Abstract
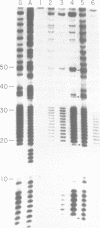
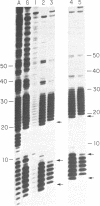
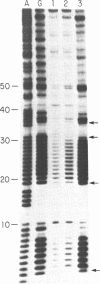
A synthetic DNA fragment was constructed to determine the effect of 5' and 3' neighbors of guanine runs on the binding of chemical carcinogens. Determinations were made on the relative intensity of reactivity between aflatoxin B1 or benzo(a)pyrene and methylnitrosourea or 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea with various guanine positions in an endlabeled DNA fragment of known sequence. After reaction, the fragments were depurinated to produce strand breaks to allow Maxam and Gilbert sequencing for guanine positions. Relative reaction intensities were compared densitometrically. 3' neighbors exerted greater influence on carcinogen binding than did 5' neighbors, the influence extended only to the adjacent guanine and depended upon the chemical nature of the carcinogen. In addition, the presence of one carcinogen adduct in the guanine run influenced the formation of a subsequent adduct when the DNA was exposed to a second carcinogen, and this effect also depended on the nature of the second carcinogen. The results suggest that DNA adduct formation in the presence of multiple carcinogens is more complicated than an additive mechanism would suggest.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benasutti M., Ejadi S., Whitlow M. D., Loechler E. L. Mapping the binding site of aflatoxin B1 in DNA: systematic analysis of the reactivity of aflatoxin B1 with guanines in different DNA sequences. Biochemistry. 1988 Jan 12;27(1):472–481. doi: 10.1021/bi00401a068. [DOI] [PubMed] [Google Scholar]
- Boles T. C., Hogan M. E. High-resolution mapping of carcinogen binding sites on DNA. Biochemistry. 1986 May 20;25(10):3039–3043. doi: 10.1021/bi00358a045. [DOI] [PubMed] [Google Scholar]
- Briscoe W. T., Anderson S. P., May H. E. Base sequence specificity of three 2-chloroethylnitrosoureas. Biochem Pharmacol. 1990 Sep 15;40(6):1201–1209. doi: 10.1016/0006-2952(90)90384-w. [DOI] [PubMed] [Google Scholar]
- Briscoe W. T., Cotter L. E. DNA sequence has an effect on the extent and kinds of alkylation of DNA by a potent carcinogen. Chem Biol Interact. 1985 Dec 31;56(2-3):321–331. doi: 10.1016/0009-2797(85)90014-6. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Haseltine W. A. Modification of DNA by aflatoxin B1 creates alkali-labile lesions in DNA at positions of guanine and adenine. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4120–4124. doi: 10.1073/pnas.75.9.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan M. E., Oplinger M., Pegg A. E. Sequence specificity of guanine alkylation and repair. Carcinogenesis. 1988 Nov;9(11):2139–2143. doi: 10.1093/carcin/9.11.2139. [DOI] [PubMed] [Google Scholar]
- Hartley J. A., Gibson N. W., Kohn K. W., Mattes W. B. DNA sequence selectivity of guanine-N7 alkylation by three antitumor chloroethylating agents. Cancer Res. 1986 Apr;46(4 Pt 2):1943–1947. [PubMed] [Google Scholar]
- Jeffrey A. M., Weinstein I. B., Jennette K. W., Grzeskowiak K., Nakanishi K., Harvey R. G., Autrup H., Harris C. Structures of benzo(a)pyrene--nucleic acid adducts formed in human and bovine bronchial explants. Nature. 1977 Sep 22;269(5626):348–350. doi: 10.1038/269348a0. [DOI] [PubMed] [Google Scholar]
- King H. W., Osborne M. R., Brookes P. The in vitro and in vivo reaction at the N7-position of guanine of the ultimate carcinogen derived from benzolalpyrene. Chem Biol Interact. 1979 Mar;24(3):345–353. doi: 10.1016/0009-2797(79)90082-6. [DOI] [PubMed] [Google Scholar]
- Kohn K. W., Hartley J. A., Mattes W. B. Mechanisms of DNA sequence selective alkylation of guanine-N7 positions by nitrogen mustards. Nucleic Acids Res. 1987 Dec 23;15(24):10531–10549. doi: 10.1093/nar/15.24.10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Loeb L. A. Apurinic sites as mutagenic intermediates. Cell. 1985 Mar;40(3):483–484. doi: 10.1016/0092-8674(85)90191-6. [DOI] [PubMed] [Google Scholar]
- Mattes W. B., Hartley J. A., Kohn K. W. DNA sequence selectivity of guanine-N7 alkylation by nitrogen mustards. Nucleic Acids Res. 1986 Apr 11;14(7):2971–2987. doi: 10.1093/nar/14.7.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Pullman A., Pullman B. Molecular electrostatic potential of the nucleic acids. Q Rev Biophys. 1981 Aug;14(3):289–380. doi: 10.1017/s0033583500002341. [DOI] [PubMed] [Google Scholar]
- Richardson F. C., Boucheron J. A., Skopek T. R., Swenberg J. A. Formation of O6-methyldeoxyguanosine at specific sites in a synthetic oligonucleotide designed to resemble a known mutagenic hotspot. J Biol Chem. 1989 Jan 15;264(2):838–841. [PubMed] [Google Scholar]
- Riggs A. D., Jones P. A. 5-methylcytosine, gene regulation, and cancer. Adv Cancer Res. 1983;40:1–30. doi: 10.1016/s0065-230x(08)60678-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulsky G., Johnson R. L., Shockcor J. P., Taylor L. C., Stark A. A. Properties of aflatoxin-DNA adducts formed by photoactivation and characterization of the major photoadduct as aflatoxin-N7-guanine. Carcinogenesis. 1990 Apr;11(4):519–527. doi: 10.1093/carcin/11.4.519. [DOI] [PubMed] [Google Scholar]
- Shieh J. C., Song P. S. Photochemically induced binding of aflatoxins to DNA and its effects on template activity. Cancer Res. 1980 Mar;40(3):689–695. [PubMed] [Google Scholar]
- Warpehoski M. A., Hurley L. H. Sequence selectivity of DNA covalent modification. Chem Res Toxicol. 1988 Nov-Dec;1(6):315–333. doi: 10.1021/tx00006a001. [DOI] [PubMed] [Google Scholar]
- Wurdeman R. L., Gold B. The effect of DNA sequence, ionic strength, and cationic DNA affinity binders on the methylation of DNA by N-methyl-N-nitrosourea. Chem Res Toxicol. 1988 May-Jun;1(3):146–147. doi: 10.1021/tx00003a003. [DOI] [PubMed] [Google Scholar]