Abstract
Background
Costimulation of murine macrophages with immune complexes (ICs) and TLR ligands leads to alternative activation. Studies on human myeloid cells, however, indicate that ICs induce an increased pro-inflammatory cytokine production. This study aimed to clarify the effect of ICs on the pro- versus anti-inflammatory profile of human polarized macrophages.
Materials and Methods
Monocytes isolated from peripheral blood of healthy donors were polarized for four days with IFN-γ, IL-4, IL-10, GM-CSF, M-CSF, or LPS, in the presence or absence of heat aggregated gamma-globulins (HAGGs). Phenotypic polarization markers were measured by flow cytometry. Polarized macrophages were stimulated with HAGGs or immobilized IgG alone or in combination with TLR ligands. TNF, IL-6, IL-10, IL-12, and IL-23 were measured by Luminex and/or RT-qPCR.
Results
HAGGs did not modulate the phenotypic polarization and the cytokine production of macrophages. However, HAGGs significantly altered the TLR-induced cytokine production of all polarized macrophage subsets, with the exception of MΦIL-4. In particular, HAGGs consistently enhanced the TLR-induced IL-10 production in both classically and alternatively polarized macrophages (M1 and M2). The effect of HAGGs on TNF and IL-6 production was less pronounced and depended on the polarization status, while IL-23p19 and IL-12p35 expression was not affected. In contrast with HAGGs, immobilized IgG induced a strong upregulation of not only IL-10, but also TNF and IL-6.
Conclusion
HAGGs alone do not alter the phenotype and cytokine production of in vitro polarized human macrophages. In combination with TLR-ligands, however, HAGGs but not immobilized IgG shift the cytokine production of distinct macrophage subsets toward IL-10.
Introduction
Macrophages play an important role in a wide variety of physiological and pathological processes including host defence, acute and chronic inflammation, and tissue homeostasis and remodelling. These pleiotropic cells can scavenge debris, sense microbial dangers signals, process and present antigens, and produce an array of pro- and anti-inflammatory mediators. Macrophage function, including the production of key cytokines such as TNF and IL-10, is not only determined by their activation but also by previous exposure to cytokines, growth factors, and other mediators during their differentiation from monocyte to macrophage. This so-called polarization process was originally proposed to distinguish classically activated macrophages (M1), which drive pro-inflammatory responses, from alternatively activated macrophages (M2), which steer immunoregulation and/or tissue remodelling [1]–[4]. Subsequent studies with mice and, to a lesser extent, human myeloid cells have lead to several more complex polarization models [5]–[7]. Using here the nomenclature proposed by Mantovani et al [5], the best characterized subsets are M1, M2a, and M2c, which are induced by IFN-γ, IL-4, or IL-10, respectively. Functional differences are accompanied by distinct phenotypic profiles, and we recently validated in vitro a number of specific phenotypic markers for each of these three macrophage subsets [8].
Of particular interest in the model proposed by Mantovani [5], are the so-called M2b macrophages, which result from polarization with ICs in combination with TLR ligands, such as LPS. Original studies showed that stimulation of mouse macrophages with ICs resulted in enhanced production of IL-10 and prostaglandins, especially PGE2 [9], while IL-6, IL-1, and TNF levels were not affected [10]–[12]. Polarization of mouse bone-marrow derived macrophages (BMDMs) with IFN-γ, followed by stimulation with ICs and LPS resulted also in an increased IL-10 production, which led to the conclusion that ICs modulate the macrophage cytokine production profile towards alternative activation, in a similar fashion as IL-10, TGF-β, or glucocorticoids [5], [7], [13]–[15].
Although this model has been confirmed by several studies, two important aspects remain incompletely understood. Firstly, it is unclear whether ICs induce macrophage polarization to a distinct subset or rather modulate the function of polarized macrophages. The previously mentioned experiments using IFN-γ polarized BMDMs could suggest namely either that M1 polarization can be reversed by ICs, or that ICs modulate the function of macrophages irrespective of their polarization status. Secondly, most of these experiments were performed in mice and only few studies analyzed the effects of ICs on human myeloid cells. In human monocytes, cross-linking of FcγRs decreased IL-12 and increased IL-1ra, IL-10, and PGE2 production, which is in agreement with the M2 profile in mice [16], [17]. The increased IL-10 production was not only observed after monocyte stimulation with artificial ICs, but also with ICs from SLE sera [18]. At the same time, however, the production of pro-inflammatory factors such as TNF, GM-CSF, IL-6, IL-8, and IL-1β by monocytes was also increased by FcγR cross-linking [19]–[24]. This was not only observed in human monocytes, since we demonstrated previously that costimulation of human monocyte-derived DCs with ICs and TLR ligands leads to increased production of TNF and IL-6 [25]. Similarly, stimulation of M-CSF polarized human macrophages (MΦM-CSF) with immobilized HAGGs (iHAGGs) or ACPA-containing ICs induced higher TNF production [26], [27].
In order to clarify the effect of ICs on human macrophages and to assess whether the existing discrepancies in the literature are due to interspecies differences or to specific polarization conditions, we systematically studied the effect of HAGGs in the presence or absence of TLR stimuli on the phenotype and cytokine production of human polarized macrophages.
Materials and Methods
Ethics statement
This study was conducted with the approval of the Medical Ethical Committee of the Academic Medical Center/University of Amsterdam and all blood donors gave their written informed consent.
Monocyte isolation and in vitro polarization
Monocyte isolation and in vitro polarization were performed as previously described [8]. Briefly, monocytes from peripheral blood of healthy volunteers were isolated by gradient centrifugation with Lymphoprep (Axis-Shield PoPAS, Oslo, Norway) and, subsequently, Percoll gradient separation (GE Healthcare, Uppsala, Sweden). Monocytes were cultured at a concentration of 0.5×106/ml in Iscove's Modified Dulbecco's Medium (Invitrogen, Breda, The Netherlands) supplemented with 10% fetal calf serum (FCS) (PAA Laboratories, Cölbe, Germany) in 6 well culture plates (Corning Incorporated, New York, NY, USA). Cells were cultured in medium alone or polarized with human recombinant IFN-γ (50 ng/ml; R&D Systems, Abingdon, UK), IL-4 (40 ng/ml; Miltenyi Biotec, Bergisch Gladbach, Germany), IL-10 (50 ng/ml; R&D Systems), GM-CSF (50 ng/ml, R&D Systems), or M-CSF (50 ng/ml, R&D Systems) for 4 days. Polarization with LPS (100 ng/ml, E. coli 0111:B4; Sigma Aldrich, Zwijndrecht, The Netherlands) was additionally used for the phenotypic experiments. Human HAGGs (50 µg/ml), prepared as previously described [28], were added to each polarizing condition.
Flow cytometric analysis
Cultured macrophages were recovered by scraping of the plate. Surface marker expression was analyzed by flow cytometry (BD FACS Calibur Flow Cytometer, Erembodegem, Belgium) using fluorochrome-labeled monoclonal antibodies against CD14 (clone 61D3, eBioscience, San Diego, CA), CD16 (clone DJ130c, AbD Serotec, Düsseldorf, Germany), CD32 (clone AT10, abcam, Cambridge, UK), CD64 (clone 10.1, BioLegend, Uithoorn, The Netherlands), CD80 (clone L307.4, BD Pharmingen, Breda, Nederland), CD163 (clone GHI/61, BD Pharmingen), CD200R (clone OX108, AbD Serotec), TLR2 (clone T2.5, abcam) and TLR4 (clone 76B357.1, abcam). Equivalent concentrations of matched isotype controls were included in all experiments. Before staining, Fc receptors were blocked with 10% human serum (Lonza, Cologne, Germany). Data were analyzed with Flow Jo Flow Cytometry Analysis software (Tree Star, Ashland, OR) after gating on the myeloid population in the FSC/SSC window. Values were expressed as the ratio of the geometric mean fluorescence intensity (gMFI) of the marker of interest over the gMFI of the isotype control.
Cytokine production
To assess the cytokine production of polarized macrophages, the in vitro polarized cells were harvested and extensively washed at day 4. Macrophages were subsequently activated with HAGGs (50 µg/ml) and/or the following TLR ligands: LPS (100 ng/ml; Sigma Aldrich), Pam3CSK4 (5 µg/ml; EMC microcollections, Tübingen, Germany), or R848 (2 µg/ml; InvivoGen, San Diego, CA). In order to study the effect of immobilized IgG, macrophages were also cultured on IgG coated plates (96-well plate, Corning). The supernatant of these cultures was recovered after 20 hours of stimulation and analyzed using commercially available Luminex kits (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. Cytokine levels of TNF, IL-6, and IL-10 were measured and analyzed with the Bio-Plex system (Bio-Rad). The sensitivity of the cytokine assay was <5 pg/ml for all measured cytokines.
Quantitative real-time PCR
Total RNA was isolated from in vitro polarized macrophages using GenElute™ Mammalian Total RNA Miniprep Kit (Sigma Aldrich) and reverse transcribed using RevertAid™ H Minus First Strand cDNA Synthesis Kit (Fermentas, St. Leon-Rot, Germany). RNA concentration was determined with the Nanodrop (Nanodrop Technologies, Wilmington, DE). Quantitative real-time PCR was performed using StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA). Each 12 µl reaction was performed in a 96-well format with 5 ng of cDNA, 10 µl of SYBR green PCR Master Mix (Applied Biosystems) and a concentration of 50 nmol of each primer. The primers comprised IL-10 (forward 5′-GATGCCTTCAGCAGAGTGAA, reverse 5′-CCCAGGTAACCCTTAAAGTCC), IL-23p19 (forward 5′-TTCTCTGCTCCCTGATAGCC, reverse 5′-CCTCAGGCTGCAGGAGTT), and IL-12p35 (forward 5′- ACCAGGTGGAGTTCAAGACC, reverse 5′-TGGCACAGTCTCACTGTTGA), respectively. All reactions were performed in duplicate. The mRNA expression levels were normalized to those of the human housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Oligonucleotide primers were designed using the online tool for Real-time PCR (TaqMan) Primer Design (Genscript) and obtained from Invitrogen.
Statistics
Statistical analysis was performed using Prism software (GraphPad, La Jolla, CA). Data were expressed as mean ± SEM. ANOVA followed by Bonferroni post test were used for comparisons between more than 2 groups. For comparisons between 2 groups (stimulation with and without HAGGs) we used the Wilcoxon matched pair test. A P value of less than 0.05 was considered to be statistically significant.
Results
Soluble ICs do not alter phenotypic polarization of human macrophages
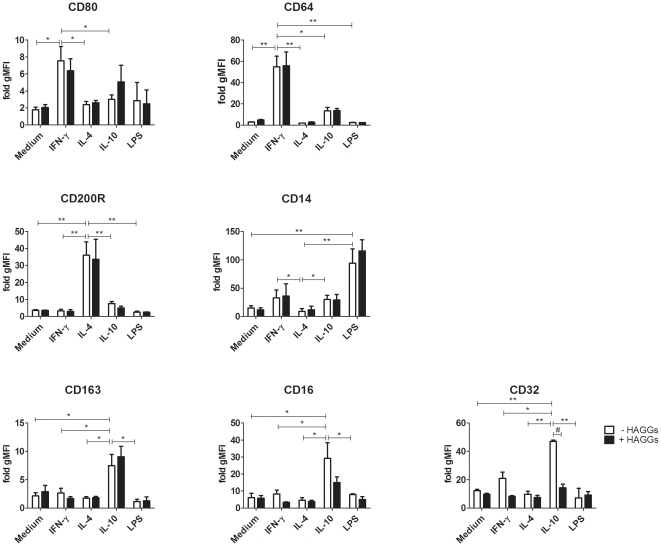
Several reports indicate that stimulation of murine macrophages with ICs in combination with TLR ligands induces alternative macrophage polarization [7], [10], [12]–[14], [29]. Therefore, our first aim was to investigate whether HAGGs had a similar polarizing effect on human macrophages. We tested whether the expression of phenotypic markers for specific polarized macrophage subsets was altered by HAGGs alone, in combination with LPS, or in combination with IFN-γ, IL-4, or IL-10 as major polarizing cytokines [5], [7]. IFN-γ polarization resulted in upregulation of CD80 (p<0.05 compared to medium, IL-4 and IL-10) and CD64 (p<0.05 compared to IL-10, and p<0.01 compared to medium, IL-4 and LPS), IL-4 upregulated CD200R (p<0.01 for all comparisons) and downregulated CD14 (p<0.05 compared to IFN-γ and IL-10, and p<0.01 compared to LPS), while IL-10 induced a higher expression of CD163 (p<0.05 for all comparisons), CD16 (p<0.05 for all comparisons), and CD32 (p<0.05 compared to IFN-γ, and p<0.01 compared to medium, IL-4 and LPS), respectively [8] (Figure 1). LPS used as polarizing stimulus strongly increased the expression of CD14 (p<0.01), as described previously [30]. Stimulation with HAGGs alone or in combination with LPS did not lead to upregulation of specific phenotypic markers, in particular those associated with IL-4 and IL-10 polarization (Figure 1). Additionally, HAGGs did not influence the phenotypic effects of IFN-γ, IL-4, or IL-10, with the exception of CD32 downregulation on MΦIL-10 (p<0.01) (Figure 1). Taken together, these experiments did not show any polarizing effect of HAGGs on the phenotype of human macrophage subsets.
Figure 1. Expression of phenotypic markers on in vitro polarized human macrophages.
Healthy peripheral blood monocytes were cultured for 4 days in medium or in medium supplemented with IFN-γ, IL-4, IL-10, or LPS in the absence (white bars) or presence (black bars) of HAGGs. Expression of MΦIFN-γ markers CD80 and CD64, MΦIL-4 markers CD200R and CD14, and MΦIL-10 markers CD163, CD16 and CD32 was measured by flow cytometry. Bars represent the mean ± SEM of 4 independent experiments. *p<0.05, **p<0.01, #p<0.01.
Macrophage polarization determines the TLR-induced production of pro- and anti-inflammatory cytokines
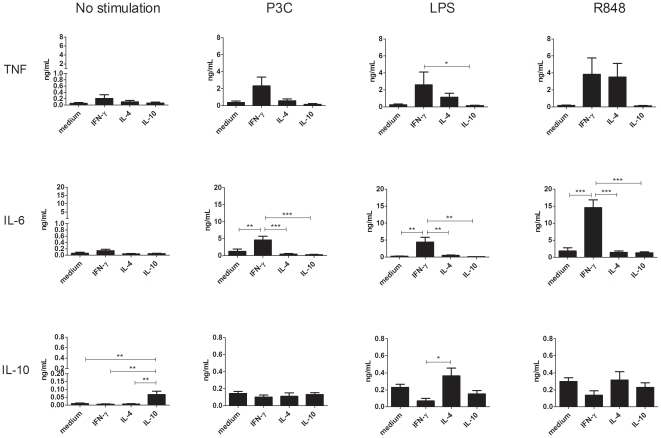
Numerous reports indicate that, besides phenotype, a main difference between distinct polarized macrophage subsets is the production of key cytokines such as TNF, IL-6, IL-10, IL-12 and IL-23 upon activation [5], [7], [13], [15], [31]–[33]. Before evaluating the effect of HAGGs on the cytokine production of polarized human macrophages, we first determined the cytokine production profile of MΦIFN-γ, MΦIL-4, and MΦIL-10 upon TLR stimulation. As depicted in Figure 2, the production of TNF, IL-6 and IL-10 was very low for all macrophage subsets in the absence of TLR stimulation. In these basal conditions, MΦIL-10 produced significantly more IL-10 than all other macrophage subsets (p<0.01). Following TLR stimulation, MΦIFN-γ produced more IL-6 than all other macrophage subsets (p<0.001 for the P3C and R848 stimulations, and p<0.01 for the LPS stimulation). There was a similar albeit less pronounced trend for TNF (p<0.05 versus MΦIL-10 for the LPS stimulation), which was partially due to the fact that MΦIL-4 also produced more TNF, especially after LPS and R848 stimulation. IL-10 levels were similar in all polarized subsets, with the exception of a higher production by MΦIL-4 versus MΦIFN-γ after LPS stimulation (p<0.05). Despite the fact that some comparisons did not reach statistical significance, these findings confirm previous reports of a pro-inflammatory profile of MΦIFN-γ versus a more neutral to anti-inflammatory profile for MΦIL-4 and MΦIL-10 upon TLR stimulation [5], [7], [13], [15].
Figure 2. TLR-induced production of TNF, IL-6, and IL-10 by MΦIFN-γ, MΦIL-4, and MΦIL-10.
Healthy peripheral blood monocytes were cultured for 4 days in medium or in medium supplemented with IFN-γ, IL-4, or IL-10. Polarized macrophages were not stimulated, or stimulated for 20 hours with P3C, LPS, or R848. Bars represent the mean ± SEM of 6 independent experiments. *p<0.05, **p<0.01, ***p<0.001.
Soluble ICs enhance the TLR-induced IL-10 production by polarized macrophages
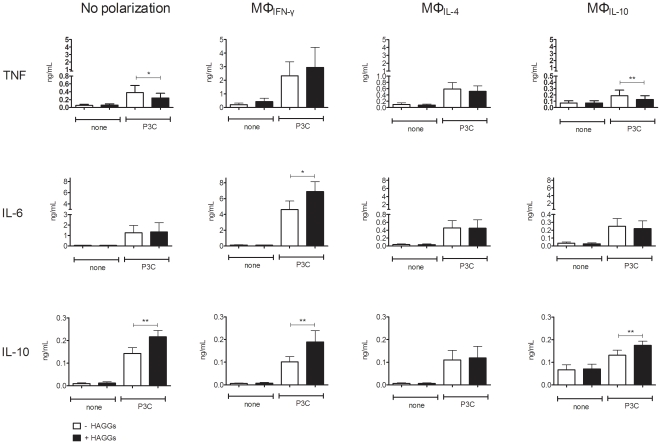
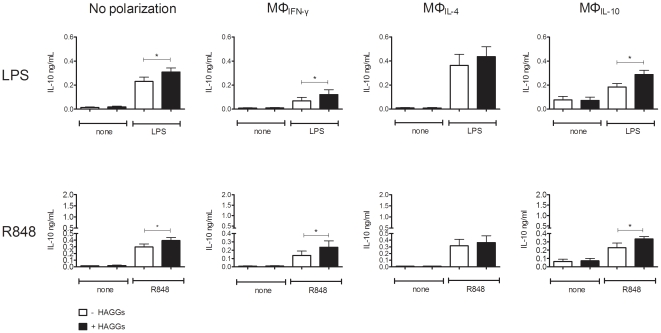
Since ICs were described to induce an immunoregulatory function in mouse macrophages [10], [18], [29], [34], [35], we next investigated whether HAGGs alter the pro- versus anti-inflammatory cytokine production of differentially polarized human macrophages. In the absence of TLR stimulation, HAGGs did not alter the low basal production of TNF, IL-6, and IL-10 by any of the macrophage subsets (Figure 3). Using P3C as prototypical TLR stimulation, costimulation with HAGGs had variable effects on the production of pro-inflammatory cytokines, with a decrease in TNF production by unpolarized macrophages and MΦIL-10, and an increased IL-6 production by MΦIFN-γ (p<0.05 compared to P3C alone for all 3 comparisons) (Figure 3). In contrast, HAGGs consistently enhanced IL-10 production by all macrophage subsets, with the exception of MΦIL-4 (p<0.01 for all comparisons) (Figure 3). In particular, HAGGs almost doubled the IL-10 secretion by MΦIFN-γ. These data were confirmed by repeating the experiments with other TLR stimuli. HAGGs together with LPS or R848 induced a modest and variable effect on TNF and IL-6 production (data not shown), but a consistent and significant increase in IL-10 production by all macrophage subsets, with the exception of MΦIL-4 (p<0.05 compared to TLR stimulation without HAGGs) (Figure 4). Thus, costimulation of in vitro polarized human macrophages with HAGGs and TLR ligands resulted in an increased IL-10 production by all polarized macrophage subsets, excepting MΦIL-4.
Figure 3. TNF, IL-6, and IL-10 production by MΦIFN-γ, MΦIL-4, and MΦIL-10 after stimulation with HAGGs and P3C.
Healthy peripheral blood monocytes were cultured for 4 days in medium or in medium supplemented with IFN-γ, IL-4, or IL-10. Polarized macrophages were not stimulated, or stimulated for 20 hours with P3C, in the presence or absence of HAGGs. Bars represent the mean ± SEM of 9 independent experiments. *p<0.05, **p<0.01.
Figure 4. IL-10 production by MΦIFN-γ, MΦIL-4, and MΦIL-10 after stimulation with HAGGs and LPS, or R848.
Healthy peripheral blood monocytes were cultured for 4 days in medium or in medium supplemented with IFN-γ, IL-4, or IL-10. Polarized macrophages were not stimulated, or stimulated for 20 hours with LPS, or R848, in the presence or absence of HAGGs. Bars represent the mean ± SEM of 6 independent experiments. *p<0.05.
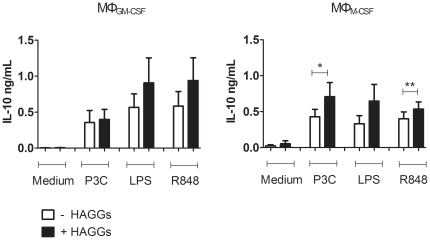
Effect of soluble ICs on the IL-10 production by MΦM-CSF and MΦGM-CSF
Both phenotypic analyses and cytokine profiling indicated that GM-CSF polarized macrophages (MΦGM-CSF) tend to resemble MΦIFN-γ, whereas M-CSF mimics the effects of alternative polarization stimuli, in particular IL-10 [8], [32], [33], [36]. We therefore investigated whether stimulation with HAGGs also modulated the IL-10 production of MΦGM-CSF and MΦM-CSF. HAGGs alone did not have any effect on the IL-10 production. However, after co-stimulation with TLR ligands, HAGGs induced an increased IL-10 production by MΦM-CSF but not MΦGM-CSF (p<0.05 for P3C, and p<0.01 for R848) (Figure 5). Concluding, MΦM-CSF showed a higher TLR-induced IL-10 production after stimulation with HAGGs.
Figure 5. IL-10 production by MΦGM-CSF and MΦM-CSF after stimulation with HAGGs and TLR ligands.
Healthy peripheral blood monocytes were cultured for 4 days in medium supplemented with GM-CSF or M-CSF. MΦGM-CSF and MΦM-CSF were not stimulated, or stimulated for 20 hours with P3C, LPS, or R848, in the presence or absence of HAGGs. Bars represent the mean ± SEM of 6 independent experiments. *p<0.05, **p<0.01.
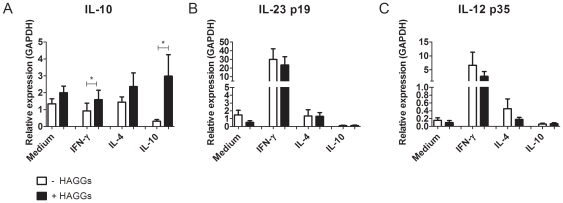
Increased LPS-induced IL-10 versus IL-23 and IL-12 expression after stimulation with soluble ICs
In order to confirm the increase in TLR-induced IL-10 production by polarized macrophages after stimulation with HAGGs, we measured the mRNA expression of IL-10 by each macrophage subset after 7 hours of stimulation with LPS, in the presence or absence of HAGGs. As shown in figure 6, HAGGs increased the expression of IL-10 in all macrophage subsets (p<0.05 for MΦIFN-γ and MΦIL-10). As IL-23 and IL-12 protein levels were undetectable in the supernatants of the previous experiments, we investigated the effect of HAGGs on these cytokines at mRNA level. In contrast with IL-10, we did not observe any significant effect of HAGGs on IL-23p19 and IL-12p35 mRNA expression. Taken together, these data confirm the specific increase in IL-10 expression after costimulation with HAGGs and TLR ligands, with the strongest effects observed in MΦIFN-γ and MΦIL-10.
Figure 6. mRNA expression of IL-10, IL-23p19, and IL-12p35 after macrophage stimulation with HAGGs and LPS.
Healthy peripheral blood monocytes were cultured for 4 days in medium or in medium supplemented with IFN-γ, IL-4, or IL-10. Polarized macrophages were stimulated for 7 hours with LPS, in the presence or absence of HAGGs. mRNA expression levels were measured by quantitative RT-PCR and were normalized to those of the human housekeeping gene GAPDH. Bars represent the mean ± SEM of 6 independent experiments. *p<0.05.
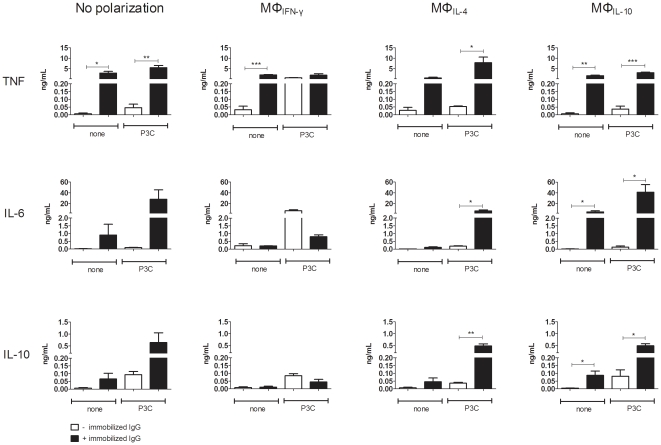
Immobilized IgG enhances the cytokine production of polarized macrophages without shifting the balance towards IL-10
As the consistent increase in IL-10 production by HAGGs in our experiments contrasted with previously published data on increased TNF production by human macrophages upon stimulation with iHAGGs or immobilized IgG [26], [27], [37], we next investigated the effect of immobilized IgG on the cytokine production of differentially polarized macrophages. In contrast with HAGGs, even in the absence of P3C stimulation, immobilized IgG increased the production of TNF (p<0.05 in unpolarized macrophages, p<0.01 in MΦIL-10, and p<0.001 in MΦIFN-γ), IL-6 (p<0.05 in MΦIL-10) and IL-10 (p<0.05 in MΦIL-10) (Figure 7). Similarly, immobilized IgG strongly augmented the TLR-induced production of TNF (p<0.05 in MΦIL-4, p<0.01 in unpolarized macrophages, and p<0.001 in MΦIL-10), IL-6 (p<0.05 in MΦIL-4 and MΦIL-10) and IL-10 (p<0.05 in MΦIL-10, and p<0.01 in MΦIL-4). Comparable results were obtained when using LPS or R848 as TLR stimuli (data not shown). These data indicate that immobilized IgG augments the spontaneous, as well as the TLR-induced cytokine production of polarized macrophages, without shifting the balance from pro-inflammatory cytokines to IL-10.
Figure 7. TNF, IL-6, and IL-10 production by MΦIFN-γ, MΦIL-4, and MΦIL-10 after stimulation with immobilized IgG and P3C.
Healthy peripheral blood monocytes were cultured for 4 days in medium or in medium supplemented with IFN-γ, IL-4, or IL-10. Polarized macrophages were not stimulated, or stimulated for 20 hours with P3C, in the presence or absence of immobilized IgG. Bars represent the mean ± SEM of 3 independent experiments. *p<0.05, **p<0.01, ***p<0.001.
Discussion
ICs in combination with TLR ligands have been described to increase the IL-10 production of mouse macrophages, leading to a distinct alternative macrophage activation type (M2b) [5]. ICs also stimulated the in vivo IL-10 production in different animal models, which induced immune regulation [34], [38]. In host defence, this mechanism could represent a physiological feed-back loop, contributing to the down-regulation of innate tissue inflammation at the moment that microorganisms are cleared by the adaptive immune system. The effect of ICs on human myeloid cells, however, is less clearly defined, as several reports indicate that they do not only increase the production of anti-inflammatory mediators such as IL-10 [16]–[18], but also strongly upregulate pro-inflammatory mediators [19]–[27]. This discrepancy may be related to differences between species, cell types (monocytes versus macrophages, or dendritic cells), or macrophage polarization conditions. In this study we show that HAGGs in the absence of TLR stimuli do not alter the phenotype and cytokine production of in vitro polarized human macrophage subsets. Costimulation with HAGGs and TLR ligands, however, resulted in markedly increased IL-10 production in most macrophage subsets, while the modulation of TNF and IL-6 production was more discrete and dependent on the macrophage polarization status. As previously shown in mouse macrophages [10], [12], [13], [29], costimulation with ICs and TLR ligands also augmented the IL-10 production of human MΦIFN-γ, which are prototypical pro-inflammatory cells.
These findings raise the critical issue of the exact relationship between polarization, phenotype and function of human macrophages. The macrophage polarization models originally refer to the production of pro- versus anti-inflammatory mediators to define classically (M1) versus alternatively activated macrophages (M2) [1]–[3], [5]–[7]. Macrophage polarization is also associated with the expression of specific phenotypic markers [4], [8], [8], [39]–[41]. Our experiments confirm that polarization affects both the phenotype and the function of macrophages, as exemplified by the distinct cytokine production of MΦIFN-γ, MΦIL-4, and MΦIL-10 upon TLR stimulation. Concerning the effect of ICs, we show that HAGGs can modulate the TLR-mediated cytokine production of human polarized macrophages, despite the lack of phenotypic alterations. The capacity of so-called ‘pro-inflammatory’ macrophages to produce significant amounts of IL-10 upon costimulation with HAGGs and TLR ligands illustrate the functional plasticity of these cells. Furthermore, our data indicate that the production of pro- versus anti-inflammatory mediators by macrophages is not only related to their polarization, but also to the type of activation and that, as a consequence, phenotypic polarization markers do not fully correlate to the macrophage function.
An important aspect in this context is the expression of FcγRs on polarized macrophage subsets. Studies using different IgG subtypes, specific FcγR blocking antibodies, or FcγR knockout animals demonstrated that the differential expression of the activating versus inhibitory FcγRs on immune cells influences their response to ICs [23], [28], [37], [42]–[45]. We previously confirmed that the high affinity FcγRI (CD64) was upregulated on MΦIFN-γ, while the low affinity FcγRII (CD32) and FcγRIII (CD16) were specifically expressed by MΦIL-10. Furthermore, the activating and inhibitory isoforms of CD32 (CD32a and CD32b, respectively) were differentially expressed on the 3 macrophage subsets, with a lower CD32a/CD32b ratio on MΦIL-4 versus MΦIL-10 and MΦIFN-γ [8]. A shifted FcγR balance towards FcγRIIb on IL-4 primed human monocytes in comparison to IFN-γ, TNF, and IL-10 primed monocytes was previously related to the failure of these cells to upregulate TNF after crosslinking by IgG [37]. Finally, also the expression of TLR2 and TLR4 was differentially modulated by polarization, with lower expression levels on MΦIL-4 versus MΦIL-10 and MΦIFN-γ (Figure S1). Although it is beyond the scope of this study to investigate the exact signal transduction in these conditions, the low expression of activating FcγRs, TLR2, and TLR4 on MΦIL-4 could at least partially explain their poor responsiveness to HAGGs.
In an attempt to clarify the discrepancy between our findings and reports indicating increased TNF production by monocytes and macrophages upon stimulation with immobilized IgG [26], [27], [37], we used immobilized IgG in the same experimental conditions and observed a number of differences with soluble HAGGs. Firstly, immobilized IgG, but not HAGG was able to stimulate cytokine production by polarized macrophages in the absence of TLR co-stimulation. Secondly, whereas MΦIL-4 appeared to be poorly responsive to HAGGs, all polarized macrophage subsets reacted strongly upon stimulation with immobilized IgG. Finally and most importantly, HAGGs consistently augmented IL-10 production, whereas immobilized IgG upregulated the production of TNF, IL-6 and IL-10. These data are consistent with previous reports in which prevention of IC phagocytosis by using immobilized IgG was shown to induce persistent ERK signaling and high TNF production by both monocytes and macrophages [26], [46], [47]. Furthermore, the TLR-induced IL-10 production by human monocytes was higher after immobilized IgG versus HAGG stimulation, and immobilized IgG was able to induce IL-10 production even in the absence of TLR ligands [48]. This data emphasize that, besides many other factors such as IgG isotype, monomeric versus polymeric IgG, antigen-antibody ratio, and natural versus artificial ICs, the capacity to internalize ICs is a key determinant of the functional outcome of IC stimulation of macrophages [16], [18]–[23], [42], [49].
The differential effects of soluble versus immobilized IgG do not only emphasize the functional plasticity of polarized macrophages, but may also be relevant for our understanding of specific pathological and therapeutic conditions. In autoimmune diseases, ICs are thought to contribute to chronic tissue inflammation when deposited in tissues, with as prototypical examples lupus nephritis and rheumatoid synovitis. In this immobilized form, ICs may promote mainly pro-inflammatory over anti-inflammatory responses by tissue macrophages. Moreover, IC binding to myeloid cells in these conditions was also reported to inhibit their responsiveness to IL-10 [50], [51]. In sharp contrast, intravenous administration of soluble immunoglobulins (IVIg) has been used for a long time in the treatment of diverse autoimmune diseases [52]–[55]. The immunosuppressive effect of macrophage FcγR ligation has been attributed to blocking of the activating FcγRs, stimulation of FcγRIIb, and increased anti- versus pro-inflammatory cytokine production [10], [12], [16], [18], [45], [56]. Our in vitro data thus support the hypothesis that soluble ICs shift the balance of pro- towards anti-inflammatory cytokine production. Interestingly, this effect may be important not only for the therapeutic efficacy of IVIg, but also of targeted biological therapies. It was indeed recently demonstrated that anti-TNF antibodies induce IL-10 producing macrophages in an Fc-dependent manner and that these immunoregulatory macrophages are involved in mucosal healing in inflammatory bowel disease [56], [57]. These studies reveal that monoclonal antibody therapy can drive anti-inflammatory responses by Fc region-dependent and target-independent modulation of macrophage function.
In conclusion, we showed here that distinct polarized macrophage subsets retain an important functional plasticity despite maintenance of their specific phenotype. In particular, we demonstrated that soluble ICs, but not immobilized IgG shifted the balance of human macrophage cytokine production towards IL-10. These findings raise the possibility of therapeutic modulation of macrophage function in the context of chronic tissue inflammation.
Supporting Information
Expression of TLR2 and TLR4 on MΦIFN-γ, MΦIL-4, and MΦIL-10. Healthy peripheral blood monocytes were cultured for 4 days in medium or in medium supplemented with IFN-γ, IL-4, or IL-10. Expression TLR2 and TLR4 was measured by flow cytometry. Bars represent the mean ± SEM of 4 independent experiments. *p<0.05, **p<0.01
(TIF)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research is supported by a VIDI grant from The Netherlands Organization for Scientific Research (NWO) and by a grant from the Dutch Arthritis Foundation (Reumafonds). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10:137–142. doi: 10.1016/s1074-7613(00)80014-x. S1074-7613(00)80014-X [pii] [DOI] [PubMed] [Google Scholar]
- 3.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. 10.1038/nri978 [doi];nri978 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. S1471-4906(04)00295-9 [pii];10.1016/j.it.2004.09.015 [doi] [DOI] [PubMed] [Google Scholar]
- 6.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 7.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. nri2448 [pii];10.1038/nri2448 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambarus CA, Krausz S, van Eijk M, Hamann J, Radstake TR, et al. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods. 2011 doi: 10.1016/j.jim.2011.10.013. S0022-1759(11)00294-8 [pii];10.1016/j.jim.2011.10.013 [doi] [DOI] [PubMed] [Google Scholar]
- 9.Bonney RJ, Naruns P, Davies P, Humes JL. Antigen-antibody complexes stimulate the synthesis and release of prostaglandins by mouse peritoneal macrophages. Prostaglandins. 1979;18:605–616. doi: 10.1016/0090-6980(79)90027-3. [DOI] [PubMed] [Google Scholar]
- 10.Gerber JS, Mosser DM. Reversing lipopolysaccharide toxicity by ligating the macrophage Fc gamma receptors. J Immunol. 2001;166:6861–6868. doi: 10.4049/jimmunol.166.11.6861. [DOI] [PubMed] [Google Scholar]
- 11.Sutterwala FS, Noel GJ, Clynes R, Mosser DM. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J Exp Med. 1997;185:1977–1985. doi: 10.1084/jem.185.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutterwala FS, Noel GJ, Salgame P, Mosser DM. Reversal of proinflammatory responses by ligating the macrophage Fcgamma receptor type I. J Exp Med. 1998;188:217–222. doi: 10.1084/jem.188.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298–1307. doi: 10.1189/jlb.0406249. jlb.0406249 [pii];10.1189/jlb.0406249 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson CF, Mosser DM. Cutting edge: biasing immune responses by directing antigen to macrophage Fc gamma receptors. J Immunol. 2002;168:3697–3701. doi: 10.4049/jimmunol.168.8.3697. [DOI] [PubMed] [Google Scholar]
- 15.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. 2692 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Berger S, Ballo H, Stutte HJ. Immune complex-induced interleukin-6, interleukin-10 and prostaglandin secretion by human monocytes: a network of pro- and anti-inflammatory cytokines dependent on the antigen∶antibody ratio. Eur J Immunol. 1996;26:1297–1301. doi: 10.1002/eji.1830260618. 10.1002/eji.1830260618 [doi] [DOI] [PubMed] [Google Scholar]
- 17.Berger S, Chandra R, Ballo H, Hildenbrand R, Stutte HJ. Immune complexes are potent inhibitors of interleukin-12 secretion by human monocytes. Eur J Immunol. 1997;27:2994–3000. doi: 10.1002/eji.1830271136. 10.1002/eji.1830271136 [doi] [DOI] [PubMed] [Google Scholar]
- 18.Ronnelid J, Tejde A, Mathsson L, Nilsson-Ekdahl K, Nilsson B. Immune complexes from SLE sera induce IL10 production from normal peripheral blood mononuclear cells by an FcgammaRII dependent mechanism: implications for a possible vicious cycle maintaining B cell hyperactivity in SLE. Ann Rheum Dis. 2003;62:37–42. doi: 10.1136/ard.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abrahams VM, Cambridge G, Lydyard PM, Edwards JC. Induction of tumor necrosis factor alpha production by adhered human monocytes: a key role for Fcgamma receptor type IIIa in rheumatoid arthritis. Arthritis Rheum. 2000;43:608–616. doi: 10.1002/1529-0131(200003)43:3<608::AID-ANR18>3.0.CO;2-G. 10.1002/1529-0131(200003)43:3<608::AID-ANR18>3.0.CO;2-G [doi] [DOI] [PubMed] [Google Scholar]
- 20.Debets JM, Van de Winkel JG, Ceuppens JL, Dieteren IE, Buurman WA. Cross-linking of both Fc gamma RI and Fc gamma RII induces secretion of tumor necrosis factor by human monocytes, requiring high affinity Fc-Fc gamma R interactions. Functional activation of Fc gamma RII by treatment with proteases or neuraminidase. J Immunol. 1990;144:1304–1310. [PubMed] [Google Scholar]
- 21.Herrmann F, De Vos S, Brach M, Riedel D, Lindemann A, et al. Secretion of granulocyte-macrophage colony-stimulating factor by human blood monocytes is stimulated by engagement of Fc gamma receptors type I by solid-phase immunoglobulins requiring high-affinity Fc-Fc gamma receptor type I interactions. Eur J Immunol. 1992;22:1681–1685. doi: 10.1002/eji.1830220703. 10.1002/eji.1830220703 [doi] [DOI] [PubMed] [Google Scholar]
- 22.Krutmann J, Kirnbauer R, Kock A, Schwarz T, Schopf E, et al. Cross-linking Fc receptors on monocytes triggers IL-6 production. Role in anti-CD3-induced T cell activation. J Immunol. 1990;145:1337–1342. [PubMed] [Google Scholar]
- 23.Mullazehi M, Mathsson L, Lampa J, Ronnelid J. Surface-bound anti-type II collagen-containing immune complexes induce production of tumor necrosis factor alpha, interleukin-1beta, and interleukin-8 from peripheral blood monocytes via Fc gamma receptor IIA: a potential pathophysiologic mechanism for humoral anti-type II collagen immunity in arthritis. Arthritis Rheum. 2006;54:1759–1771. doi: 10.1002/art.21892. 10.1002/art.21892 [doi] [DOI] [PubMed] [Google Scholar]
- 24.Polat GL, Laufer J, Fabian I, Passwell JH. Cross-linking of monocyte plasma membrane Fc alpha, Fc gamma or mannose receptors induces TNF production. Immunology. 1993;80:287–292. [PMC free article] [PubMed] [Google Scholar]
- 25.Radstake TR, van Lent PL, Pesman GJ, Blom AB, Sweep FG, et al. High production of proinflammatory and Th1 cytokines by dendritic cells from patients with rheumatoid arthritis, and down regulation upon FcgammaR triggering. Ann Rheum Dis. 2004;63:696–702. doi: 10.1136/ard.2003.010033. 10.1136/ard.2003.010033 [doi];63/6/696 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clavel C, Nogueira L, Laurent L, Iobagiu C, Vincent C, et al. Induction of macrophage secretion of tumor necrosis factor alpha through Fcgamma receptor IIa engagement by rheumatoid arthritis-specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis Rheum. 2008;58:678–688. doi: 10.1002/art.23284. 10.1002/art.23284 [doi] [DOI] [PubMed] [Google Scholar]
- 27.Laurent L, Clavel C, Lemaire O, Anquetil F, Cornillet M, et al. Fcgamma receptor profile of monocytes and macrophages from rheumatoid arthritis patients and their response to immune complexes formed with autoantibodies to citrullinated proteins. Ann Rheum Dis. 2011;70:1052–1059. doi: 10.1136/ard.2010.142091. ard.2010.142091 [pii];10.1136/ard.2010.142091 [doi] [DOI] [PubMed] [Google Scholar]
- 28.Radstake TR, Blom AB, Sloetjes AW, van Gorselen EO, Pesman GJ, et al. Increased FcgammaRII expression and aberrant tumour necrosis factor alpha production by mature dendritic cells from patients with active rheumatoid arthritis. Ann Rheum Dis. 2004;63:1556–1563. doi: 10.1136/ard.2003.016550. 63/12/1556 [pii];10.1136/ard.2003.016550 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson CF, Mosser DM. A novel phenotype for an activated macrophage: the type 2 activated macrophage. J Leukoc Biol. 2002;72:101–106. [PubMed] [Google Scholar]
- 30.Landmann R, Knopf HP, Link S, Sansano S, Schumann R, et al. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun. 1996;64:1762–1769. doi: 10.1128/iai.64.5.1762-1769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gratchev A, Kzhyshkowska J, Kothe K, Muller-Molinet I, Kannookadan S, et al. Mphi1 and Mphi2 can be re-polarized by Th2 or Th1 cytokines, respectively, and respond to exogenous danger signals. Immunobiology. 2006;211:473–486. doi: 10.1016/j.imbio.2006.05.017. S0171-2985(06)00076-3 [pii];10.1016/j.imbio.2006.05.017 [doi] [DOI] [PubMed] [Google Scholar]
- 32.Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A. 2004;101:4560–4565. doi: 10.1073/pnas.0400983101. 10.1073/pnas.0400983101 [doi];0400983101 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verreck FA, de Boer T, Langenberg DM, van der Zanden L, Ottenhoff TH. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-gamma- and CD40L-mediated costimulation. J Leukoc Biol. 2006;79:285–293. doi: 10.1189/jlb.0105015. jlb.0105015 [pii];10.1189/jlb.0105015 [doi] [DOI] [PubMed] [Google Scholar]
- 34.Shanley TP, Schmal H, Friedl HP, Jones ML, Ward PA. Regulatory effects of intrinsic IL-10 in IgG immune complex-induced lung injury. J Immunol. 1995;154:3454–3460. [PubMed] [Google Scholar]
- 35.Virgin HW, Unanue ER. Immune complexes suppress cellular immunity. Ann N Y Acad Sci. 1984;437:16–27. doi: 10.1111/j.1749-6632.1984.tb37118.x. [DOI] [PubMed] [Google Scholar]
- 36.Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol. 2007;178:5245–5252. doi: 10.4049/jimmunol.178.8.5245. 178/8/5245 [pii] [DOI] [PubMed] [Google Scholar]
- 37.Wijngaarden S, Van de Winkel JG, Jacobs KM, Bijlsma JW, Lafeber FP, et al. A shift in the balance of inhibitory and activating Fcgamma receptors on monocytes toward the inhibitory Fcgamma receptor IIb is associated with prevention of monocyte activation in rheumatoid arthritis. Arthritis Rheum. 2004;50:3878–3887. doi: 10.1002/art.20672. 10.1002/art.20672 [doi] [DOI] [PubMed] [Google Scholar]
- 38.Tripp CS, Beckerman KP, Unanue ER. Immune complexes inhibit antimicrobial responses through interleukin-10 production. Effects in severe combined immunodeficient mice during Listeria infection. J Clin Invest. 1995;95:1628–1634. doi: 10.1172/JCI117837. 10.1172/JCI117837 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chroneos Z, Shepherd VL. Differential regulation of the mannose and SP-A receptors on macrophages. Am J Physiol. 1995;269:L721–L726. doi: 10.1152/ajplung.1995.269.6.L721. [DOI] [PubMed] [Google Scholar]
- 40.Hogger P, Dreier J, Droste A, Buck F, Sorg C. Identification of the integral membrane protein RM3/1 on human monocytes as a glucocorticoid-inducible member of the scavenger receptor cysteine-rich family (CD163). J Immunol. 1998;161:1883–1890. [PubMed] [Google Scholar]
- 41.Koning N, van Eijk M, Pouwels W, Brouwer MS, Voehringer D, et al. Expression of the inhibitory CD200 receptor is associated with alternative macrophage activation. J Innate Immun. 2010;2:195–200. doi: 10.1159/000252803. 000252803 [pii];10.1159/000252803 [doi] [DOI] [PubMed] [Google Scholar]
- 42.Mathsson L, Lampa J, Mullazehi M, Ronnelid J. Immune complexes from rheumatoid arthritis synovial fluid induce FcgammaRIIa dependent and rheumatoid factor correlated production of tumour necrosis factor-alpha by peripheral blood mononuclear cells. Arthritis Res Ther. 2006;8:R64. doi: 10.1186/ar1926. ar1926 [pii];10.1186/ar1926 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. 19/1/275 [pii];10.1146/annurev.immunol.19.1.275 [doi] [DOI] [PubMed] [Google Scholar]
- 44.Wenink MH, Santegoets KC, Roelofs MF, Huijbens R, Koenen HJ, et al. The inhibitory Fc gamma IIb receptor dampens TLR4-mediated immune responses and is selectively up-regulated on dendritic cells from rheumatoid arthritis patients with quiescent disease. J Immunol. 2009;183:4509–4520. doi: 10.4049/jimmunol.0900153. jimmunol.0900153 [pii];10.4049/jimmunol.0900153 [doi] [DOI] [PubMed] [Google Scholar]
- 45.Wernersson S, Karlsson MC, Dahlstrom J, Mattsson R, Verbeek JS, et al. IgG-mediated enhancement of antibody responses is low in Fc receptor gamma chain-deficient mice and increased in Fc gamma RII-deficient mice. J Immunol. 1999;163:618–622. ji_v163n2p618 [pii] [PubMed] [Google Scholar]
- 46.Kim JW, Wierda WG, Kim YB. Immobilized IgG immune complex induces secretion of tumor necrosis factor-alpha by porcine alveolar macrophages. Am J Respir Cell Mol Biol. 1991;5:249–255. doi: 10.1165/ajrcmb/5.3.249. [DOI] [PubMed] [Google Scholar]
- 47.Gupta S, Booth JW. ERK phosphorylation and tumor necrosis factor-alpha production by monocytes are persistent in response to immobilized IgG. Biochem Biophys Res Commun. 2010;402:301–304. doi: 10.1016/j.bbrc.2010.10.020. S0006-291X(10)01871-1 [pii];10.1016/j.bbrc.2010.10.020 [doi] [DOI] [PubMed] [Google Scholar]
- 48.DiMartino SJ, Yuan W, Redecha P, Ivashkiv LB, Salmon JE. Insoluble immune complexes are most effective at triggering IL-10 production in human monocytes and synergize with TLR ligands and C5a. Clin Immunol. 2008;127:56–65. doi: 10.1016/j.clim.2007.11.014. S1521-6616(07)01413-1 [pii];10.1016/j.clim.2007.11.014 [doi] [DOI] [PubMed] [Google Scholar]
- 49.Boross P, Verbeek JS. The complex role of Fcgamma receptors in the pathology of arthritis. Springer Semin Immunopathol. 2006;28:339–350. doi: 10.1007/s00281-006-0049-9. 10.1007/s00281-006-0049-9 [doi] [DOI] [PubMed] [Google Scholar]
- 50.Ji JD, Tassiulas I, Park-Min KH, Aydin A, Mecklenbrauker I, et al. Inhibition of interleukin 10 signaling after Fc receptor ligation and during rheumatoid arthritis. J Exp Med. 2003;197:1573–1583. doi: 10.1084/jem.20021820. 10.1084/jem.20021820 [doi];jem.20021820 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan W, DiMartino SJ, Redecha PB, Ivashkiv LB, Salmon JE. Systemic lupus erythematosus monocytes are less responsive to interleukin-10 in the presence of immune complexes. Arthritis Rheum. 2011;63:212–218. doi: 10.1002/art.30083. 10.1002/art.30083 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ballow M. The IgG molecule as a biological immune response modifier: mechanisms of action of intravenous immune serum globulin in autoimmune and inflammatory disorders. J Allergy Clin Immunol. 2011;127:315–323. doi: 10.1016/j.jaci.2010.10.030. S0091-6749(10)01645-3 [pii];10.1016/j.jaci.2010.10.030 [doi] [DOI] [PubMed] [Google Scholar]
- 53.Ephrem A, Misra N, Hassan G, Dasgupta S, Delignat S, et al. Immunomodulation of autoimmune and inflammatory diseases with intravenous immunoglobulin. Clin Exp Med. 2005;5:135–140. doi: 10.1007/s10238-005-0079-y. 10.1007/s10238-005-0079-y [doi] [DOI] [PubMed] [Google Scholar]
- 54.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. S0065-2776(07)96005-8 [pii];10.1016/S0065-2776(07)96005-8 [doi] [DOI] [PubMed] [Google Scholar]
- 55.Nimmerjahn F, Ravetch JV. The antiinflammatory activity of IgG: the intravenous IgG paradox. J Exp Med. 2007;204:11–15. doi: 10.1084/jem.20061788. jem.20061788 [pii];10.1084/jem.20061788 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vos AC, Wildenberg ME, Duijvestein M, Verhaar AP, van den Brink GR, et al. Anti-tumor necrosis factor-alpha antibodies induce regulatory macrophages in an Fc region-dependent manner. Gastroenterology. 2011;140:221–230. doi: 10.1053/j.gastro.2010.10.008. S0016-5085(10)01496-4 [pii];10.1053/j.gastro.2010.10.008 [doi] [DOI] [PubMed] [Google Scholar]
- 57.Vos AC, Wildenberg ME, Arijs I, Duijvestein M, Verhaar AP, et al. Regulatory macrophages induced by infliximab are involved in healing in vivo and in vitro. Inflamm Bowel Dis. 2011 doi: 10.1002/ibd.21818. 10.1002/ibd.21818 [doi] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of TLR2 and TLR4 on MΦIFN-γ, MΦIL-4, and MΦIL-10. Healthy peripheral blood monocytes were cultured for 4 days in medium or in medium supplemented with IFN-γ, IL-4, or IL-10. Expression TLR2 and TLR4 was measured by flow cytometry. Bars represent the mean ± SEM of 4 independent experiments. *p<0.05, **p<0.01
(TIF)