Abstract
The effect of moonlight and temperature on activity of slow lorises was previously little known and this knowledge might be useful for understanding many aspects of their behavioural ecology, and developing strategies to monitor and protect populations. In this study we aimed to determine if the activity of the pygmy loris (Nycticebus pygmaeus) is affected by ambient temperature and/or moonlight in a mixed deciduous forest. We radio-collared five females and five males in the Seima Protection Forest, Cambodia, in February to May, 2008 and January to March, 2009 and recorded their behaviour at 5 minutes intervals, totalling 2736 observations. We classified each observation as either inactive (sleeping or alert) or active behaviour (travel, feeding, grooming, or others). Moon luminosity (bright/dark) and ambient temperature were recorded for each observation. The response variable, activity, was binary (active or inactive), and a logit link function was used. Ambient temperature alone did not significantly affect mean activity. Although mean activity was significantly affected by moonlight, the interaction between moonlight and temperature was also significant: on bright nights, studied animals were increasingly more active with higher temperature; and on dark nights they were consistently active regardless of temperature. The most plausible explanation is that on bright cold nights the combined risk of being seen and attacked by predators and heat loss outweigh the benefit of active behaviours.
Introduction
The sensory world of the forest at night has strongly influenced the behaviour and physiology of nocturnal mammals. In the absence of visual sensory cues, many nocturnal mammals are solitary [1], rely heavily on smell [2], and use crypsis to avoid predators [3]. Consequently, the activity of many nocturnal mammalian prey species is affected by the intensity of nocturnal illumination. Some prey animals may reduce (lunar phobic) or increase (lunar philic) activity with increasing moon luminosity depending on their vulnerability to predators under bright moonlight [4], [5], [6], [7], [8]. Other prey animals may not change their activity in response to moon luminosity (lunar neutrality) [9]. Temperature also affects the activity of nocturnal mammals [10] and also their food supply, with the abundance and activity of invertebrates being lower with decreasing temperature. Periods of torpor and reduced activity are most commonly energy saving adaptations in response to extremely hot or cold climatic fluctuations [11].
Amongst primates, some species are nocturnal specialists, and others are cathemeral, meaning their activities are distributed throughout the 24-hour cycle for at least some parts of the year [12]. Together these comprise some 35% of primate species, found in every part of their range from the Neotropics (night monkeys), Africa (galagos, pottos), Madagascar (lemurs), and Asia (tarsiers, lorises) [13]. All of these taxa are equipped with a suite of morphological traits suited to this mode of life including the presence of a tapetum lucidum (a reflecting retinal layer enhancing vision at night) or extra-enlarged eyes to allow in light; a low basal metabolic rate to thermal-regulate on cold nights; and enhanced olfactory and vocal signals to communicate in the dark [14].
Some primate species, through enhanced vision and ability to mob predators, to produce alarm calls and/or move off with speed, are better equipped to detect and avoid predators near and during full moons, and hence, do not need to reduce activity on bright nights [9], [15], [16]. Bright nights may enhance foraging efficiency in some primates, allowing them to see and catch more insect prey and ripe fruits [8]. Recent studies have reported different combinations of species-specific lunar responses in nocturnal primates [9], [17], [18], [19], [20], [21]. The spectral tarsier (Tarsius spectrum) and numerous species of galago (Galago senegalensis, G. moholi, Galagoides demidovii, G. zanzibaricus, Sciurocheirus alleni, and Euoticus elegantulus) increase foraging and/or travelling during bright moon nights [8], [22], [23], [24].
Regulation of body temperature in nocturnal primates has been shown to be of particular importance in one family of Malagasy lemurs (Cherogaleidae) [25], [26], [27], [28], [29], [30]. Torpor and hibernation have been studied extensively in these species, showing some of the most remarkable specialisations of any nocturnal mammal, such as extreme lowering of the body temperature, and heavy storage of fat in the tail [29], [30]. Torpor has also been observed in a captive sub-adult Galago moholi [31], although it was not observed in two long-term field studies of this species [15], [21], [32]. Indeed, the opposite was the case, when galagos became active in the daytime to feed on valuable gum resources [15].
The Asian lorises (subfamily Lorisinae) have classically been described to avoid predators by crypsis [33]. Ranging in body size from 120 to 2100 g, loris species are characterized by non-saltatory locomotion [34], moving slowly and deliberately through vegetation [35]. They have reduced second digits, and their limbs have retia mirabilia, allowing them to maintain grip for long periods of time [34], [36], so that they can remain utterly still in the presence of a threat. All lorises have monochromatic vision, possessing only a single functional medium/long wavelength-sensitive cone [37], [38], which affects the way they perceive predators and food in open versus dense forests.
This was clearly demonstrated by two species of South Asian slender lorises (Loris). L. lydekkerianus inhabits open dry acacia scrub forests. On bright nights it increased foraging activity for energy-rich foods, but do not alter their distance travelled [15], [24]. It whistled more frequently during dark nights than bright nights. In contrary, the rain-forest dwelling L. tardigradus whistled more frequently on bright nights than dark nights [39]. On dark nights it slept, travelled less and groomed more, reducing active behaviours [39].
The lunar response of slow lorises in South and Southeast Asia has not been previously investigated prior to the present study. Metabolic rates are extremely low in slow lorises [40], [41], [42], [43], with the greater slow loris (N. coucang) having a basal metabolic rate 60% lower than predicted [44]. Captive pygmy slow lorises (N. pygmaeus) in Northern Vietnam exhibited extensive periods of inactivity and reduced body temperatures during cooler months [45], [46]. This species was also encountered at lower rates during colder months in the dry season in Laos, suggesting its activity was reduced with lower temperatures [47].
We, therefore, chose to study the lunar response of the pygmy slow (hereafter pygmy) loris Nycticebus pygmaeus. Endemic to Vietnam, Laos, southern China and eastern Cambodia [48], [49], [50], [51], it is listed as Vulnerable in the IUCN Red List [52], and in Appendix 1 of CITES [53] based on increasing and unsustainable trade [53], habitat loss and degradation [52].
Optimal foraging theory in its most basic form suggests an animal will forage in ways that will maximise its energy intake [54], with a trade off between the risk of being seen by predators on bright nights, and the benefit of increasing food intake. The study site is located in a seasonal deciduous forest rich in species diversity of civets, pythons, monitor lizards and predatory birds, which are known predators of other small nocturnal primates including loris species [55], [56]. The site experiences low temperatures during the dry season from December to February, with a marked reduction of foliage, increasing the visibility for predators. Foraging at low temperatures would increase heat loss, and foraging during bright nights would increase the risk of predation. These conditions allow us to test how moonlight and temperature interact to affect nocturnal animal behavior.
We predict that the pygmy loris will:
be more active during dark nights when predation risk decreases;
reduce active behaviour such as travel and foraging, and increase inactive behaviours such as resting and grooming during cold periods.
Results
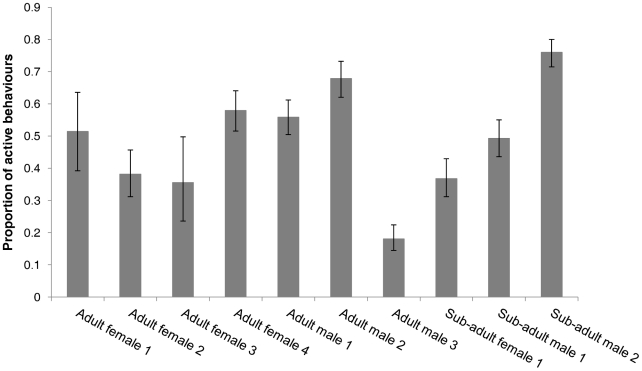
Mean activity differed significantly between the 10 studied individuals (χ2 = 234.86, d.f. = 9, p = <0.001; Figure 1). Time of the night significantly affected mean activity (χ2 = 73.72, d.f. = 4, p = <0.001; Figure 2). These individual and temporal differences were accounted for in the following test results.
Figure 1. Proportion of active behaviours across individuals in the study.
The standard error of the logit values were used to construct 95% confidence intervals indicated by the error bar.
Figure 2. Proportion of active behaviours across the night excluding periods of astronomical twilight.
The standard error of the logit values were used to construct 95% confidence intervals indicated by the error bar. Means are adjusted for other fixed effects.
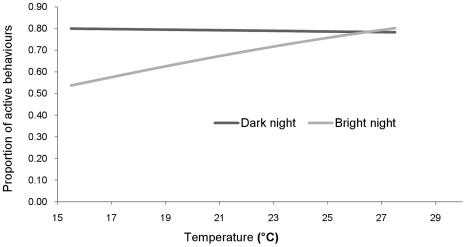
Temperature on its own did not significantly affect mean activity (χ2 = 0.06, d.f. = 1, p = 0.809). Mean activity was significantly affected by moonlight (χ2 = 5.14, d.f. = 1, p = 0.023), and the interaction between moonlight and temperature was significant (χ2 = 4.05, d.f. = 1, p = 0.044), with temperature affecting mean activity during bright nights, but not dark nights (Figure 3).
Figure 3. Interaction between moonlight and temperature on activity of the pygmy loris as predicted by the model.
Mean activity was constant across the temperature range from 16 to 28°C on dark nights, and did not reach the proportion on bright nights until the temperature reached above 26°C.
Discussion
The observed effect of moonlight on activity indicates that during the dry, cool season the pygmy loris is lunar phobic. A plausible explanation for this is that during this season the deciduous forest is sparse, providing reduced cover for animals from potential predators, resulting in a less effective anti-predator strategy. This was exacerbated by burning of the forest by local people to improve access for collecting non-timber forest products [57]. The pygmy loris uses more crypsis and concealment than faster nocturnal primates such as the slender lorises, galagos, mouse lemurs and tarsiers [9], [17], [19], [20], [21], [24]. The most plausible explanation for the pygmy loris reducing its activity with lower temperature on bright nights is that the combined risk of both predation and heat loss on bright, cold nights outweigh the benefit of active behaviours.
Avoiding predators at the study site requires multiple adaptations, as potential predators occur on the ground (small cats), in the trees (snakes), in the air (hawk-eagles), and those animals that move between these areas (monitor lizards, civets) [58]. Kenyon [pers. comm.] reports that monitor lizards and birds of prey caused high levels of mortality to reintroduced pygmy lorises in Vietnam. Decreasing activity during the light moon would confound at least several classes of these predators.
The lunar phobic behaviour observed is possibly seasonal and the pygmy loris may be more active on bright nights during the wet season when temperatures are higher and the forest provides denser vegetation cover. Other mammals are known to display seasonal variation in their lunar response [5]. Unfavourable conditions due to the lack of rain for tropical plants and invertebrates during the dry season may lower food supply and the pygmy loris may use inactivity to conserve energy in response. The heavy reliance of the species on gum during the study [59] parallels that of Galago moholi which relied on this resource during the cold winter months, when it also decreased activity [60].
It has been suggested that highly insectivorous nocturnal primates will be more lunar philic because moonlight improves their hunting success [8], [9], [24]. Although animals in our site were frequently observed to catch and consume arthropods, and a high proportion of invertebrates were found in scats throughout the study period (CS, unpub. data), our data did not indicate that this resource was important enough to select for lunar philia in the local population at the site.
A behaviour exhibited rarely by the study animals was emitting their loud long-distance whistle. Although slender lorises produced this whistle varyingly in dry versus wet environments, they still whistled multiple times per hour regardless of moonlight [24], [39]. Pygmy lorises kept in outdoor enclosures also whistle regularly [46]. Towards the end of our study, the study animals were heard to begin to whistle on occasion, and it might have been that as the forest became denser with foliage they could afford to decrease crypsis.
Ambient temperatures are known to affect the activity in a range of cathemeral [61], [62], [63], [64], [65], [66] and nocturnal [29], [67] primates. Many of these primates undergo periods of torpor or heterothermy to conserve energy to cope with low ambient temperatures [25], [26], [27], [28], [29], [30], [31]. Fernández-Duque et al. [68] reported that the activity of a lunar philic primate was reduced during cooler temperatures, despite luminance. Some species are known to adapt their behaviours as a coping mechanism. For example, at lower temperatures, G. moholi displayed diurnal activity such as moving into the sunlight, or feeding during the day [60], [69], [70]. Our study animals became inactive on cool nights only when the moon was up, however it is plausible the species may undergo periods of torpor or heterothermy in cooler parts of their range such as Northern Vietnam, where nightly temperatures are known to reach 5°C [46], [71]. Female pygmy lorises experience late pregnancy, birth and begin lactation during the cool season [72], which would further increase their energy demands, and may result in further inactivity during times of food shortage and low ambient temperatures.
Field surveys have indicated that densities of pygmy lorises in Cambodia are low [73], and an effective monitoring method is required. Our data suggest that monitoring of this species should occur on dark nights, or warm bright nights during the dry season to maximize the chance of encountering animals. Enforcement initiatives may also focus on patrolling the forest during these nights when pygmy lorises are more active, and, therefore, more likely to be encountered by poachers.
Methods
Ethics statement
This research involved work with wild non-human primates. Animal ethics approval for this project (Approval number: SAS/696/07/PhD) was approved by the University of Queensland Animal Ethics Committee. All experiments comply with the current laws of the country where they were performed.
Animals were hand captured, fitted with a radio-collar and immediately released at the point of capture in the field to minimise stress in accordance with the recommendations of the Weatherall report, “The use of non-human primates in research”. Direct observations of study animals were used to collect behavioural data, and we attempted to retrieve all radio-collars on completion of the project to minimise discomfort of the study animals. We were unable to recapture 2 individuals despite numerous attempts; however the collars were made of thin leather bands which would eventually break free from the animals.
Study site
The study was conducted in the Seima Protection Forest (PF), in southern Mondulkiri province, Cambodia (Figure 4). This conservation area encompasses 292,690 hectares. The study was conducted in two periods: from 12 February to 31 May 2008; and from 9 January to 24 March 2009.
Figure 4. Location of Seima Protection Forest, Cambodia.
The study site is indicated on the map.
In Mondulkiri dry season extends from November to April and the rainy season from May to October and the mean annual rainfall is approximately 2000–2500 mm [74]. Rainfall levels in the southern, more mountainous part of the province are considerably higher, with an annual mean of over 3,200 mm. The conservation area lies between 100–700 m asl on the western slopes of the Sen Monorom plateau, and in the south is part of the Annamite Range [57].
The Seima PF consists of a mosaic of forest types, including semi-evergreen, mixed deciduous, deciduous dipterocarp, and evergreen forests [75]. The mixed deciduous forest is dominated by Lagerstroemia sp., a deciduous tree species. This study occurred in mixed deciduous forest, which had patches of bamboo throughout. We selected this forest type for our study for two reasons. First, spotlight surveys identified higher encounter rates in this habitat type [73], and second, it was easier to catch, collar and observe animals in mixed deciduous forests as they were less dense, making it easier to pursue animals quietly. We were unable to collect data during the wet season due to heavy rains and inaccessibility to the site during these months.
Study animals
Animals were located using Petzl Zoom 4.5v headlamps (Petzl, Crolles, France) with a red filter and hand captured. Each captured animal was fitted with a two-stage transmitter (Sirtrack®, Havelock North, New Zealand) and released at the point of capture. Ten individuals were tracked on foot, using a 6-element Yagi antenna (Sirtrack®, Havelock North, New Zealand), and a portable radio receiver (ICOM IC-R10 receiver; Icom Inc. ®, Osaka, Japan). Data were collected on nightly follows of collared animals between dusk and dawn, over two shifts: 18:00–00:00 and 00:00–06:00. Each individual had at least one all-night follow from 18:00–06:00. We captured and collared four adult females, three adult males, one sub-adult female, and two sub-adult males. Sub-adults were generally smaller, and were recognizable as observed by Wiens and Zitzmann [76] as having teeth that were white and unworn, little or no wear on the inner surfaces of nails, longer, and paler fur when compared to adult animals, and females were nulliparous.
Behavioural observation
Focal animal sampling and instantaneous sampling methods were used to collect behavioural data [77], [78], and scan samples were taken at 5-min intervals, recording data at the end of each observation [78], [79]. We adapted behavioural categories from Gursky [8] and Nekaris [80]. Our categories were alert (eyes were open, the animal was not moving), sleeping (eyes were closed), travel, feed (we pooled data on hunting and foraging in to this category), activity unknown, auto-grooming, and others (less common behaviours such as scent marking and allo-grooming were pooled in to this category). Very few observations were made in the category ‘others’. A total of 2736 (1233 active, 1503 inactive) observation points were collected from 10 individuals. The number of observations made on bright and dark nights are listed for individual study animals in Table 1.
Table 1. Number of observations collected on bright and dark nights for individuals.
| Individual ID | Bright nights | Dark nights |
| Adult female 1 | 11 | 54 |
| Adult female 2 | 110 | 76 |
| Adult female 3 | 174 | 78 |
| Adult female 4 | 164 | 104 |
| Adult male 1 | 105 | 245 |
| Adult male 2 | 166 | 153 |
| Adult male 3 | 411 | 113 |
| Sub-adult female 1 | 152 | 123 |
| Sub-adult male 1 | 50 | 60 |
| Sub-adult male 2 | 165 | 222 |
Analysis
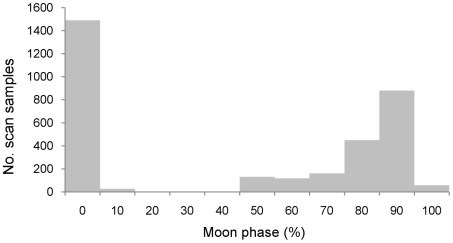
The response variable, activity, was binary, and a logit link function was used. The model allowed baseline activity to vary between individuals. Inactive behaviour was either sleeping or being alert. All other categories of behaviours were defined as active behaviour. The model consisted of individual ID, time of night, temperature, moonlight and the interaction between temperature and moonlight. Time of the night was classified (taking into account astronomical twilight) by five time bands: <21:00; 21:00–22:59; 23:00–00:59; 01:00–02:59; and >03:00. Observations collected during the twilight periods were excluded from the analysis to avoid the possible effect of sunlight. Nearly all observations were collected when the moon was either below the horizon and had a phase value of 0% (dark nights), or a phase value from 50% to 100% (bright nights). Preliminary analyses of the data indicated that classifying moonlight by dark/bright nights was more parsimonious than using an illumination index given nearly all observations were collected on either dark or bright nights (Figure 5). Illumination indices were used in some previous studies of primate activity [81], [82]. This index was used in earlier models during preliminary analysis but was not used in the final model to improve the parsimony of the model. Given the small number of individuals observed, we did not investigate the effect of sex or age class on activity. The analyses were performed using the GENMOD Procedure in SAS® version 8.2.
Figure 5. Frequency distribution of observations across moon phases.
Moon phase is expressed as the illuminated percentage of the portion of moon. When the moon was not above the horizon the moon phase was given a value of 0.
Temperature
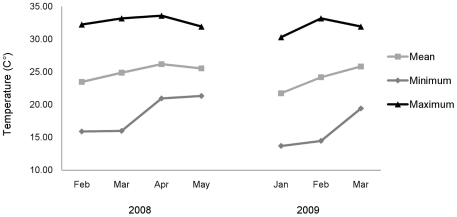
Ambient temperature was collected by a HOBO Pro series data logger (Onset®, Massachusetts, US) at the Seima PF base camp. The logger was set to record at 15 minutes intervals. Linear interpolation was used to calculate temperature for 5 minute intervals. Temperature fluctuated between dusk and dawn across the study period (Figure 6).
Figure 6. Mean, minimum and maximum nightly temperatures in the Seima Protection Forest during the study period.
Moon
Sunrise, sunset, moonrise, moonset, and moon phase were sourced from Geoscience Australia (http://www.ga.gov.au/geodesy/astro/) for the time at which each observation was collected.
Acknowledgments
We are grateful to Mr Men Soriyun and staff from Cambodian Forestry Administration's Department of Wildlife and Biodiversity Forestry Administration, the Ministry of Environment for permission to work in the site. Staff from the Wildlife Conservation Society, World Wide Fund for Nature, and Fauna and Flora International - Cambodia programmes provided logistical assistance and support for this study. This would not have been possible without the help of many local people and field assistants; in particular, we wish to thank Hao, Sam At, Eam Sam Un, Som Piseth, Melissa Jenson, and Richard Moore. Allan Lisle assisted with the analysis.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Kays RW, Gittleman JL. The social organisation of the kinkajou Potos flavus (Procyonidae). Journal of Zoology London. 2001;253:491–504. [Google Scholar]
- 2.Wang G, Shi P, Zhu Z, Zhang Y. More functional V1R genes occur in nest-living and nocturnal terricolous mammals. Genome Biology and Evolution. 2010;2:277–283. doi: 10.1093/gbe/evq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman C, Buesching CD, Wolff JO. The function of facial masks in “midguild” carnivores. Oikos. 2005;108:623–633. [Google Scholar]
- 4.Bowers M. Exploitation of seed aggregates by the Merriam's kangaroo rats: harvesting rates and predatory risk. Ecology. 1990;71:2334–2344. [Google Scholar]
- 5.Lockard R, Davies N. Seasonal variation in moonlight avoidance by bannertail kangaroo rats. Journal of Mammalogy. 1974;55:189–193. [PubMed] [Google Scholar]
- 6.Kramer K, Birney E. Effect of light intensity on activity patterns of Patagonian Leaf-eared mice, Phyllotis xanthopygus. Journal of Mammalogy. 2001;82:535–544. [Google Scholar]
- 7.JulienLaferriere D. The influence of moonlight on activity of woolly opossums (Caluromys philander). Journal of Mammalogy. 1997;78:251–255. [Google Scholar]
- 8.Gursky S. Lunar Phillia in a nocturnal primate. International Journal of Primatology. 2003;24:351–367. [Google Scholar]
- 9.Nash LT. Moonlight and Behavior in Nocturnal and Cathemeral Primates, Especially Lepilemur leucopus: Illuminating Possible Anti-Predator Efforts. In: Gursky SL, Nekaris KAI, editors. Primate Anti-Predator Strategies. New York, United States: Springer; 2007. pp. 173–205. [Google Scholar]
- 10.O'Farrell MJ. Seasonal activity patterns of rodents in a sagebrush community. Journal of Mammalogy. 1974;55:809–823. [Google Scholar]
- 11.Regal PJ. Behavioural differences between reptiles and mammals. In: Greenberg N, MacLean PD, editors. Behaviour and neurology of lizards. Washington, DC: Department of Health, Education and Welfare; 1978. pp. 183–202. [Google Scholar]
- 12.Tattersall I. Cathemeral activity in primates: a definition. Folia Primatologica. 1987;49:200–202. [Google Scholar]
- 13.Campbell C, Fuentes A, Mackinnon KC, Panger M, Bearder SK, et al. Primates in Perspective. Oxford: Oxford University Press; 2011. [Google Scholar]
- 14.Donati G, Borgognini S. From darkness to daylight: cathemeral activity in primates. Journal of Anthropological Sciences. 2006;84:7–32. [Google Scholar]
- 15.Bearder SK, Nekaris KAI, Curtis DJ. A re-evaluation of the role of vision in the activity and communication of nocturnal primates. Folia Primatologica. 2006;77:50–71. doi: 10.1159/000089695. [DOI] [PubMed] [Google Scholar]
- 16.Allman J. Evolution of the visual system in the early primates. Progress in pyschobiology and physiological psychology. 1977;7:1–53. [Google Scholar]
- 17.Rahlfs M, Fichtel C. Anti-Predator Behaviour in a Nocturnal Primate, the Grey Mouse Lemur (Microcebus murinus). Ethology. 2010;116:429–439. [Google Scholar]
- 18.Fichtel C. Avoiding predators at night: antipredator strategies in red-tailed sportive lemurs (Lepilemur ruficaudatus). American Journal of Primatology. 2007;69:611–624. doi: 10.1002/ajp.20363. [DOI] [PubMed] [Google Scholar]
- 19.Gursky S. Function of snake mobbing in spectral tarsiers. American Journal of Physical Anthropology. 2006;129:601–608. doi: 10.1002/ajpa.20364. [DOI] [PubMed] [Google Scholar]
- 20.Eberle M, Kappeler P. Mutualism, reciprocity, or kin selection? Cooperative rescue of a conspecific from a boa in a nocturnal solitary forager the gray mouse lemur. American Journal of Primatology. 2008;70:410–414. doi: 10.1002/ajp.20496. [DOI] [PubMed] [Google Scholar]
- 21.Bearder SK. Gursky SL, Nekaris KAI, editors. A Comparison of Calling Patterns in Two Nocturnal Primates, Otolemur crassicaudatus and Galago moholi as a Guide to Predation Risk. Primate Anti-Predator Strategies: Springer. 2007. pp. 206–221.
- 22.Nash L. Influence of moonlight level on traveling and calling patterns in two sympatric species of Galago in Kenya. In: Taub D, King F, editors. Current perspectives in primate social dynamics. New York: Van Norstrand Reinhold; 1986. pp. 357–367. [Google Scholar]
- 23.Charles-Dominique P. Ecology and Behaviour of Nocturnal Primates. London: Duckworth Press; 1977. [Google Scholar]
- 24.Bearder SK, Nekaris KAI, Buzzell CA. Dangers of the night: are some primates afraid of the dark? In: Miller LE, editor. Eat or Be Eaten: Predator Sensitive Foraging in Primates. Cambridge: Cambridge University Press; 2002. pp. 21–43. [Google Scholar]
- 25.Dausmann KH. Hypometabolism in primates: torpor and hibernation. In: Lovegrove BG, McKechnie AE, editors. Hypometabolism in animals: hibernation, torpor and cryobiology. Pietermaritzburg, South Africa: Interpak; 2008. pp. 327–336. [Google Scholar]
- 26.Ortmann S, Heldmaier G, Schmid J, Ganzhorn JU. Spontaneous daily torpor in Malagasy mouse lemurs. Naturwissenschaften. 1997;84:28–32. doi: 10.1007/s001140050344. [DOI] [PubMed] [Google Scholar]
- 27.Schmid J, Ruf J, Heldmaier G. Metabolism and temperature regulation during daily torpor int he smallest primate, the pygmy mouse lemur (Microcebus myoxinus) in Madagascar. Journal of Comparative Physiology [B] 2000;170:59–68. doi: 10.1007/s003600050008. [DOI] [PubMed] [Google Scholar]
- 28.Schmid J. Daily torpor in the gray mouse lemur (Microcebus murinus) in Madagascar: energetic consequences and biological significance. Oecologica. 2000;123:175–183. doi: 10.1007/s004420051003. [DOI] [PubMed] [Google Scholar]
- 29.Dausmann KH, Glos J, Ganzhorn JU, Heldmaier G. Hibernation in the tropics: lessons from a primate. Journal of Comparative Physiology B-Biochemical Systemic and Environmental Physiology. 2005;175:147–155. doi: 10.1007/s00360-004-0470-0. [DOI] [PubMed] [Google Scholar]
- 30.Schulke O, Ostner J. Physiological ecology of cheirogaleid primates: variation in hibernation and torpor. Acta Ethologica. 2007;10:13–21. [Google Scholar]
- 31.Nowack J, Mzilikazi N, Dausmann KH. Torpor on demand: heterothermy in the non-lemur primate Galago moholi. PLoS ONE. 2010;5:e10797. doi: 10.1371/journal.pone.0010797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pullen SL, Bearder SK, Dixson AF. Preliminary observations on sexual behaviour and the mating system in free-ranging lesser galagos (Galago moholi). American Journal of Primatology. 2000;51:79–88. doi: 10.1002/(SICI)1098-2345(200005)51:1<79::AID-AJP6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 33.Nekaris KAI, Pimley ER, Ablard K. Anti-predator behaviour of lorises and pottos. In: Gursky SG, Nekaris KAI, editors. Primate Anti-predator Strategies. New York: Springer Press; 2007. pp. 220–238. [Google Scholar]
- 34.Osman-Hill WC. Primates, Comparative Anatomy and Taxonomy. Edinburgh: Edinburgh University Press; 1953. [Google Scholar]
- 35.Ishida H, Hirasaki E, Matano S. Locomotion of the slow loris between discontinuous substrates. In: Matano S, Tuttle RH, Ishida H, Goodman M, editors. Topics in Primatology, vol 3, Evolutionary Biology, Reproductive Endocrinology, and Virology. Tokyo: University of Tokyo Press; 1992. pp. 139–152. [Google Scholar]
- 36.McArdle JE. Functional anatomy of the hip and thigh of the Lorisidae: correlations with behaviour and ecology. In: Chivers DJ, editor. Recent Advances in Primatology. London: Academic Press; 1981. pp. 123–137. [Google Scholar]
- 37.Kawamura S, Kubotera N. Ancestral loss of short wave-sensitive cone visual pigment in lorisiform prosimians, contrasting with its strict conservation in other prosimians. Journal of Molecular Evolution. 2004;58:314–321. doi: 10.1007/s00239-003-2553-z. [DOI] [PubMed] [Google Scholar]
- 38.Tan Y, Yoder AD, Yamashita N, Li WH. Evidence from opsin genes rejects nocturnality in ancestral primates. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14712–14716. doi: 10.1073/pnas.0507042102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernede L. Social organisation, ecology and conservation of Loris tardigradus tardigradus [PhD Thesis] Oxford: Oxford Brookes University; 2009. [Google Scholar]
- 40.Wiens F, Zitzmann A, Hussein NA. Fast food for slow lorises: is low metabolism related to secondary compounds in high-energy plant diet? Journal of Mammalogy. 2006;87:790–798. [Google Scholar]
- 41.Müller EF. Energy metabolism, thermoregulation and water budget in the slow loris (Nycticebus coucang, Boddaert 1789). Comparative Biochemistry and Physiology. 1979;64A:109–119. [Google Scholar]
- 42.Müller EF, Nieschalk U, Meier B. Thermoregulation in the slender loris (Loris tardigradus). Folia Primatologica. 1985;44:216–226. doi: 10.1159/000156215. [DOI] [PubMed] [Google Scholar]
- 43.Ramussen DT. Life history and behaviour of slow lorises and slender lorises. Durham, NC: Duke University; 1986. [Google Scholar]
- 44.Wiens F. Behaviour and Ecology of Wild Slow Lorises (Nycticebus coucang): Social Organisation, Infant Care System, and Diet [PhD thesis] Bayreuth: Bayreuth University; 2002. 117 [Google Scholar]
- 45.Streicher U. Seasonal changes in colouration and fur patterns in the pygmy loris (Nycticebus pygmaeus). In: Nadler T, Streicher U, Thang Long H, editors. Conservation of Primates in Vietnam. Hanoi, Vietnam: Haki Publishing; 2004. [Google Scholar]
- 46.Streicher U. Aspects of Ecology and Conservation of the Pygmy Loris Nycticebus pygmaeus in Vietnam [PhD thesis] Muenchen: Ludwig-Maximilians Universitaet; 2004. [Google Scholar]
- 47.Evans TD, Duckworth JW, Timmins RJ. Field observations of larger mammals in Laos, 1994–1995. Mammalia. 2000;64:55–100. [Google Scholar]
- 48.Fooden J. Zoogeography of Vietnamese Primates. International Journal of Primatology. 1996;17:845–899. [Google Scholar]
- 49.Brandon-Jones D, Eudey AA, Geissmann T, Groves CP, Melnick DJ, et al. Asian primate classification. International Journal of Primatology. 2004;25:97–164. [Google Scholar]
- 50.Nisbett RA, Ciochon RL. Primates in North Vietnam: a review of the ecology and conservation status of extant species, with a note on Pleistocene localities. International Journal of Primatology. 1993;14:765–795. [Google Scholar]
- 51.Ratajszczak R. Taxonomy, distribution and status of the lesser slow loris Nycticebus pygmaeus and their implications for captive management. Folia Primatologica. 1998;69:171–174. [Google Scholar]
- 52.Streicher U, Vu TN, Nadler T, Timmins RJ, Nekaris A. Nycticebus pygmaeus. 2008. IUCN Red List of Threatened Species v 20102.
- 53.Nekaris KAI, Nijman V. CITES proposal highlights rarity of Asian nocturnal primates (lorisidae: Nycticebus). Folia Primatologica. 2007;78:211–214. doi: 10.1159/000102316. [DOI] [PubMed] [Google Scholar]
- 54.Pyke GH. Optimal Foraging Theory A Critical Review. In: Johnston RF, editor. Annual Review of Ecology and Systematics. USA: Annual Reviews; 1984. pp. 523–575. [Google Scholar]
- 55.Radespiel U, Ehresmann P, Zimmermann E. Species-specific usage of sleeping sites in two sympatric mouse lemur species (Microcebus murinus and M. ravelobensis) in northwestern Madagascar. American Journal of Primatology. 2003;59:139–151. doi: 10.1002/ajp.10071. [DOI] [PubMed] [Google Scholar]
- 56.Wiens F, Zitzmann A. Predation on a wild slow loris (Nycticebus coucang) by a reticulated python (Python reticulatus). Folia Primatologica. 1999;70:362–364. doi: 10.1159/000021719. [DOI] [PubMed] [Google Scholar]
- 57.Evans TD, Hout P, Phet P, Hang M. A study of resin-tapping and livelihoods in Southern Mondulkiri. Cambodia and implications for conservation and forest management. Phnom Penh, Cambodia: Wildlife Conservation Society, Cambodia program; 2003. [Google Scholar]
- 58.Hagey LR, Fry BG, Fitch-Snyder H. Talking defensively, a dual use for the brachial gland exudates of slow and pygmy lorises. In: Gursky SL, Nekaris KAI, editors. Primate Anti-predator Strategies. New York: Springer Press; 2007. pp. 253–272. [Google Scholar]
- 59.Nekaris KAI, Starr CR, Collins RL, Nararro-Montes A. Comparative ecology of exudate feeding by Asian slow lorises (Nycticebus). In: Burrows A, Nash LT, editors. The Evolution of Exudativory in Primates. New York: Springer; 2010. [Google Scholar]
- 60.Bearder SK, Martin RD. Acacia gum and its use by bushbabies, Galago senegalensis (Primates: Lorisidae). International Journal of Primatology. 1980;1:103–128. [Google Scholar]
- 61.Overdorff DJ, Rasmussen MA. Determinants of nighttime activity in ‘diurnal’ lemurid primates. In: Alterman LG, Doyle GA, Izard K, editors. Creatures of the Dark: The Nocturnal Prosimians. New York: Plenum Press; 1995. [Google Scholar]
- 62.Curtis DJ, Zaramody A, Martin RD. Cathemerality in the mongoose lemur, Eulemur mongoz. American Journal of Primatology. 1999;47:279–298. doi: 10.1002/(SICI)1098-2345(1999)47:4<279::AID-AJP2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 63.Donati G, Lunardini A, Kappeler P. Cathemeral activity of red-fronted brown lemurs (Eulemur fulvus rufus) in the Kirindy Forest/CFPF. In: Rakotosamimanana B, Rasamimanana H, Ganzhorn JU, Goodman SM, editors. New Directions in Lemur Studies. New York: Plenum Press; 1999. pp. 119–137. [Google Scholar]
- 64.Kappeler P, Erkert HG. On the move around the clock: correlates and determinants of cathemeral activity in the wild redfronted lemurs (Eulemur fulvus rufus). Behavioural Ecology and Sociobiology. 2003;54:359–369. [Google Scholar]
- 65.Fernandez-Duque E. Influences of moonlight, ambient temperature, and food availability on the diurnal and nocturnal activity of owl monkeys (Aotus azarai). Behavioural Ecology and Sociobiology. 2003;54:359–369. [Google Scholar]
- 66.Mutschler T. Folivory in a small-bodied lemur: the nutrition of the Alaotran gentle lemur (Hapalemur griseus alaotrensis). In: Rakotosamimanana B, Rasamimanana H, Ganzhorn JU, Goodman SM, editors. New Directions in Lemur Studies. New York: Plenum Press; 1999. [Google Scholar]
- 67.Fietz J, Ganzhorn JU. Feeding ecology of the hibernating primate Cheirogaleus medius: how does it get so fat? Oecologica. 1999;121:157–164. doi: 10.1007/s004420050917. [DOI] [PubMed] [Google Scholar]
- 68.Fernández-Duque E, Iglesia HDL, Erkert H. Moonstruck primates: owl monkeys (Aotus) need moonlight for nocturnal activity in their natural environment. PLoS ONE. 2010;5:1–6. doi: 10.1371/journal.pone.0012572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bearder SK, Doyle GA. Field and laboratory studies of social organisation in bushbabies (Galago senegalensis). Journal of Human Evolution. 1974;3:37–50. [Google Scholar]
- 70.Bearder SK. Semi-natural Conditions and in the Field [MSc thesis] Johannesburg: University of the Witwatersrand; 1967. Territorial and Intergroup Behaviour of the Lessor Bushbaby, Galago sengalensis moholi (A. Smith), [Google Scholar]
- 71.Nguyen KV, Nguyen TH, Phan KL, Nguyen TH. Bioclimatic Diagrams of Vietnam. Hanoi: Vietnam National University Publishing House; 2000. [Google Scholar]
- 72.Fitch-Snyder H, Schulze H, Larson Le. Management of Lorises in Captivity. A Husbandry Manual for Asian Lorisines (Nycticebus and Loris ssp.) San Diego: Centre for Reproduction in Endangered Species (CRES), Zoological Society of San Diego; 2001. [Google Scholar]
- 73.Starr CR, Nekaris KAI, Streicher U, Leung LK-P. Field surveys of the Vulnerable pygmy slow loris Nycticebus pygmaeus using local knowledge in Mondulkiri Province, Cambodia. Oryx. 2011;45:135–142. [Google Scholar]
- 74.Javier EL. Rice systems and varieties. In: Nesbitt HJ, editor. Rice Production in Cambodia. Manila, Phillippines: International Rice Research Institute; 1997. pp. 39–81. [Google Scholar]
- 75.Walston J, Davidson P, Soriyun M. A Wildlife Survey of southern Mondulkiri Province, Cambodia. Phnom Penh, Cambodia: Wildlife Conservation Society- Cambodia Programme; 2001. [Google Scholar]
- 76.Wiens F, Zitzmann A. Social structure of the solitary slow loris Nycticebus coucang (Lorisidae). Journal of Zoology (London) 2003;261:35–46. [Google Scholar]
- 77.Altmann J. Observational study of behaviour: sampling methods. Behaviour. 1974;49:227–265. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- 78.Martin P, Bateson P. Measuring Behaviour. An Introductory Guide 3rd edition. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 79.Martin P, Bateson P. Recording methods. Measuring Behaviour: An Introductory Guide. Cambridge: Cambridge University Press; 1993. pp. 84–100. [Google Scholar]
- 80.Nekaris KAI. Activity budget and positional behavior of the Mysore slender loris (Loris tardigradus lydekkerianus): Implications for slow climbing locomotion. Folia Primatologica. 2001;72:228–241. doi: 10.1159/000049942. [DOI] [PubMed] [Google Scholar]
- 81.Donati G, Borgognini-Tarli SM. Influence of abiotic factors on cathemeral activity: the case of Eulemur fulvus collaris in the littoral forest of Madagascar. Folia Primatologica. 2006;77:104–122. doi: 10.1159/000089698. [DOI] [PubMed] [Google Scholar]
- 82.Donati G, Lunardini A, Kappeler P, Borgognini-Tarli SM. Nocturnal activity in the cathemeral red-fronted lemur (Eulemur fulvus rufus), with observations during a lunar eclipse. American Journal of Primatology. 2001;53:69–78. doi: 10.1002/1098-2345(200102)53:2<69::AID-AJP2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]