ABSTRACT
Loss-of-function mutations in the autoimmune regulator (AIRE) gene are responsible for autoimmune polyglandular syndrome type 1 (APS-1), which commonly manifests as infertility in women. AIRE is a transcriptional regulator that promotes expression of tissue-restricted antigens in the thymus, including antigens specific to the ovary. Thymic expression of ovarian genes under AIRE's control may be critical for preventing ovarian autoimmune disease. Because mice lacking Aire are an important APS-1 model, we examined the reproductive properties of female Aire-deficient (Aire−/−) mice. Female Aire−/− mice on the BALB/c background were examined for reproductive parameters, including fertility, litter sizes, and ovarian follicular reserves. Although delayed puberty was observed in Aire−/− mice, all mice entered puberty and exhibited mating behavior. Only 50% of Aire−/− females gave an initial litter, and only 16% were able to produce two litters. Ovarian histopathologic examination revealed that 83% of previously bred females lost all ovarian follicular reserves. Among virgin females, follicular depletion was observed in 25% by 8 wk, and by 20 wk, 50%–60% of mice lost all follicles. This was associated with elevated serum follicle-stimulating hormone level and ovarian infiltration of proliferating CD3+ T lymphocytes. Ovulation rates of 6-wk-old Aire−/− mice were reduced by 22%, but this difference was not statistically significant. Finally, transplantation experiments revealed that follicular loss depended on factors extrinsic to the ovary. These results suggest that immune-mediated ovarian follicular depletion is a mechanism of infertility in Aire−/− mice. The results have important implications in the pathogenesis of ovarian autoimmune disease in women.
Keywords: AIRE, autoimmune disease, autoimmune regulator, female infertility, follicle, immunology, infertility, ovarian follicles, ovary
The autoimmune regulator is required to maintain fertility in female mice.
INTRODUCTION
The immune and reproductive systems are often at cross-purposes. Immunological disease can contribute to infertility in women through inappropriate targeting of self-proteins, leading to endocrine dysfunction, destruction of germ cells, and miscarriage. Of the causes of autoimmune-mediated infertility, the ovary is the best-studied target. Approximately 1.5 million women each year undergo premature ovarian failure, defined as menopause at younger than 40 years of age, and roughly a third of these are attributable to autoimmune disease [1, 2].
The thymus orchestrates both the development of T cells and the establishment of immunological tolerance to self. In contrast to “peripheral” tolerance, which occurs via interaction of lymphocytes with self-antigens within tissues and peripheral lymphoid organs, such as the spleen and lymph nodes, “central” tolerance is established in the thymus [3]. Induction of central tolerance is accomplished in the thymic medulla via presentation of self-antigens in the context of class I and class II major histocompatibility complex (MHC) molecules on medullary thymic epithelial cells (mTEC) and resident dendritic cells. Upon encountering of high-avidity self-antigen/MHC, the developing self-reactive thymocytes undergo clonal deletion, thus barring them from the periphery, where they might otherwise instigate autoimmune disease [4]. It was not understood how central tolerance could account for deletion of T cells reactive to antigens for which expression is confined to tissues other than the thymus until the discovery that mTECs express many tissue-restricted proteins. The initial discovery that several pancreas-specific genes, including insulin and glucagon, are expressed in rare cells of the thymic medulla and, further, that transgenic expression of a model tissue-restricted antigen was sufficient to confer tolerance to that antigen suggested that autoreactive T cells against tissue-specific antigens are subjected to the mechanisms of central tolerance [5, 6]. Subsequent studies of global gene expression revealed that mature mTECs, which display high levels of class II MHC and CD80, stochastically express tissue-specific antigens to represent nearly every organ [7]. Direct evidence of a critical role for thymic expression of tissue-restricted antigens in central tolerance was recently provided through the demonstration that absence of the tissue-specific antigens insulin [8] or interphotoreceptor retinoid-binding protein [9] is sufficient to cause autoimmunity against their respective organs, the pancreas and retina.
Autoimmune regulator (AIRE) is a 545-amino-acid glycoprotein that is expressed predominantly in mTECs, where it promotes the ectopic expression of tissue-restricted antigens [10]. The precise molecular mechanism of action is unknown, but it is generally agreed that AIRE functions as a transcriptional coactivator, acting with other proteins to control expression of chromosomally clustered target genes [11, 12]. In addition, studies using transgenic expression of model self-antigens have shown that AIRE induces the negative selection of self-reactive T cells and that failure of AIRE-induced expression of tissue-restricted antigens permits the escape of autoreactive T cells into the periphery, followed by autoimmune disease [13–15].
Autoimmune polyglandular syndrome type 1 (APS-1) is a monogenic multiorgan disease that is caused by functional mutations in the AIRE gene and is diagnosed by the presence of two of three clinical symptoms: 1) chronic mucocutaneous candidiasis, 2) hypoparathyroidism, and 3) Addison disease [16, 17]. Other autoimmune manifestations of APS-I can include type 1 diabetes mellitus, alopecia, and hepatitis [18, 19]. In addition, many female patients with APS-1 are infertile or experience early reproductive senescence associated with high rates of primary ovarian insufficiency, delayed puberty, and miscarriage [18].
Targeted Aire mutations in mice produce autoimmune disease that often mirrors APS-1. Ectopic gene expression by mTECs in mice includes antigens associated with the ovary, uterus, and fetus [12]. The finding of reduced fertility together with the presence of antiovarian antibodies and ovarian mononuclear cell infiltration in female Aire-deficient (Aire−/−) mice has been reported [10, 20, 21]. To our knowledge, however, the onset, progression, and underlying reasons for this female infertility have not been investigated. In the present study, we examined the lesions in fertility in mice lacking Aire. Consequences resulting from mutations in human AIRE and its murine orthologue form the basis for the overall hypothesis that thymic expression of AIRE-regulated female reproductive tissue antigens is critical for fertility.
MATERIALS AND METHODS
Animals
All protocols using animals were approved by the Institutional Animal Care and Use Committee at the University of Kansas Medical Center and were in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals. Aire-deficient mice were backcrossed onto the BALB/c genetic background (more than eight generations), and heterozygous breeders were generously donated to our laboratory by C. Benoist and D. Mathis (Harvard Medical School, Boston, MA) [10, 21]. These mice were bred at the Laboratory Animal Resources facility at the University of Kansas Medical Center under pathogen-free conditions. All mice were provided autoclaved food and water ad libitum and were exposed to a 12L:12D photoperiod. Wild-type BALB/c male and female mice were obtained from the Jackson Laboratory. Genotypes were confirmed by PCR as per protocols described by the Jackson Laboratory. Separate PCR reactions were performed using two primer sets: 1-FWD, 5′ GTCATGTTGACGGATCCAGGGTAGAAAGT 3′ (oIMR2096); 1-REV, 5′ AGACTAGGTGTTCCCTCCCAACCTCAG 3′ (oIMR2097); 2-FWD, 5′TCCGTCTGAAGGAGAAGGAA 3′ (oIMR3721); and 2-REV, 5′ AAGCCGTCCAGGATGCTAT3′ (oIMR3722). Primer set 1 yields a 1200-bp product in wild-type mice and a 750-bp product in Aire-deficient mice. Primer set 2 yields a 160-bp product in wild-type mice and no product in Aire-deficient mice.
Fertility Studies
Virgin Aire-deficient (n = 14) and wild-type control (n = 11) mice were examined daily for vaginal opening, a sign of approaching sexual maturation, beginning on Postnatal Day 20. To evaluate fertility, Aire-deficient (n = 12) and wild-type control (n = 11) mice were monitored across two cycles of mating to proven wild-type BALB/c male mice. Beginning at 6 wk of age, female Aire-deficient or wild-type control females were cohabitated with BALB/c wild-type males until the day of copulatory plug detection. All plugged mice were allowed to have two litters and were euthanized during the third pregnancy. Females that failed to become pregnant were bred for four consecutive cycles and then euthanized. Female mice that failed to exhibit a copulatory plug were housed with the males for 15 consecutive days, followed by euthanasia to harvest tissues.
Tissue Collection and Histology
Virgin wild-type and Aire-deficient mice were euthanized at 1, 4, 8, 12, 16, and 20 wk of age (n = 7–8 per group), and blood was collected. One ovary from each mouse was fixed overnight in 4% paraformaldehyde, dehydrated, embedded in paraffin, and serially sectioned (thickness, 8 μm). Ovarian follicular reserves were calculated as previously described [22]: Sections were stained with hematoxylin and eosin, and of these, every fifth section was evaluated by counting healthy follicles at 400× magnification. Total follicular reserves were calculated as the total follicles counted multiplied by five.
Ovulation and Fertilization Rates
Ovulation rates were determined by collecting oocytes following copulation as previously described [23]. Six-week-old Aire-deficient (n = 17) and wild-type control (n = 11) females were mated to wild-type BALB/c males and euthanized on the evening of copulatory plug detection. Oocytes were flushed from the oviducts using mouse embryo culture medium (EmbryoMax FHM HEPES-Buffered Medium; Millipore) and were incubated in 0.3 mg/ml of hyaluronidase (Sigma-Aldrich) for 10 min to remove cumulus cells. Oocytes were counted using a dissecting microscope.
Subrenal Transplant Surgeries
To determine whether observed effects resulted from AIRE intrinsic or extrinsic to the ovary, wild-type ovaries were transplanted into the kidney capsule of either Aire-deficient or wild-type control female mice. At 8–10 wk of age, wild-type (n = 3) or Aire-deficient (n = 6) recipient mice were anesthetized with 2,2,2-tribromoethanol (250 mg/kg i.p.; Sigma-Aldrich). Following exteriorization of the kidney, the renal capsule was perforated, and an ovary from an age-matched wild-type mouse was maneuvered to the opposite pole of the kidney, after which the kidney was returned to the body cavity. The transplant recipient was euthanized after 10 or 20 days, and the transplanted ovary together with the kidney and overlying fat as well as the recipient's endogenous ovary were collected for histopathology.
Immunohistochemistry
Immunohistochemical detection of CD3+ T cells was performed on ovarian tissues from Aire-deficient (n = 7–8 per group) and wild-type control (n = 5–6 per group) mice at 1, 4, 8, 12, 16, and 20 wk of age. Ovaries were fixed in 4% paraformaldehyde overnight, dehydrated through a series of increasing concentrations of ethanol, and embedded in paraffin. Sections (thickness, 8 μm) were placed onto slides and rehydrated before staining as previously described [24]. Tissue sections were subjected to antigen retrieval using Reveal buffer (Biocare Medical) according to the manufacturer's protocol, and nonspecific antibody binding was blocked in 10% goat serum (Sigma-Aldrich). CD3 expression was detected using a polyclonal rabbit anti-mouse CD3 antibody (catalog no. ab16669; Abcam) at a dilution of 1:100 incubated with the sections overnight at 4°C. As a negative control, rabbit immunoglobulin (Ig) G (ChromPure, Jackson ImmunoResearch) was used at the same concentration. After incubation with biotinylated secondary antibody (goat anti-rabbit IgG BA-1000; Vector Laboratories), depletion of endogenous peroxidases, and addition of horseradish peroxidase (HRP)-streptavidin conjugate (Invitrogen), staining was developed using aminoethyl carbazole (AEC; AEC Substrate Kit; Invitrogen). Sections were counterstained with hematoxylin and viewed by light microscopy using 200× and 400× magnification. To assess proliferation of CD3+ T cells, a polyclonal rabbit anti-mouse MKI67 antibody (catalog no. ab15580; Abcam) at a concentration of 10 μg/ml was used on ovarian tissues from 4-wk-old Aire-deficient (n = 7) and wild-type control (n = 5) mice. Staining was developed as described above.
To evaluate whether antiovarian antibodies are present in the sera of Aire-deficient mice, ovaries from Rag1 (recombination-activating gene 1)-deficient mice (Taconic), which lack endogenous antibodies, were flash-frozen in 2-methylbutane (Sigma-Aldrich), cooled in dry ice for 5 min, and embedded in Tissue-Tek O.C.T. Compound (Sakura). Sections (thickness, 8 μm) were prepared using a cryostat, air-dried for 30 min at room temperature, and postfixed for 5 min in 100% cold acetone. After three washes in PBS (pH 7.4), blocking for 1 h at room temperature with 2% bovine serum albumin (Sigma-Aldrich) in 0.03% Triton X-100 (Fisher) supplemented with PBS, and treatment for endogenous peroxidase activity (0.5% H2O2 in methanol), the sections were incubated overnight at 4°C with Aire-deficient or wild-type mouse serum (1:40 dilution). The following morning, sections were washed in PBS and incubated at room temperature for 45 min with a biotinylated anti-mouse IgG (Vector Laboratories). An HRP-streptavidin conjugate was applied before color development with AEC. Finally, the sections were washed in tap water, counterstained with hematoxylin (Sigma-Aldrich) and coverslipped.
Evaluation of Serum Follicle-Stimulating Hormone
Mouse serum follicle-stimulating hormone (FSH) was measured by radioimmunoassay at the Ligand Assay and Analysis Core Laboratory at the University of Virginia Center for Research in Reproduction (Charlottesville, VA). The sensitivity of the assay is 2.0 ng/ml, and the range is 4.3–75 ng/ml.
Statistical Analysis
Comparisons of age at puberty and litter sizes between Aire-deficient and wild-type control mice were performed using two-tailed Student t-tests. Proportions of wild-type and Aire-deficient mice bearing litters and exhibiting follicular depletion and T cell infiltration were compared by chi-square analysis. An alpha level of 0.05 was considered to be statistically significant.
RESULTS
Aire-Deficient Mice Exhibit Delayed Onset of Puberty and Reduced Fertility
Previous studies have suggested that reproductive function is compromised in female Aire-deficient mice [10, 20]. Our aim, therefore, was to characterize reproductive parameters of mice lacking this gene. First, to determine whether the absence of Aire influenced the onset of puberty, Aire-deficient and wild-type control mice were observed daily to assess vaginal opening beginning at Postnatal Day 20. In Aire-deficient mice, vaginal opening was delayed, occurring at 27.71 ± 1.14 (mean ± SEM) days of age as compared to 22.09 ± 0.82 days in wild-type controls (P = 0.0009).
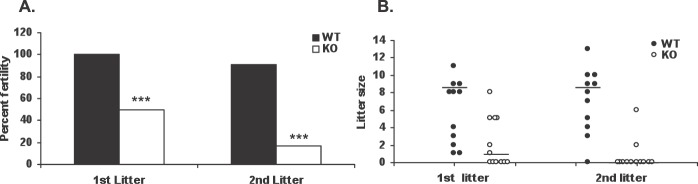
We next monitored Aire-deficient and wild-type control mice across two cycles of mating. Wild-type male BALB/c mice were used as sires for these studies so that any phenotype observed could be attributed solely to the female. Females were housed with males at a 1:1 ratio beginning at 6 wk of age and were monitored each morning for copulation plugs. Although all Aire-deficient and control females exhibited mating behavior within 1 wk of placement with a male, only 50% of Aire-deficient mice (n = 12) (Fig. 1A) delivered a litter. However, among Aire-deficient mice that successfully delivered litters, litter size was not different from that of controls (range, 1–8; mean, 4.3 ± 1.02; P = 0.39) (Fig. 1B). Of the six animals that failed to deliver pups, five (83%) subsequently showed signs of impaired estrous cyclicity, as suggested by failure to obtain a copulatory plug during prolonged cohabitation with a male. The remaining animal mated with a male on multiple occasions but nonetheless failed to produce a litter. As expected, all control mice delivered pups (n = 11), and none displayed impaired cyclicity.
FIG. 1.
Influence of Aire deficiency on female fertility. Wild-type (WT) and Aire-deficient (KO) mice were monitored across two consecutive litters as described in Materials and Methods. A) Percentage of WT control and KO mice giving rise to viable litters. B) Litter size. Each circle represents the litter size of an individual dam. Bars represent the median. ***P < 0.001 by chi-square analysis.
Only 17% (2 of 12) of Aire-deficient mice were able to deliver a second litter, whereas 90% of control females delivered a second litter (Fig. 1A). Similar to first litters, the size of second litters of Aire-deficient mice remained within the range seen for the controls (4.0 ± 2.0 vs. 7.8 ± 0.97 pups, respectively; P = 0.13) (Fig. 1B). In addition, three of the six mice that delivered an initial litter and one of the two that delivered a second litter developed signs of impaired cyclicity following delivery. Neither of the Aire-deficient mice that produced two litters were pregnant when euthanized, despite continued cohabitation with a male and, in one case, repeated observations of a copulatory plug.
Despite the effects of Aire deficiency on fertility, females who delivered litters were able to rear their pups to weaning normally. Survival rate to weaning (20 days) of the pups of Aire-deficient mothers was 73% (19 of 26 total pups born) and100% (8 of 8) in the first and second litters, respectively, as compared to 89% (57 of 64) and 90% (70 of 78) in first and second litters, respectively, of control females (P > 0.05 for both litters). Also, gestational length was comparable between Aire-deficient and control mice (first litter, 20.16 ± 0.47 vs. 19.37 ± 0.26 days, respectively; P > 0.1 for both litters).
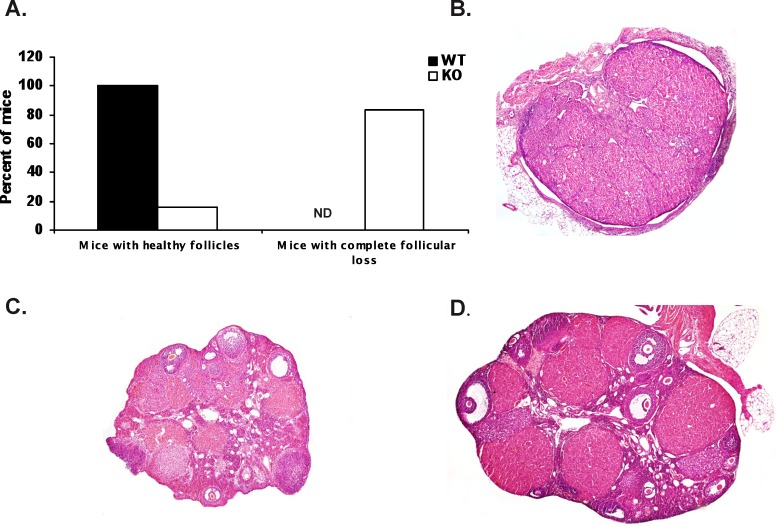
Because many of the Aire-deficient mice displayed a failure to mate, we examined the ovaries from the mice used for the breeding trials described above. Of the 10 Aire-deficient mice (n = 12 total) that failed to deliver either a first or second litter, eight exhibited a complete loss of ovarian follicles (Fig. 2, A and B). The two Aire-deficient mice that delivered a second litter also had depleted follicular reserves. Two Aire-deficient mice, on the other hand, possessed histologically normal ovaries, showing multiple stages of follicles as well as corpora lutea (Fig. 2C). With the exception of one Aire-deficient mice, the observed ovarian phenotype was correlated with their failure to mate. As expected, all of the wild-type control mice possessed histologically normal ovaries (Fig. 2, A and D).
FIG. 2.
Ovarian phenotype in parous Aire-deficient (KO) mice. Mice were followed for two consecutive litters, and at euthanization, ovaries were harvested and processed for histological analysis. A) Percentage of KO and wild-type (WT) mice with intact and depleted follicular reserves. B–D) Histological sections of ovaries from representative KO (B and C) and WT (D) mice exhibiting follicular loss (B) and intact follicles (C and D). ND, nondetected. Original magnification ×100.
Follicular Loss and Lymphocyte Infiltration in the Ovaries of Aire-Deficient Mice
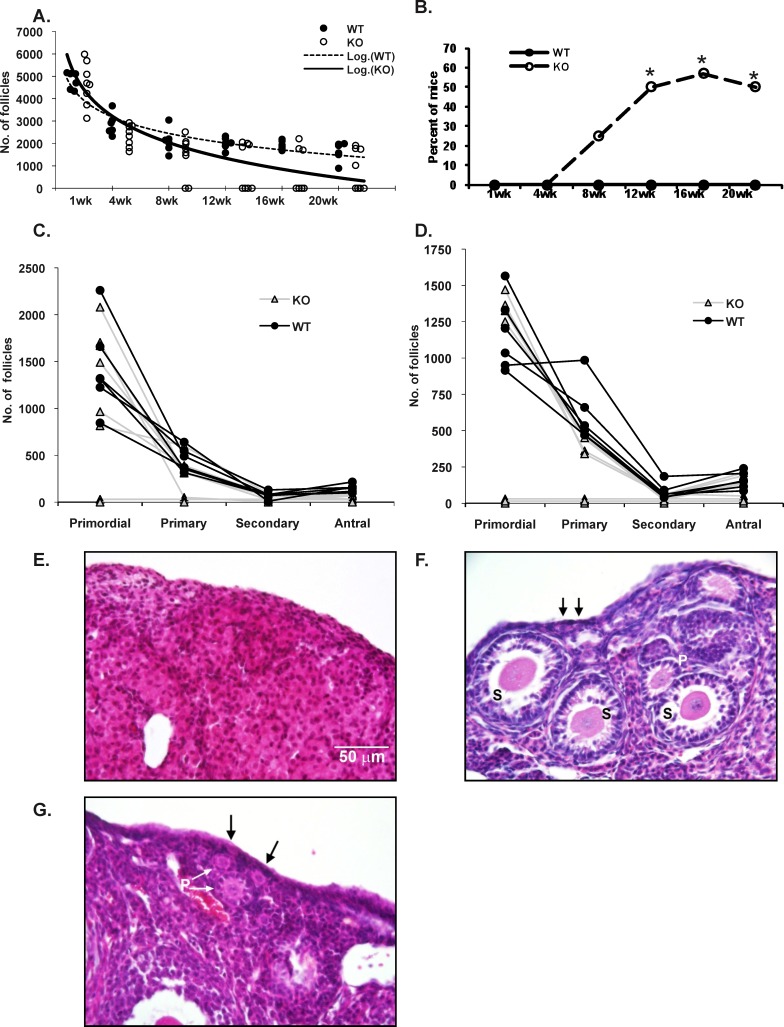
Because we noticed that many previously bred mice exhibited depletion of ovarian follicular reserves, we assessed the influence of Aire depletion on this parameter in detail. Virgin wild-type and Aire-deficient female mice were euthanized at 1, 4, 8, 12, 16, and 20 wk of age, and follicular reserve was quantified by counting healthy oocytes in histological sections. Of all Aire-deficient females examined, approximately 30% (14 of 47) displayed loss of ovarian follicles at or before 20 wk of age, whereas the remaining 70% displayed numbers of healthy follicles comparable to those of the wild-type controls (Fig. 3, A, B, and F). Follicular loss was evident in 25% (2 of 8) of animals at 8 wk of age, and the proportion of mice exhibiting follicular depletion increased with advancing age thereafter, reaching a plateau of 50%–60% at 12–16 wk (Fig. 3B). Loss of primordial and primary follicles paralleled these findings (Fig. 3, C–F), suggesting that one or both of these early stage follicles are targeted by the immune system. As expected, none of the wild-type control mice exhibited ovaries with loss of follicular reserves.
FIG. 3.
Impact of Aire deficiency on ovarian follicular reserves. A) Ovarian follicular reserves were counted from virgin Aire-deficient (KO) and wild-type (WT) mice as described in Materials and Methods. Data are expressed as numbers of healthy follicles per ovary. Each circle represents the number of follicles from an individual mouse. Dashed and solid lines represent trend lines for the WT control and KO groups, respectively. The x-axis represents age of mice in weeks. B) Percentage of WT and KO mice exhibiting complete follicular loss as a function of age. C and D) Numbers of follicles of different stages found in WT and KO mice at 8 wk (C) and 12 wk (D) of age. Each line represents an individual mouse. E–G) Representative images of ovaries from 8-wk-old, nulliparous KO (E and F) and WT (G) mice. Arrows indicate primordial follicles. P, primary follicles; S, secondary follicles. *P < 0.05. Bar = 50 μm.
Inflammatory infiltrates could often be observed in or around the ovaries of Aire-deficient mice. To determine whether the inflammatory infiltrates observed in Aire-deficient females were T lymphocytes, we performed immunohistochemistry for CD3, a marker of T lymphocytes, on the ovarian tissues harvested. Similar to the pattern of follicular depletion, ovarian CD3+ staining increased with age in Aire-deficient animals (Fig. 4). CD3+ T cell infiltration was observed in 57% of Aire-deficient mice as early as 4 wk of age, before any evidence for the onset of follicular depletion. By 20 wk of age, T cells were observed in 86% of Aire-deficient mice. The T cells in these ovaries were found to surround follicles, infiltrate corpora lutea, or organize into aggregates (Fig. 5). Of the control mice, only 1 of 32 (3%) in the wild-type group displayed detectable CD3+ lymphocytes. To determine whether CD3+ cells within Aire-deficient ovaries proliferate, we performed immunohistochemistry for the proliferation marker Ki67 on adjacent ovarian tissues of 4-wk-old Aire-deficient and wild-type control mice. Of the five Aire-deficient mice that exhibited CD3+ T cell staining within the ovaries, two revealed strongly positive MKI67 staining within the T cell-infiltrated areas (Fig. 6).
FIG. 4.
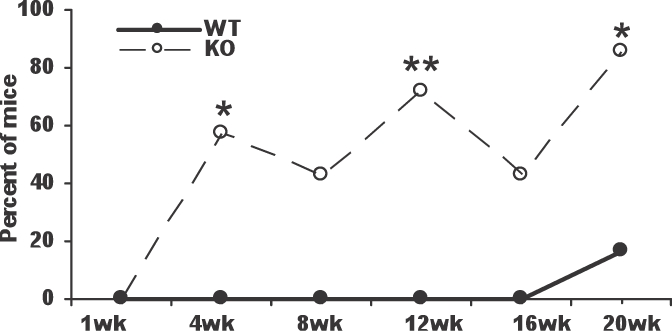
Proportion of mice exhibiting CD3+ T cell infiltration into ovaries of Aire-deficient (KO) and wild-type (WT) mice. *P < 0.05, **P < 0.01 as compared to WT controls.
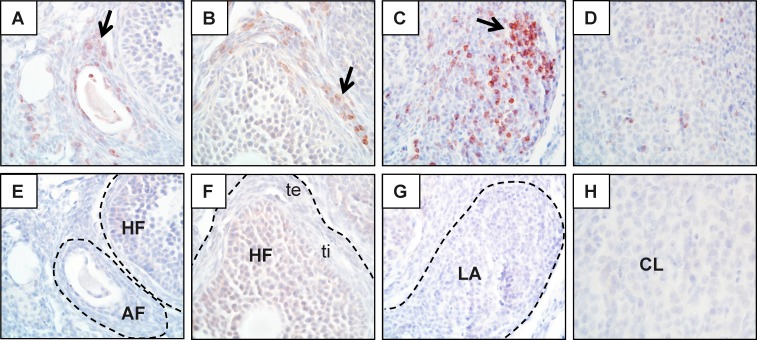
FIG. 5.
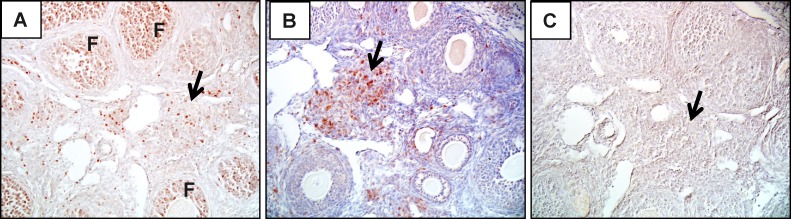
Infiltration of CD3+ T cells into ovaries of Aire-deficient mice. Ovaries of wild-type (not shown) and Aire-deficient (A–H) mice were stained by immunohistochemistry using an anti-mouse CD3 antibody. Reddish-brown staining represents positive staining (arrows); sections were counterstained with hematoxylin (blue). T cells are seen surrounding atretic follicles (AF; A), surrounding healthy follicles (HF; B), organized into lymphoid aggregates (LA; C), and infiltrating corpora lutea (CL; D). Corresponding isotype controls (E–H) are also shown. te, theca externa; ti, theca interna. Original magnification ×600.
FIG. 6.
CD3+ cells proliferate in situ within the ovary. Serial sections of ovaries of Aire-deficient (A–C) and wild-type (not shown) mice were stained with MKI67 (A), CD3 (B), and isotype control (C) antibodies. Arrows show areas of intense staining by both antibodies. F, follicles. Original magnification ×200 (A and B) and ×400 (C).
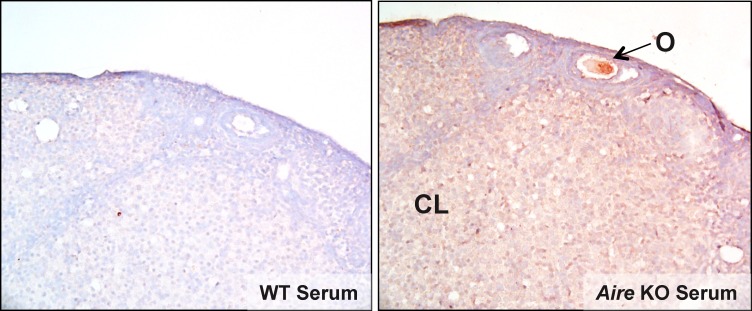
We next evaluated sera from Aire-deficient mice for the presence of antiovarian antibodies by indirect immunostaining of frozen ovary sections from Rag1-deficient mice. By using ovaries from Rag1-deficient mice, any antiovarian antibodies produced by Aire-deficient mice can bind to ovarian target antigens without interference by endogenously produced antibodies. We found that a high percentage (5 of 6) of the Aire-deficient mice possessed antiovarian autoantibodies, whereas none of the wild-type control mice (n = 3) did. These results confirm the work of others [10, 20]. Autoantibody immunoreactivity revealed a range of autoreactive targets, including stromal cells, luteal cells, and the oocyte (Fig. 7). Autoantibodies could be observed in Aire-deficient mice as early as 7 wk of age and occurred in both virgin and parous females.
FIG. 7.
Ovary-reactive autoantibodies are produced in Aire-deficient mice. Ovaries from Rag1-deficient mice were stained by immunohistochemistry using serum from wild-type or Aire-deficient mice. CL, corpus luteum; O, oocyte. Original magnification ×20.
We also examined FSH in sera from Aire-deficient (n = 17) and wild-type control (n = 17) mice. Overall, 29% of Aire-deficient mice had elevated serum FSH levels across all ages (Fig. 8). Serum FSH concentrations for the remaining Aire-deficient females were comparable with those for wild-type controls. Elevated serum FSH concentrations were associated with follicular loss: All animals with follicular loss also had elevated FSH, and all animals with elevated FSH exhibited follicular loss.
FIG. 8.
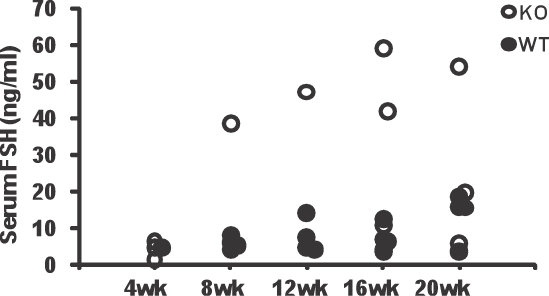
Serum FSH concentrations (ng/ml) in Aire-deficient (KO) and wild-type (WT) mice. Each circle represents FSH concentration in an individual mouse. The x-axis represents age in weeks.
Ovulation and Fertilization Rates Do Not Differ Between Aire-Deficient and Control Mice
We next investigated whether the reduced fertility rates observed in Aire-deficient mice could be attributed to a failure in ovulation or fertilization. Aire-deficient or wild-type control females were mated to BALB/c males, and on the day of copulatory plug detection, oocytes were flushed from the oviduct. Copulation plugs were detected in all Aire-deficient and control mice. Eleven of 17 (65%) Aire-deficient and 9 of 11 (82%) control mice ovulated healthy oocytes. Although this represents a 22% reduction in ovulation compared to controls, the ovulation rates were not statistically different by chi-square analysis (0.1 < P < 0.2). The mean number of oocytes recovered also did not differ significantly between groups (4.45 ± 1.11 and 3.00 ± 0.8 oocytes in wild type and Aire-deficient mice, respectively; P = 0.29). Finally, the average rate of fertilization (number of oocytes fertilized/number of oocytes ovulated) did not differ between Aire-deficient and wild-type control mice (75% vs. 80%, respectively; P = 0.87).
AIRE-Sufficient Ovaries in an Immune Environment Lacking AIRE
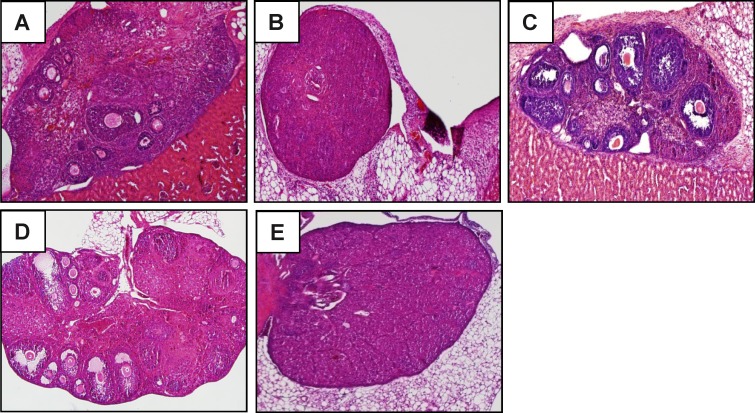
A function for AIRE in the normal physiology of the ovary has been suggested, because AIRE mRNA can be detected in the ovary [10, 25]. Therefore, we cannot rule out the possibility that follicular depletion occurs independently of the immune system. To test this, ovaries from wild-type mice were transplanted into the subrenal capsule of either Aire-deficient (n = 6) or wild-type control female (n = 2) mice, and transplantation sites were examined 10 days later. In addition, a transfer from wild-type to wild-type mice was performed and analyzed after 20 days. As expected, ovaries transplanted into wild-type mice appeared healthy and did not show signs of follicular depletion (Fig. 9C). Two Aire-deficient recipients, neither of which had undergone endogenous follicular depletion, bore healthy engrafted ovaries (Fig. 9, A and D). However, the remaining four Aire-deficient females that received wild-type ovaries underwent rapid and complete follicular depletion together with leukocytic infiltration (Fig. 9B). All four of these recipients also displayed follicular depletion of their own endogenous ovaries (Fig. 9E). These results are consistent with a principal role of the extraovarian immune system in mediating follicular loss in Aire-deficient mice.
FIG. 9.
Follicular loss is independent of AIRE expression intrinsic to the ovary. Aire-deficient (A and B) and wild-type (C) mice were engrafted with a donor wild-type ovary under the kidney capsule and euthanized after 10 days. Grafts and endogenous ovaries were harvested for histological analysis. A–C represent the grafted ovary; D and E represent the endogenous ovary of the Aire-deficient recipients. Original magnification ×100.
DISCUSSION
The identification of the AIRE gene as the causative agent in the human autoimmune syndrome APS-1 [16] together with the finding that the thymus ectopically expresses thousands of tissue-restricted antigens under the control of AIRE [7, 10] have transformed our appreciation of the mechanisms that lead to tissue-specific immune tolerance. Mice lacking Aire have become an important tool to facilitate this work, because these mice exhibit autoimmune disease mirroring APS-1 in many respects, including female infertility or subfertility [10, 20]. In the present study, we characterized the reproductive properties of Aire-deficient mice and showed that this gene is critical for the prevention of early reproductive senescence. In addition, our results strongly suggest that impaired fertility in Aire-deficient mice is due, at least in part, to an immune-mediated loss of ovarian follicular reserves.
Breeding trials revealed that fertility of Aire-deficiency on the BALB/c genetic background is compromised from an early reproductive age. At 6 wk of age, fertility is already reduced to 50% as compared to wild-type control mice. Infertility increases with advancing age of Aire-knockout mice: Only 17% of mice examined in the present study were able to produce two litters. Because many of the infertile mice lost breeding behavior over time, and because mice and humans with AIRE mutations have previously been documented to show ovarian pathology [10, 21], we looked more closely at the ovarian histopathology of the mice. Indeed, of the 12 mice examined in the present study, 83% exhibited complete depletion of follicular reserves.
Examination of the time course of follicular loss in virgin animals over time suggested that lymphocytic infiltration precedes follicular loss in Aire-knockout mice. T cell accumulation preceded follicular loss, occurring in 60% of animals as early as 4 wk of age. Follicular depletion was not seen at 4 wk of age but was observed in a minority of animals at 6 wk (data not shown) and 8 wk (Fig. 3B) of age. Thus, immunological recognition of ovarian targets often appears to begin at an early stage, independently of gonadotropin stimulation of follicular growth and ovulation, which is consistent with findings in the neonatal thymectomy model of ovarian autoimmune disease [26, 27]. The reason we did not observe 100% penetrance of the follicular destruction phenotype is not clear, but it is possible that immune effectors (antibodies or T cells) mediating follicular destruction simply had not yet developed in these apparently unaffected mice. Indeed, we observed an age-related increase in ovary-infiltrating T cells: By 20 wk of age, 96% of Aire-deficient mice showed ovarian T cell infiltration. It seems likely that these mice would later exhibit follicular loss.
In previously bred mice, follicular depletion was observed in 83% of mice that were euthanized at ages ranging from 12 to 20 wk. This contrasted with the approximately 52% rate of follicular depletion in virgin mice at this age range. Thus, pregnancy itself may further predispose these mice to ovarian autoimmunity. The reasons for this are unclear, but it is possible that the progesterone-dominant hormone environment of pregnancy impacts immunological targeting of the ovary. Alternatively, the expression of ovarian antigens provoking the immune system may be altered during pregnancy.
Indeed, the antigenic target proteins of the ovary remain a question. Previous studies have noted the presence of autoantibodies reactive against oocytes and granulosa cells in the serum of Aire-deficient female mice [10]. Several AIRE-regulated genes are restricted to the ovary, and malexpression of these genes occurs in the Aire-deficient thymus. This is likely followed by escape of ovary-reactive T cells into the periphery and, further, production of antiovarian antibodies; evidently, peripheral mechanisms of tolerance are unable to compensate. Ovary-specific genes expressed in murine mTECs include Zp2 and Zp3, the latter of which is regulated by AIRE, as demonstrated by the presence and absence of its transcripts in wild-type and Aire-deficient murine thymi, respectively [10, 12] (B. Warren and M. Petroff, unpublished results). Both are oocyte-specific. Other potential antigens include ALDH1A2, BCAT1, INHB, and an unknown protein, AU020206, the latter of which is reported to be strongly expressed in mice with high fertility [28]. In women, hypogonadism associated with APS-1 may be caused, in addition to targeting of ovary-specific antigens, by autoantibodies directed against steroidogenic enzymes. These include CYP11A1 and CYP17, which are also found in the adrenal gland [29, 30]. Intriguingly, these studies also identify placental syncytiotrophoblast as an autoimmune target due to the presence of these antibodies, suggesting that AIRE deficiency could also result in immune-mediated pregnancy loss. Future studies will reveal the identity of antigenic targets of the ovary and other tissues of the reproductive tract and, further, will show the cell types that mediate autoimmune damage.
A second question is what physiological events in the ovary lead to the display of ovarian antigens to the immune system. The ovary has a rich lymphatic supply, a route for antigens to access the draining regional lymph nodes [31] (B. Warren and M. Petroff, unpublished results). The observation that T cells traffic to the ovary before any evidence of ovulation argues against ovulation as the trigger for autoimmunity. An intriguing alternative is that the marked oocyte cell death that occurs early in life serves as a source of antigen to resident macrophages and other antigen-presenting cells, which subsequently traffic to and present antigen within the draining lymph nodes. Our finding that all stages of follicles (primordial, preantral, and antral) are depleted suggests that none of these is protected from the immune system; it does not, however, reveal the stage of follicle that serves as the initial antigenic target.
Surprisingly, ovulation and fertilization rates in Aire-deficient mice were not statistically different from those in wild-type controls, despite the early onset of lymphocyte infiltration and later degeneration of follicles in these animals. Further studies will be required to determine the developmental potential of fertilized oocytes from Aire-deficient mice; however, preliminary results suggest that implantation rates are reduced in Aire-knockout females bred to wild-type males. Thus, degenerative processes that impair the ability to be fertilized and/or the developmental capacity of the zygote may be initiated before ovulation. Alternatively, immune tolerance to the pre- or postimplantation embryo may be compromised. Indeed, AIRE-regulated genes specific to the blastocyst, placenta, and fetus have been reported in mTECs. Studies are currently underway to further determine the mechanisms of pre- and postimplantation loss.
A limitation of the present study is the inability to completely rule out other causative factors as the mediators of follicular destruction. The observation that wild-type ovaries undergo similar follicular depletion in knockout hosts following subrenal transplantation, the infiltration and proliferation of CD3+ lymphocytes into the ovary, and the presence of ovarian autoantibodies all argue strongly for a primary role of the immune system rather than an ovary-endogenous function of AIRE. In addition, thymus graft experiments from Anderson et al. [10] show that although Aire is transcribed in peripheral organs, including the ovary, albeit at low levels, thymic rather than peripheral expression of Aire is required for preventing autoimmune disease in multiple organs. Finally, using a commercially available anti-AIRE antibody, we were unable to detect AIRE protein in the mouse ovary, despite highly specific reactivity in the thymus (S. Jasti, M. Farahbakhsh and M. Petroff, unpublished results). Collectively, these data strongly argue that the defect in ovarian follicles and fertility in Aire-deficient mice is autoimmune-mediated. Studies using Rag1-deficient mice and phenotypic analyses are underway to further evaluate the role of specific subsets of responsible lymphocytes.
The discovery of Aire and its function has provided a unifying model for how central tolerance prevents autoimmune disease against a multitude of organs. In the present study, we show the effects of Aire deletion in mice on the female reproductive system. Because AIRE controls expression of ovary-specific antigens in the thymus, loss of AIRE and its targets appear to lead to failure of central tolerance to the ovary, resulting in progressive follicular depletion and premature ovarian failure. This follicular depletion was correlated with hypergonadotropism and infertility. The demonstration of the mechanisms of female infertility in AIRE deficiency provided here may have implications not only for patients with APS-1 but also provide broader insight into autoimmune-mediated infertility.
ACKNOWLEDGMENT
The authors thank Drs. Christophe Benoist and Dianne Mathis (Department of Pathology, Harvard Medical School, Boston, MA) for their kind donation of Aire-deficient mice and Joseph Juscius for excellent technical assistance and genotyping.
Footnotes
Supported by NIH R21HD062879 (M.G.P.), the KUMC Research Institute Lied Pilot Grant Program (M.G.P. and B.K.P.), K-INBRE grant P20RR016475, and NIH R01HD14846 (W.K.). S.J. was supported by Biomedical Research Training Grant Program. The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by NICHD (SCCPIR) grant U54HD28934.
REFERENCES
- Forges T, Monnier-Barbarino P, Faure GC, Bene MC. Autoimmunity and antigenic targets in ovarian pathology. Hum Reprod Update 2004; 10: 163 175 [DOI] [PubMed] [Google Scholar]
- Conway GS, Kaltsas G, Patel A, Davies MC, Jacobs HS. Characterization of idiopathic premature ovarian failure. Fertil Steril 1996; 65: 337 341 [DOI] [PubMed] [Google Scholar]
- Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol 2006; 24: 571 606 [DOI] [PubMed] [Google Scholar]
- Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol 2003; 21: 139 176 [DOI] [PubMed] [Google Scholar]
- Smith KM, Olson DC, Hirose R, Hanahan D. Pancreatic gene expression in rare cells of thymic medulla: evidence for functional contribution to T cell tolerance. Int Immunol 1997; 9: 1355 1365 [DOI] [PubMed] [Google Scholar]
- Jolicoeur C, Hanahan D, Smith KM. T-cell tolerance toward a transgenic beta-cell antigen and transcription of endogenous pancreatic genes in thymus. Proc Natl Acad Sci U S A 1994; 91: 6707 6711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol 2001; 2: 1032 1039 [DOI] [PubMed] [Google Scholar]
- Fan Y, Rudert WA, Grupillo M, He J, Sisino G, Trucco M. Thymus-specific deletion of insulin induces autoimmune diabetes. EMBO J 2009; 28: 2812 2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoss J, Hou Y, Johannes K, Lu W, Liou GI, Rinn J, Chang H, Caspi RR, Fong L, Anderson MS. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med 2006; 203: 2727 2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science 2002; 298: 1395 1401 [DOI] [PubMed] [Google Scholar]
- Abramson J, Giraud M, Benoist C, Mathis D. Aire's partners in the molecular control of immunological tolerance. Cell 2010; 140: 123 135 [DOI] [PubMed] [Google Scholar]
- Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med 2005; 202: 33 45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of aire control of T cell tolerance. Immunity 2005; 23: 227 239 [DOI] [PubMed] [Google Scholar]
- Liston A, Gray DHD, Lesage S, Fletcher AL, Wilson J, Webster KE, Scott HS, Boyd RL, Peltonen L, Goodnow CC. Gene dosage-limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J Exp Med 2004; 200: 1015 1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol 2003; 4: 350 354 [DOI] [PubMed] [Google Scholar]
- Aaltonen J, Bjorses P, Perheentupa J, Horelli-Kuitunen N, Palotie A, Peltonen L, Lee YS, Franscis F, Henning S, Thiel C, Leharach H, Yaspo M-L. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger proteins. Nat Genet 1997; 17: 399 403 [DOI] [PubMed] [Google Scholar]
- Björses P, Aaltonen J, Horelli-Kuitunen N, Yaspo M-L, Peltonen L. Gene defect behind APECED: a new clue to autoimmunity. Hum Mol Genet 1998; 7: 1547 1553 [DOI] [PubMed] [Google Scholar]
- Perheentupa J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab 2006; 91: 2843 2850 [DOI] [PubMed] [Google Scholar]
- Mathis D, Benoist C. Aire. Annu Rev Immunol 2009; 27: 287 312 [DOI] [PubMed] [Google Scholar]
- Ramsey C, Winqvist O, Puhakka L, Halonen M, Moro A, Kampe O, Eskelin P, Pelto-Huikko M, Peltonen L. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet 2002; 11: 397 409 [DOI] [PubMed] [Google Scholar]
- Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med 2005; 202: 805 815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly JL. Ovarian follicle counts—not as simple as 1, 2, 3. Reprod Biol Endocrinol 2003; 1: 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis LK, Albertini DF, Kinsey WH. Localized activation of Src-family protein kinases in the mouse egg. Dev Biol 2007; 306: 241 254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holets LM, Hunt JS, Petroff MG. Trophoblast CD274 (B7-H1) is differentially expressed across gestation: influence of oxygen concentration. Biol Reprod 2006; 74: 352 358 [DOI] [PubMed] [Google Scholar]
- Halonen M, Pelto-Huikko M, Eskelin P, Peltonen L, Ulmanen I, Kolmer M. Subcellular location and expression pattern of autoimmune regulator (Aire), the mouse orthologue for human gene defective in autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED). J Histochem Cytochem 2001; 49: 197 208 [DOI] [PubMed] [Google Scholar]
- Alard P, Thompson C, Agersborg SS, Thatte J, Setiady Y, Samy E, Tung KSK. Endogenous oocyte antigens are required for rapid induction and progression of autoimmune ovarian disease following day-3 thymectomy. J Immunol 2001; 166: 4363 4369 [DOI] [PubMed] [Google Scholar]
- Nair S, Mastorakos G, Raj S, Nelson LM. Murine experimental autoimmune oophoritis develops independently of gonadotropin stimulation and is primarily localized in the stroma and theca. Am J Reprod Immunol 1995; 34: 132 139 [DOI] [PubMed] [Google Scholar]
- Vanselow J, Nurnberg G, Koczan D, Langhammer M, Thiesen H-J, Reinsch N. Expression profiling of a high-fertility mouse line by microarray analysis and qPCR. BMC Genomics 2008; 9: 307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Sawicka J, Betterle C, Powell M, Prentice L, Volpato M. Rees Smith B, Furmaniak J. Autoantibodies to steroidogenic enzymes in autoimmune polyglandular syndrome, Addison's disease, and premature ovarian failure. J Clin Endocrinol Metab 1996; 81: 1871 1876 [DOI] [PubMed] [Google Scholar]
- Uibo R, Aavik E, Peterson P, Perheentupa J, Aranko S, Pelkonen R, Krohn KJ. Autoantibodies to cytochrome P450 enzymes P450scc, P450c17, and P450c21 in autoimmune polyglandular disease types I and II and in isolated Addison's disease. J Clin Endocrinol Metab 1994; 78: 323 328 [DOI] [PubMed] [Google Scholar]
- Brown HM, Robker RL, Russell DL. Development and hormonal regulation of the ovarian lymphatic vasculature. Endocrinology 2010; 151: 5446 5455 [DOI] [PubMed] [Google Scholar]