ABSTRACT
Uterine leiomyomas (fibroids) are the most common benign tumors in women of reproductive age. These tumors are three to four times more prevalent in African American women, who also have a 10 times higher incidence of hypovitaminosis D than white women. Recent studies have demonstrated the antitumor effects of 1,25-dihydroxyvitamin D3 on several cancers, but its effects on uterine leiomyomas are still unknown. To determine the antitumor and therapeutic effects of 1,25-dihydroxyvitamin D3 on uterine leiomyomas, female Eker rats (14–16 mo old) harboring uterine leiomyomas were randomized into control and experimental groups and were given vehicle versus 1,25-dihydroxyvitamin D3 (0.5 μg/kg per day) subcutaneously for 3 wk, respectively. At the end of the experiment, the rats were euthanized, and the leiomyoma tumors were analyzed. Treatment with 1,25-dihydroxyvitamin D3 significantly reduced leiomyoma tumor size in Eker rats. It also reduced leiomyoma size by suppressing cell growth and proliferation-related genes (Pcna, cyclin D1 [Ccnd1], Myc, Cdk1, Cdk2, and Cdk4), antiapoptotic genes (Bcl2 and Bcl2l1 [Bcl-x]), and estrogen and progesterone receptors. Additionally, immunohistochemistry revealed decreased expression of PCNA and MKI67 (a marker of proliferation) and increased expression of caspase 3 in 1,25-dihydroxyvitamin D3-treated Eker rat leiomyomas. Toxicity analyses using serum samples showed similar levels of SGOT, SGPT, calcium, and total bilirubin in 1,25-dihydroxyvitamin D3-treated and vehicle-treated control Eker rats. These results support that 1,25-dihydroxyvitamin D3 is an antitumor agent that may be a potential safe, nonsurgical therapeutic option for the treatment of uterine leiomyomas.
Keywords: 1,25-dihydroxyvitamin D3; Eker rats; fibroids; leiomyoma; radioimmunoassay
The steroid 1,25-dihydroxyvitamin D3 is a potent antitumor agent that shrinks uterine leiomyoma tumors in the Eker rat model and may be a useful non-surgical treatment option for uterine leiomyomas.
INTRODUCTION
Uterine leiomyomas (fibroids) are the most common benign tumors in women of reproductive age [1–3]. Leiomyomas are associated with significant symptoms, such as anemia, excessive vaginal bleeding, pelvic pain, pressure-related bowel and bladder dysfunction, recurrent miscarriage, and preterm labor [4, 5]. Leiomyomas are also associated with infertility and recurrent abortion [4–6]. These tumors are the most commonly cited indication for hysterectomy in the United States [7]. Although fibroids negatively affect women's health, few conservative treatment options are available to women with symptomatic uterine fibroids [8, 9]. Surgery, in the form of myomectomy or hysterectomy, is currently the primary treatment for uterine fibroids [9, 10], with uterine artery embolization and focused ultrasound ablation as emerging, less-invasive options [11]. In addition to the associated morbidity and mortality of these procedures [12], most of these options will potentially preclude or diminish future fertility [12, 13]. At present, available options for leiomyoma treatment are far from satisfactory; therefore, the development of a safe, effective oral treatment method would appeal to many women with symptomatic uterine leiomyomas. The initiating factors that lead to the development of fibroids are not well understood. However, ample evidence supports that ovarian steroids, such as estrogen and progesterone, are important factors for leiomyoma growth [14, 15]. The pivotal role of estrogen in leiomyoma development and growth has been established through clinical observations and research studies [16, 17]. The effects of estrogen in leiomyoma cells are mediated mainly via estrogen receptor alpha (ESR1) [17, 18]. We and others have reported that leiomyomas express ESR1 at a higher level compared with adjacent normal myometrium [19–21]. Leiomyomas are three to four times more prevalent in African American women than white women [22]. Furthermore, hypovitaminosis D is approximately 10 times more prevalent in African Americans (40%–45%) than in whites (4%) [23] because of the high melanin content of their skin and various dietary factors [24].
1,25-Dihydroxyvitamin D3 is the biologically active form of vitamin D3 and is known as a strong growth inhibitor that induces apoptosis in human breast cancer cells [25]. 1,25-Dihydroxyvitamin D3 suppresses proliferation of both normal and malignant cells, and it induces differentiation and apoptosis [26]. 1,25-Dihydroxyvitamin D3 analogs have also been shown to potentiate antitumor activity in a murine squamous cell carcinoma model [27]. Studies have demonstrated that an adequate amount of vitamin D intake decreased the risk of developing some types of cancer, type 1 diabetes, and multiple sclerosis [24]. We recently demonstrated that 1,25-dihydroxyvitamin D3 inhibited the proliferation of human uterine leiomyoma cells in part by inhibiting catechol-O-methyltransferase, an estrogen-metabolizing enzyme that is overexpressed in human uterine leiomyomas [28]. Additionally, our recent results demonstrated that 1,25-dihydroxyvitamin D3 reduced TGFB3-induced fibrosis-related gene expressions in leiomyoma cells [29]. The effect of 1,25-dihydroxyvitamin D3 on expression of profibrotic factors has recently been shown in mesenchymal multipotent cells [30]. Bläuer et al. [31] confirmed the effect of 1,25-dihydroxyvitamin D3 on the inhibition of human myometrial and leiomyoma cell growth [31]. Potent vitamin D analogs have been shown to have antitumor activity in a mouse model of squamous cell carcinoma [27]. Another synthetic analog of vitamin D, EB1089, has also been shown to induce apoptosis in breast cancer cells in vitro and in vivo [32]. However, the in vivo effects of 1,25-dihydroxyvitamin D3 on uterine leiomyomas in animal models have yet to be investigated.
In the present study, we evaluated the efficacy and safety of 1,25-dihydroxyvitamin D3 for the potential treatment of leiomyoma using the immune-competent Eker rat model. The Eker rat has been widely characterized as an excellent in vivo animal model for uterine leiomyomas [33]. Eker rats carrying a germ line mutation in the tuberous sclerosis 2 (Tsc2) tumor suppressor gene develop leiomyomas in their uteri with a frequency of 65% between ages 12 and 16 mo [33]. These leiomyoma lesions share many similar anatomic, histologic, and biologic features with human leiomyomas. Therefore, Eker rats serve as an excellent preclinical model to screen for and evaluate the therapeutic potential of candidate compounds for nonsurgical treatment of uterine leiomyomas.
MATERIALS AND METHODS
Reagents and Antibodies
1,25-Dihydroxyvitamin D3 was purchased from Sigma Biochemicals (St. Louis, MO). Miniosmotic pumps were purchased from Alzet osmotic pumps. The following antibodies were purchased: anti-PCNA, anti-CDK1, anti-CDK2, anti-CDK4, anti-MYC, anti-cyclin D1, anti-BCL2, anti-BCL2L1, anti-BAD, anti-ESR1, anti-VDR, anti-PGR-A, and anti-PGR-B purchased from Santa Cruz Biotechnology; anti-caspase 3 from Calbiochem; anti-collagen type 1 from Fitzgerald; and anti-fibronectin and anti-β-actin from Sigma.
Experimental Animals and Animal Care
Female Eker rats (approximately 14–16 mo old) were obtained as a gift from Eker breeders from a local Eker rat colony that was started by Dr. Cheryl Walker at MD Anderson Cancer Center in Smithville, TX. The animals were cared for and handled in accordance with National Institutes of Health guidelines and were housed in facilities accredited by the Association for the Accreditation of Laboratory Animal Care. All animal experiments were approved by the Institutional Animal Care and Use Committee, Meharry Medical College, Nashville, TN. Throughout the experimentation period, food (normal) and water were available to animals ad libitum.
Eker Rat Leiomyoma Model and 1,25-Dihydroxyvitamin D3 Treatment
The presence of uterine leiomyoma tumors in the Eker rats was confirmed by direct inspection via a limited laparotomy. Rats harboring visible leiomyoma tumors were randomized into two groups, with six rats in each group: 1) the control group was administered ethylene glycol (vehicle), and 2) the treatment group was given 1,25-dihydroxyvitamin D3 delivered by micro-osmotic pumps (Alzet) implanted into the dorsal subcutaneous space at a rate of 0.5 μg/kg per day for 3 wk. Animals were observed daily for any posttreatment complications or adverse events. At the end of the 3-wk experiment, animals were euthanized, and the following tissues were collected: blood, uterus (myometrium and leiomyoma), ovaries, fallopian tubes, liver, kidney, lung, heart, and brain. Leiomyoma tumor size was measured in three diameters (length, width, and height) by an electronic slide caliper, and tumor volume was determined using the following ellipsoid formula: volume = (length × width × depth × 0.52) as previously described [34].
Safety and Toxicity Studies in Eker Rats
All experimental animals were examined for any visual signs of toxicity, including lethargy, reduction in body weight, difficulty in mobility, change in food and water intake, and unexplained death. Additionally, autopsies were performed at the end of the experiment to examine for gross evidence of toxicity. Tissue samples from various organs, including the uterus (myometrium and endometrium), ovaries, fallopian tubes, cervix, liver, spleen, heart, lung, kidneys, and brain, were examined macroscopically and microscopically by an animal pathologist who was blinded to the treatment group assignments. In particular, the liver and kidney tissues were histologically examined after hematoxylin and eosin (H&E) staining to verify any significant toxicity associated with the treatment. Liver function was evaluated by determining the serum levels of serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), and total bilirubin (TB) from vehicle-treated control and 1,25-dihydroxyvitamin D3-treated Eker rats using standard techniques as previously described [35]. Additionally, the level of serum calcium was evaluated as previously described [36].
Histology of Leiomyoma Tumors
Leiomyoma tumor tissues collected from vehicle-treated control and 1,25-dihydroxyvitamin D3-treated Eker rats were divided into two parts: the first was immediately snap frozen in liquid nitrogen and then stored at −80°C, and the second was fixed in 10% buffered formalin for 15–20 h, embedded with paraffin, and subjected to immunohistochemistry. Paraffin-embedded tissue sections (4 μm) were deparaffinized and rehydrated by being passed through xylene and graded ethanol solutions as previously described [37]. Cell proliferation was analyzed by staining with immunoreactive proliferating cell nuclear antigen (PCNA) and MKI67 (a cell proliferating marker) via polyclonal anti-PCNA and anti-MKI67 antibodies (Santa Cruz Biotechnology) at 1:100 dilutions. Apoptosis in leiomyoma tumors was evaluated using histochemical staining with anti-caspase 3 antibody and subsequent staining with peroxidase-conjugated secondary antibodies.
Western Blot Analysis
Sections of the frozen leiomyoma tumor tissues were homogenized and sonicated in SDS-PAGE lysis buffer. Preparation of protein lysates and Western blot analyses were performed as previously described [38]. Briefly, the crude lysates were centrifuged at 13 000 rpm for 20 min at 4°C. The clear protein lysates were transferred into new Eppendorf tubes, and protein concentrations were determined using Bio-Rad protein assay reagents according to the manufacturer's instructions. Equal amounts of each protein lysate (30–40 μg) were resolved by 10% SDS-PAGE and transferred onto polyvinylidene fluoride membrane. Western blot analyses were performed to assess the expressions of cell growth and proliferation markers using anti-PCNA (1:500), anti-CDK1 (1:1000), anti-CDK2 (1:1000), anti-CDK4 (1:1000), anti-MYC (1:500), and anti-cyclin D1 (1:500); antiapoptotic markers using anti-BCL2 (1:300) and anti-BCL2L1 (1:300), proapoptotic markers using anti-BAD (1:300); and apoptotic markers using caspase 3 antibodies. Steroid hormone receptors and vitamin D receptor (VDR) expressions were verified using anti-ESR1 (1:300), anti-PGR-A, anti-PGR-B (1:300), and anti-VDR (1:500) antibodies. The antigen-antibody complex was detected with an enhanced chemiluminescence detection system (Amersham). Specific protein bands were visualized after exposure to autoradiography films and development using an automatic x-ray developer.
Vitamin D3 Assay
At the end of the treatment phase, all experimental Eker rats were euthanized, and their blood was collected via cardiac puncture. Blood samples were allowed to clot at room temperature for approximately 15 min and then placed on ice. Blood samples were centrifuged at 2000 rpm at 4°C for 15 min. Following centrifugation, the serum was separated and stored at −20°C. The determination of serum concentration of 1,25-dihydroxyvitamin D3 (vitamin D3) was performed by R. L. Horst at Heartland Assays Inc. using a radioimmunoassay (RIA) as previously described [39].
Statistical Analysis
Data were expressed as mean ± SD unless otherwise stated. All statistical analyses were performed using paired t-tests. The paired t-test was used to assess the significance of differences in vehicle-treated control and 1,25-dihydroxyvitamin D3-treated data points. Values were considered to be statistically significant when P was less than 0.05 (P < 0.05).
RESULTS
Higher Levels of Serum 1,25-Dihydroxyvitamin D3 Were Detected in Treated Eker Rats
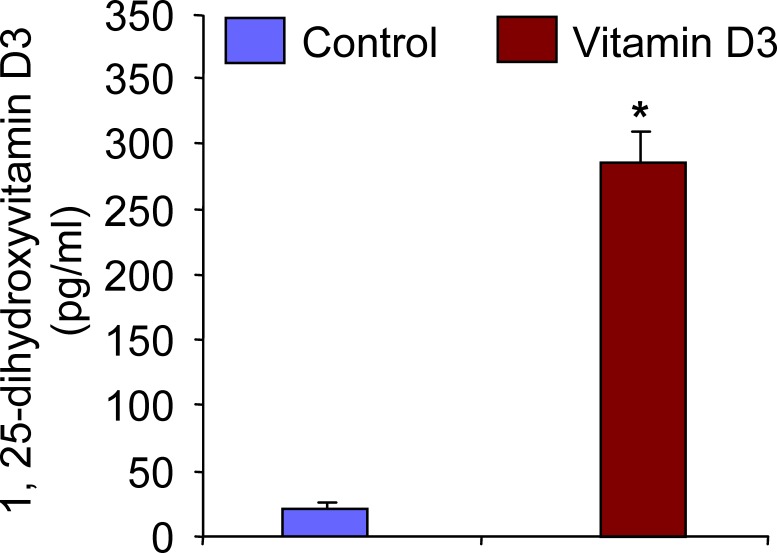
In an attempt to first confirm whether the micro-osmotic pumps delivered 1,25-dihydroxyvitamin D3 into Eker rats effectively, we measured serum levels of 1,25-dihydroxyvitamin D3 in both vehicle-treated control and 1,25-dihydroxyvitamin D3-treated Eker rats. Serum levels of 1,25-dihydroxyvitamin D3 were measured using the standard RIA technique as described in Materials and Methods. We observed that there were significantly higher levels of serum 1,25-dihydroxyvitamin D3 in treated Eker rats compared with vehicle-treated control rats (Fig. 1). Therefore, these data suggest that the exogenous 1,25-dihydroxyvitamin D3 was efficiently delivered from the micro-osmotic pumps, which subsequently led to shrinkage of uterine leiomyoma tumors in Eker rats.
FIG. 1. .
Serum levels of 1,25-dihydroxyvitamin D3 (vitamin D3) in treated Eker rats. Serum samples from vehicle-treated control and vitamin D3-treated Eker rats were used to determine the concentration of vitamin D3 using RIA. Elevated levels of serum vitamin D3 were found in treated Eker rats compared with vehicle-treated control rats (n = 6 each group). *P < 0.05 when compared with corresponding control.
1,25-Dihydroxyvitamin D3 at the Dose of 0.5 μg/kg per Day Is Safe and Well Tolerated in Eker Rats
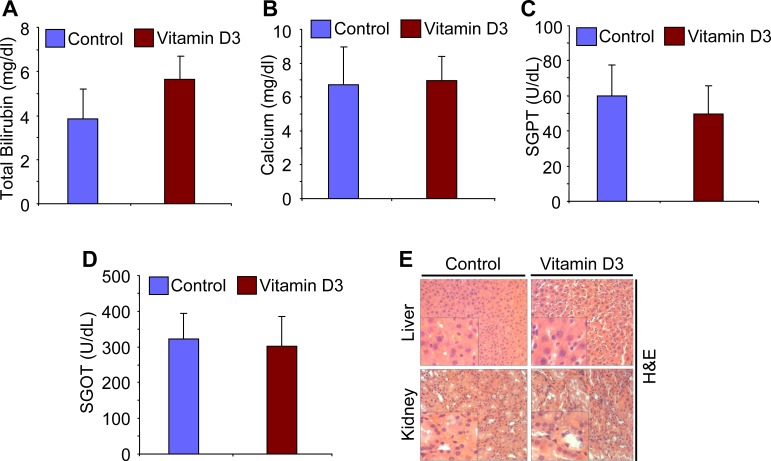
To evaluate the safety of 1,25-dihydroxyvitamin D3 in the treatment of uterine leiomyoma in female Eker rats, we performed additional histological and laboratory testing on 1,25-dihydroxyvitamin D3-treated and vehicle-treated control rats. Our daily monitoring indicated that all animals tolerated the 1,25-dihydroxyvitamin D3 dose and survived during the course of the experiment. There were no apparent signs of toxicity, such as lethargy, body weight reduction, difficulty in mobility, or change in food and water intake, and no unexplained deaths. Additionally, there was no evidence of any gross toxicity, necrosis, or changes in the morphology of the vital organs on macroscopic examination, which included the liver, kidneys, lung, spleen, brain, uterus, ovaries, and brain. Histopathological examination of these organs was normal, with no evidence of tissue damage (data not shown). The H&E staining of tissue sections, especially liver and kidney tissues, from vehicle-treated control and 1,25-dihydroxyvitamin D3-treated Eker rats was examined by an animal pathologist blinded from treatment assignments. These tissues revealed no signs of tissue damage or necrosis (Fig. 2E). To further evaluate liver function, we measured serum levels of SGOT, SGPT, and TB. 1,25-Dihydroxyvitamin D3 did not cause any significant change in the Eker rat liver function tests 21 days after treatment started compared with vehicle-treated control rats (Fig. 2). Serum levels of both SGOT and SGPT were not significantly different between vehicle-treated control and 1,25-dihydroxyvitamin D3-treated Eker rats (Fig. 2, C and D). A slight increase was observed in TB levels in 1,25-dihydroxyvitamin D3-treated Eker rats compared with vehicle-treated control rats; however, that slight increase was not statistically significant (P > 0.05; Fig. 2A). We further investigated whether 1,25-dihydroxyvitamin D3 treatment could affect serum calcium levels in Eker rats. On analysis, the serum levels of calcium were similar in both vehicle-treated control and 1,25-dihydroxyvitamin D3-treated Eker rats (Fig. 2B), suggesting that the 1,25-dihydroxyvitamin D3 dose (0.5 μg/kg per day) did not cause a significant increase in serum calcium. Therefore, these results demonstrate that 1,25-dihydroxyvitamin D3 at the dose of 0.5 μg/kg per day did not show observable signs of toxicity in Eker rats.
FIG. 2. .
Effect of 1,25-dihydroxyvitamin D3 (vitamin D3) treatment on liver function and serum calcium levels in Eker rats. To verify the possible toxicity associated with vitamin D3 treatment in Eker rats, the serum levels of total bilirubin (A), calcium (B), SGPT (C), and SGOT (D) in vehicle-treated control and vitamin D3-treated Eker rats were analyzed (n = 6 rats per group). Total serum bilirubin level was slightly elevated in vitamin D3-treated samples, but this elevation was not statistically significant (*P > 0.05). No significant changes in serum calcium level or SGPT and SGOT levels were found between vitamin D3-treated versus vehicle-treated control Eker rats. E) The H&E staining of vehicle-treated control and vitamin D3-treated liver and kidney tissues demonstrated no signs of toxicity associated with the treatment in the Eker rats. All images were captured at original magnification ×100. A high magnification of the region of the bottom left corner is shown in each image.
1,25-Dihydroxyvitamin D3 Treatment Reduced Uterine Leiomyoma Tumor Size in Eker Rats
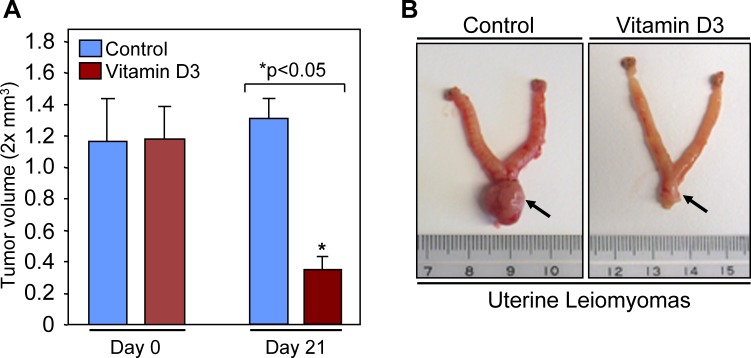
The Eker rat is a unique model of uterine leiomyoma that is characteristically similar to human fibroids and provides an excellent spontaneous, preclinical model for evaluation of potential treatments of uterine leiomyomas. To evaluate the potential for 1,25-dihydroxyvitamin D3 to shrink preexisting uterine leiomyoma tumors, female Eker rats harboring visible uterine leiomyomas were randomized into control and treatment groups and then treated with either vehicle or 1,25-dihydroxyvitamin D3 for 3 wk. As shown in Figure 3A, 1,25-dihydroxyvitamin D3 dramatically shrank (approximately 75% ± 3.85%; P < 0.05) uterine leiomyoma tumor size in Eker rats compared with the vehicle-treated control group. At 21 days, uterine leiomyoma tumors in vehicle-treated control Eker rats were slightly larger compared with tumor volumes at Day 0, which was not statistically significant and was consistent with the slow growth nature of uterine leiomyomas. Figure 3B is a representative picture of a uterus that showed a reduction of uterine leiomyoma tumor size following 1,25-dihydroxyvitamin D3 treatment. The uterine horns in the vehicle-treated control rats were distended and appear large because of inflammation and fluid entrapment secondary to the large fibroid lesion in the cervical region, a common feature of Eker rats [33]. After 1,25-dihydroxyvitamin D3 treatment, the major reduction in the fibroid's size appears to have allowed fluid to be released from the horns and thus resulted in the apparently smaller size of the uterine horns (data not shown). These results suggest that 1,25-dihydroxyvitamin D3 has the potential to reduce growth of uterine leiomyoma tumors in the preclinical Eker rat model.
FIG. 3. .
1,25-Dihydroxyvitamin D3 (vitamin D3) shrinks uterine leiomyoma tumors in Eker rats. A) Female Eker rats having leiomyoma tumors were randomized into control and treatment groups. The control group of rats was given ethylene glycol that served as a vehicle control. Vitamin D3 was given to the treatment group at the rate of 0.5 μg/kg per day for 3 wk. At the end of 3 wk, all rats (six rats per group) were euthanized, and leiomyoma tumors were measured as described in Materials and Methods. The reduction in uterine leiomyoma tumor sizes in vitamin D3-treated rats was statistically significant (*P < 0.05). B) Representative pictures of leiomyoma tumors in the uterus of vehicle-treated control (left) and vitamin D3-treated (right) Eker rats are shown. The arrow points to the leiomyoma lesions at the uterine-cervical junction area.
1,25-Dihydroxyvitamin D3 Suppresses Cell Proliferation and Apoptosis-Related Protein Expressions in Leiomyoma Tumors in Eker Rats
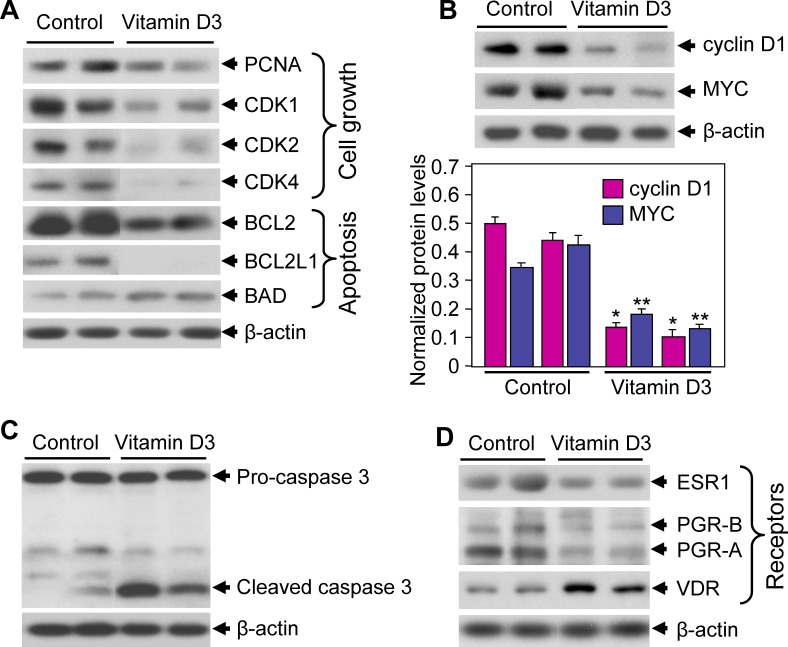
To determine the effects of 1,25-dihydroxyvitamin D3 on protein expression associated with leiomyoma growth, we performed Western blot analyses using uterine leiomyoma tumor lysates obtained from both vehicle-treated control and 1,25-dihydroxyvitamin D3-treated Eker rats. We observed that 1,25-dihydroxyvitamin D3 reduced the expression levels of cell proliferation marker PCNA compared with vehicle-treated control rats (Fig. 4A). 1,25-Dihydroxyvitamin D3 also significantly reduced the expression levels of cell cycle regulatory proteins, such as CDK1, CDK2, and CDK4, in leiomyoma tumors (Fig. 4A). Next, the effects of 1,25-dihydroxyvitamin D3 treatment on cellular apoptosis-related protein expression were verified using Western blot analyses using the previously mentioned leiomyoma tumor lysates. 1,25-Dihydroxyvitamin D3 treatment significantly reduced the antiapoptotic BCL2 and BCL2L1 protein expressions in leiomyoma tumors in Eker rats (Fig. 4A). The expression of the proapoptotic Bad protein was slightly increased in leiomyoma tumors in Eker rats treated with 1,25-dihydroxyvitamin D3 compared with vehicle-treated controls (Fig. 4A). The cell proliferation marker cyclin D1 and the proto-oncogene MYC have been reported to be overexpressed in uterine leiomyomas compared with normal myometrium [40, 41]. We evaluated the effects of 1,25-dihydroxyvitamin D3 on the expression of cyclin D1 and MYC proteins using Western blot analyses. 1,25-Dihydroxyvitamin D3 treatment significantly reduced both cyclin D1 and MYC protein expression in Eker rat leiomyoma tumors (Fig. 4B). Additionally, 1,25-dihydroxyvitamin D3 treatment induced the caspase 3-cleaved product in leiomyoma tumors in the treatment group compared with vehicle-treated control rats (Fig. 4C), suggesting the activation of the caspase signaling cascade. Together, these results demonstrate strong antiproliferative and proapoptotic functions of 1,25-dihydroxyvitamin D3 in the regulation of leiomyoma growth in Eker rats.
FIG. 4. .
Effect of 1,25-dihydroxyvitamin D3 (vitamin D3) on cell proliferation and apoptosis-related protein expressions in Eker rats. A) Lysates were prepared from leiomyoma tumors from vitamin D3-treated and vehicle-treated control Eker rats. Equal amounts of tumor lysates were analyzed using Western blot analyses with antibodies against PCNA, CDK1, CDK2, CDK4, BCL2, BCL2L1, and BAD. A Western blot analysis using anti-β-actin antibody served as the loading control. B) Above tumor lysates were analyzed using Western blot analyses with anti-cyclin D1 and anti-MYC antibodies. A Western blot analysis using anti-β-actin antibody served as the loading control (top). Protein band intensities were quantified by image analysis software and normalized with corresponding β-actin (bottom). *P < 0.05 and **P < 0.05 when compared with their corresponding controls. C) Leiomyoma tumor lysates were analyzed for activation of apoptosis signaling using anti-caspase 3 antibody. A Western blot analysis using anti-β-actin antibody served as the loading control. D) Leiomyoma tumor lysates were also analyzed for expression of steroid hormone receptors using Western blot analyses with anti-ESR1, anti-PGR-A, anti-PGR-B, and anti-VDR antibodies. A Western blot analysis using anti-β-actin antibody served as the loading control.
1,25-Dihydroxyvitamin D3 Treatment Reduced ESR1, PGR-A, and PGR-B and Induced VDR Expressions in Eker Rats
Both estrogen and progesterone are known to play major roles in leiomyoma growth and function through their nuclear receptors [15, 42, 43]. In an attempt to verify whether 1,25-dihydroxyvitamin D3 treatment affects these hormone functions via regulating their levels of receptor expression, we performed Western blot analyses using leiomyoma tumor lysates from vehicle-treated control and 1,25-dihydroxyvitamin D3-treated Eker rats. These analyses revealed that 1,25-dihydroxyvitamin D3 treatment reduced ESR1, PGR-A, and PGR-B protein expressions in Eker rat leiomyoma tumors (Fig. 4D). Prior reports have suggested that 1,25-dihydroxyvitamin D3 treatment induced its own nuclear VDR expression in other organ systems [30]. Using Western blot analyses, we further assessed whether 1,25-dihydroxyvitamin D3 treatment changed the expression levels of VDR in leiomyoma tumors. We observed that 1,25-dihydroxyvitamin D3 significantly induced VDR expression in leiomyoma tumors compared with vehicle-treated control rats (Fig. 4D). These results demonstrated that 1,25-dihydroxyvitamin D3 reduced leiomyoma tumor size by decreasing the protein expressions of ESR1, PGR-A, and PGR-B, and increasing the VDR expression in Eker rats.
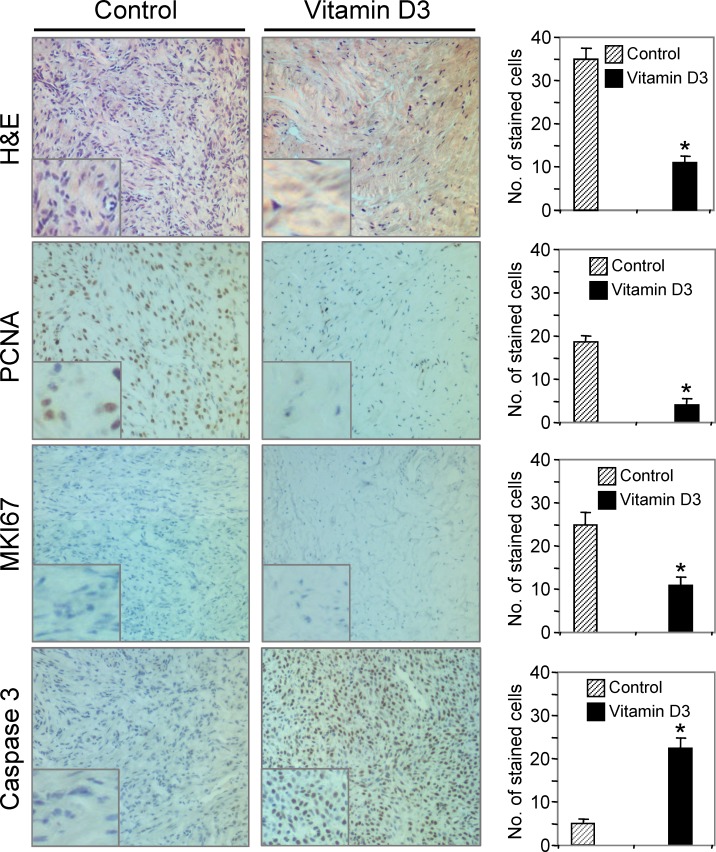
1,25-Dihydroxyvitamin D3 Reduced PCNA and MKI67 and Induced Caspase 3 Expression in Eker Rat Leiomyoma Tumors
To test the expression and localization of cell growth and proliferation markers, such as PCNA and MKI67, we performed immunohistochemical analyses on leiomyoma tumor samples from 1,25-dihydroxyvitamin D3-treated Eker rats and vehicle-treated controls. The H&E staining revealed a reduced number of tumor cells in 1,25-dihydroxyvitamin D3-treated Eker rats compared with vehicle-treated control rats (Fig. 5). Cell proliferation marker PCNA was expressed in the nucleus of cells in leiomyoma tumors of vehicle-treated control Eker rats. However, the expression of PCNA was significantly lower (P < 0.05) in leiomyoma tumors obtained from 1,25-dihydroxyvitamin D3-treated Eker rats compared with vehicle-treated control rats (Fig. 5). Staining for another cell proliferation marker, MKI67, was also significantly reduced (P < 0.05) in leiomyoma tumors obtained from 1,25-dihydroxyvitamin D3-treated Eker rats (Fig. 5). To further understand the effects of 1,25-dihydroxyvitamin D3 on apoptosis signaling, we performed immunohistochemistry with the anti-caspase 3 antibody. Significantly higher levels of caspase 3-immunoreactive cells were observed in leiomyoma tumors derived from 1,25-dihydroxyvitamin D3-treated Eker rats compared with vehicle-treated control rats (Fig. 5). Together, these results suggest that 1,25-dihydroxyvitamin D3 shrinks uterine leiomyoma tumor size in the Eker rat model by reducing cell growth and proliferation-associated PCNA and MKI67 protein expressions and by activating the intrinsic apoptosis pathway.
FIG. 5. .
Immunohistochemical analyses of 1,25-dihydroxyvitamin D3 (vitamin D3)-treated and vehicle-treated control leiomyoma tissues derived from Eker rats. The H&E staining demonstrated a reduced number of tumor cells in vitamin D3-treated leiomyoma tumor relative to vehicle-treated control. The cell proliferation marker MKI67 nuclear staining (blue) was much lower in leiomyoma tumor in Eker rats treated with vitamin D3. Additionally, the cell growth and proliferation marker PCNA staining (brown) was significantly reduced in leiomyoma tumor in vitamin D3-treated Eker rats. The apoptosis-related marker caspase 3 staining (brown) was significantly higher in leiomyoma tumor derived from vitamin D3-treated Eker rats. All images were captured at original magnification ×100. The number of stained cells was counted in six random fields in the representative vehicle-treated control and vitamin D3-treated Eker rats, and the average number of cells is presented (right). A high magnification of the region of the bottom left corner is shown in each image. Similar results were also observed in other Eker rats. The representative data showed the changes in staining cells in vitamin D3-treated Eker rats were statistically significant (*P < 0.05).
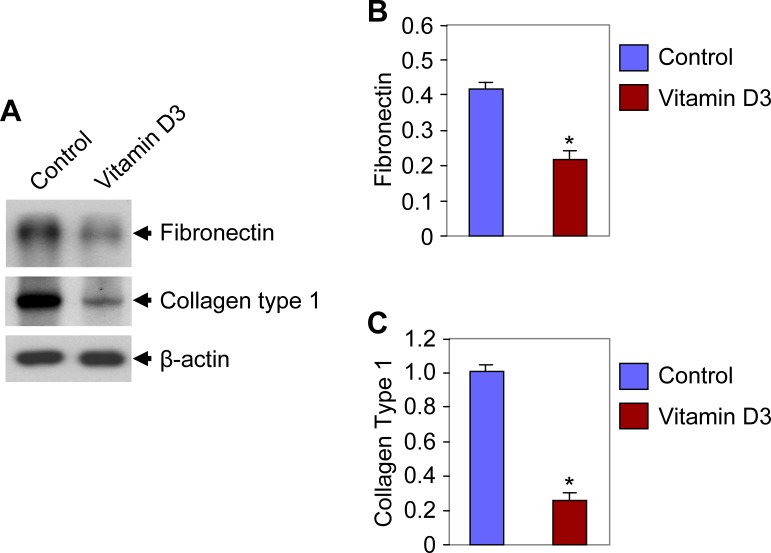
1,25-Dihydroxyvitamin D3 Reduced Extracellular Matrix-Associated Protein Expressions in Eker Rat Leiomyoma Tumors
Uterine leiomyomas are characterized by the presence of an abundance of extracellular matrix (ECM), which is mainly composed of collagen and fibronectin [8]. Previous reports have demonstrated that genes that encode ECM proteins are expressed abnormally in leiomyomas [44, 45]. These ECM-related genes, such as collagen type 1 and fibronectin, were found to be overexpressed in uterine leiomyomas [46, 47]. The effect of 1,25-dihydroxyvitamin D3 on ECM-related protein expression was investigated using Western blot analyses on tissue lysates from treated and vehicle-treated control rats. Decreased levels of fibronectin expression (2-fold) were observed in lysates of leiomyoma tumors obtained from the 1,25-dihydroxyvitamin D3-treated Eker rats compared with the vehicle-treated control rats (Fig. 6). Another ECM-related protein, collagen type 1, was also studied in the above tumor lysates. Similarly, we observed a significantly reduced expression of collagen type 1 (4-fold) in the lysates of leiomyoma tumors from Eker rats treated with 1,25-dihydroxyvitamin D3 (Fig. 6). These results suggest that 1,25-dihydroxyvitamin D3 has the potential to reduce leiomyoma tumor size in Eker rats by suppressing the ECM-associated fibronectin and collagen type 1 protein expressions.
FIG. 6. .
Effect of 1,25-dihydroxyvitamin D3 (vitamin D3) on fibronectin and collagen type 1 protein expression in Eker rat leiomyoma tumors. Protein lysates were prepared from Eker rat leiomyoma tumors and analyzed using Western blot analyses with anti-fibronectin and anti-collagen type 1 antibodies. A Western blot analysis using anti-β-actin antibody served as the loading control. Protein band intensities were quantified by image analysis software and normalized with corresponding β-actin. Similar results were also observed in other Eker rats. The representative data showed the reductions in fibronectin and collagen type 1 protein expression in vitamin D3-treated rats were statistically significant (*P < 0.05).
DISCUSSION
The management of uterine leiomyomas is challenging given their high incidence in premenopausal women and the lack of an effective and safe nonsurgical treatment. It is a complicated disease process that exerts an enormous burden on health care resources in this country and around the globe [48, 49]. Currently, hysterectomy remains the main option for treatment of uterine leiomyoma, with more than 600 000 procedures done each year in United States alone, making it one of the most commonly performed surgeries, second only to cesarean sections [50]. Nevertheless, this surgical approach is not always a favorable choice, especially in women who desire to preserve their future fertility and those who are not good surgical candidates. Therefore, the development of a safe, effective, and nonsurgical therapeutic strategy is a critical need in women's health care.
1,25-Dihydroxyvitamin D3 is a biologically active hormone [24]. We have recently presented preliminary data suggesting an association between lower serum levels of either 25-hydroxyvitamin D3 or 1,25-dihydroxyvitamin D3 and an increased risk of symptomatic uterine fibroids [51]. We and others have also recently demonstrated that 1,25-dihydroxyvitamin D3 induced leiomyoma growth inhibition [28, 31] and that it induced apoptosis in human leiomyoma cell lines in vitro [26]. Although these studies have demonstrated the effect of 1,25-dihydroxyvitamin D3 on leiomyoma growth in vitro, no in vivo study has been conducted to evaluate the effects of vitamin D3 on uterine leiomyomas. The direct association between uterine leiomyoma growth in vivo and vitamin D3 is not documented. Therefore, the present study was conducted to evaluate the therapeutic efficacy of 1,25-dihydroxyvitamin D3 on leiomyoma tumors using the Eker rat model.
The Eker rat is a unique model for the evaluation of the therapeutic efficacy of nonsurgical treatment of uterine leiomyoma [12]. The Eker rat contains Tsc2 gene mutations that spontaneously lead to the development of uterine leiomyoma lesions when they are 12–16 mo old [33]. Uterine leiomyomas in Eker rats share many phenotypic characteristics with their cognates in humans [33]. Therefore, to evaluate the therapeutic utility of 1,25-dihydroxyvitamin D3, we used this model, which is the only immunocompetent, preclinical animal model that spontaneously develops orthotopic uterine leiomyoma lesions [33]. The objective of this study was to evaluate the safety and efficiency of 1,25-dihydroxyvitamin D3 in preparation for a pilot clinical trial for women with symptomatic uterine leiomyomas in the future.
Vitamin D plays a key role in maintaining the skeleton by regulating calcium and phosphate homeostasis, and its deficiency leads to common bone diseases, such as rickets, in children and osteomalacia in adults. It also influences other components of the skeleton, notably the cells that control bone turnover. A recent study showed that 1,25-dihydroxyvitamin D3 has an effect on the proliferation and differentiation on bone-forming osteoblasts through the suppression of mammalian target of rapamycin (MTOR) signaling [52]. The MTOR is a member of the phosphoinositol kinase-related kinase family whose induction is regulated by phosphorylation by protein kinase B (AKT) in response to insulin, growth factor, and nutrient stimulation. The MTORC1 is induced by AKT, which phosphorylates and inhibits TSC1/2, leading to downstream effects on protein synthesis and cell proliferation. The MTOR pathway is a crucial master regulator of cell function, and a study has shown that 1,25-dihydroxyvitamin D3 suppressed MTOR function by inducing the expression of DNA-damage-inducible transcript 4 (DDIT4), a specific inhibitor of MTOR. Additionally, the upregulation of DDIT4 results in the activation of TSC1/2, leading to suppression of MTOR activity and decreased cell proliferation [52], suggesting a protective role for vitamin D3 in the regulation of MTOR signaling involved in cell proliferation. At present, there is limited information available to support that human leiomyomas that do not exhibit aberrance in the MTOR pathway respond to vitamin D3. However, we and others have shown that vitamin D3 can suppress proliferation of human normal myometrial cells as well as uterine leiomyoma cells [28, 31]. It is possible that uterine leiomyoma cells that do not have an aberrant MTOR pathway may still respond to vitamin D3.
A previous study demonstrated a dose-dependent inhibition of human liver cancer by 1,25-dihydroxyvitamin D3 in an in vivo nude mouse model [36]. In that study the authors employed three dosages of 1,25-dihydroxyvitamin D3 (0.02, 0.1, and 0.5 μg/kg per day) for 21 days in the treatment of human liver cancer. However, they observed that the inhibition of tumor growth was most evident in animals treated with the 0.5 μg/kg per day dosage of the drug. Additionally, this dosage did not induce any significant increase in serum calcium levels. Therefore, based on this published result we hypothesized that 1,25-dihydroxyvitamin D3 at the dosage of 0.5 μg/kg per day would be the better choice to effectively reduce uterine leiomyoma in Eker rats without the toxic effect of the drug. We first addressed the safety issue regarding the exogenous 1,25-dihydroxyvitamin D3 dose, which was given subcutaneously to the Eker rats. We measured the serum levels of 1,25-dihydroxyvitamin D3 in both treated and untreated Eker rats and observed significantly higher levels of serum 1,25-dihydroxyvitamin D3 in treated Eker rats compared with vehicle-treated control rats, as shown in Figure 1. To further evaluate any toxicity associated with 1,25-dihydroxyvitamin D3 treatment dosing, we screened liver enzymes, including SGOT and SGPT, as well as serum levels of TB, SGOT, and SGPT, to provide an index of hepatocyte integrity. Leakage of these liver enzymes into the extracellular compartment causes a subsequent rise in their serum levels and reflects hepatocyte damage. Studies have shown that these enzymes are significantly elevated in a number of conditions that cause liver injury [53]. Our results did not reveal any significant change in serum SGOT and SGPT levels in 1,25-dihydroxyvitamin D3-treated Eker rats, as shown in Figure 2. These results clearly demonstrate that the 1,25-dihydroxyvitamin D3 dose used for leiomyoma treatment in Eker rats was therapeutically safe and nontoxic. 1,25-Dihydroxyvitamin D3 is involved in calcium homeostasis, and it enhances active calcium absorption in the small intestine and stimulates calcium reabsorption in the kidney [54, 55], which is a key nutritional role for 1,25-dihydroxyvitamin D3 in supporting healthy bones. Higher levels of 1,25-dihydroxyvitamin D3 can cause hypercalcemia, a condition that is known to be associated with renal stones [56]. We measured the effect of 1,25-dihydroxyvitamin D3 on serum levels of calcium in both vehicle-treated control and 1,25-dihydroxyvitamin D3-treated Eker rats. Our results indicate that the serum levels of calcium were not affected by 1,25-dihydroxyvitamin D3 (Fig. 2B). Therefore, these results demonstrate that 1,25-dihydroxyvitamin D3 treatment was therapeutically effective, safe, and well tolerated for the treatment of uterine leiomyomas in Eker rats.
Vitamin D3 (cholecalciferol) is generally considered to be the primary form of dietary vitamin D. Vitamin D3 is produced naturally in human skin exposed to ultraviolet B light at 285- to 320-nm wavelengths. Vitamin D3 itself is biologically inactive, and it must be metabolized to its biologically active forms. After it is consumed in the diet or synthesized in the epidermis of skin, vitamin D3 enters into the circulation and is transported to the liver, where it is hydroxylated to form 25-hydroxyvitamin D3, the major circulating form of vitamin D3. Increased exposure to sunlight or increased dietary intake of vitamin D3 increases serum levels of 25-hydroxyvitamin D3, a useful indicator of vitamin D nutritional status. In the kidney, the 25-hydroxyvitamin D3 enzymatically catalyzed to the biologically active 1,25-dihydroxyvitamin D3. Most of the physiological effects of vitamin D3 in the body are related to the activity of 1,25-dihydroxyvitamin D3 [57]. Vitamin D is found naturally in fatty fish such as mackerel, salmon, and sardines, fish liver oils, and eggs from hens that have been fed vitamin D. Ordinary dietary sources usually provide approximately 2.5 μg/day vitamin D, but can reach as high as 5–10 μg with the use of fortified foods [58], which is still a small amount compared with the recommended tolerable upper intake level of 250 μg [58]. Accumulating epidemiologic and clinical intervention trial data suggest that increased vitamin D status may decrease the risk of cancer, particularly colorectal adenomas, decrease the prevalence of diabetes mellitus, help in maintaining physical strength in the elderly, and also be protective against falls [58]. However, the safety is always an important consideration when formulating recommendations for nutrient intake. Vitamin D is usually nontoxic in a physiologic dose, which is 30 ng/ml for 25-hydroxyvitamin D3, the level usually obtained by 2000 IU/day. Chronic or acute administration of higher doses of vitamin D3 leads to hypervitaminosis D and induces abnormally high serum calcium levels (hypercalcemia), which could result in bone loss, kidney stones, and calcification of organs like the heart and kidneys. Hypercalcemia has been reported following daily doses of greater than 50 000 IU of vitamin D3. Studies demonstrated that vitamin D toxicity is very unlikely in healthy people at intake levels lower than 10 000 IU/day [59, 60]. However, the Food and Nutrition Board of the Institute of Medicine conservatively set a tolerable upper intake level of 4000 IU/day (100 μg/day) for all adults [58]. In our study we have used a 0.5 μg/kg per day dosage of vitamin D3, which is equivalent to 1400 IU for an adult having a body weight of 70 kg. We have calculated the dose of vitamin D3 using the conversion equation in which 1 μg of vitamin D3 is equivalent to 40 IU [61, 62]. Recently, there are no available published data evaluating the effect of vitamin D3 on reduction of uterine leiomyoma in humans. However, based on our preclinical data in an Eker rat leiomyoma model (Fig. 3), we believe that the 1400 IU is an effective dose of vitamin D3 with no toxicity in the treatment of human diseases like uterine leiomyoma. A clinical study is warranted to evaluate the therapeutic efficacy of the dose of vitamin D3 in the treatment of human uterine leiomyoma.
Our study revealed that 1,25-dihydroxyvitamin D3 was able to significantly shrink uterine leiomyoma tumor volumes in the Eker rats (Fig. 3). The shrinkage of uterine leiomyoma lesions reached a maximum of 75% in the 1,25-dihydroxyvitamin D3-treated group after 21 days. Furthermore, we evaluated the expression of genes controlling cell growth, proliferation, and apoptosis. These data provide potential mechanisms of positive 1,25-dihydroxyvitamin D3-mediated therapeutic effects on uterine leiomyoma and explain the observed rapid and notable tumor shrinkage in the Eker rat model (Figs. 3 and 4). In particular, we observed that this treatment approach was safe and did not induce any macroscopic or microscopic tissue damage or significant changes in serum liver function tests or calcium levels (Fig. 2).
To further understand the potential mechanisms of tumor shrinkage by 1,25-dihydroxyvitamin D3, we evaluated the cellular signaling pathways that are associated with uterine leiomyoma pathogenesis, including cell growth and proliferation (PCNA, CDK1, CDK2, CDK4), apoptosis (BCL2, BCL2L1, BAD, caspase 3), and steroid hormone receptors, such as ESR1 and progesterone receptors (PGR-A and PGR-B). The evidence indicates that leiomyoma growth is regulated in part by the balance between genes controlling proliferation and apoptosis [63]. Decreased levels of PCNA, CDK1, CDK2, CDK4, cyclin D1, and MYC expressions were observed in the tumors of the 1,25-dihydroxyvitamin D3-treated rats compared with vehicle-treated control rats (Fig. 4); this suggests the inhibition of leiomyoma proliferation by 1,25-dihydroxyvitamin D3 treatment. PCNA is a known marker for cell proliferation [64], and its expression was higher in uterine leiomyoma compared with normal myometrium [63]. Our results (Figs. 4 and 5) showed markedly reduced PCNA expression by 1,25-dihydroxyvitamin D3 in Eker rats, which might reveal the cause for cell cycle arrest. The proto-oncogene MYC was found to be overexpressed in uterine leiomyoma tumors [41]. Our observations suggest that 1,25-dihydroxyvitamin D3 notably reduced MYC protein expression in leiomyoma tumors (Fig. 4B), suggesting an antitumor effect for 1,25-dihydroxyvitamin D3. Similarly, MKI67 is also a known cell proliferation marker. Our immunohistochemical analyses showed that there was reduced nuclear MKI67 staining in Eker rat leiomyoma tumors in the 1,25-dihydroxyvitamin D3 treatment group (Fig. 5), suggesting that 1,25-dihydroxyvitamin D3 has the potential to inhibit leiomyoma proliferation. To further verify whether 1,25-dihydroxyvitamin D3 induced apoptosis signaling in Eker rat leiomyoma, we performed Western blot analyses on several apoptosis factors. Our results showed that 1,25-dihydroxyvitamin D3 induced apoptosis by reducing the expression of antiapoptotic BCL2 and BCL2L1 and by increasing proapoptotic BAD expression in leiomyoma tumors, as shown in Figure 4A. Additionally, higher levels of caspase 3 cleaved product were detected in leiomyoma tumors in the 1,25-dihydroxyvitamin D3-treated Eker rats (Fig. 4C), indicating that 1,25-dihydroxyvitamin D3 treatment reduced leiomyoma growth by activating cellular apoptosis pathways in Eker rats. Together, our results demonstrate that 1,25-dihydroxyvitamin D3 treatment shrinks Eker rat uterine leiomyoma in part through the induction of apoptosis and the inhibition of proliferation in leiomyoma cells.
Prior studies have demonstrated that both estrogen and progesterone play pivotal roles in uterine leiomyoma growth [15, 42, 43, 65]. ESR expression was found to be higher in human uterine leiomyoma compared with adjacent normal myometrium [19–21]. The effects of estrogen are mediated via ESR1, which is a nuclear receptor belonging to a superfamily of ligand-regulated transcription factors [66]. Both estrogen and progesterone transmit signals through their nuclear receptors. We observed significantly reduced levels of both estrogen and progesterone receptors in 1,25-dihydroxyvitamin D3-treated Eker rat leiomyoma tumors, as shown in Figure 4D. Additionally, an induced level of VDR expression was also detected in the treated Eker rat leiomyoma tumors, suggesting the functional implication of 1,25-dihydroxyvitamin D3 treatment. We hypothesized that there is a balance between VDR levels and ESR1 and PGR-A/B in normal cells. In the absence of VDR protein expression or with reduced levels of VDR protein, ESR1 as well as PGR-A/B carry out their growth-promoting functions in uterine leiomyoma cells. Vitamin D3 induces VDR expression, which further combines with the retinoid X receptor (RXRA) to form a heterodimer complex, VDR-RXRA. This VDR-RXRA complex binds to specific vitamin D response elements (VDREs) in Esr1 gene or other VDR target gene promoters, which in turn negatively or positively regulates gene transcription. The steroid receptor coactivators (SRCs) family of proteins play important roles in cancer cell growth and are functionally associated with ESR1 in cancer cells [67]. These SRC family proteins were also upregulated in uterine leiomyomas [68]. The transcriptional activation of ESR1 requires NCOA1 (SRC1), NCOA2 (SRC2), and NCOA3 (SRC3) to interact with the VDR-RXRA complex. Vitamin D3 induces the VDR-RXRA complex in uterine leiomyoma cells. Additionally, vitamin D3 treatment can reduce the expression of the SRC family of transcription coactivators in human uterine leiomyoma cells (data not shown), thereby reducing the transcription of ESR1 as well as PGR-A/B or other vitamin D3 target genes, which can lead to a blockage of cell cycle progress and can induce apoptosis and possibly other anticancer mechanisms. Thus, our results clearly demonstrate that 1,25-dihydroxyvitamin D3 targets estrogen and progesterone functions by suppressing the expression levels of their receptors to shrink uterine leiomyoma tumors in Eker rats.
In healthy adults, bone mass declines with age in both men and women because of decreasing estrogen levels. The decline in circulating estrogen levels leads to an imbalance favoring bone resorption over new bone formation, and that leads to osteoporosis, a systemic disease that causes low bone mass and increases bone fragility and susceptibility to fracture. A published study demonstrated that the agonists of gonadotropin-releasing hormone (GnRH) are often given to the women who have estrogen-dependent diseases, such as endometriosis and uterine leiomyomas [69]. Because estrogen deficiency causes bone loss, a concern about premature osteoporosis has prevented long-term therapy with GnRH agonists. However, the administration of parathyroid hormone with GnRH agonists can prevent bone loss in women with estrogen deficiency caused by the treatment with GnRH agonists [69]. Bone loss is a common issue during the treatment of cancers with GnRH agonists, and higher doses of vitamin D3 can also cause bone loss. However, supplementation with calcium and vitamin D3 is recommended in order to prevent bone loss during treatment with GnRH agonists as cancer therapy [70]. Therefore, vitamin D3 could be a useful therapeutic for the treatment of uterine leiomyomas in humans, and the recommended dose of vitamin D3 for Eker rat leiomyoma treatment may not have a significant effect on bone loss during the treatment of human uterine leiomyomas. It is important that medical professionals are aware of the risks and complications associated with GnRH agonists or high-dose vitamin D3 therapy that may induce bone loss, and that they are monitored for bone-related complications to facilitate appropriate treatment.
Uterine leiomyomas are characterized by an excessive accumulation of ECM proteins and proteoglycans that leads to fibrosis [8]. Prior reports have demonstrated that the ECM-associated proteins collagen type 1 and fibronectin were found to be overexpressed and play important roles in the pathophysiology of uterine leiomyomas [46, 47]. We observed that 1,25-dihydroxyvitamin D3 treatment reduced protein expression of collagen type 1 and fibronectin in Eker rat leiomyoma tumors, as shown in Figure 6. These results suggest that 1,25-dihydroxyvitamin D3 potentially reduced ECM-associated collagen type 1 and fibronectin proteins, which might lead to the shrinkage of uterine leiomyoma volume in Eker rats.
In conclusion, 1,25-dihydroxyvitamin D3 treatment notably shrinks uterine leiomyoma tumors in the Eker rat model. This tumor shrinkage effect of 1,25-dihydroxyvitamin D3 is mediated in part through the modulation of several cell growth genes that control apoptosis and proliferation. 1,25-dihydroxyvitamin D3 also reduced estrogen and progesterone receptor levels as well as ECM-associated fibronectin and collagen type 1 protein expression, which may also contribute to uterine leiomyoma tumor growth in Eker rats. In summary, this study provides the first preclinical data on the use of 1,25-dihydroxyvitamin D3-based therapy as an alternative, safe, and nonsurgical treatment option for uterine leiomyoma and suggests the need for a future pilot clinical trial to verify the utility of this approach in women with symptomatic uterine fibroids.
ACKNOWLEDGMENT
We would like to thank Dr. Cheryl L. Walker, University of Texas MD Anderson Cancer Center, Smithville, TX, for kindly providing the Eker rat breeders. We would also like to thank Dr. Kevin G. Osteen, Department of Obstetrics and Gynecology, Vanderbilt University School of Medicine, Nashville, TN, for mentorship and intellectual advice to S.K.H.
Footnotes
Supported by the Vanderbilt Clinical and Translational Science Award grant UL1 RR024975 from the National Center for Research Resources/National Institutes of Health (NCRR/NIH) to S.K.H.; NIH/National Institute of Child Health and Human Development 1 R01 HD046228 to A.A.-H.; and Research Centers in Minority Institutions pilot 2 G12 RR003032-26, Wal-Mart award (study WPP2009-01), and Meharry Translational Research Center/Clinical Research Center pilot award RE: 202142-535001-20 to S.K.H.
REFERENCES
- Maruo T, Matsuo H, Samoto T, Shimomura Y, Kurachi O, Gao Z, Wang Y, Spitz IM, Johansson E. Effects of progesterone on uterine leiomyoma growth and apoptosis. Steroids 2000; 65: 585 592 [DOI] [PubMed] [Google Scholar]
- Walker CL, Burroughs KD, Davis B, Sowell K, Everitt JI, Fuchs-Young R. Preclinical evidence for therapeutic efficacy of selective estrogen receptor modulators for uterine leiomyoma. J Soc Gynecol Investig 2000; 7: 249 256 [PubMed] [Google Scholar]
- Stewart EA. Uterine fibroids. Lancet 2001; 357: 293 298 [DOI] [PubMed] [Google Scholar]
- Farhi J, Ashkenazi J, Feldberg D, Dicker D, Orvieto R, Ben Rafael Z. Effect of uterine leiomyomata on the results of in-vitro fertilization treatment. Hum Reprod 1995; 10: 2576 2578 [DOI] [PubMed] [Google Scholar]
- Surrey ES, Lietz AK, Schoolcraft WB. Impact of intramural leiomyomata in patients with a normal endometrial cavity on in vitro fertilization-embryo transfer cycle outcome. Fertil Steril 2001; 75: 405 410 [DOI] [PubMed] [Google Scholar]
- Eldar-Geva T, Meagher S, Healy DL, MacLachlan V, Breheny S, Wood C. Effect of intramural, subserosal, and submucosal uterine fibroids on the outcome of assisted reproductive technology treatment. Fertil Steril 1998; 70: 687 691 [DOI] [PubMed] [Google Scholar]
- Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988–1990. Obstet Gynecol 1994; 83: 549 555 [DOI] [PubMed] [Google Scholar]
- Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science 2005; 308: 1589 1592 [DOI] [PubMed] [Google Scholar]
- Bachmann G. Expanding treatment options for women with symptomatic uterine leiomyomas: timely medical breakthroughs. Fertil Steril 2006; 85: 46 47; discussion 48–50 [DOI] [PubMed] [Google Scholar]
- Lefebvre G, Allaire C, Jeffrey J, Vilos G, Arneja J, Birch C, Fortier M. SOGC clinical guidelines. Hysterectomy. J Obstet Gynaecol Can 2002; 24: 37 61; quiz 74–76 [PubMed] [Google Scholar]
- Stein K, Ascher-Walsh C. A comprehensive approach to the treatment of uterine leiomyomata. Mt Sinai J Med 2009; 76: 546 556 [DOI] [PubMed] [Google Scholar]
- Al-Hendy A, Salama S. Gene therapy and uterine leiomyoma: a review. Hum Reprod Update 2006; 12: 385 400 [DOI] [PubMed] [Google Scholar]
- Vilos GA, Daly LJ, Tse BM. Pregnancy outcome after laparoscopic electromyolysis. J Am Assoc Gynecol Laparosc 1998; 5: 289 292 [DOI] [PubMed] [Google Scholar]
- Wilson EA, Yang F, Rees ED. Estradiol and progesterone binding in uterine leiomyomata and in normal uterine tissues. Obstet Gynecol 1980; 55: 20 24 [PubMed] [Google Scholar]
- Rein MS, Barbieri RL, Friedman AJ. Progesterone: a critical role in the pathogenesis of uterine myomas. Am J Obstet Gynecol 1995; 172: 14 18 [DOI] [PubMed] [Google Scholar]
- Parazzini F, Negri E, La Vecchia C, Chatenoud L, Ricci E, Guarnerio P. Reproductive factors and risk of uterine fibroids. Epidemiology 1996; 7: 440 442 [DOI] [PubMed] [Google Scholar]
- Benassayag C, Leroy MJ, Rigourd V, Robert B, Honore JC, Mignot TM, Vacher-Lavenu MC, Chapron C, Ferre F. Estrogen receptors (ERalpha/ERbeta) in normal and pathological growth of the human myometrium: pregnancy and leiomyoma. Am J Physiol 1999; 276: E1112 E1118 [DOI] [PubMed] [Google Scholar]
- Wang Y, Matsuo H, Kurachi O, Maruo T. Down-regulation of proliferation and up-regulation of apoptosis by gonadotropin-releasing hormone agonist in cultured uterine leiomyoma cells. Eur J Endocrinol 2002; 146: 447 456 [DOI] [PubMed] [Google Scholar]
- Al-Hendy A, Salama SA. Ethnic distribution of estrogen receptor-alpha polymorphism is associated with a higher prevalence of uterine leiomyomas in black Americans. Fertil Steril 2006; 86: 686 693 [DOI] [PubMed] [Google Scholar]
- Brandon DD, Bethea CL, Strawn EY, Novy MJ, Burry KA, Harrington MS, Erickson TE, Warner C, Keenan EJ, Clinton GM. Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am J Obstet Gynecol 1993; 169: 78 85 [DOI] [PubMed] [Google Scholar]
- Brandon DD, Erickson TE, Keenan EJ, Strawn EY, Novy MJ, Burry KA, Warner C, Clinton GM. Estrogen receptor gene expression in human uterine leiomyomata. J Clin Endocrinol Metab 1995; 80: 1876 1881 [DOI] [PubMed] [Google Scholar]
- Baird DD, Dunson DB. Why is parity protective for uterine fibroids Epidemiology 2003; 14: 247 250 [DOI] [PubMed] [Google Scholar]
- Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 2002; 76: 187 192 [DOI] [PubMed] [Google Scholar]
- Holick MF. Too little vitamin D in premenopausal women: why should we care Am J Clin Nutr 2002; 76: 3 4 [DOI] [PubMed] [Google Scholar]
- Ylikomi T, Laaksi I, Lou YR, Martikainen P, Miettinen S, Pennanen P, Purmonen S, Syvälä H, Vienonen A, Tuohimaa P. Antiproliferative action of vitamin D. Vitam Horm 2002; 64: 357 406 [DOI] [PubMed] [Google Scholar]
- Mathiasen IS, Lademann U, Jaattela M. Apoptosis induced by vitamin D compounds in breast cancer cells is inhibited by Bcl-2 but does not involve known caspases or p53. Cancer Res 1999; 59: 4848 4856 [PubMed] [Google Scholar]
- Light BW, Yu WD, McElwain MC, Russell DM, Trump DL, Johnson CS. Potentiation of cisplatin antitumor activity using a vitamin D analogue in a murine squamous cell carcinoma model system. Cancer Res 1997; 57: 3759 3764 [PubMed] [Google Scholar]
- Sharan C, Halder SK, Thota C, Jaleel T, Nair S, Al-Hendy A. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil Steril 95: 247 253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder SK, Goodwin JS, Al-Hendy A. 1,25-Dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J Clin Endocrinol Metab 96: E754 E762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artaza JN, Norris KC. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J Endocrinol 2009; 200: 207 221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bläuer M, Rovio PH, Ylikomi T, Heinonen PK. Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertil Steril 2009; 91: 1919 1925 [DOI] [PubMed] [Google Scholar]
- James SY, Mercer E, Brady M, Binderup L, Colston KW. EB1089, a synthetic analogue of vitamin D, induces apoptosis in breast cancer cells in vivo and in vitro. Br J Pharmacol 1998; 125: 953 962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt JI, Wolf DC, Howe SR, Goldsworthy TL, Walker C. Rodent model of reproductive tract leiomyomata. Clinical and pathological features. Am J Pathol 1995; 146: 1556 1567 [PMC free article] [PubMed] [Google Scholar]
- Hassan MH, Salama SA, Zhang D, Arafa HM, Hamada FM, Fouad H, Walker CC, Al-Hendy A. Gene therapy targeting leiomyoma: adenovirus-mediated delivery of dominant-negative estrogen receptor gene shrinks uterine tumors in Eker rat model. Fertil Steril 2010; 93: 239 250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbrana E, Xiao SY, Popov VL, Newman PC, Tesh RB. Experimental yellow fever virus infection in the golden hamster (Mesocricetus auratus) III. Clinical laboratory values. Am J Trop Med Hyg 2006; 74: 1084 1089 [PubMed] [Google Scholar]
- Pourgholami MH, Akhter J, Lu Y, Morris DL. In vitro and in vivo inhibition of liver cancer cells by 1,25-dihydroxyvitamin D3. Cancer Lett 2000; 151: 97 102 [DOI] [PubMed] [Google Scholar]
- Al-Hendy A, Auersperg N. Applying the herpes simplex virus thymidine kinase/ganciclovir approach to ovarian cancer: an effective in vitro drug-sensitization system. Gynecol Obstet Invest 1997; 43: 268 275 [DOI] [PubMed] [Google Scholar]
- Halder SK, Beauchamp RD, Datta PK. Smad7 induces tumorigenicity by blocking TGF-beta-induced growth inhibition and apoptosis. Exp Cell Res 2005; 307: 231 246 [DOI] [PubMed] [Google Scholar]
- Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol 1997; 282: 174 186 [DOI] [PubMed] [Google Scholar]
- Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev 1993; 7: 812 821 [DOI] [PubMed] [Google Scholar]
- Jeffers MD, Richmond JA, Macaulay EM. Overexpression of the c-myc proto-oncogene occurs frequently in uterine sarcomas. Mod Pathol 1995; 8: 701 704 [PubMed] [Google Scholar]
- Chegini N, Verala J, Luo X, Xu J, Williams RS. Gene expression profile of leiomyoma and myometrium and the effect of gonadotropin releasing hormone analogue therapy. J Soc Gynecol Investig 2003; 10: 161 171 [DOI] [PubMed] [Google Scholar]
- Al-Hendy A, Lee EJ, Wang HQ, Copland JA. Gene therapy of uterine leiomyomas: adenovirus-mediated expression of dominant negative estrogen receptor inhibits tumor growth in nude mice. Am J Obstet Gynecol 2004; 191: 1621 1631 [DOI] [PubMed] [Google Scholar]
- Catherino WH, Leppert PC, Stenmark MH, Payson M, Potlog-Nahari C, Nieman LK, Segars JH. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosomes Cancer 2004; 40: 204 217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsibris JC, Segars J, Coppola D, Mane S, Wilbanks GD, O'Brien WF, Spellacy WN. Insights from gene arrays on the development and growth regulation of uterine leiomyomata. Fertil Steril 2002; 78: 114 121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab 1994; 79: 900 906 [DOI] [PubMed] [Google Scholar]
- Ding L, Xu J, Luo X, Chegini N. Gonadotropin releasing hormone and transforming growth factor beta activate mitogen-activated protein kinase/extracellularly regulated kinase and differentially regulate fibronectin, type I collagen, and plasminogen activator inhibitor-1 expression in leiomyoma and myometrial smooth muscle cells. J Clin Endocrinol Metab 2004; 89: 5549 5557 [DOI] [PubMed] [Google Scholar]
- Downes E, Sikirica V, Gilabert-Estelles J, Bolge SC, Dodd SL, Maroulis C, Subramanian D. The burden of uterine fibroids in five European countries. Eur J Obstet Gynecol Reprod Biol 2010; 152: 96 102 [DOI] [PubMed] [Google Scholar]
- Mauskopf J, Flynn M, Thieda P, Spalding J, Duchane J. The economic impact of uterine fibroids in the United States: a summary of published estimates. J Womens Health (Larchmt) 2005; 14: 692 703 [DOI] [PubMed] [Google Scholar]
- Martel KM, Ko AC, Christman GM, Stribley JM. Apoptosis in human uterine leiomyomas. Semin Reprod Med 2004; 22: 91 103 [DOI] [PubMed] [Google Scholar]
- Halder SK, Sharan C, Harirah H, Al-Hendy A. Lower serum levels of vitamin D3 is a risk factor for uterine fibroids in African Americans. Presented at the 57th Annual Scientific Meeting, Society of Gynecologic Investigation (SGI), March 24–27, 2010, Orlando, Florida: [Google Scholar]
- Lisse TS, Liu T, Irmler M, Beckers J, Chen H, Adams JS, Hewison M. Gene targeting by the vitamin D response element binding protein reveals a role for vitamin D in osteoblast mTOR signaling. FASEB J 2011; 25: 937 947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin Chem 2000; 46: 2027 2049 [PubMed] [Google Scholar]
- Brown AJ, Dusso A, Slatopolsky E. Vitamin D. Am J Physiol 1999; 277: F157 F175 [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Müller D, Van Der Kemp AW, Hartog A, Suzuki M, Ishibashi K, Imai M, Sweep F, Willems PH, Van Os CH, Bindels RJ. Calcitriol controls the epithelial calcium channel in kidney. J Am Soc Nephrol 2001; 12: 1342 1349 [DOI] [PubMed] [Google Scholar]
- Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int 2001; 59: 2290 2298 [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D: a millenium perspective. J Cell Biochem 2003; 88: 296 307 [DOI] [PubMed] [Google Scholar]
- Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr 2007; 85: 6 18 [DOI] [PubMed] [Google Scholar]
- Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 2003; 77: 204 210 [DOI] [PubMed] [Google Scholar]
- Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr 2001; 73: 288 294 [DOI] [PubMed] [Google Scholar]
- Dietary Reference Intake Tables, Health Canada, 2005. World Wide Web (URL: http://dietarysupplementdatabase.usda.nih.gov/ingredient_calculator/help.php#q9). (July 21, 2011)
- Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF. The role of vitamin D in cancer prevention. Am J Public Health 2006; 96: 252 261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D, Flake GP, Moore AB, He H, Haseman JK, Risinger JI, Lancaster JM, Berchuck A, Barrett JC, Robboy SJ. Cell proliferation and apoptosis in human uterine leiomyomas and myometria. Virchows Arch 2002; 441: 53 62 [DOI] [PubMed] [Google Scholar]
- Kurki P, Vanderlaan M, Dolbeare F, Gray J, Tan EM. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp Cell Res 1986; 166: 209 219 [DOI] [PubMed] [Google Scholar]
- Sozen I, Arici A. Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil Steril 2002; 78: 1 12 [DOI] [PubMed] [Google Scholar]
- Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev 2001; 81: 1535 1565 [DOI] [PubMed] [Google Scholar]
- Li HJ, Haque Z, Lu Q, Li L, Karas R, Mendelsohn M. Steroid receptor coactivator 3 is a coactivator for myocardin, the regulator of smooth muscle transcription and differentiation. Proc Natl Acad Sci U S A 2007; 104: 4065 4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein-Fikret S, Fuller PJ, Gargett CE. Expression of steroid receptor coactivators in cultured cells from paired myometrial and fibroid tissues. J Soc Gynecol Investig 2005; 12: 445 451 [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Klibanski A, Schaefer EH, Hornstein MD, Schiff I, Neer RM. Parathyroid hormone for the prevention of bone loss induced by estrogen deficiency. N Engl J Med 1994; 331: 1618 1623 [DOI] [PubMed] [Google Scholar]
- Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C, Jakesz R, Seifert M, Hubalek M, Bjelic-Radisic V, Samonigg H, Tausch C. et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med 2009; 360: 679 691 [DOI] [PubMed] [Google Scholar]