ABSTRACT
Retinoic acid (RA) is a meiosis-inducing factor. Primordial germ cells (PGCs) in the developing ovary are exposed to RA, resulting in entry into meiosis. In contrast, PGCs in the developing testis enter mitotic arrest to differentiate into prospermatogonia. Sertoli cells express CYP26B1, an RA-metabolizing enzyme, providing a simple explanation for why XY PGCs do not initiate meios/is. However, regulation of entry into mitotic arrest is likely more complex. To investigate the mechanisms that regulate male germ cell differentiation, we cultured XX and XY germ cells at 11.5 and 12.5 days postcoitus (dpc) with an RA receptor inhibitor. Expression of Stra8, a meiosis initiation gene, was suppressed in all groups. However, expression of Dnmt3l, a male-specific gene, during embryogenesis was elevated but only in 12.5-dpc XY germ cells. This suggests that inhibiting RA signaling is not sufficient for male germ cell differentiation but that the male gonadal environment also contributes to this pathway. To define the influence of Sertoli cells on male germ cell differentiation, Sertoli cells at 12.5, 15.5, and 18.5 dpc were aggregated with 11.5 dpc PGCs, respectively. After culture, PGCs aggregated with 12.5 dpc Sertoli cells increased Nanos2 and Dnmt3l expression. Furthermore, these PGCs established male-specific methylation imprints of the H19 differentially methylated domains. In contrast, PGCs aggregated with Sertoli cells at late embryonic ages did not commit to the male pathway. These findings suggest that male germ cell differentiation is induced both by inhibition of RA signaling and by molecule(s) production by embryonic age-specific Sertoli cells.
Keywords: de novo DNA methylation, male germ cell differentiation, primordial germ cells, Sertoli cells, sex differentiation
The commitment to the male pathway of primordial germ cells is induced both by inhibiting retinoic acid signaling and by embryonic age-specific Sertoli cells.
INTRODUCTION
In vertebrates, gonad formation is a dynamic process which develops from a sexually bipotential primordium consisting of germ cell and somatic cell lineages. The presence or absence of the Y chromosome-encoded male-determining Sry gene directs the developing gonad to differentiate into either a testis or an ovary, respectively [1, 2]. In mice, Sry expression occurs in the XY supporting cells between 10.5 and 12.0 days post coitus (dpc), leading to up-regulation of the transcription factor gene Sox9 [3–9]. Both Sry and Sox9 expression are necessary and sufficient to differentiate the supporting cell lineage into Sertoli cells [5–8]. Sertoli cells are believed to organize cells of the male gonad and orchestrate the differentiation of all other cell types [9]. The morphological signs of testicular differentiation appear in the mouse by 12.5 dpc. Sertoli cells polarize and begin to aggregate around clusters of primordial germ cells (PGCs) to form testis cords, which are in turn surrounded by a layer of peritubular myoid cells [9, 10]. In contrast, few morphological changes are apparent in the female gonad until near birth, when ovarian follicles begin to form in the ovarian cortex [10].
Before sex differentiation, both XX and XY PGCs are sexually bipotent. The fate of PGCs is regulated by the gonadal environment, not by their chromosomal sex (XX, XY). PGCs differentiate either as prospermatogonia or as oogonia, depending on the sex of gonadal somatic cells [11]. Once sex differentiation occurs, in the developing ovary at approximately 13.5 dpc, oogonia differentiate into primary oocytes, and most of them proceed to the diplotene stage around the time of birth [12, 13]. In the developing testis, XY PGCs enclosed in the testis cord differentiate into prospermatogonia and enter mitotic arrest by 13.5–15.5 dpc. Spermatogonia remain at the G0 stage of the cell cycle until after birth and can subsequently initiate meiosis several days later [14]. During the G0 stage, male germ cells initiate de novo methylation of imprinted loci and establish male-specific methylation imprints at around birth [15–17].
As the initiation or prevention of meiosis has often been used to monitor sex-specific differentiation of germ cells, studies have focused on whether a feminizing meiosis-inducing substance and a masculinizing meiosis-preventing substance direct sex-specific behavior in the embryonic gonads [18]. In 2006, retinoic acid (RA) was found to act as a meiosis-inducing substance in female embryonic gonads [19, 20]. In the developing ovary, RA induces meiosis of female germ cells at around 13.5 dpc. In the developing testis, in contrast, male germ cells do not enter meiosis. Because Sertoli cells of the testis cord produce the retinoid-degrading enzyme CYP26B1, the germ cells inside of the testis cord are prevented from entry into meiosis during this period [19–21]. In Cyp26b1-deficient mouse testes, XY germ cells eventually enter meiosis as they do in the female-specific pathway [19, 21]. Subsequently, there have been studies suggesting that the inhibition of the RA signaling induces germ cells to enter mitotic arrest (G0 stage), as in the male-specific pathway [22, 23]. CYP26B1 has been recognized as a meiosis-preventing substance in male embryonic gonads [19, 20]. However, it remains uncertain whether male differentiation of germ cells occurs simply by inhibiting entry into meiosis. Recently, it was reported that a factor(s) other than CYP26B1 produced in the male gonad influences male germ cell differentiation [24–27]. Therefore, the prevention of meiosis may not be the only mechanism that induces the male-specific pathway in germ cells.
In this study, we investigated the contribution of the PGC niche to the male differentiation pathway. In the testis, Sertoli cells associate with germ cells and nurture their development into sperm. Therefore, we focused on the influence of Sertoli cells on male germ cell differentiation. Our studies indicate that differentiation of PGCs to the male pathway is induced both by inhibition of the RA signaling and by the male factor(s) produced by stage-specific Sertoli cells.
MATERIALS AND METHODS
Mice
Transgenic mice expressing germ cell-specific green fluorescent protein (GFP) driven by the Pou5f1 gene promoter/enhancer (Tg OG2) were generated by microinjecting (CBA/Caj × C57BL/6J) F2 zygotes (a generous gift from Dr. J.R. Mann, University of Melbourne, Melbourne, Australia) [28]. Sox9-EGFP knock-in mice were generated by introduction of an IRES-EGFP-pA cassette into the 3′ untranslated region of the endogenous Sox9 gene by gene targeting in mouse embryonic stem cells [29]. Sox9-EGFP homozygous mice were generated on a C57BL/6J-129/SvEv-Swiss mixed genetic background. Subsequent experiments were performed by mating CD1 females with Tg OG2 homozygous males or Sox9-EGFP knock-in homozygous males. GFP-positive germ cells were obtained from (CD1 × Tg OG2) F1 hybrid fetuses, whereas GFP-positive pre-Sertoli cells were collected from (CD1 × Sox9-EGFP knock-in) F1 fetuses. All relevant experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Hawaii.
Media
Urogenital ridges were dissected in HEPES-Dulbecco modified Eagle medium (DMEM) solution (Invitrogen, Carlsbad, CA) with 20% fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT). Isolated PGCs, aggregates, and urogenital ridges were cultured in high-glucose DMEM supplemented with 0.1 mM nonessential amino acids, 0.1 mM 2-mercaptoethanol, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 15% FBS. For culture of isolated 11.5-dpc PGCs, 100 ng/ml murine c-Kit ligand (R&D Systems, Minneapolis, MN), 50 ng/ml human bone morphogenetic protein 4 (Invitrogen), 20 ng/ml murine stromal cell-derived factor 1 (R&D Systems), 20 ng/ml human basic fibroblast growth factor (R&D Systems), and 5 μM forskolin (Sigma-Aldrich) were added to the medium to increase cell viability [30].
Isolated Germ Cell Culture and [PGCs + Sertoli Cells] Aggregation Culture
To isolate germ cells, XX and XY urogenital ridges were obtained from (CD1 × Tg OG2) F1 hybrid fetuses at 11.5 and 12.5 dpc. The sexes of 11.5-dpc fetuses were determined by PCR amplification of genomic DNA for the Sry gene, using specific primers (5′-CTGTGTAGGATCTTCAATCTCT-3′ and 5′-GTGGTGAGAGGCACAAGTTGGC-3′) [31]. After urogenital ridges were dissected, they were incubated in Accumax (Innovate Cell Technology, San Diego, CA) at 37°C for 10 min and then gently pipetted to prepare a single-cell suspension. GFP-positive germ cells were sorted using an Aria model fluorescence-activated cell sorter (BD Biosciences). To isolate Sertoli cells, urogenital ridges were obtained from (CD1 × Sox9-EGFP knock-in) F1 fetuses at 12.5, 15.5, and 18.5 dpc. GFP-positive Sertoli cells were sorted as described above. Purity of germ cells and Sertoli cells was more than 99.8% and 99.9%, respectively. GFP intensities of the germ cells and Sertoli cells were very different. Isolated PGCs had very strong GFP expression, whereas isolated Sertoli cells showed relatively faint GFP fluorescence at the stages examined. According to differences in intensity of GFP fluorescence and morphology, we could distinguish germ cells from Sertoli cells. Using immunostaining for germ cell-specific markers (POU5F1, DNMT3L, SYCP3), we also confirmed that no germ cell contamination occurred in the Sertoli cell population after sorting.
For germ cell culture without gonadal somatic cells, about 10 000 isolated PGCs were cultured on collagen-coated mesh inserts (Corning Life Sciences, Lowell, MA) [17]. To produce aggregates of PGCs and Sertoli cells, we mixed and centrifuged 5000 PGCs and 50 000 Sertoli cells at 2000 rpm for 5 min at 4°C. The pellet was incubated at 37°C for 12 h and then transferred onto 2% agar block for culture. Urogenital ridges from (CD1 × Tg OG2) F1 hybrid fetuses at 11.5 dpc were also cultured on agar blocks as positive controls. Isolated germ cell [PGCs + Sertoli cells] aggregates and urogenital ridges were cultured with or without retinoic acid receptor (RAR) inhibitor (product no. Ro41-5253; Calbiochem, San Diego, CA) at 0, 10−7, or 10−6 M. This inhibitor is a selective RARA antagonist (concentration required to inhibit 50% of specific retinoic acid binding is 60 nM for RARA, 2400 nM for RARB, and 3300 nM for RARG).
Germ Cell Collection after Culture
After 4–6 days of culture, [PGCs + Sertoli cells] aggregates, and urogenital ridges were treated with Accumax to generate single-cell suspensions. For all experiments, germ cells were collected manually using a fluorescence microscope equipped with a micropipette. In aggregates, the intensity of GFP fluorescence in the germ cells was much higher than that in Sertoli cells. Therefore, germ cells were isolated based on their high GFP fluorescence intensity.
Quantitative Gene Expression
Quantitative PCR analysis was performed using MyiQ single-color real-time PCR detection system (Bio-Rad). cDNA was synthesized from isolated germ cells, using the Superscript III Cells Direct cDNA synthesis kit (Invitrogen). Three experiments were performed independently for each treatment group. Sequences of forward and reverse, respectively, primers were 5′-GTTTCCTGCGTGTTCCACAAG-3′ and 5′-CACCCGAGGCTCAAGCTTC-3′ for Stra8; 5′-GTGCGGGTACTGAGCCTTTTTAGA-3′ and 5′-CGACATTTGTGACATCTTCCACGTA-3′ for Dnmt3l; and 5′-CCTGTATGCCTCTGGTCGTA-3′ and 5′-CCATCTCCTGCTCGAAGTCT-3′ for Actb. The relative expression of target mRNAs was calculated from target CT values and Actb CT values, using the standard curve method. Results were normalized to Actb gene expression.
Immunohistochemistry
After being cultured for 4–7 days, aggregates and urogenital ridges were fixed with 4% paraformaldehyde (Sigma-Aldrich) at 4°C for 12 h. Fixed tissues were washed with PBS containing 7% sucrose for 12 h and embedded in tissue freezing medium (Triangle Biomedical Sciences, Durham, NC). Ten-micrometer sections were stored at −70°C until immunostaining. Sections were pretreated with 1% bovine serum albumin (BSA) and then 0.1% Triton X-100 in PBS for 10 min and then treated with 10% goat serum in PBS for 1 h at room temperature. Sections were incubated with primary antibodies at 4°C overnight. Primary antibodies used for immunostaining were mouse monoclonal anti-POU5F1 (1:2 dilution; Abcam, Cambridge, MA), mouse monoclonal anti-human AMH (1:40 dilution; AbD Serotec, Oxford, U.K.), mouse monoclonal anti-mouse CDKN2A (p16INK4a; 1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal anti-mouse DDX4 (MVH; 1:200 dilution; Abcam), and rabbit polyclonal anti-mouse SYCP3 (1:200 dilution; Abcam). Rabbit polyclonal anti-mouse DNMT3L (1:200 dilution) and rabbit polyclonal anti-mouse NANOS2 (1:100) were kindly provided by Drs. S. Tajima and Y. Saga, respectively. Thereafter, slides were incubated with secondary antibodies (Alexa Fluor 488 goat anti-mouse immunoglobulin G [IgG; 1:250 dilution] and Alexa Fluor 546 goat anti-rabbit IgG [1:250 dilution] [Invitrogen]) in PBS containing 1% BSA for 1 h at room temperature. Slides were subsequently incubated with Hoechst 33342 stain (Invitrogen) for 15 min and mounted in Vectashield (Vector Laboratories Inc., Burlingame, CA). Immunofluorescence was visualized using Axioskop 2 plus and AxioVision version 3.1 software (Carl Zeiss Microimaging, Thornwood, NY). For NANOS2 detection, aggregates were embedded in tissue freezing medium after culture for 4 days. Sections were cut and stored at −70°C. Before being incubated with antibodies, sections were fixed with 4% paraformaldehyde in PBS for 10 min and treated with 10% goat serum in PBS for 1 h at room temperature. Incubation with antibodies, mounting, and observation were as described above.
Immunocytochemistry
Germ cells were placed on glass slides and fixed with 4% paraformaldehyde for 15 min. Cells were washed with PBS (calcium and magnesium free; Invitrogen) and then permeabilized with 0.25% Triton X-100, 1% BSA in PBS. After cells were washed with PBS, blocking was performed with 5% goat serum, 1% BSA in PBS. Cells were incubated with primary antibodies at 4°C overnight. Primary antibodies used were mouse monoclonal anti-mouse p16INK4a (1:250 dilution) and rabbit polyclonal anti-mouse NANOS2 (1:200 dilution). Thereafter, slides were incubated with secondary antibodies in PBS containing 1% BSA for 30 min at room temperature. Slides were then incubated with Hoechst 33342 stain, mounted, and observed as described above.
Bisulfite Genomic Sequencing
Methylation patterns in the differentially methylated domains (DMDs) of the paternally imprinted H19 gene were determined using bisulfite genomic sequencing as previously described [17], with some modifications. After 8 days of culture, [PGCs + Sertoli cells] aggregates were dissociated into single cells and manually collected GFP-positive germ cells. Genomic DNA prepared from 500 GFP-positive germ cells was used for PCR amplification. Bisulfate modification of DNA was performed using EZ DNA methylation direct kit according to the manufacturer's instruction (Zymo Research, Orange, CA). Bisulfate-converted DNA was subjected to PCR amplification of the H19 DMDs. PCR primers and condition were described previously [32]. PCR products were subcloned into the pGEM-T Easy vector (Promega, Madison, WI) and sequenced. After sequencing, the methylated percentage of all CpG sites combined was calculated.
RESULTS
Sex Differentiation of Germ Cells Cultured with an RAR Inhibitor
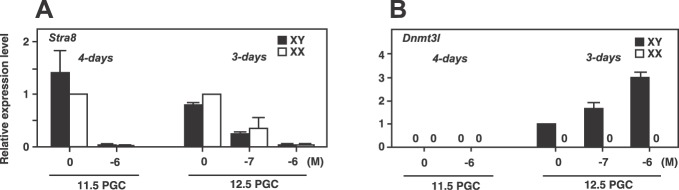
First, we examined the effect of an RAR inhibitor on the inhibition of entry into meiosis in fetal germ cells. Isolated XX and XY germ cells at 11.5 and 12.5 dpc were cultured with or without an RAR inhibitor and examined for Stra8 expression. Stra8 is essential for initiating meiosis, and its expression occurs only in female germ cells before birth [33, 34]. In the absence of the RAR inhibitor, all four groups expressed Stra8, regardless of their sex chromosome genotype (Fig. 1A). In the presence of the RAR inhibitor at a concentration of 10−6 M, Stra8 expression was suppressed in all groups, regardless of their sex or stage. These data suggest that the RAR inhibitor directly blocks entry of germ cells into meiosis. Next, we determined whether fetal germ cells can enter the male pathway in the presence of the RAR inhibitor. Dnmt3l is a gene that regulates de novo DNA methylation to establish sex-specific methylation imprints [35, 36]. In mice, only male germ cells initiate de novo methylation at around 15.5 dpc and establish male-specific imprints at around birth. Therefore, Dnmt3l expression is male-specific during embryogenesis. After being cultured without RAR inhibitor, XX and XY PGCs at 11.5 dpc and XX germ cells at 12.5 dpc did not express Dnmt3l (Fig. 1B). These three groups did not express Dnmt3l even in the presence of RAR inhibitor in culture (Fig. 1B). However, XY germ cells at 12.5 dpc (prospermatogonia) expressed low levels of Dnmt3l after 3 days of culture without inhibitor (Fig. 1B). When these cells were cultured with inhibitor, the Dnmt3l expression was increased in a dose-dependent manner and reached 3.0-fold higher expression level than that of the control at a concentration of 10−6 M. These results suggest that inhibiting RA signals is not sufficient for male differentiation of germ cells and that the male gonadal environment at 12.5 dpc imposes male differentiation on the germ cells.
FIG. 1.
Stra8 and Dnmt3l expressions in germ cells cultured with RAR inhibitor. Isolated XX and XY germ cells at 11.5 and 12.5 dpc were cultured on mesh inserts with RAR inhibitor (at 0, 10−6, and 10−7 M) for 4 and 3 days, respectively. Stra8 expression levels were decreased in all germ cell groups in the presence of the RAR inhibitor (A). Dnmt3l expression was detected only in XY germ cells at 12.5 dpc (B). Gene expression levels are shown relative to those of an untreated control (which is set as 1 for each gene). Normalization of expression was achieved against the amplification of Actb. Values are means ± SEM from 3 separate experiments.
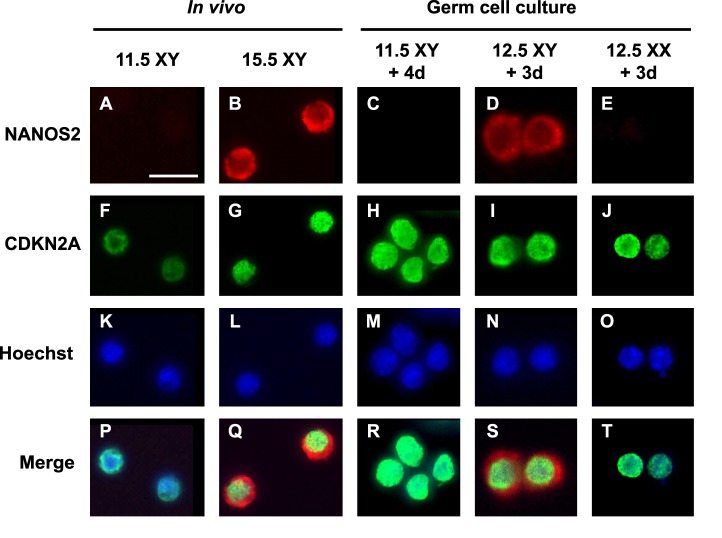
To further confirm the effects of inhibiting RA signals on male germ cell differentiation, we next performed immunostaining for NANOS2, another male-specific marker [37]. At 11.5 dpc, XY PGCs did not produce NANOS2 protein (Fig. 2A). After XY germ cells entered the male-specific pathway in vivo, NANOS2 localization was clearly detected in the cytoplasm of prospermatogonia at 15.5 dpc (Fig. 2B). When XY PGCs at 11.5 dpc were cultured with RAR inhibitor for 4 days, NANOS2 expression was not detected (Fig. 2C). In contrast, XY germ cells at 12.5 dpc became NANOS2-positive after 3 days of culture with RAR inhibitor (Fig. 2D), and protein localization in the cytoplasm was very similar to that of in vivo prospermatogonia at 15.5 dpc (Fig. 2B). XX germ cells at 12.5 dpc showed no staining for NANOS2 after culture, even in the presence of RAR inhibitor (Fig. 2E). Entry into mitotic arrest (G0) is one of the male-specific characteristics after sex differentiation. Using the cell cycle arrest marker CDKN2A (p16INK4a) [38], we also confirmed male differentiation in cultured PGCs. In in vivo controls, XY PGCs became CDKN2A-positive after sex differentiation (Fig. 2, F and G). Nuclei of XX and XY germ cells isolated at 11.5 and 12.5 dpc became CDKN2A-positive after being cultured with RAR inhibitor (Fig. 2, H–J), suggesting that these germ cells entered mitotic arrest by the inhibition of RA, regardless of XX or XY genotype.
FIG. 2.
NANOS2 and CDKN2A expression in germ cells cultured with RAR inhibitor. Isolated XX and XY germ cells at 11.5 and 12.5 dpc were cultured for 4 or 3 days, respectively, with 10−6 M RAR inhibitor, followed by immunostaining for NANOS2 (A–E) and CDKN2A (p16INK4a) (F–J). As in vivo controls, XY germ cells at 11.5 (A, F, K, P) and 15.5 dpc (B, G, L, Q) were also immunostained. After culture, only XY germ cells at 12.5 dpc expressed NANOS2, localized in their cytoplasm (D, S), similar to that of XY germ cells at 15.5 dpc (B, Q). DNA was stained with Hoechst 33342 stain (K–O). Merged images correspond to the overlay of NANOS2, CDKN2A, and Hoechst 33342 signals (P–T). Bar = 20 μm.
These data suggest that inhibiting the RA signaling in germ cells prevents entry into meiosis but is not sufficient to induce male germ cell differentiation. Furthermore, it is clear that once PGCs are enveloped in the testis cords and differentiate into prospermatogonia, these cells commit to male differentiation without the male gonadal environment. This suggests that male gonadal somatic cells at approximately 12.5 dpc produce a molecule(s) that directs germ cells toward male differentiation.
Sex Differentiation of PGCs Aggregated with Sertoli Cells
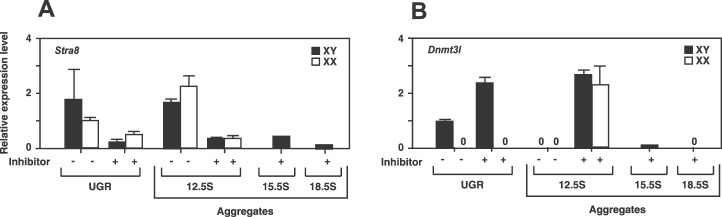
In the developing testis, Sertoli cells form the testis cords and communicate with germ cells and other somatic cells. To investigate the influence of Sertoli cells on male germ cell differentiation, we developed a [PGC + Sertoli cell] aggregation assay. Sexually undifferentiated PGCs at 11.5 dpc were aggregated with Sertoli cells isolated at different embryonic ages (12.5, 15.5, and 18.5 dpc). After being centrifuged, the Sertoli cells at 12.5 dpc tightly adhered to each other to generate solid aggregates. In contrast, the cells at later stages (15.5 and 18.5 dpc) formed less solid aggregates. These aggregates were cultured on agar gels with or without RAR inhibitor for 6 days. As controls, XX and XY urogenital ridges at 11.5 dpc were cultured for the same period. First, we examined the influence of Sertoli cells on entry of germ cells into meiosis (Fig. 3A). Without RAR inhibitor, PGCs in the [PGCs + 12.5-dpc Sertoli cells] aggregates expressed Stra8 at high levels, regardless of their sex chromosome genotype. In the presence of RAR inhibitor, in contrast, XX and XY PGCs of the [PGCs + 12.5-dpc Sertoli cells] aggregates suppressed Stra8 expression levels during culture. These results were the same as those for germ cells derived from organ culture of the 11.5-dpc XX and XY genital ridges (Fig. 3A) and 11.5-dpc PGCs cultured without gonadal somatic cells (Fig. 1A). When the aggregates of [PGCs + 15.5-dpc Sertoli cells] and [PGCs + 18.5-dpc Sertoli cells] were cultured in the presence of the RAR inhibitor, Stra8 expression levels in these PGCs were also suppressed to very low levels after 6 days of culture (Fig. 3A). Next, we determined the influence of Sertoli cells on male germ cell differentiation (Fig. 3B). When [PGCs + 12.5-dpc Sertoli cells] aggregates were cultured without RAR inhibitor for 6 days, the PGCs did not express Dnmt3l, regardless of their sex chromosome genotype. In the presence of RAR inhibitor, both XX and XY PGCs in the [PGCs + 12.5-dpc Sertoli cells] aggregates significantly increased Dnmt3l expression after culture (Fig. 3B). In contrast, PGCs in the [PGCs + 15.5-dpc Sertoli cells] and [PGCs + 18.5-dpc Sertoli cells] aggregates did not up-regulate Dnmt3l expression, even in the presence of RAR inhibitor (Fig. 3B). These results suggest that Sertoli cells at 12.5 dpc are directly able to induce male differentiation of germ cells in the presence of the RAR inhibitor; however, this function is apparently lost in Sertoli cells in later stages, by 15.5 dpc.
FIG. 3.
Stra8 and Dnmt3l expressions in PGCs aggregated with Sertoli cells. XX and XY PGCs at 11.5 dpc were aggregated with Seroli cells at 12.5, 15.5, or 18.5 dpc and cultured with RAR inhibitor (0, 10−6 M) for 6 days. As controls, XX and XY 11.5-dpc urogenital ridges (UGR) were cultured under the same conditions. In the presence of RAR inhibitor, Stra8 expression levels were decreased in the germ cells of all groups (A). In contrast, Dnmt3l expression levels were detected only in PGCs aggregated with 12.5-dpc Sertoli cells in the presence of RAR inhibitor (B). Stra8 expression levels are shown relative to those of XX UGR cultured for 4 days. The expression level of XY UGR cultured for 4 days is set at 1 to show the relative expression levels of Dnmt3l in each group. Only XY PGCs were used for making aggregates with 15.5- and 18.5-dpc Sertoli cells. Normalization of expression was achieved relative to the amplification of Actb. Values are mean ± SEM from 3 separate experiments.
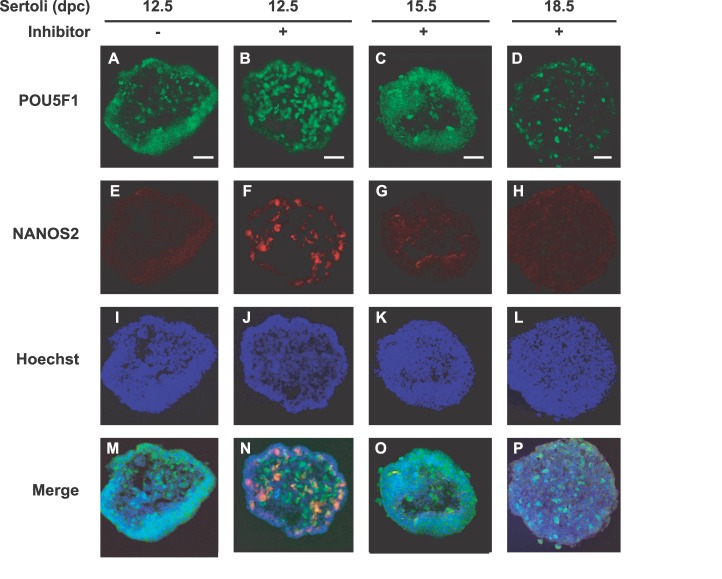
Using an immunohistochemical staining, we investigated the direct influence of Sertoli cells on male germ cell differentiation (Fig. 4). Sertoli cells at different embryonic ages (12.5, 15.5, and 18.5 dpc) were aggregated with XY PGCs at 11.5 dpc and cultured with or without RAR inhibitor. After 4 days of culture, we prepared frozen sections of the aggregates to detect male-specific NANOS2. Germ cells were recognized as POU5F1-positive cells in all aggregates (Fig. 4, A–D). In the absence of RAR inhibitor, PGCs aggregated with 12.5 dpc Sertoli cells did not express NANOS2 after culture (Fig. 4E). When the same aggregates of 12.5-dpc Sertoli cells were cultured with RAR inhibitor, some PGCs turned NANOS2-positive in the aggregates (Fig. 4F). However, PGCs aggregated with Sertoli cells at late embryonic ages (15.5 and 18.5 dpc) did not express NANOS2 even in the presence of RAR inhibitor (Fig. 4, G and H).
FIG. 4.
NANOS2 expression in PGCs aggregated with Sertoli cells. XY PGCs at 11.5 dpc were aggregated with Sertoli cells at 12.5, 15.5, or 18.5 dpc and cultured with RAR inhibitor (B–D, F–H, J–L, N–P) or without RAR inhibitor (A, E, I, M). After 4 days of culture, immunostaining for POU5F1 (A–D) and NANOS2 (E–H) was performed. Only PGCs aggregated with 12.5-dpc Sertoli cells expressed NANOS2 in the presence of RAR inhibitor (F, N). DNA was stained with Hoechst 33342 stain (I–L). Merged images correspond to the overlay of POU5F1, NANOS2, and Hoechst 33342 signals (M–P). Bar = 50 μm.
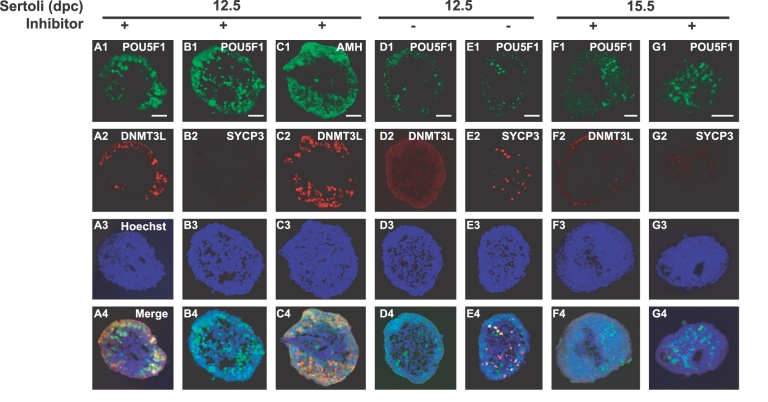
We also analyzed male-specific DNMT3L and meiosis-specific SYCP3 expression in PGCs aggregated with 12.5- or 15.5-dpc Sertoli cells (Fig. 5). After 7 days of culture, a large population of PGCs aggregated with 12.5-dpc Seroli cells became DNMT3L-positive (Fig. 5, A2 and C2) but SYCP3-negative in the presence of RAR inhibitor (Fig. 5B2). In the absence of RAR inhibitor, however, no DNMT3L-positive cells were observed in the same aggregates with 12.5-dpc Sertoli cells (Fig. 5D2). Instead, some PGCs became SYCP3-positive cells, indicating entry into meiosis (Fig. 5E2). These data suggest that the 12.5-dpc Sertoli cells influenced PGCs to commit to male differentiation when RA signaling was inhibited. Under the same culture condition, most POU5F1-positive cells in the [PGCs + 15.5 dpc Sertoli cells] aggregates were neither DNMT3L- nor SYCP3-positive (Fig. 5, F1–G4). Therefore, these PGCs did not appear to have entered either the male or female pathway after culture. In most cases, POU5F1-positive cells were located at the periphery of the aggregates. To determine the distribution of the Sertoli cell population, we immunostained the aggregates for the Sertoli cell-specific marker AMH. We found that AMH-positive cells were localized in the periphery (Fig. 5C1), overlapping with the distribution of the PGC population (Fig. 5C2). This result indicates that the interaction between Sertoli cells and PGCs was productive in the peripheral regions of the aggregates.
FIG. 5.
DNMT3L and SYCP3 localization in PGCs aggregated with Sertoli cells. XY PGCs at 11.5 dpc aggregated with Sertoli cells at 12.5 (A1–E4) or 15.5 dpc (F1–G4) were cultured with RAR inhibitor (A1–C4, F1–G4) or without RAR inhibitor (D1–E4). After 4 days of culture, immunostaining for POU5F1 (A1, B1, D1, E1, F1, G1), DNMT3L (A2, C2, D2, F2), SYCP3 (B2, E2, G2), and AMH (C1) was performed. In the presence of RAR inhibitor, PGCs aggregated with 12.5-dpc Sertoli cells expressed DNMT3L (A2, C2) but not SYCP3 (B2). In the absence of RAR inhibitor, PGCs aggregated with 12.5 dpc did express SYCP3 (E2) but not DNMT3L (D2). PGCs aggregated with 15.5-dpc Sertoli cells did not show either DNMT3L (F2) or SYCP3 (G2) expression, even in the presence of RAR inhibitor. DNA was stained with Hoechst 33342 stain (A3, B3, C3, D3, E3, F3, G3). Merged images correspond to the overlay of signals (A4, B4, C4, D4, E4, F4, G4). Bar = 50 um.
The ratios of DNMT3L- and SYCP3-positive cells in the aggregates are summarized in Table 1. When 11.5-dpc XY PGCs aggregated with 12.5-dpc Sertoli cells were cultured with RAR inhibitor for 4 and 7 days, approximately 18% and 53% of the POU5F1-positive cells became DNMT3L-positive, respectively. Under the same culture conditions, approximately 66% of the XX PGCs aggregated with 12.5-dpc Sertoli cells eventually turned DNMT3L-positive after 7 days of culture. In the absence of RAR inhibitor, XY PGCs in the [PGCs + 12.5-dpc Sertoli cells] aggregates did not express DNMT3L. Instead, most of these PGCs entered meiosis (88% SYCP3-positive of the POU5F1-positive cells), suggesting commitment to the female-specific pathway. When undifferentiated PGCs were aggregated with Sertoli cells at 15.5 or 18.5 dpc, almost none of the POU5F1-positive cells became DNMT3L-positive, even in the presence of RAR inhibitor. Interestingly, these PGCs did not express SYCP3 either. These data suggest that the RAR inhibitor blocks entry into meiosis and that some factor(s) from Sertoli cells induces male differentiation of germ cells in a stage-specific manner.
TABLE 1.
Percentages of DNMT3L-positive PGCs after cultured with different stages of Sertoli cells.a
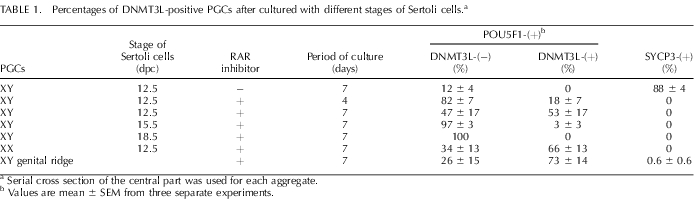
Serial cross section of the central part was used for each aggregate.
Values are mean ± SEM from three separate experiments.
Establishment of Male-Specific Methylation Imprints in PGCs Aggregated with Sertoli Cells
To determine whether Sertoli cells induce undifferentiated PGCs to establish male-specific methylation imprints, we performed a bisulfite genomic sequencing assay. XY PGCs at 11.5 dpc were aggregated with Sertoli cells at 12.5 or 15.5 dpc and cultured with RAR inhibitor. After being cultured for 8 days, isolated germ cells were used for DNA methylation analysis of the DMDs of the H19 paternally imprinted gene. We found that 42% of the total CpG sites in the H19 DMDs were methylated in PGCs aggregated with 12.5-dpc Sertoli cells after culture (Fig. 6). This suggests that nearly half the aggregated PGCs initiated male-specific de novo methylation of the H19 DMDs. When PGCs were aggregated with Sertoli cells at 15.5 dpc, only 9% of the total CpGs in the H19 DMDs were methylated, even in the presence of RAR inhibitor (Fig. 6). These results indicate that some factor(s) produced by Sertoli cells in an embryonic age-specific manner influences PGCs to establish male-specific methylation imprints.
FIG. 6.
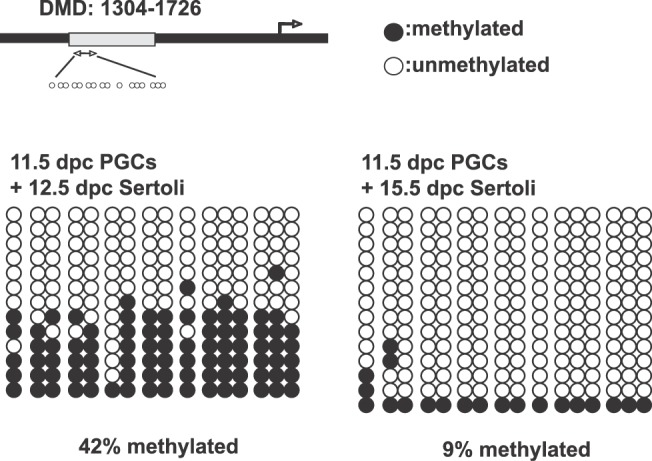
DNA methylation status of the H19 DMDs in PGCs aggregated with Sertoli cells. XY PGCs at 11.5 dpc aggregated with Sertoli cells at 12.5 or 15.5 dpc were cultured with RAR inhibitor. After 8 days culture, GFP-positive germ cells were collected to analyze DNA methylation status of the H19 DMDs by bisulfate genomic sequencing. In PGCs aggregated with 12.5-dpc Sertoli cells, 42% of the CpG sites of the H19 DMDs were methylated. In contrast, only 9% of CpG sites of the H19 DMDs were methylated in the PGCs aggregated with 15.5-dpc Sertoli cells. Closed circles represent methylated CpGs, and open circles indicate unmethylated CpGs.
DISCUSSION
In the fetal ovary, RA induces meiosis in XX germ cells to enter oogenesis [19, 20]. However, in the fetal testis, RA signaling is suppressed by an RA catalyzing enzyme, CYP26B1, produced by Sertoli cells [19, 20]. As a result, XY germ cells enclosed by Sertoli cells enter mitotic arrest and establish male-specific methylation imprints. However, the mechanisms for male germ cell differentiation are likely more complex. In this study, we first investigated whether the male differentiation of germ cells occurs simply by inhibition of the RA signaling. We also determined a direct effect of Sertoli cells on male germ cell differentiation. Using germ cell culture [17, 39] and [PGCs + Sertoli cells] aggregation assay systems, we demonstrated that (1) inhibiting RA signaling is not sufficient to induce male germ cell differentiation and that (2) Sertoli cells produce a molecule(s) which directly influences PGCs to initiate male differentiation in an embryonic age-specific manner.
When isolated 11.5- and 12.5-dpc germ cells were cultured in the presence of an RAR inhibitor, none of these cell types initiated meiosis regardless of their sex chromosomes (XX, XY) and stages. In contrast, only XY germ cells at 12.5 dpc entered the male differentiation pathway under the same culture conditions. These results suggest that inhibiting RA signaling in germ cells suppresses entry into meiosis but is not sufficient to induce male differentiation. Furthermore, our result showed that once PGCs are enclosed in the testis cord to differentiate into prospermatogonia at 12.5 dpc, these male germ cells commit to the male pathway without the male gonadal environment. Adams and McLaren [40] previously demonstrated that 12.5-dpc XY germ cells developed as prospermatogonia even in the female gonadal environment, whereas 11.5-dpc XY PGCs developed into oogonia under the same conditions. Together with our present results, this suggests that the testis cord provides the male-specific environment that imposes male differentiation on germ cells by 12.5 dpc.
Both male and female germ cells in the developing gonads express RA receptors such as RARA, RARB, and RARG [19]. To inhibit RA signaling in germ cells, we used selective RA receptor α (RARA) antagonist. This RAR inhibitor directly suppressed Stra8 expression in the isolated germ cells regardless of their sex. Moreover, PGCs aggregated with Sertoli cells did not enter meiosis in the presence of RARA antagonist. These results suggest that the meiotic process induced by RA signaling is mediated by RARA. Our results are supported by a previous report that all of the effects of RA on development of the fetal testis are mediated by RARA [41]. In addition, RARA is selectively implicated in the RA-induced decrease in the number of germ cells in the fetal ovary [42]. However, it is important to note that mutant mice lacking each of the RAR isotypes do not show any obvious defects in the ovary, probably due to compensation by the other remaining RARs [43]. This does not conflict with the fact that Stra8 expression was suppressed by an agonist of the three RARs (RARA, RARB, and RARG) in the cultured testis [20]. However, another report suggested that RA exerts its effects on male germ cells by RARA-retinoid X receptor α (RXRA) heterodimers, with RARA as an active partner [44]. Taken together, our results suggest that RARA is dominantly involved in RA signaling to induce meiosis in the germ cells.
Our aggregation experiments revealed that Sertoli cells directly imposed the male differentiation of germ cells without the testis cord structure. Sexually undifferentiated PGCs aggregated with 12.5-dpc Sertoli cells expressed male-specific genes and established male-specific imprints of the H19 DMDs. In the developing testis, Sertoli cells directly surround PGCs to form the testis cord. Once testis cord formation occurs, the enclosed XY germ cells initiate male differentiation at approximately 12.5 dpc [40]. Therefore, it has been suggested that the formation of the testis cord should support male germ cell differentiation [18]. In contrast, PGCs that accidentally end up in the testis interstitium between the testis cords still differentiate into prospermatogonia, suggesting the testis cord structure is not essential for male germ cell differentiation [45, 46]. Interestingly, our results showed that late Sertoli cells at 15.5 and 18.5 dpc had little or no effect on male germ cell differentiation in the same aggregation system. Therefore, Sertoli cells might produce a diffusible factor(s) at the time of sex differentiation to impose the male-specific pathway. FGF9 is a well-known diffusible factor that regulates testis differentiation [47]. In Fgf9-deficient XY gonads, Sertoli precursors fail to proliferate and differentiate into Sertoli cells, resulting in male-to-female sex reversal [47, 48]. Recently it has been reported that FGF9 directly suppresses meiosis and promotes male germ cell differentiation [25, 26]. Fgf9 is initially expressed in both XX and XY gonadal supporting cells, but its expression is restricted only in the male gonad by 12.5 dpc, followed by gradual loss by 14.5 dpc [26, 47]. The expression profile of Fgf9 in the male gonad is coincident with our proposed male factor(s) which is produced by Sertoli cells in an embryonic age-specific manner. Therefore, diffusible signal(s) such as FGF9 might be the candidate molecule that induces male germ cell differentiation. However, we still do not know the identity of the molecule(s) for male differentiation of germ cells. Using a coculture system, Guerquin et al. [27] demonstrated that diffusible molecules in the developing testes from 13.5 dpc and neonates induced male differentiation of the XX germ cells in an undifferentiated gonad. They suggested that the interstitial cell population such as peritubular and Leydig cells, but not Sertoli cells, had potential to induce the male-specific pathway in germ cells. Tmem184a (also known as Sdmg1) encodes a transmembrane protein expressed in Sertoli cells and regulates membrane trafficking pathways such as localizing a secretory SNARE STX2 correctly [24]. The membrane trafficking pathway seems important for the Sertoli cells to secrete male factors to germ cells during testis development, because inhibiting secretion and post-Golgi body trafficking in the XY urogenital ridges causes male-to-female sex reversal of the germ cells [24]. Taken together, we speculate that the diffusible and secretory factor(s) produced by Sertoli cells and other somatic cells positively induce the male differentiation of germ cells in the developing testis.
It is important to note that PGCs aggregated with 12.5-dpc Sertoli cells entered meiosis in the absence of RAR inhibitor. This appears to be inconsistent with the in vivo observation that Sertoli cells express Cyp26b1 to inhibit RA signaling of PGCs [19]. This may be due to structural differences between the intact testis cords and the reconstructed aggregates. In vivo, Sertoli cells form the testis cord with the peritubular myoid cells and enclose the PGC population to inhibit RA signaling by CYP26B1. In our aggregates of the PGCs and Sertoli cells, Sertoli cells formed multilayer structures that tightly interacted with PGCs or themselves. This unusual structure might negatively affect the inhibition of RA signaling in germ cells. Also, it is known that CYP26B1 is produced not only by Sertoli cells but also by other somatic cells during embryogenesis. In developing testes, Cyp26b1 is up-regulated as early as 11.5 dpc and is expressed by both Sertoli cells and interstitial cells [19, 21]. Very recently, it was revealed that Cyp26b1 is up-regulated by SF1 in Leydig cells residing in the interstitial space and by SF1 and SOX9 in Sertoli cells in the developing testes [49]. Therefore, in the aggregates of PGCs and Sertoli cells without the Leydig cell contribution, the quantity of Cyp26b1 generated by Sertoli cells might not be sufficient to inhibit germ cells from entry into meiosis. Another possible reason why PGCs aggregated with Sertoli cells enter meiosis without the RAR inhibitor is that the culture medium may contain RA. We found that our culture medium supplemented with 15% FBS contained approximately 0.1 ng/ml (0.33 nM) RA measured by HPLC analysis (data not shown). Therefore, PGCs might be exposed to RA in the culture medium before Sertoli cells tightly interact with PGCs in the aggregates. Indeed, XY PGCs in the 11.5-dpc urogenital ridges often entered meiosis in the same culture medium without the RAR inhibitor (unpublished data), whereas these cells entered the male-specific pathway in the presence of the RAR inhibitor.
When isolated 12.5-dpc XY germ cells were cultured with RAR inhibitor, Dnmt3l expression levels increased in a dose-dependent manner. Although we still do not know the exact relationship between inhibition of RA signaling and male germ cell differentiation, our result suggests that male germ cells at 12.5 dpc maintain their sexual bipotency and respond to RA. We previously showed that isolated male germ cells at 13.5 dpc autonomously undergo male differentiation in vitro [17]. These male germ cells expressed male-specific genes and established male-specific imprints of the H19 DMDs after 6 days of culture without the gonadal environment. Interestingly, our recent study has revealed that male germ cells at 13.5 dpc maintain their sexual bipotency, because they entered meiosis in response to the exogenous RA [39]. Later, the response to RA was gradually lost, and the male germ cells at 15.5 dpc completely committed to the male-specific pathway regardless of the presence of RA in culture [39]. In contrast, undifferentiated XX and XY PGCs at 11.5 dpc readily entered meiosis in the general medium without the exogenous RA [30, 31, and our unpublished data], probably because sexually undifferentiated PGCs responded to the very small amounts of RA in the medium. It is possible that PGCs change their sensitivity to RA according to their differentiation. Sexually undifferentiated PGCs maintain high sensitivity to RA, but once they commit to male differentiation, they gradually lose their response to RA, until the cells enter the G0 cell cycle stage by 15.5 dpc [39]. As suggested by Suzuki and Saga [37], Nanos2 may be involved in this process. Nanos2 expression initiates in male germ cells by 13.5 dpc and appears to have a role in meiotic suppression by preventing Stra8 expression, especially after Cyp26b1 begins to decrease around 14.5 dpc. Thus, CYP26B1 inhibits RA signaling, which suppresses the entry of the male germ cells into meiosis and may provide the conditions required for expression of male-specific Nanos2 [37]. The ectopic expression of Nanos2 induced Dnmt3l expression [37], suggesting that Nanos2 is an upstream regulator of Dnmt3l. Because Dnmt3l regulates the establishment of sex-specific methylation imprints, it is possible that the molecule(s) generated by Sertoli cells triggers Nanos2 expression in the male germ cells, followed by Dnmt3l expression, to complete the male-specific imprints as prospermatogonia (Fig. 7).
FIG. 7.
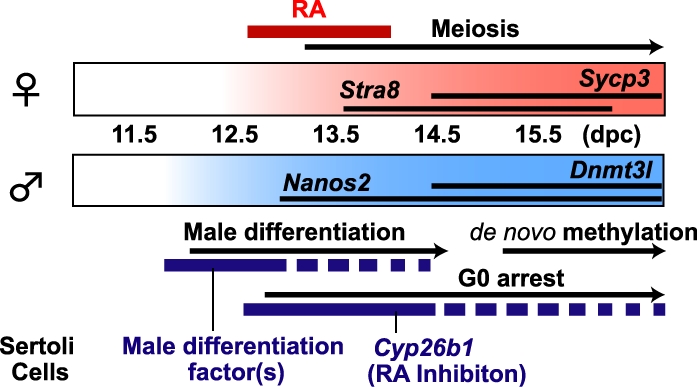
Male and female pathways of sex differentiation in mouse fetal germ cells. The male pathway is induced by the following two-step mechanism of Sertoli cells: (1) inhibition of the RA signaling in germ cells to prevent entry into meiosis by CYP26B1 enzymatic activity, and (2) induction of male-specific differentiation of germ cells by a molecule(s) produced at approximately 12.5 dpc.
In conclusion, this study demonstrates that Sertoli cells at approximately 12.5 dpc can induce PGC male differentiation. This suggests that Sertoli cells play two crucial roles in the male differentiation pathway of germ cells. One role is inhibition of RA signaling to prevent entry into meiosis by generating CYP26B1 enzyme. Another role is the active imposition of male-specific differentiation by generating the molecule(s) at approximately the time that sex differentiation occurs in germ cells. Coordination of these spatiotemporal factors is likely important for regulating germ cells to commit to male differentiation. Future study to identify the factor(s) for male germ cell differentiation should provide new insights into the molecular mechanisms of sex differentiation in germ cells.
ACKNOWLEDGMENT
We thank Dr. Jeff Mann for generously providing OG2 transgenic mice. We are grateful to Drs. Shoji Tajima and Yumiko Saga for kindly providing the DNMT3L and NANOS2 antibodies, respectively. We also thank Alexandra Gurary for technical advice and assistance with FACS sorting.
Footnotes
Supported by National Institutes of Health (NIH) grants P20RR024206 and G12RR003061 to Y.Y., NIH grant HD30284 to R.R.B., and a Japan Society for the Promotion of Science postdoctoral fellowship for research abroad to K.O. The fluorescence microscope at the Kakaako Imaging Core was supported by NIH grants G12RR003061 and P20RR016453.
REFERENCES
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 1990; 346: 240 244 [DOI] [PubMed] [Google Scholar]
- Koopman P, Münsterberg A, Capel B, Vivian N, Lovell-Badge R. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature 1990; 348: 450 452 [DOI] [PubMed] [Google Scholar]
- Sekido R, Bar I, Narváez V, Penny G, Lovell-Badge R. SOX9 is up-regulated by the transient expression of SRY specifically in Sertoli cell precursors. Dev Biol 2004; 274: 271 279 [DOI] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 2008; 453: 930 934 [DOI] [PubMed] [Google Scholar]
- Lovell-Badge R, Robertson E. XY female mice resulting from a heritable mutation in the primary testis-determining gene, Tdy. Development 1990; 109: 635 646 [DOI] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature 1991; 351: 117 121 [DOI] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, de Rooij DG, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet 2001; 28: 216 217 [DOI] [PubMed] [Google Scholar]
- Chaboissier MC, Kobayashi A, Vidal VI, Lützkendorf S, van de Kant HJ, Wegner M, de Rooij DG, Behringer RR, Schedl A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development 2004; 131: 1891 1901 [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol Rev 2007; 87: 1 28 [DOI] [PubMed] [Google Scholar]
- Brennan J, Capel B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet 2004; 5: 509 521 [DOI] [PubMed] [Google Scholar]
- McLaren A. Germ cells and germ cell sex. Philos Trans R Soc Lond B Biol Sci 1995; 350: 229 233 [DOI] [PubMed] [Google Scholar]
- Borum K. Oogenesis in the mouse: a study of the meiotic prophase. Exp Cell Res 1961; 24: 495 507 [DOI] [PubMed] [Google Scholar]
- McLaren A. Meiosis and differentiation of mouse germ cells : Evans CW, Dickinson HG. (eds.), Controlling Events in Meiosis: 38th Symposium of the Society For Experimental Biology. Cambridge: Company of Biologists; 1984; 7 23 [PubMed] [Google Scholar]
- Hilscher B, Hilscher W, Bülthoff-Ohnolz B, Krämer U, Birke A, Pelzer H, Gauss G. Kinetics of gametogenesis. Cell Tissue Res 1974; 154: 443 470 [DOI] [PubMed] [Google Scholar]
- McLaren A. Primordial germ cells in the mouse. Dev Biol 2003; 262: 1 15 [DOI] [PubMed] [Google Scholar]
- Allegrucci C, Thurston A, Lucas E, Young L. Epigenetics and the germline. Reproduction 2005; 129: 137 149 [DOI] [PubMed] [Google Scholar]
- Iwahashi K, Yoshioka H, Low EW, McCarrey JR, Yanagimachi R, Yamazaki Y. Autonomous regulation of sex-specific developmental programming in mouse fetal germ cells. Biol Reprod 2007; 77: 697 706 [DOI] [PubMed] [Google Scholar]
- Kocer A, Reichmann J, Best D, Adams IR. Germ cell sex determination in mammals. Mol Hum Reprod 2009; 15: 205 213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science 2006; 312: 596 600 [DOI] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A 2006; 103: 2474 2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean G, Li H, Metzger D, Chambon P, Petkovich M. Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology 2007; 148: 4560 4567 [DOI] [PubMed] [Google Scholar]
- Trautmann E, Guerquin MJ, Duquenne C, Lahaye JB, Habert R, Livera G. Retinoic acid prevents germ cell mitotic arrest in mouse fetal testes. Cell Cycle 2008; 7: 656 664 [DOI] [PubMed] [Google Scholar]
- Li H, MacLean G, Cameron D, Clagett-Dame M, Petkovich M. Cyp26b1 expression in murine Sertoli cells is required to maintain male germ cells in an undifferentiated state during embryogenesis. PLoS One 2009; 4: e7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best D, Sahlender DA, Walther N, Peden AA, Adams IR. Sdmg1 is a conserved transmembrane protein associated with germ cell sex determination and germline-soma interactions in mice. Development 2008; 135: 1415 1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios F, Filipponi D, Pellegrini M, Paronetto MP, Di Siena S, Geremia R, Rossi P, De Felici M, Jannini EA, Dolci S. Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J Cell Sci 2010; 123: 871 880 [DOI] [PubMed] [Google Scholar]
- Bowles J, Feng CW, Spiller C, Davidson TL, Jackson A, Koopman P. FGF9 suppresses meiosis and promotes male germ cell fate in mice. Dev Cell 2010; 19: 440 449 [DOI] [PubMed] [Google Scholar]
- Guerquin MJ, Duquenne C, Lahaye JB, Tourpin S, Habert R, Livera G. New testicular mechanisms involved in the prevention of fetal meiotic initiation in mice. Dev Biol 2010; 346: 320 330 [DOI] [PubMed] [Google Scholar]
- Szabó PE, Hübner K, Schöler H, Mann JR. Allele-specific expression of imprinted genes in mouse migratory primordial germ cells. Mech Dev 2002; 115: 157 160 [DOI] [PubMed] [Google Scholar]
- Nel-Themaat L, Vadakkan TJ, Wang Y, Dickinson ME, Akiyama H, Behringer RR. Morphometric analysis of testis cord formation in Sox9-EGFP mice. Dev Dyn 2009; 238: 1100 1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farini D, Scaldaferri ML, Iona S, La Sala G, De Felici M. Growth factors sustain primordial germ cell survival, proliferation and entering into meiosis in the absence of somatic cells. Dev Biol 2005; 285: 49 56 [DOI] [PubMed] [Google Scholar]
- Chuma S, Nakatsuji N. Autonomous transition into meiosis of mouse fetal germ cells in vitro and its inhibition by gp130-mediated signaling. Dev Biol 2001; 229: 468 479 [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Low EW, Marikawa Y, Iwahashi K, Bartolomei MS, McCarrey JR, Yanagimachi R. Adult mice cloned from migrating primordial germ cells. Proc Natl Acad Sci U S A 2005; 102: 11361 11366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet 2006; 38: 1430 1434 [DOI] [PubMed] [Google Scholar]
- Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci U S A 2008; 105: 14976 14980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourc'his D, Xu GL, Lin CS, Bollman B, Bestor TH. Dnmt3L and the establishment of maternal genomic imprints. Science 2001; 294: 2536 2539 [DOI] [PubMed] [Google Scholar]
- Kaneda M, Okano M, Hata K, Sado T, Tsujimoto N, Li E, Sasaki H. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature 2004; 429: 900 903 [DOI] [PubMed] [Google Scholar]
- Suzuki A, Saga Y. NANOS2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev 2008; 22: 430 435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western PS, Miles DC, van den Bergen JA, Burton M, Sinclair AH. Dynamic regulation of mitotic arrest in fetal male germ cells. Stem Cells 2008; 26: 339 347 [DOI] [PubMed] [Google Scholar]
- Ohta K, Lin Y, Hogg N, Yamamoto M, Yamazaki Y. Direct effects of retinoic acid on entry of fetal male germ cells into meiosis in mice. Biol Reprod 2010; 83: 1056 1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams IR, McLaren A. Sexually dimorphic development of mouse primordial germ cells: switching from oogenesis to spermatogenesis. Development 2002; 129: 1155 1164 [DOI] [PubMed] [Google Scholar]
- Livera G, Rouiller-Fabre V, Habert R. Retinoid receptors involved in the effects of retinoic acid on rat testis development. Biol Reprod 2001; 64: 1307 1314 [DOI] [PubMed] [Google Scholar]
- Livera G, Rouiller-Fabre V, Valla J, Habert R. Effects of retinoids on the meiosis in the fetal rat ovary in culture. Mol Cell Endocrinol 2000; 165: 225 231 [DOI] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Chambon P. Function of retinoic acid receptors during embryonic development. Nucl Recept Signal 2009; 7: e002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulogne B, Habert R, Levacher C. Regulation of the proliferation of cocultured gonocytes and Sertoli cells by retinoids, triiodothyronine, and intracellular signaling factors: differences between fetal and neonatal cells. Mol Reprod Dev 2003; 65: 194 203 [DOI] [PubMed] [Google Scholar]
- Buehr M, Gu S, McLaren A. Mesonephric contribution to testis differentiation in the fetal mouse. Development 1993; 117: 273 281 [DOI] [PubMed] [Google Scholar]
- Yao HH, Capel B. Disruption of testis cords by cyclopamine or forskolin reveals independent cellular pathways in testis organogenesis. Dev Biol 2002; 246: 356 365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahl J, Kim Y, Colvin JS, Ornitz DM, Capel B. Fgf9 induces proliferation and nuclear localization of FGFR2 in Sertoli precursors during male sex determination. Development 2004; 131: 3627 3636 [DOI] [PubMed] [Google Scholar]
- Colvin JS, Green RP, Schmahl J, Capel B, Ornitz DM. Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell 2001; 104: 875 889 [DOI] [PubMed] [Google Scholar]
- Kashimada K, Svingen T, Feng CW, Pelosi E, Bagheri-Fam S, Harley VR, Schlessinger D, Bowles J, Koopman P. Antagonistic regulation of Cyp26b1 by transcription factors SOX9/SF1 and FOXL2 during gonadal development in mice. FASEB J 2011; 25: 3561 3569 [DOI] [PMC free article] [PubMed] [Google Scholar]