bound’ drug.
Figure 1. Intracellular doxorubicin associates with a slowly-releasing intracellular binding site.
(A) HCT116 cells superfused with Hepes/Mes buffer at pH = 7.4, 37°C. Doxorubicin (DOX; 50 µM) was applied transiently by switching rapidly between drug-free and drug-containing solution (average of 25 cells, ±SEM). (B) Proposed model with equilibria involving free and bound doxorubicin. (C) Mathematical simulation showing the fast rise of intracellular doxorubicin upon exposure, and its slow release upon reversal of the trans-membrane concentration gradient.
Low extracellular pH decreases the rate of doxorubicin uptake and accumulation in isolated cells
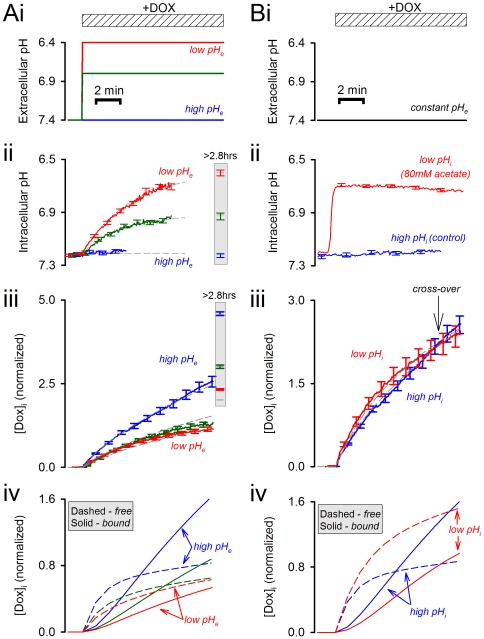
HCT116 cells were exposed to doxorubicin at different superfusate pH (Fig. 2A) to measure the drug's initial uptake rate and its steady-state accumulation as a function of the trans-membrane pH gradient. Figure 2Ai illustrates the experimental protocol and Figure 2Aii shows the pHi response to changes in pHe. For measurements of the initial rate of drug uptake, it was assumed that starting pHi is 7.2. Analyses of doxorubicin accumulation at steady-state must, however, take into account the slow, pHe-evoked changes in pHi.
Figure 2. Effect of changing extracellular and intracellular pH on doxorubicin uptake.
Time-courses show the average (±SEM) of at least 25 cells. (A) (i) Extracellular pH was changed by switching to superfusates titrated to pH 6.8 or 6.4, simultaneously with the application of 50 µM doxorubicin. (ii) Intracellular pH measured in separate experiments using carboxy-SNARF-1. (iii) Intracellular doxorubicin, normalized to its extracellular signal. Inset shows intracellular fluorescence at steady state, attained after 2.8 hours of drug-exposure. (iv) Simulated doxorubicin time-courses. (B) (i) Intracellular pH was reduced to 6.7 at constant extracellular pH by superfusing cells with 80 mM acetate in the presence of the Na+/H+ exchange inhibitor, dimethyl amiloride (DMA; 30 µM). Doxorubicin was applied once pHi attained a steady-state. (ii) Intracellular pH measured with carboxy-SNARF-1. (iii) Intracellular doxorubicin fluorescence, showing the cross-over of time-courses for pHi = 7.2 and 6.7. (iv) Simulation of doxorubicin-time-courses.
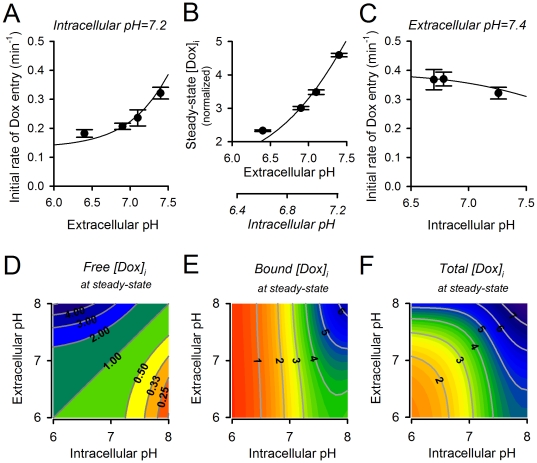
In agreement with pH-partition theory, doxorubicin uptake was slowest at acidic pHe (Fig. 2Aiii). The mathematical model was used to best-fit these experimental data for the permeability constants of the protonated and unprotonated forms of drug (PHDox; PDox). If only the unprotonated form of drug were able to enter cells, drug uptake at pHe = 6.4 should be ∼9-fold lower than at pHe = 7.4. Our data suggest that doxorubicin uptake is less pHe-sensitive than this: uptake at pHe = 6.4 (ΔpH = −0.8) was only 1.7-fold lower than at pHe = 7.4 (ΔpH = +0.2) (Fig. 3A). This would be expected if the protonated drug could also permeate across cell-membranes. The pHe-sensitivity of drug uptake can be simulated with a PDox of 0.041 s−1 and a 12-fold lower PHDox. In summary, doxorubicin protonation does not render it completely membrane-impermeant. Consequently, extracellular acidification reduces the initial rate of drug uptake to a smaller degree than predicted by pH-partition theory.
Figure 3. Effect of intracellular and extracellular pH on drug uptake and accumulation.
(A) Initial rate of doroxubicin uptake, measured at constant intracellular pH, over a range of extracellular pH values (data from Fig. 2A) with best-fit. (B) Intracellular doxorubicin at steady-state, normalized to extracellular concentration, over a range of extracellular pH values. Secondary axis plots steady-state pHi attained at given pHe. (C) Initial rate of doxorubicin uptake, measured at constant extracellular pH, over a range of intracellular pH values (data from Fig. 2B) with best-fit. Model predictions for the steady-state relationship between intracellular pH, extracellular pH and either (D) free, (E) bound or (F) total doxorubicin. Contour labels denote total intracellular doxorubicin concentration, normalized to its extracellular concentration (50 µM).
Intracellular doxorubicin accumulation was measured after 2.8, 3.3 and 7.5 hours and was found to attain a steady-state by 2.8 hours. Steady-state drug accumulation was lowest at acidic pHe (Fig. 2Aiii). During the course of this experiment, the trans-membrane pH gradient gradually decreases because pHi tends towards pHe (Fig. 2Aii). According to pH-partition theory, the time-courses of doxorubicin accumulation measured at different pHe should gradually converge, having attained the greatest separation initially, before the onset of the secondary pHi change. The experimental data are contrary to this (see Fig. 3A vs 3B): the initial rate of drug uptake was 1.7-fold lower at pHe = 6.4 (ΔpH = −0.8) than at pHe = 7.4 (ΔpH = +0.2), but at steady-state, drug accumulation was 2.0-fold lower at the more acidic pHe (despite the narrowing of ΔpH to −0.2). The on-going intracellular acidification at low pHe reduces doxorubicin accumulation in cells (Fig. 2Aiii). While this is incompatible with pH-partition theory, it could be explained by reduced doxorubicin binding to ‘receptors’ at acidic pHi. These data fit best to a model with pHi-dependence of binding capacity Bmax (i.e. non-competitive model) rather than of affinity (i.e. competitive model). The acid-dissociation constant (KH) that best describes the data was estimated to be 10−7.44 (Fig. 2Aiv).
Low intracellular pH increases the rate of doxorubicin uptake in isolated cells
pH-partition theory was tested further by comparing drug-uptake at reduced pHi but unchanged pHe (Fig. 2Bi–ii). This was attained by applying superfusates (pH = 7.4) containing 80 mM sodium acetate (to acid-load the cytoplasm) and 30 µM DMA (to block acid-extrusion that would otherwise ensue). Acidification, when confined to the intracellular compartment, increased the initial rate of drug uptake, as expected from pH-partition theory (Fig. 3C). However, after 11 min of exposure, drug accumulation became larger at higher pHi values (Fig. 2Biii). An analogous cross-over was observed with 40 mM sodium acetate at 14 minutes. This behaviour is not predicted by the pH-partition theory, but could be explained by reduced drug-binding to the intracellular ‘receptor’ at low pHi, as proposed herein. Model simulations were again in good agreement with the data (Fig. 2Biii).
Intracellular pH is a principal determinant of doxorubicin accumulation in its slowly-releasable form
The parameterised mathematical model was used to simulate the steady-state concentration of ‘free’ and ‘bound’ doxorubicin as a function of pHi and pHe (Fig. 3D, E, F) The trans-membrane distribution of free doxorubicin is in agreement with pH-partition theory, being greatest at low pHi and high pHe (Fig. 3E). The concentration of bound doxorubicin increases with pHe (due to increased influx of drug) and with pHi (due to increased binding of drug). Overall, optimal doxorubicin uptake is attainable at high pHi and pHe. This was tested further using flow cytometry [27].
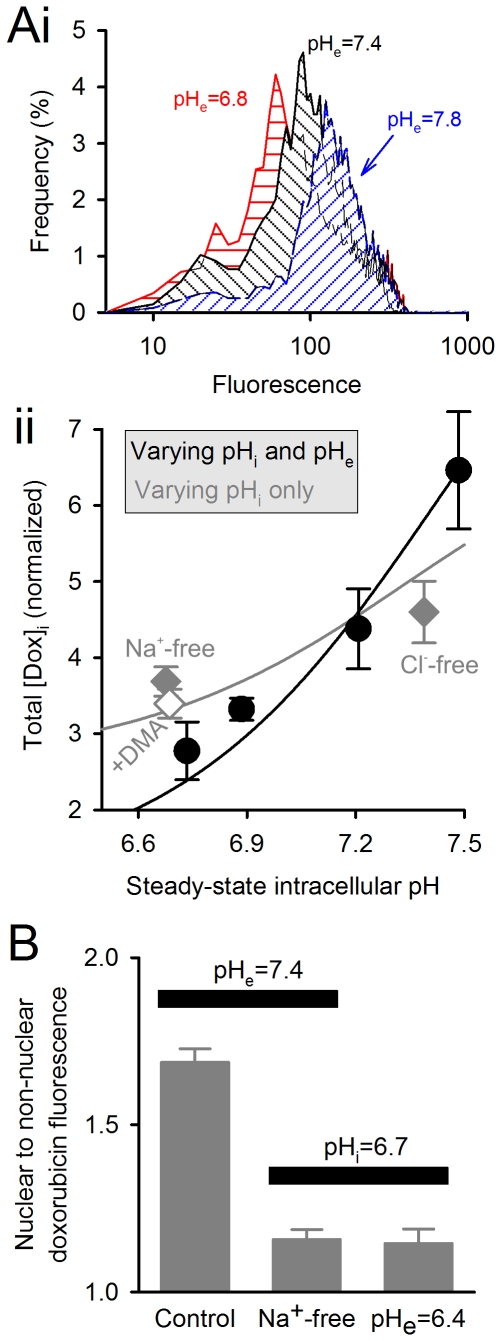
The pHi of cells was manipulated by changing pHe or by altering the balance between acid-loading and acid-extruding membrane transporters, at constant pHe. In Hepes/Mes-buffered (i.e. CO2/HCO3 −-free) media, resting pHi in HCT116 cells is set by the balance of acid-extruding Na+/H+ exchange (NHE) and acid-loading Cl−/OH− exchange (CHE) [22]. By replacing extracellular Na+ isosmotically with N-methyl-D-glucamine or by including the NHE inhibitor DMA (50 µM), CHE-flux drives pHi to a more acidic level. Conversely, pHi can be driven in the alkaline direction by NHE, when CHE is blocked by replacing extracellular Cl− isosmotically with gluconate. After allowing 1 hour for the attainment of a new steady-state pHi, HCT116 cells were treated with 50 µM doxorubicin for 2 hours. Figure 4Ai shows doxorubicin fluorescence histograms for three individual flow cytometric experiments. Parallel experiments were performed in the absence of doxorubicin, on cells loaded with carboxy-SNARF-1 to measure steady-state pHi. Figure 4Aii plots the measured and simulated relationship between pHi and total intracellular doxorubicin. In agreement with the mathematical predictions, but in contradiction to pH-partition theory, doxorubicin accumulation increases with pHi, and is further augmented by raised pHe.
Figure 4. Importance of intracellular pH in determining doxorobucin accumulation.
(A) (i) Specimen histogram of intracellular doxorubicin fluorescence (from >5000 cells) at different extracellular pH. (ii) Plot of intracellular doxorubicin (±coefficient of variation) versus intracellular pH. Black circles: intracellular pH manipulated by varying extracellular pH. Grey symbols: intracellular pH manipulated at constant extracellular pH. (B) Data from HCT116 monolayers treated with doxorobicin and Hoechst 33342. Ratio of doxorubicin fluorescence in nuclear (Hoechst 33342 positive) and non-nuclear regions quantifies the degree of drug accumulation in the nucleus.
Doxorubicin, like other anthracyclines, targets DNA [15], [25], [28], [29]. The possibility that the nucleus hosts the pHi-sensitive, slowly-releasing ‘receptor’ was tested by measuring doxorubicin fluorescence in nuclear and extra-nuclear regions. Nuclear regions were identified by using the DNA-binding stain, Hoechst 33342 (Figure S2). HCT116 monolayers were equilibrated with solutions at pHe = 7.4 (the control), pHe = 6.4 (to reduce pHi and pHe) and Na+-free solution at pHe = 7.4 (to reduce pHi at constant pHe). Monolayers were then treated with doxorubicin and Hoechst 33342 for two hours and then imaged confocally. Doxorubicin was distributed near-uniformly in non-nuclear regions, suggesting that the degree of sequestration into acidic compartments is small in HCT116 cells. Nuclear sequestration of doxorubicin, expressed as a ratio of total doxorubicin fluorescence in nuclear to non-nuclear regions, was markedly reduced at low pHi, irrespective of whether this was attained by reducing pHe or removing extracellular Na+ (Fig. 4B). In summary, the distribution of doxorubicin between cytoplasm and the nucleus is pHi-sensitive.
Imaging uptake of doxorubicin in multi-cellular spheroids
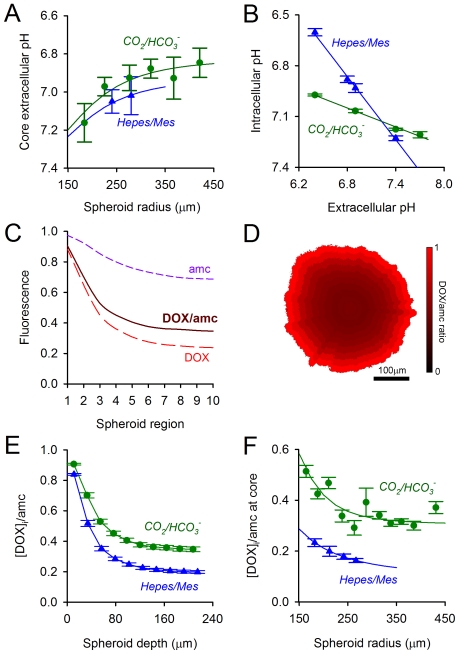
HCT116 cells were cultured as multi-cellular 3-D spheroids of radius 170 to 450 µm. The extracellular spaces within the spheroid-mass restrict the diffusion of metabolically-produced acids. This establishes radial gradients of pHi and pHe, with the lowest levels attained at the spheroid-core. Figure 5A shows the relationship between spheroid radius and its core-pHe (measured with fluorescein sulphonic acid). The extracellular space was buffered by CO2/HCO3 − or a mixture of Hepes+Mes, supplied by the superfusate as 5% CO2/22 mM HCO3 − or 20 mM Hepes+20 mM Mes, respectively. These solutions provide a similar level of mobile buffering capacity, resulting in comparable pHe gradients (Fig. 5A). However, the presence of CO2/HCO3 − buffer activates additional acid-base transporters (Na+-HCO3 − co-transport and Cl−/HCO3 − exchange) which provide tighter pHi control. This is illustrated by the relationship between pHe and pHi measured in single cells under the two buffering regimes (Fig. 5B). Replacement of Hepes/Mes buffer with CO2/HCO3 − will raise pHi at the pHe levels recorded in spheroids [17], [18]. pH-partition theory predicts that this should decrease doxorubicin uptake.
Figure 5. Doxorubicin accumulation in multi-cellular spheroids.
(A) Extracellular pH measured at the core of spheroids, as a function of radius, under two buffering regimes: 20 mM Hepes/Mes and 5% CO2/22 mM HCO3 −. (B) Relationship between extracellular and intracellular pH in single cells, under the two buffering regimes. (C) Radial gradients of doxorubicin and 7-amino-4-methyl coumarin: the ratio of these signals provides a measure of doxorubicin uptake corrected for light-path and absorbance. (D) Ratiometric image showing doxorubicin accumulation in a HCT116 spheroid, averaged in ten concentric layers. (E) Doxorubicin accumulation decreases with spheroid depth, as expected from the depth-dependent decrease in extracellular pH. Doxorubicin uptake is lower in Hepes/Mes-treated spheroids, i.e. at lower intracellular pH. (F) Doxorubicin uptake at the core of spheroids decreases with spheroid-size and is further reduced by replacing CO2 /HCO3 − buffer with Hepes/Mes.
To measure doxorubicin uptake, spheroids were treated with drug for 2 hours and then imaged for doxorubicin fluorescence. Imaging alternately with 7-amino-4-methyl coumarin (AMC) corrected for the depth-dependent loss of signal (Fig. 5C, D). Drug uptake decreased with spheroid-depth, as expected from the radially-decreasing pHe (Fig. 5E, F). In contradiction to pH-partition theory, replacement of Hepes/Mes with CO2/HCO3 − increased drug uptake for size-matched spheroids (Fig. 5E, F). This observation is, however, consistent with the notion that alkaline pHi facilitates doxorubicin accumulation onto nuclear binding sites.
Effect of intracellular and extracellular pH on doxorubicin efficacy
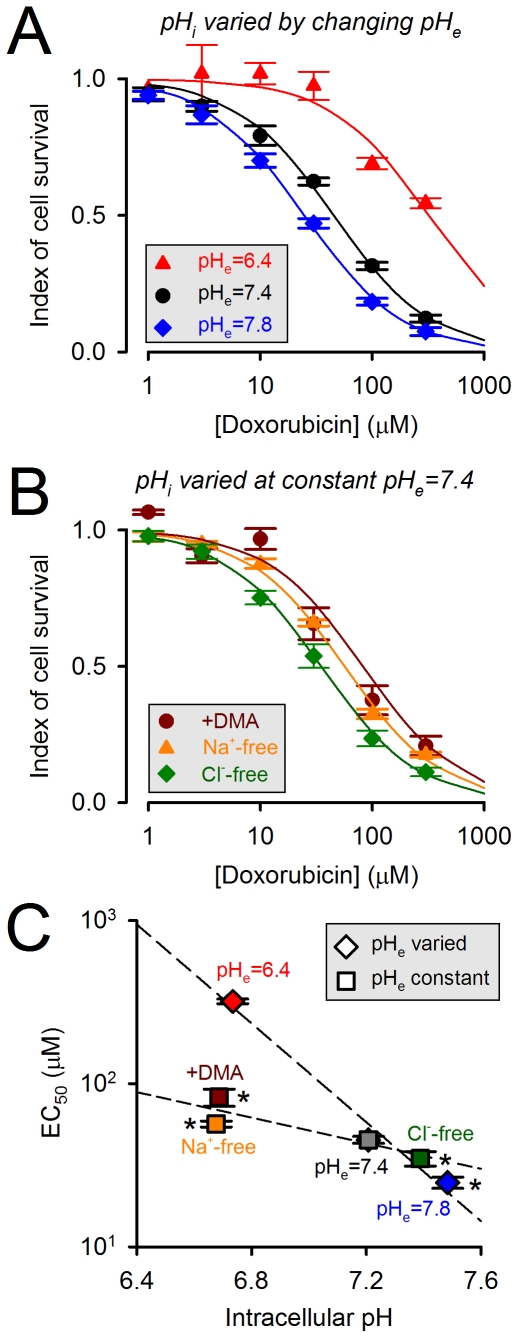
Doxorubicin efficacy was quantified in terms of the dose required to reduce cell proliferation by 50% (EC50). The effect of intra- and extracellular pH on EC50 was determined in cells equilibrated in solutions that alter pHi with pHe, or pHi alone. For control experiments, cells were incubated in normal Tyrode at pH 7.4. To alter pHe and pHi, cells were incubated in normal Tyrode titrated to pH 6.4 or 7.8. To alter pHi alone (pHe constant at 7.4), cells were incubated in normal Tyrode containing 50 µM DMA or in solutions lacking Na+ or Cl− salts.
Figure 6A show the effect of changing pHi and pHe on the doxorubicin dose-response curve. The efficacy of doxorubicin as a cytotoxic drug increases (i.e. EC50 falls) as pHe and pHi are raised in tandem. Under pH-partition theory, changes in pHi and pHe should exert opposite effects on EC50. However, changes in pHe evoke changes in pHi that are parallel but of smaller magnitude (Fig. 5B). Consequently, the effects on EC50, shown in Figure 6A, are likely to be dominated by the pHe-effect, and are consistent with pH-partition theory (i.e. increasing pHe increases doxorubicin membrane-permeability).
Figure 6. Doxorubicin efficacy as a function of intracellular pH.
Cell proliferation was measured using the CellTiter blue assay kit (quantified as a ratio of absorbance at 562 nm and 595 nm). Doxorubicin efficacy was determined from dose-response curves as the concentration which results in a 50% decrease in proliferation (EC50). EC50 was measured under six different conditions that change pHi, with or without an associated change in pHe. (A) Determining EC50 under incubation with normal Tyrode solutions titrated to pH 7.4 (the control), 6.4 or 7.8. Incubation under these conditions also changes pHi (n = 8 each). (B) Determining EC50 under incubation with solutions at pHe = 7.4 containing 50 µM DMA or lacking Na+ salts or Cl− salts (n = 8 each). These manoeuvres change pHi by altering the balance of acid/base fluxes across membranes (but do not alter pHe significantly because of the dilution effect into the large extracellular volume). (C) EC50 plotted against pHi (see Fig. 4Aii). Alkaline pHi increases doxorubicin efficacy (decreases EC50).
According to pH-partition theory, an increase in pHi (at constant pHe) should decrease doxorubicin efficacy (i.e. raise EC50). However, the experimental data shown in Figure 6B are contrary to this and, instead, are supportive of the model proposed herein featuring pHi-dependent binding of doxorubicin to its nuclear target. Figure 6C plots EC50 values as a function of pHi attained with the six experimental protocols, and shows that doxorubicin efficacy increases (i.e. EC50 falls) as pHi is raised. This relationship was steeper in experiments where pHi and pHe were changed in parallel. This is because changes in pHi and pHe have synergistic effects on doxorubicin uptake and its efficacy. In summary, doxorubicin's efficacy as a cytotoxic drug depends on pHi and pHe in a manner predicted by the present model. These data also illustrate the inadequacy of pH-partition theory in predicting the effects of pHi on drug efficacy.
Discussion
Evaluation of pH-partition theory
pH-partitioning has been the principal theoretical framework for describing the uptake of weakly acidic or basic drugs into cells. Its predictions have been applied, with success, to explain doxorubicin resistance in cancer cells. Doxorubicin efficacy has been shown to increase with a rise in pHe, although some investigations have suggested that the increase is less than predicted from pH-partitioning alone [10]. Doxorubicin flux on SLC22A16 [30] and MDR proteins [31] may produce discrepancies from pH-partitioning, particularly if these fluxes are pH-sensitive. However, the present work tested pH-partition theory on colon HCT116 cells, a cell line with only modest SLC22A16 and MDR1 expression [19], [20], [21].
Our experimental data are in partial agreement with pH-partition theory. The initial rate of doxorubicin uptake (before significant intracellular binding can occur) is favoured by a positive trans-membrane pH gradient (pHe−pHi>0; Fig. 3D). However, the measured uptake rates are less pHe-sensitive than expected (Fig. 3A, C). We have inferred this to be evidence that the protonated form of drug is not absolutely membrane-impermeant, but instead can enter cells, albeit 12-fold slower than the unprotonated drug. More substantial discrepancies between pH-partitioning predictions and our data arise from drug-binding to slowly-reversible (Fig. 1), pHi-sensitive (Fig. 2A) ‘receptors’ that are most likely nuclear (Fig. 4B). Our estimated affinity constant for doxorubicin (7.2 µM) is in the range determined previously for DNA [28], [29] and the estimated maximal binding capacity is equivalent to 3×108 base-pairs (every tenth base-pair of human DNA). Our data cannot determine unequivocally if these binding sites are selective for either protonated or unprotonated drug, but the considerable difference between estimated receptor pKH (7.44) and doxorubicin pK (8.2) argues against selectivity. Based on the goodness-of-fit to model simulations, our experimental data support a non-competitive model of pH-sensitivity (i.e. H+ ions reducing receptor availability for drug-binding). Since nuclear pH is in communication with cytoplasmic pH, drug-binding will respond to changes in pHi rather than ΔpH. Importantly, the analysis presented in Figure 3F suggests that drug accumulation is similarly sensitive to changes in either pHi or pHe from ‘physiological’ levels of 7.2 and 7.4. The importance of pHi in determining doxorubicin efficacy is illustrated experimentally in the cell proliferation assay (Fig. 6). The evidence for increased drug uptake and efficacy at alkaline pHi is contradictory to pH-partitioning and emphasises the necessity of implementing our novel model for describing the effect of pH on doxorubicin resistance.
Limitations of using fluorescence as a measure of concentration
The use of fluorescence intensity as a measure of doxorubicin concentration may introduce errors due to dye self-quenching or pH-sensitivity of excitation or emission spectra. These potential artefacts are unlikely to affect the interpretation of our data. Firstly, confocal imaging of cells at the base of a superfusion chamber minimises self-quenching in the optical slice. Nonetheless, if doxorubicin fluorescence were to saturate at high drug-concentrations, it would exacerbate the discrepancy between our data (Fig. 2) and pH-partition theory, and support the herein proposed model. Secondly, doxorubicin fluorescence is only weakly pH-sensitive (Figure S1A). Fluorescence intensity may change independently of concentration when doxorubicin dissolves into a medium of different dielectric constant, such as a non-aqueous environment. The doxorubicin emission spectrum carries a signature, quantified in terms of the 580 nm/640 nm fluorescence ratio, that correlates with the properties of the solvent [32]. Our measurements of this ratio under different bathing conditions show no significant pH-related change in the drug's environment (Figure S1B). Collectively, these findings suggest that fluorescence is a reasonable measure of doxorubicin concentration.
Conclusions
The efficacy of doxorubicin (and potentially other related drugs) is pH-dependent because of (i) pH-partitioning across cell-surface and vesicular membranes (which determines drug entry and its organellar distribution) and (ii) the pH-sensitivity of binding to nuclear sites (which sequesters doxorubicin in a slowly-releasable form). Our study shows that changes to pHi exert opposite effects on trans-membrane drug entry and on drug accumulation in nuclear sites, but the latter emerges as dominant (per pH-unit) and can determine overall efficacy, at least in HCT116 cells. These influences should be considered carefully when addressing resistance to chemotherapy. There have been three proposed pH-interventions for increasing doxorubicin efficacy. The first is alkalinisation of the extracellular space (e.g. titration to a higher pHe or infusion of HCO3 −) which raises pHe and pHi [1], [8]. The second manoeuvre is to inhibit surface membrane-bound proteins that acidify the extracellular space, such as extracellular-facing carbonic anhydrases, Na+/H+ exchangers or Na+-HCO3 − co-transporters [33], [34] and thereby increase pHe. The third intervention proposes to inhibit vesicular acidification, which reduces drug sequestration into endosomes [12], [13]. pH-partition theory predicts that the second and third manoeuvres should produce the more powerful effect on overcoming pH-related chemo-resistance, because these raise pHe or pHv and decrease pHi (i.e. tending to reverse the trans-membrane pH-gradient). However, the present findings argue that the first manoeuvre will have a more potent effect on improving doxorubicin uptake because of the parallel rise in pHi and pHe. It is noteworthy that inhibitors of acid-extruding proteins, proposed as anti-cancer drugs [33], may decrease doxorubicin efficacy by reducing pHi, if the associated change in pHe is relatively small.
Supporting Information
Characterising doxorubicin fluorescence. (A) Doxorubicin (50 µM) fluorescence was measured in Hepes/Mes buffered solution over a range of pH. The pH-sensitivity of fluorescence emission is only mildly pH-sensitivity (<5% per pH unit). (B) Intracellular doxorubicin fluorescence ratio measured flow cytometrically at 580 nm and 640 nm. This ratio carries a signature that describes changes to the ambient environment of doxorubicin. The constancy of the ratio suggests that the drug remains in an aqueous environment.
(TIF)
Measuring the nuclear versus non-nuclear doxorubicin accumulation. HCT116 monolayers were grown to confluency and then incubated in buffer solution (of desired pH and salt composition). Monolayers were then loaded with Hoechst 33342 and doxorubicin. (A) Doxorubicin fluorescence (488 nm excitation) recorded confocally, showing signal in nuclear and non-nuclear regions. (B) Hoechst 33342 fluorescence (405 nm excitation) used to identify nuclei. (C) Doxorubicin fluorescence in nuclear regions identified on the basis of supra-threshold Hoechst 33342 signal. The ratio of doxorubicin fluorescence in nuclear and non-nuclear regions was determined as (nuclear doxorubicin fluorescence) divided by (total doxorubicin fluorescence minus nuclear doxorubicin fluorescence).
(TIF)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This works was supported by the Royal Society (PS), Medical Research Council (PS), British Heart Foundation (RDV-J) and Cancer Research UK (ALH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Raghunand N, He X, van Sluis R, Mahoney B, Baggett B, et al. Enhancement of chemotherapy by manipulation of tumour pH. Br J Cancer. 1999;80:1005–1011. doi: 10.1038/sj.bjc.6690455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerweck LE, Kozin SV, Stocks SJ. The pH partition theory predicts the accumulation and toxicity of doxorubicin in normal and low-pH-adapted cells. Br J Cancer. 1999;79:838–842. doi: 10.1038/sj.bjc.6690134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996;56:1194–1198. [PubMed] [Google Scholar]
- 4.Stubbs M, McSheehy PM, Griffiths JR, Bashford CL. Causes and consequences of tumour acidity and implications for treatment. Mol Med Today. 2000;6:15–19. doi: 10.1016/s1357-4310(99)01615-9. [DOI] [PubMed] [Google Scholar]
- 5.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 6.Raghunand N, Zhang S, Sherry AD, Gillies RJ. In vivo magnetic resonance imaging of tissue pH using a novel pH-sensitive contrast agent, GdDOTA-4AmP. Acad Radiol. 2002;9(Suppl 2):S481–483. doi: 10.1016/s1076-6332(03)80270-2. [DOI] [PubMed] [Google Scholar]
- 7.Smalley RV, Lefante J, Bartolucci A, Carpenter J, Vogel C, et al. A comparison of cyclophosphamide, adriamycin, and 5-fluorouracil (CAF) and cyclophosphamide, methotrexate, 5-fluorouracil, vincristine, and prednisone (CMFVP) in patients with advanced breast cancer. Breast Cancer Res Treat. 1983;3:209–220. doi: 10.1007/BF01803563. [DOI] [PubMed] [Google Scholar]
- 8.Gerweck LE, Vijayappa S, Kozin S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol Cancer Ther. 2006;5:1275–1279. doi: 10.1158/1535-7163.MCT-06-0024. [DOI] [PubMed] [Google Scholar]
- 9.Mahoney BP, Raghunand N, Baggett B, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics. I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem Pharmacol. 2003;66:1207–1218. doi: 10.1016/s0006-2952(03)00467-2. [DOI] [PubMed] [Google Scholar]
- 10.Raghunand N, Mahoney BP, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics. II. pH-dependent partition coefficients predict importance of ion trapping on pharmacokinetics of weakly basic chemotherapeutic agents. Biochem Pharmacol. 2003;66:1219–1229. doi: 10.1016/s0006-2952(03)00468-4. [DOI] [PubMed] [Google Scholar]
- 11.Noel G, Peterson C, Trouet A, Tulkens P. Uptake and subcellular localization of daunorubicin and adriamycin in cultured fibroblasts. Eur J Cancer. 1978;14:363–368. doi: 10.1016/0014-2964(78)90206-2. [DOI] [PubMed] [Google Scholar]
- 12.Schindler M, Grabski S, Hoff E, Simon SM. Defective pH regulation of acidic compartments in human breast cancer cells (MCF-7) is normalized in adriamycin-resistant cells (MCF-7adr). Biochemistry. 1996;35:2811–2817. doi: 10.1021/bi952234e. [DOI] [PubMed] [Google Scholar]
- 13.Lee CM, Tannock IF. Inhibition of endosomal sequestration of basic anticancer drugs: influence on cytotoxicity and tissue penetration. Br J Cancer. 2006;94:863–869. doi: 10.1038/sj.bjc.6603010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boron WF. Regulation of intracellular pH. Adv Physiol Educ. 2004;28:160–179. doi: 10.1152/advan.00045.2004. [DOI] [PubMed] [Google Scholar]
- 15.Cutts SM, Nudelman A, Rephaeli A, Phillips DR. The power and potential of doxorubicin-DNA adducts. IUBMB Life. 2005;57:73–81. doi: 10.1080/15216540500079093. [DOI] [PubMed] [Google Scholar]
- 16.Cullinane C, Cutts SM, van Rosmalen A, Phillips DR. Formation of adriamycin–DNA adducts in vitro. Nucleic Acids Res. 1994;22:2296–2303. doi: 10.1093/nar/22.12.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swietach P, Patiar S, Supuran CT, Harris AL, Vaughan-Jones RD. The role of carbonic anhydrase 9 in regulating extracellular and intracellular ph in three-dimensional tumor cell growths. J Biol Chem. 2009;284:20299–20310. doi: 10.1074/jbc.M109.006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swietach P, Wigfield S, Cobden P, Supuran CT, Harris AL, et al. Tumor-associated carbonic anhydrase 9 spatially coordinates intracellular pH in three-dimensional multicellular growths. J Biol Chem. 2008;283:20473–20483. doi: 10.1074/jbc.M801330200. [DOI] [PubMed] [Google Scholar]
- 19.Long BH, Wang L, Lorico A, Wang RC, Brattain MG, et al. Mechanisms of resistance to etoposide and teniposide in acquired resistant human colon and lung carcinoma cell lines. Cancer Res. 1991;51:5275–5283. [PubMed] [Google Scholar]
- 20.Wu L, Smythe AM, Stinson SF, Mullendore LA, Monks A, et al. Multidrug-resistant phenotype of disease-oriented panels of human tumor cell lines used for anticancer drug screening. Cancer Res. 1992;52:3029–3034. [PubMed] [Google Scholar]
- 21.Aouida M, Poulin R, Ramotar D. The human carnitine transporter SLC22A16 mediates high affinity uptake of the anticancer polyamine analogue bleomycin-A5. J Biol Chem. 2010;285:6275–6284. doi: 10.1074/jbc.M109.046151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hulikova A, Vaughan-Jones RD, Swietach P. Dual role of CO2/HCO3(-) formula buffer in the regulation of intracellular pH of three-dimensional tumor growths. J Biol Chem. 2011;286:13815–13826. doi: 10.1074/jbc.M111.219899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rzymski T, Milani M, Pike L, Buffa F, Mellor HR, et al. Regulation of autophagy by ATF4 in response to severe hypoxia. Oncogene. 2010;29:4424–4435. doi: 10.1038/onc.2010.191. [DOI] [PubMed] [Google Scholar]
- 24.Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- 25.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 26.Durand RE, Olive PL. Flow cytometry studies of intracellular adriamycin in single cells in vitro. Cancer Res. 1981;41:3489–3494. [PubMed] [Google Scholar]
- 27.Luk CK, Tannock IF. Flow cytometric analysis of doxorubicin accumulation in cells from human and rodent cell lines. J Natl Cancer Inst. 1989;81:55–59. doi: 10.1093/jnci/81.1.55. [DOI] [PubMed] [Google Scholar]
- 28.Schneider YJ, Baurain R, Zenebergh A, Trouet A. DNA-binding parameters of daunorubicin and doxorubicin in the conditions used for studying the interaction of anthracycline-DNA complexes with cells in vitro. Cancer Chemother Pharmacol. 1979;2:7–10. doi: 10.1007/BF00253097. [DOI] [PubMed] [Google Scholar]
- 29.Bryn SR, Dolch GD. Analysis of binding of daunorubicin and doxorubicin to DNA using computerized curve-fitting procedures. J Pharm Sci. 1978;67:688–693. doi: 10.1002/jps.2600670532. [DOI] [PubMed] [Google Scholar]
- 30.Okabe M, Unno M, Harigae H, Kaku M, Okitsu Y, et al. Characterization of the organic cation transporter SLC22A16: a doxorubicin importer. Biochem Biophys Res Commun. 2005;333:754–762. doi: 10.1016/j.bbrc.2005.05.174. [DOI] [PubMed] [Google Scholar]
- 31.Roninson IB, Chin JE, Choi KG, Gros P, Housman DE, et al. Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci U S A. 1986;83:4538–4542. doi: 10.1073/pnas.83.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karukstis KK, Thompson EH, Whiles JA, Rosenfeld RJ. Deciphering the fluorescence signature of daunomycin and doxorubicin. Biophys Chem. 1998;73:249–263. doi: 10.1016/s0301-4622(98)00150-1. [DOI] [PubMed] [Google Scholar]
- 33.Huber V, De Milito A, Harguindey S, Reshkin SJ, Wahl ML, et al. Proton dynamics in cancer. J Transl Med. 2010;8:57. doi: 10.1186/1479-5876-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luciani F, Spada M, De Milito A, Molinari A, Rivoltini L, et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst. 2004;96:1702–1713. doi: 10.1093/jnci/djh305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterising doxorubicin fluorescence. (A) Doxorubicin (50 µM) fluorescence was measured in Hepes/Mes buffered solution over a range of pH. The pH-sensitivity of fluorescence emission is only mildly pH-sensitivity (<5% per pH unit). (B) Intracellular doxorubicin fluorescence ratio measured flow cytometrically at 580 nm and 640 nm. This ratio carries a signature that describes changes to the ambient environment of doxorubicin. The constancy of the ratio suggests that the drug remains in an aqueous environment.
(TIF)
Measuring the nuclear versus non-nuclear doxorubicin accumulation. HCT116 monolayers were grown to confluency and then incubated in buffer solution (of desired pH and salt composition). Monolayers were then loaded with Hoechst 33342 and doxorubicin. (A) Doxorubicin fluorescence (488 nm excitation) recorded confocally, showing signal in nuclear and non-nuclear regions. (B) Hoechst 33342 fluorescence (405 nm excitation) used to identify nuclei. (C) Doxorubicin fluorescence in nuclear regions identified on the basis of supra-threshold Hoechst 33342 signal. The ratio of doxorubicin fluorescence in nuclear and non-nuclear regions was determined as (nuclear doxorubicin fluorescence) divided by (total doxorubicin fluorescence minus nuclear doxorubicin fluorescence).
(TIF)