Abstract
Members of the Asteroidea (phylum Echinodermata), popularly known as starfish or sea stars, are ecologically important and diverse members of marine ecosystems in all of the world's oceans. We present a comprehensive overview of diversity and phylogeny as they have figured into the evolution of the Asteroidea from Paleozoic to the living fauna. Living post-Paleozoic asteroids, the Neoasteroidea, are morphologically separate from those in the Paleozoic. Early Paleozoic asteroid faunas were diverse and displayed morphology that foreshadowed later living taxa. Preservation presents significant difficulties, but fossil occurrence and current accounts suggests a diverse Paleozoic fauna, which underwent extinction around the Permian-Triassic interval was followed by re-diversification of at least one surviving lineage. Ongoing phylogenetic classification debates include the status of the Paxillosida and the Concentricycloidea. Fossil and molecular evidence has been and continues to be part of the ongoing evolution of asteroid phylogenetic research. The modern lineages of asteroids include the Valvatacea, the Forcipulatacea, the Spinlosida, and the Velatida. We present an overview of diversity in these taxa, as well as brief notes on broader significance, ecology, and functional morphology of each. Although much asteroid taxonomy is stable, many new taxa remain to be discovered with many new species currently awaiting description. The Goniasteridae is currently one of the most diverse families within the Asteroidea. New data from molecular phylogenetics and the advent of global biodiversity databases, such as the World Asteroidea Database (http://www.marinespecies.org/Asteroidea/) present important new springboards for understanding the global biodiversity and evolution of asteroids.
Introduction
Introduction to Basic Biology and Morphology
The class Asteroidea (also known as starfish or sea stars) is one of the most diverse groups within the phylum Echinodermata, including nearly 1900 extant species grouped into 36 families, and approximately 370 extant genera. Asteroids occur at all depths from the intertidal to the abyssal (to approximately 6000 m) and are present throughout all of the world's oceans, but they are most diverse in the tropical Atlantic and Indo-Pacific regions [1], [2], [3]
All living asteroids have been regarded as members of the post-Paleozoic Asteroidea [4], [5], which have a Triassic (early Mesozoic) fossil first occurrence [6]. The taxonomy uses the term “Neoasteroidea” recognizing the modern Asteroidea (i.e., the post-Paleozoic Asteroidea) [5], [6]. Certain late Paleozoic asteroids show similar and intermediate morphology with the crown group, and these similarities have been treated differently [4], [5], [6].
Asteroids are dorsoventraly flattened with five to 50 rays projecting from a central disk. Each arm possesses a series of paired J-shaped ambulacral ossicles that occur along each arm radius. Tube feet emerge from pores present between ambulacral ossicles into a large ventrally facing open groove. These grooves all converge on the mouth, present on the bottom-facing side of the disk. Although supported as members of the asteroid lineage, concentricycloids (represented by the monotypic Xyloplax) show a highly divergent morphology that has suggested separation of Xyloplax from the other Asteroidea. This includes unpaired, non-overlapping ambulacral ossicles, tube feet in a single row, and adambulacral plates forming a peripheral disk series [7], [8]. As outlined below, this divergent morphology has led to a highly contentious discussion over the classification of Xyloplax within the Echinodermata.
In spite of the common names “sea star” and “starfish,” asteroids possess highly varied body shapes, including those that are sphaerical (e.g., Podosphaeraster), those that are pentagonal (e.g., Sphaeriodiscus) and others that are strongly stellate with very long arms and a nearly non-existent disk (e.g., Zoroaster). Body shapes range from highly inflated and cushion shaped (e.g., Culcita) to extremely dorso-ventral flattened with paper-thin bodies (e.g., Anseropoda). In many asteroids, a thick, fleshy (e.g., Porania) to gelatinous (e.g., Hymenaster) covering/layer has obscured the skeleton. Adult animal size varies from the tiny stichasterid Allostichaster palmula [9] with a disk to arm radius of about two to ten mm to immense members of the Asteriidae, such as Evasterias echinosoma and Pisaster brevispinus, which have both been recorded with armtip to armtip diameter of nearly 90 cm.
Other aspects of asteroid biology are diverse and are only briefly touched upon herein. Generalized overviews of asteroid biology can be found in [10], [11], [12], [13]. Jangoux [14] and Sloan [15] reviewed feeding biology and nutrition. Chia [16] and Koss and Rowe et al. [17] reviewed microscopic anatomy in asteroids and concentricycloids, respectively. Lawrence [18] reviewed eponymous structures in echinoderms, including several present in asteroids. Flammang [19], [20], Flammang et al. [21], [22] and Santos et al. [23] have provided several significant new contributions to our understanding of tube foot adhesion physiology. Valentincic [24] reviewed asteroid behavioral and responses to external stimuli. Chia and Walker [25] reviewed reproduction in asteroids. McEdward and Miner [26] reviewed larval and life cycle patterns.
Importance
Asteroids occupy substantive ecological roles and are widely used subjects in developmental and experimental biology. Asteroids such as the North Pacific Pisaster have been important in ecological studies addressing the role of competition, reproduction [27], [28], [29], [30], [31] and community structure [32], [33], [34]. Paine [32] idealized Pisaster as the textbook example of a keystone species. Pisaster ochraceus has been seminal in revealing the importance of photoperiodic control of reproduction in marine animals [35], [36], [37], [38], [39]. Cold (e.g., Asterias, Leptasterias) and temperate-water (e.g., Meyenaster, Coscinasterias) asteriids continue to occupy prominent roles as model organisms in the fields of community structure [30] and feeding ecology [40]. Asterias amurensis is an introduced invasive [41], [42], [43], [44] and is perceived as a threat to Australia's shellfish industries.
Population outbreaks of the tropical corallivore Acanthaster planci, also known as the Crown-of-Thorns Starfish, led to widespread concern by coral reef conservation authorities as living reefs were devoured by massive numbers of A. planci [45], [46], [47]. Corresponding to their ecological importance, asteroids are also study subjects in marine pollution and toxicological studies. Uptake of toxic metals, PCBs, and the effects of oil have been tested on several genera, including Asterias, Evasterias, and Coscinasterias [48], [49], [50], [51]. Taxa in the Asterinidae have occupied a primary place of importance in developmental and reproductive studies [52], [53]. Additionally, sea stars have been used in a diversity of disciplines, including immunology [48], physiology [54], biochemistry [55], cryogenics [56], and parasitology [57]. Several asteroid species have become subjects in global warming and ocean acidification studies [58], [59], [60].
Materials and Methods
Morphological terms and definitions follow Clark and Downey [2] and Blake [61]. Classifications begin with the morphological-based phylogenetic work of Blake [4]. Taxonomic diversity counts and conventions for species were obtained from the World Asteroidea Database [62] and from the Asteroid Names List [63], [64], [65], [66]. The classification used for this paper is present on Table 1. Images and data from the U.S Antarctic Research Program were also included [67].
Table 1. Breakdown of living taxa among the Neoasteroidea from Foltz and Mah [69], [181].
| Superorder | Order | Family | # genera | # species |
| Forcipulatacea | Forcipulatida | Asteriidae | 35 | 178 |
| Heliasteridae | 2 | 9 | ||
| Stichasteridae | 9 | 28 | ||
| “Pedicellasteridae” | 7 | 32 | ||
| Zoroasteridae | 7 | 36 | ||
| Total Forcipulatida | 60 | 283 | ||
| Brisingida | Brisingidae | 10 | 63 | |
| Freyellidae | 7 | 47 | ||
| TOTAL Brisingida | 17 | 110 | ||
| TOTAL Forcipulatacea | 77 | 393 | ||
| Spinulosida | Echinasteridae | 8 | 133 | |
| TOTAL Spinulosida | 8 | 133 | ||
| Valvatacea | Poraniidae | 7 | 22 | |
| Valvatida | Acanthasteridae | 1 | 2 | |
| Archasteridae | 1 | 3 | ||
| “Asterinidae” | 25 | 147 | ||
| Asterodiscididae | 4 | 20 | ||
| Asteropseidae | 5 | 6 | ||
| Chaetasteridae | 1 | 4 | ||
| Ganeriidae | 9 | 21 | ||
| Goniasteridae | 65 | 256 | ||
| Leilasteridae | 2 | 4 | ||
| Mithrodiidae | 2 | 7 | ||
| Odontasteridae | 6 | 28 | ||
| Ophidiasteridae | 27 | 106 | ||
| Oreasteridae | 20 | 74 | ||
| Podospherasteridae | 1 | 6 | ||
| Solasteridae | 9 | 51 | ||
| Caymanostellidae | 2 | 6 | ||
| TOTAL Valvatida | 187 | 763 | ||
| Valvatacea | Paxillosida | Astropectinidae | 26 | 243 |
| Benthopectinidae | 8 | 69 | ||
| Ctenodiscidae | 1 | 5 | ||
| Goniopectinidae | 3 | 10 | ||
| Luidiidae | 1 | 49 | ||
| Porcellanasteridae | 12 | 30 | ||
| Radiasteridae | 1 | 5 | ||
| Pseudarchasteridae | 4 | 29 | ||
| TOTAL Paxillosida | 56 | 439 | ||
| TOTAL Valvatacea | 243 | 1224 | ||
| Velatida | Korethrasteridae | 3 | 7 | |
| Myxasteridae | 3 | 9 | ||
| Pterasteridae | 8 | 116 | ||
| Concentricycloidea | Xyloplacidae | 1 | 3 | |
| TOTAL Species | 343 | 1890 |
“Quotation marks” indicate groups that were not supported as monophyletic.
Boldface indicates groups with large numbers of taxa.
We utilize “lineage” throughout the text as a general term to indicate a species or taxon and its nominal ancestor (and/or sister taxa where applicable) as opposed to the more context-driven term “clade”, which implies a distinct suite of synapomorphies for a branch taken from a specific phylogenetic hypothesis that may or may not exist for a specific clade.
Results
Taxonomic Diversity and Diversity Trends
In terms of total number of species, the Asteroidea (n = 1890 species) (Table 1) and the Ophiuroidea (n = 2064 species) [68] comprise the two most diverse classes within the living Echinodermata. Species counts and names utilized are those nominally accepted by the World Asteroidea Database as valid (or “accepted” by the database). Following Blake's [4] classification with modification by Mah and Foltz [69] the Valvatacea (Valvatida+Paxillosida) includes the greatest number of species (n = 1224), followed by the Forcipulatacea (n = 393 species), the Velatida (n = 145 species) and finally the Spinulosida (Echinasteridae), which includes 135 species (Table 1) [70]. Mah and Foltz [69] changed the composition of the Valvatacea to include the Solasteridae, but even with this difference (n = 51 species), from Blake [4], prior versions of the Valvatida included more genera and species than the Paxillosida [71], [64].
Species diversity is disproportionately distributed among the 36 families of living Asteroidea (Table 1). Seven families, Ophidiasteridae, Pterasteridae, Echinasteridae, Asterinidae, Asteriidae, Goniasteridae and Astropectinidae, each include more than 100 species. The Goniasteridae (n = 256) and the Astropectinidae (n = 243) include the largest number of species within the Asteroidea.
Species are not evenly distributed among genera. Within the Astropectinidae, Astropecten alone includes 43% (104/243) of the total number of species in the family [72]. The Goniasteridae includes 65 genera, most of which include multiple species [73]. At least eight goniasterid genera include more than 10 species. Several genera possess disproportionately high numbers of species relative to other genera within the family. Henricia includes some 68% (91/133) of the total known species in the Echinasteridae [70]. Pteraster (n = 45) and Hymenaster (n = 50) together account for 82% of the total number of species (n = 116) in the Pterasteridae [74]. The aforementioned illustrate the extreme cases, but several more examples of disproportionately high numbers of species/family exist. In nearly every instance of a genus with a disproportionately high numbers of species, these taxa include a global or widely distributed range. Astropecten is limited largely to tropical and temperate settings, but Henricia, Pteraster, and Hymenaster all have cosmopolitan distributions in cold to temperate water settings.
Undescribed Biodiversity
It is of course difficult to evaluate how many living species remain to be discovered, but one estimate can be based on the rate of reognition in the relatively well-known and widely studied Goniasteridae, which contains the largest number of nominal genera and species in the Asteroidea (Table 1). Out of the total number of nominal genera (n = 65) and species (n = 256) in the Goniasteridae, approximately 12% (n = 31) of species and 14% (n = 9) of genera were discovered in the 21st Century (2001 to present). Based on identified but undescribed museum goniasterid material (C. Mah, unpublished data), this would raise the total number of newly discovered genera to 37% and the number of species to 32%. This does not reflect a comprehensive survey of all museum collections but does suggest that a substantial number of asteroid taxa remain undescribed.
Another potential source of undiscovered/undescribed biodiversity is to be found in cryptic species. Several asteroid taxa, outlined in the “Diversity Trends”sections below, have now been identified as containing cryptic species, which are discrete lineages that are distinguished primarily based on molecular data that were not immediately recognizable from gross morphology. Widespread species are not uncommon among asteroids and it seems likely that this will further result in the identification of additional species diversity.
Diversity Trends
Table 2 broadly categorizes asteroid families as occurring in “cold,” “temperate,” or “tropical” settings. These zones are broadly based on sea-surface temperatures, as outlined in Duxbury et al [75], with “cold” temperatures ranging between 0 and 5°C, “temperate” ranging between 5 and 15°C, and ‘tropical’ at 15° and higher. Deep-sea settings (below 200 m) are treated herein as part of “cold” temperatures. Assignment of taxa to these categories is based on occurrence data from the World Asteroidea Database [62] and other sources [63], [64], [65], [66]. However, given the wide-ranging distributions of taxa, some of these categories are continuous and/or display overlap.
Table 2. Cold-Temperate-Tropical Water Asteroid Occurrence.
| Cold Settings Only | Benthopectinidae, Brisingidae, Caymanostellidae, Ctenodiscidae, Freyellidae, *Ganeriidae, Goniopectinidae, Korethrasteridae, Leilasteridae, Myxasteridae, *Odontasteridae, Pedicellasteridae, Podosphaerasteridae, *Poraniidae, Porcellanasteridae, *Pseudarchasteridae, Radiasteridae, Xyloplacidae, Zoroasteridae |
| Primarily Cold w/minority shallow Tropical and/or Temperate Members | *Astropectinidae, *Goniasteridae, *Pterasteridae, *Solasteridae |
| Temperate & Cold-Water Occurrence | *Chaetasteridae, *Stichasteridae |
| Temperate, Cold & Tropical Occurrence | *Asteriidae, *Asterinidae, *Echinasteridae, Heliasteridae, Luidiidae, |
| Tropical Shallow Water Settings Only | Acanthasteridae, Archasteridae, Mithrodiidae |
| Primarily Tropical w/minority Cold-Water Members | Asteropseidae, Asterodiscididae, *Ophidiasteridae, Oreasteridae |
Bold indicates groups exclusively found in deep-sea settings (>200 m).
indicates those with deep-sea members.
Out of the 36 families of living Asteroidea, 23 of those occur either exclusively or primarily in cold-water settings, six families occurred in temperate environments and seven were present primarily or exclusively in tropical water habitats. Taxa defined as “exclusively” cold-water were those families that occurred entirely in cold-water settings, such as the deep-sea or at high-latitudes. Those identified as “primarily” cold water have families that include 85% of taxa present in cold-water.
Tropical Diversity Trends
Those families that are primarily or exclusively tropical, including the Acanthasteridae, the Archasteridae, the Asteropseidae, the Asterodiscididae, the Mithrodiidae, the Oreasteridae, and the Ophidiasteridae, are all members of the Valvatida, as observed by Blake [71] and Mah and Foltz [69]. The Ophidiasteridae and the Oreasteridae are the most taxonomically diverse asteroid groups throughout the tropical shallow-water Atlantic, and Indo-Pacific [2], [3]. Blake [71] argued that valvatidans, which prey on colonial or encrusting food items, are most diverse in the tropics as a result of defensive structures, such as armor and spines that protect against predators. Blake [1], [71] also posited that predatory asteroids, such as the Asteriidae that feed on active or non-colonial prey have morphological features associated with predation (e.g., wide tube foot grooves) that make them more vulnerable to predation in the tropics.
In a phylogenetic analysis of the Valvatacea, Mah and Foltz [69] found that some valvatidan clades, such as the Oreasteridae plus the Asteropseidae and Acanthasteridae, show diversification into the tropics relative to a temperate or cold-water water sister taxon (Petricia). Other sister taxon relationships (e.g., Fromia and Lithosoma) are similar.
Other asteroid genera, such as Linckia, Nardoa, Ophidiaster, Tamaria (Ophidiasteridae) and Mithrodia (Mithrodiidae) form “tropicopolitan” species complexes that occur in the tropical-shallow water Atlantic and Indo-Pacific [2], [3]. Preliminary data also suggest that genera such as Echinaster are widely distributed species complexes [76]. Taxonomic and geographic distribution data including, but not limited to, Archaster (Archasteridae), Asteropsis (Asteropseidae), Fromia (Goniasteridae), Nardoa (Ophidiasteridae), and Pentaceraster (Oreasteridae), suggest that they form widespread species networks across the Indo-Pacific/East Pacific region.
Some phylogeographic analyses of populations within a single tropical species have been performed. Linckia laevigata shows distinction between Indian and Pacific Ocean populations [77], [78], [79], [80]. Distinct lineages have been recognized in populations of the Indo-Pacific Crown-of-Thorns Starfish, Acanthaster planci, [81], [82] suggesting that multiple cryptic species are present throughout itswidespread distribution. Zulliger and Lessios [83] sampled 40 of the 150 speices in the widespread tropical genus Astropecten and discovered species complexes and likely cryptic species.
Temperate Diversity Trends
Temperate water asteroids make up a minority of the total number of asteroids (Table 2) but nearly all families possess some representation, but even these genera mostly overlap with occurrence in either cold or tropical settings. For example, Waters and Roy [84] presented a global phylogeography of the temperate-water (but also tropical), fissiparous asteriid Coscinasterias. Waters' work also suggests the possibility of cryptic speciation in Coscinasterias muricata [85] and the ongoing divergence of populations (leading to species) in Patiriella regularis [86]. The asteriid Leptasterias occurs in temperate waters but has overlapping occurrence in cold-water setting. Full treatment of the Leptasterias species complex is below under the “Cold-Water Diversity Trends” section.
Brooding seems to be present in several temperate water taxa and has been included in several molecular phylogeographic studies. Naughton and O'Hara [87] presented a molecular phylogeographic analysis of the goniasterid Tosia. Their results identified a new species, T. neossia, which was independently supported by differences in reproductive behavior and larval mode. External morphological differences between T. neossia and T. australis were described, but had been overlooked in prior studies of the wider-ranging and variable species T. australis.
Cold-Water Diversity Trends
A majority of asteroid taxa occur in cold-water and cold-temperate settings (Table 2), which include deep-sea and high-latitude habitats. Nineteen families occur exclusively in cold-water settings, and most of those are found exclusively in the deep-sea. Four families include genera that occur mostly in the deep-sea although some species occur in more temperate to tropical regions (e.g, Astropecten in the Astropectinidae or Euretaster in the Pterasteridae). Several asteroid groups with high numbers of species also range across different habitats. For example, the Goniasteridae, which shows the highest number of genera (n = 65) and the second highest number of species (n = 256), occurs widely in cold water (e.g., Ceramaster, Evoplosoma), temperate (e.g., Tosia) and tropical habitats (e.g., Fromia, Neoferdina).
Many abyssal asteroid taxa are widely distributed, and several genera show a global distribution [88]. Porcellanaster and other members of the Porcellanasteridae, for example, occur at abyssal depths in the Atlantic, Pacific, Indian, and Southern Oceans [2], [89]. Other taxa, such as Freyella and Freyastera spp. (Freyellidae, Brisingida) also occur at abyssal depths in the Atlantic, Pacific, Indian, and Southern Oceans [66], [90].
Some evidence suggests that at least some modern asteroid taxa have occurred in the past in shallower environments. Blake and Zinsmeister [91] described Eocene Zoroaster aff. fulgens fossils from shallow-water littoral sediments of Seymour Island, Antarctica. Zoroasterids are absent from the modern Antarctic asteroid fauna but Zoroaster spp. occurs in the Atlantic, Pacific, and Indian oceans to depths of nearly 5000 m [92]. Villier et al. [93] describes Cretaceous pterasterid ossicles from shallow-water sediments. Most modern pterasterids occur today in deep-sea settings. Although several members of living deep-sea asteroid groups are present in the fossil record [93], [94] from shallow-water sediments, there are few records of living asteroid groups with fossil occurrence in deep-sea sediments. Villier et al. [95] describes velatidans and forcipulataceans from deep-water sediments of the Jurassic Lagerstätte of La Volute-Sur-Rhône. The Japanese Miocene Morozaki formation is a Lagerstätte contains several well-preserved asteroid fossils [96].
Many widely distributed cold-water asteroid taxa show relatively conservative morphology and display relatively few discrete differences between species. Historical distinctions have often been based on continuous characters [2], [89], [90], [97]. However, studies addressing genetic divergence in the widespread Atlantic deep-sea species Zoroaster fulgens using COI and 16S regions of the mitochondrial genome [98] have found at least three different bathymetrically separated morphotypes that are reproductively isolated. Based on these results, it seems likely that determinations of deep-sea and especially abyssal asteroid diversity are likely underestimated.
Continuing taxonomic studies suggest widespread occurrence of several cold-water taxa, which were originally described as species occuring only in localized regions. For example, certain species of Hippasteria, including H. trojana and H. hyadesi were described as distinct species occurring in New Zealand and the Patagonian sub-Antarctic, respectively. Newer taxonomic accounts now regard these as widely occuring members of Hippasteria phrygiana [2], [99], [100]. Other cold water taxa that have widespread distributions and which show a pattern similar to Hippasteria include Solaster and Lophaster (both in the Solasteridae), Henricia (Echinasteridae), and Pteraster (Pterasteridae). This is in no way a complete list but merely touches on the most species-rich genera that would benefit from further study. These taxa suggest at least the possibility of cryptic species and the need to re-evaluate past synonymies with molecular phylogenetic methods.
Asteroids at high-latitudes in both the Arctic and the Antarctic include taxa that form diverse species complexes that show morphological intergradation along the taxon's range. For example, in the Arctic and adjacent Atlantic and Pacific regions, the asteriid Leptasterias includes approximately 38 nominal species [101], [102], [103], which show phylogeographic evidence of relatively recent trans-Arctic diversification and interchange [104], [105], [106]. The asteriid Asterias also shows this pattern [107].
Although asteroid diversity in the Antarctic is higher [108], there is less phylogeographic data available for species complexes present in the Southern Ocean. Janosik and Halanych [109] and Janosik et al. [110] have recently outlined new species and reconstructed phylogeographic relationships for the abundant and commonly encountered Odontaster, which occurs throughout the Antarctic region.
Discussion
Fossil History
Recent views on the most likely Paleozoic source for post-Paleozoic asteroids differ significantly [4], [5], [6], [111], but authors agree that the Paleozoic-Mesozoic transition marked a time of major extinction and re-diversification, thereby allowing separation in this paper based on time. Although the paper focuses on the Asteroidea, it is necessary to touch briefly on the origins and diversification of all early stellate echinoderms.
Subdivisions of Paleozoic stellate echinoderms
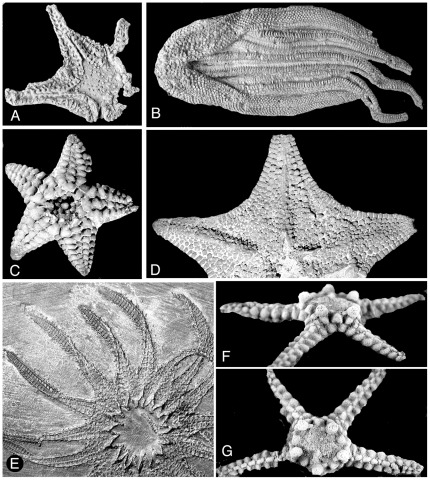
All three recognized groups of radiate echinoderms or “Asterozoa,” the surviving Asteroidea (Fig. 1B–G) and Ophiuroidea and the extinct Somasteroidea (Fig. 1A) [112], first appeared in the fossil record during a comparatively brief interval of the Early Ordovician. Similarities among certain early members have led most paleontologists to think of asterozoans as monophyletic but based on differences among living representatives, some authors have favored disparate ancestries. This discussion treats only data from the fossil record and no attempt is made to resolve differences.
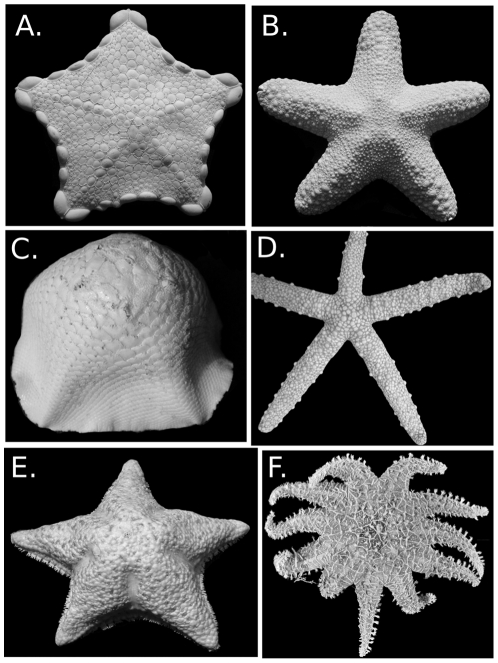
Figure 1. Paleozoic stem-group somasteroid and asteroids.
A. Ophioxenikos langenheimi (Somasteroidea) Blake & Guensburg, X-4751. B. Urasterella grandis (Meek) USNM 40885. Ordovician. C. Hudsonaster incomptus (Meek) USNM 40882 Ordovician. D. Jugiasspeciosus (Miller and Dyer). MCS 10806. Ordovician. E. Helianthaster rhenanus Roember . PWL 1983-21, Devonian. F and G. Paleaster clarki Clarke and Swartz USNM 144825. Devonian.
When named, the Somasteroidea was proposed as ancestral to both asteroids and ophiuroids. Since then, somasteroids have been seen as taxonomically cohesive [113] but their phylogenetic position has been both challenged [114], [115] and reaffirmed [115], [116]. Somasteroids can be separated from the surviving groups primarily on the basis of presence of a series of simple rod-like ossicles, so-called “virgals,” radiating laterally from each ambulacral ossicle. The first virgal is simple in all but one known somasteroid whereas it (or its equivalent) is differentiated as an “adambulacral” in asteroids and as a “lateral” in ophiuroids. The ambulacral column of asteroids is vaulted to form a permanent furrow and that of ophiuroids is vaulted only near the mouth frame. Based on ossicular configuration, the ambulacral column of somasteroids lies in the ventral plane, although it might have been capable of temporary vaulting to form a furrow [117]. Skeletal configurations appear to allow phylogenetic transformation from somasteroids to asteroids and ophiuroids, but conclusive evidence of sequencing is elusive.
The Importance of Preservation in Understanding Asteroid Phylogeny
For a number of reasons, asterozoans are rare as fossils as compared with e.g., mollusks and brachiopods. Aspects of preservation and preservation and fossil preparation have been treated in many papers, including those of Jagt [94], Lehmann [118], Spencer [112], LeClair [119], and Villier [120] although general discussions are uncommon. Schuchert [121], Ubaghs [122], and Spencer and Wright [113] described constraints on asterozoan fossilization, and for Paleozoic representatives, Schuchert [121] included total then-known occurrences for each geologic period as well as number of species of various genera recorded from different modern nations.
Here, reasons why asteroids are poor candidates for preservation are discussed first, followed by consideration of whether or not the limited record might reflect limited diversity through geologic time. A skeleton of discrete, unfused elements, largely exposed life modes, and the limited paeloenvironmental range sampled in the rock record combine to work against asteroid preservation.
Asteroids today occur at all water depths and on indurated as well as particulate substrates; the fossil record is biased toward shelf habitats with particulate substrates, hence today many asteroids occur in settings only sparsely sampled in the geologic record. Asteroids are mostly epifaunal organisms and even for those living in favored habitats, preservation requires unconsolidated sediments for burial. Fossils can be found beneath storm deposits or within and beneath submarine sediment flows. Earthquakes trigger many sediment flows but downslope movement can be gravity-induced even on relatively low slopes.
The asteroid skeleton consists of a large number of proportionately small, unfused ossicles; this construction allows flexibility of movement. The dermal-skeletal layer of many asteroids can be tough enough to provide some resistance to dissociation, but once breached, decay rapidly proceeds and ossicles are dispersed. Soft organs in the proportionately large asteroid coelom doubtless attract scavengers, leading to typically relatively rapid destruction even among buried individuals. Intact asteroid preservation demands prompt burial without later disturbance. Most skeletally intact specimens are more or less collapsed, the comparatively tough body wall apparently prevented infiltration of sediment after internal organ decay.
Dense accessory arrays typical of asteroids present their own problems of interpretation. Accessories obscure the arrangement of the taxonomically important foundation ossicles but these smaller elements are also of taxonomic significance, and data are lost where they have been lost. Expressions of delicate pedicellariae are important in the taxonomy of many extant asteroids, but few are known from the Paleozoic, perhaps only because of loss during preservation.
Both small accessories and body wall ossicles obscure interior arrangement of the ambulacral column and especially of the mouth frame. As a result, internal appearance of the mouth frame is known for few fossil species. Specimen collapse under the weight of overlying sediment displaces skeletal elements and obscures relationships.
Preservation reflects selectivity for the more skeletally robust. For the better-known post-Paleozoic crown group fauna, the Astropectinidae and Goniasteridae dominate the fossil record, and many of the better-known Paleozoic representatives are also comparatively robust. Different authors have suggested predation pressure and burrowing intensity have changed through geologic time, and an increase in burrowing activity would be detrimental to preservation of the relatively delicate asterozoans.
Major geologic settings also bias samples. Certain of the more important Paleozoic European asterozoan faunas (e.g. Montaign Noire of France) accumulated in fine-grained, clastic sedimentary settings whereas many of the more important North American occurrences (e.g., Cincinnatian of Eastern United States) sampled carbonate-rich settings. Such depositional differences have preservational as well as paleoecological implications.
The many preservational constraints indicate that it is reasonable to interpret the fossil record of all asterozoans as a deeply biased sampling of what once existed. However, a second argument, the taxonomic diversity of known fossils, is available. The extant fauna provides a measuring tool for crown group (i.e. post-Paleozoic) occurrences. Although this record is dominated by the skeletally robust, known fossils record most of the more important living families, reflecting enduring diversity.
Paleozoic faunas, all belonging to stem groups, cannot be directly compared to a modern equivalent. Useful to their interpretation is the Early Devonian Hunsrück Slate fauna of Germany [118]. The Hunsrück Slate accumulated under geologically unique conditions [123], [124]. The asterozoan fauna includes both large and delicate species, many unknown elsewhere. Although a single occurrence, the Hunsrück diversity range is (at least subjectively) parallel to if not greater than that of the modern fauna.
Fossil preservation differs significantly among specimens, and important features are not available in all specimens. A sampling of the diversity of Paleozoic somasteroids and asteroids is illustrated in Figure 1.
Origins of the Asteroids
Ancestry of the asteroids has been sought in two groups of early echinoderms, the extinct Edrioasteroidea and the Crinoidea (however, early crinoids were quite different from surviving representatives). The edrioasteroid hypothesis has been generally preferred; Smith and Jell [125] provided a recent perspective, and Zhao et al. [126] published reconstructions of certain edrioasteroids that might be suggestive of an asteroid ancestor. The crinoid hypothesis of Fell [127] received some early support but it was soon challenged [128] based on morphologic discontinuities, although recent discoveries appear to narrow differences [129], [130]. Mooi [131] reviews several different echinoderm phylogenetic hypotheses.
Like asteroids, edrioasteroids and crinoids have skeletons constructed of a large number of small, radially aligned plates or ossicles, and these similarities offer fertile ground for phylogenetic speculation. However, no known fossil bridges a morphological gap that begins with a skeleton of closely abutted elements and progresses to a flexible asteroid descendent. Further, the life-habit transition from a sessile or attached edrioasteroid or crinoid living with its mouth directed into the water column to a free-living descendent living with the mouth directed to the substrate is not bridged. Asteroid ancestry might lie within either edrioasteroids or crinoids, but much remains to be learned. Although the work of Fell [117], [127] was then not yet available, G. Ubaghs, one of the most important students of early echinoderms during the 20th century, found asterozoans to be of uncertain derivation [122], and his assessment remains sound.
Efforts at locating an asteroid ancestor of necessity focus on available fossils, but the comparatively very few yet very significant discoveries of early echinoderms of Guensburg and Sprinkle [129], [130], which were based on more than twenty years of intensive field research, clearly testify to the importance of what remains unknown. Further, both the biased fossil sampling of crown group asteroids as well as the echinoderm composition of the Early Devonian fauna of the unique Hunsrück Slate of Germany [123], [124], including many taxa unknown from other localities, attest to incomplete overall sampling. Reconstruction of the origins and early diversification of stellate echinoderms must be based on very limited and biased evidence with much early history likely to remain forever unknown.
Paleozoic Asterozoa: Important Classification Schemes
The meager fossil record has led to comparatively few taxonomic arrangements of Paleozoic asterozoans. For ordinal-level taxa, Spencer [112] provides the starting point. In this paper and following his own monographic work [132], Spencer purposed the extinct Somasteroidea as the ancestor of the surviving ophiuroids and asteroids.
Ubaghs [122] used terminology and concepts taken from Spencer [112], including the Somasteroidea. H. B. Fell [117], [127], [133], [134] proposed Platasterias, as a surviving somasteroid genus, although this interpretation is no longer generally accepted [135], [136]. Fell also posited a crinoid ancestry for living asterozoans, and he argued that extant asteroids can be used to help infer an ancient transition between crinoids and asteroids. Spencer and Wright [113] used the subordinal Paleozoic terminology of Spencer [112] as well as some new terms, and they accepted the phylogenetic ideas of Fell. In a survey treatment emphasizing German fossils, Müller [137] endorsed the three-fold subdivision of Spencer as well as the incorporation of Paleozoic fossils into extant orders. R. V. Kesling [138], [139], [140], [141], [142], [143], [144], [145] revisited the interpretations of Spencer and Wright [113]; these authors treated family through subclass rankings as well as a number of genera, some of them new. They also evaluated certain of the difficulties in the recognition of ossicular homologies. In a brief study, McKnight [146] treated the full history of asteroids and somasteroids based on collections of extant taxa and the literature for fossils; ophiuroids were not included. This author focused on projecting characters of living asteroids onto groupings of Paleozoic fossils, including soft-tissues and ontogenetic data, as well as certain skeletal expressions. He subdivided asteroids into two new superorders, both ranging from the Paleozoic that show the strong influence of the ideas of Fell [117] and of Spencer and Wright [113]. Shackleton [114] provided a phylogenetic analysis and classification of all asterozoans, but limited her treatment to Ordovician representatives. This author did not use subdivisions between the class and familial levels for either asteroids or ophiuroids.
The coverage of Ubaghs [122] was comprehensive for Paleozoic genera whereas his treatment of post-Paleozoic taxa was less complete. Spencer and Wright [113] provided a comprehensive listing of known fossil and extant genera. The compilation of Schuchert [147] provides valuable data for any survey of Paleozoic genera.
The Paleozoic Asteroidea: Complexities of Classification
Palaeontologists have traditionally regarded the Asterozoa as monophyletic but treatment within the group has varied significantly. Schuchert [121] recognized asteroids and ophiuroids as subclasses of Stelleroidea, and both used the asteroid terminology of Sladen [148], “Phanerozonia” (enlarged marginal ossicles) and “Cryptozonia” (reduced marginal ossicles). Schuchert [121] stressed his usage as descriptive subdivisions rather than as evolutionary markers. Schuchert [121] concluded that designation of taxa between the subclass and familial levels was premature. Schöndorf [149] recognized a class Auluroidea, on par with asteroids and ophiuroids. Kesling [139] embraced the auluroid concept whereas other workers have assigned these genera to the Ophiuroidea.
In their publications, W.K. Spencer, G. Ubaghs, H.B. Fell, R.V. Kesling, and D.G. McKnight all wove their arrangements of Paleozoic asteroids into the existing ordinal-level classification of the crown group. Spencer and Wright [113] included a historical summary of major papers leading to their arrangement.
Cited stratigraphic ranges and phylogenetic diagrams of Spencer and Wright [113] and especially of Ubaghs [122] indicate skepticism on the part of these authors over ranges extended from Paleozoic into the Mesozoic. Ubaghs [122] recognized only one such family, the Arthrasteridae. He assigned Carboniferous Calliasterella and Protarthraster to the Arthrasteridae, along with Cretaceous Arthraster, but he then dotted his range chart, seemingly questioning the arrangement. Ubaghs [122] treatment of predominantly crown-group asteroids was brief, but he did include Devonian Jaekelaster Sturtz and Mississippian Compsaster Worthen and Miller in the modern order Forcipulatida; he did not suggest familial assignments for these genera and his range chart does not clearly reflect his text suggestion.
Spencer and Wright [113] were somewhat more assertive in their arrangement. These authors recognized 12 suborders, five of which were thought to span the Paleozoic-Mesozoic boundary. They extended ranges of three small families (Palasterinidae, Calliasterellidae, Compsasteridae) across this boundary; however none of the three likely represents a monophyletic cluster [4], [6], [150]. The other two suborders of Spencer and Wright [113] were represented by families found on one side of the Paleozoic-Mesozoic boundary or the other, but not spanning it.
Shackleton [114] did not use taxon levels between the class and familial levels and no ranges crossing the Paleozoic-Mesozoic boundary were recognized. Although differing on stemward events in the crown group, Separate authors [1], [4], [5], [6], [111] have agreed that no extant ordinal-level taxon should be extended downward into the Paleozoic. Basic asteroid configuration and behavior have endured since early in class history, allowing much evolutionary convergence through geologic time.
Beginning with Paleozoic representatives, Blake and Hagdorn [6] proposed the subclass Ambuloasteroidea based primarily on presence of podial pores between successive ambulacral ossicles and offset placement of ambulacrals and adambulacrals, the former gradually emerging in different Paleozoic lineages, the latter extremely rare; the Neoasteroidea was treated as an infraclass within the Ambuloasteroidea. The Ambuloasteroidea provides an objective starting point in the search for the progenitors of the crown group.
Life Modes of Paleozoic Asteroidea
Rigorous data on ancient life modes are few. Paleozoic asteroids have been collected exclusively from marine rocks, including both quiet and more active depositional settings, and from both soft and firm substrates. All ancient asteroids appear to have been bottom-dwelling organisms. Certain living asteroids bury themselves at shallow depths beneath the surface, and Spencer [112] suggested that somasteroids were burrowing organisms; however, no asteroid exhibits a bilateral shape typical of active burrowing organisms such as irregular echinoids. Many living asteroids have been observed partially or fully covered with sediment and it seems plausible that Paleozoic asteroids behaved in a similar fashion.
Modern asteroids include suspension-feeders, detrital feeders, and predators on varied prey. Blake and Guensburg [151] reported the Paleozoic Promoplaeaster with its arms wrapped around a pelecypod in a manner similar to modern day asteriids, suggesting an early occurrence of this feeding behavior. Herringshaw et al. [152] provided useful summary of life habits of multiarmed species and the difficulties of their interpretation. Blake and Rozhnov [153] argued ancient asteroids likely were capable of broad ranges of behavior comparable to those found today.
Classification and Phylogeny of Post-Paleozoic Asteroids
Classification
Relatively few of the early syntheses of asteroid classification integrated fossil and living members in a phylogentic context [113], [122], [154]. Clark and Downey [2] presented the latest historical review of asteroid classification, emphasizing Atlantic taxa.
The late 19th and early 20th centuries were the “classic” period of morphologically based monographic studies of the systematics of modern asteroids. Authors consistently separated the forcipulate groups as recognized here from the remainder, and the Paxillosida gradually emerged as well, although there has been some instability of assignment (e.g., Radiaster, Pseudarchaster). The remaining groups proved more controversial, and remain so. Most influential were the ordinal concepts of Perrier [155], whose work was embraced in the widely cited Treatise of Spencer and Wright [113].
Concepts of modern higher classification among the living Asteroidea began with Viguier [156] and Perrier [155], [157] with subsequent contributions by Sladen [148] and Fisher [158]. Viguier established early groupings based on the nature of the skeletal mouth frame. Perrier [155] heavily emphasized pedicellariae as diagnostic for his four groups, the Forcipulatae, Spinulosae, Valvatae, and Paxillosae. Sladen [148] developed a different classification that largely emphasized marginal plates and regrouped the higher classification into the Phanerozonia, which included several families displaying prominent marginal plate series versus those in the Cryptozonia, which included those families that displayed more inconspicuous marginal plate series. Fisher [158] modified Sladen's classification and established three orders, the Phanerozonia, the Spinulosa, and the Forcipulata, which were in turn each subdivided into several suborders (e.g, the Paxillosa, Valvata, Notomyota) which accommodated previous classification schemes established by Perrier [155] and others and came to be heavily used throughout the 20th Century.
Phylogeny Inferred from Morphology
One of the earliest and best-known discussions of asteroid phylogeny began as a heated exchange between Mortensen [159], [160] and MacBride [161], [162], [163]. Their debate focused on the identity of the ancestral asteroid taxon. Mortensen assigning the “ancestral condition” to the Astropectinidae in part based on the absence of both a brachiolaria stage and suckered tube feet and MacBride arguing essentially that these are derived features in both astropectinid and luidiids reflected their occurrence on shallow, unconsolidated bottoms. Other workers surveyed by Mortensen [159] found that not only were the Paxillosida thought of as the “primitive” group, but also the Asterinidae and the “Spinulosa.” MacBride's contentious position did not definitively provide an alternative taxon as the ancestral asteroid but demonstrated the difficulty of interpreting “ancestral” versus “derived” characters.
The Mortensen-MacBride debate laid the foundation for the subsequent hypotheses of Fell [117], [133], [134], which suggested that the luidiid Platasterias was a living member of the Paleozoic Somasteroidea. This supported interpretation of the Paxillosida as the “primitive” or ancestral asteroid taxon and was embraced by Spencer and Wright [113]. Subsequent work [4], rejected Fell's interpretation of Platasterias and the Luidiidae as ancestral, but the debate over the Paxillosida as the group displaying the most “primitive” characters continued into modern discussions of asteroid phylogeny.
Although the “Paxillosida is primitve” discussion remains one of the best-known phylogenetic debates, there are several examples of other, less prominent, pre-cladistic, evolutionary hypotheses within the Asteroidea. Döderlein [164], [165] provided phylogenetic hypotheses for various species groups within both Astropecten and Luidia. H.L. Clark [166] provided early ideas on relationships among the Heliaster species complex in the tropical East Pacific. Madsen [167] presented ideas and an evolutionary tree regarding the interrelationships of the deep-sea Porcellanasteridae.
The modern phylogenetic paradigm for the Asteroidea begins with the cladistic-based hypotheses of Blake [4] and Gale [5]. Although the phylogenetic hypotheses significantly differ from one another, both show a well-supported modern Asteroidea as a discrete post-Paleozoic clade. In some respects, the work of Blake and Gale mirror those of MacBride [161], [162], [163] and Mortensen [159], [160] in that Gale [5], [111] advocates a primitive Paxillosida (Mortensen's position) whereas Blake argued that these characters should be interpreted as derived (MacBride's position).
An important distinction between the two phylogenetic hypotheses is that whereas Gale presented the Paxillosida as primitive, Blake emphasized the ambiguity of identifying any extant asteroid group as basal is misleading [4] (p. 515). Paleozoic lineages of asterozoans and early asteroids suffered extinction during the Permian-Triassic transition interval. Twitchett and Oji [168] summarized that all living echinoderms (including asteroids) underwent an important evolutionary bottleneck during this interval with subsequent recovery and diversification within the Triassic. Fossils are few but offer important insight [6], [169]. Extinction is an important component of understanding the early history of crown-group asteroids. Thus, our knowledge of early lineages within the Neoasteroidea is very poorly understood and the determination of a “primitive” taxon, such as the Paxillosida, is misleading and is an oversimplification of a complex but obscure history for which multiple taxa were likely present [6], [169], [170] but not reconciled within the reconstruction of a phylogeny which has only surveyed available living and fossil taxa.
Blake [4] showed the Forcipulatacea as the sister taxon to the remainder of the surviving asteroids, a separation that has been historically observed in primary asteroid monographs [148], [155], [158]. However Blake [4], [6] has emphasized that even those tree topologies that incorporate available fossils depends on the sampling of a scanty fossil record. It is important to note that divergence might be such that the common ancestor of all surviving asteroids would no more be assignable to a surviving taxon grouping below the class level than is the early Paleozoic common ancestor.
Phylogeny Inferred from Molecular Studies
Early molecular studies, such as that published by Wada et al. [171] and the combined analysis of Lafay et al. [172] are consistent with Gale's [5], [111] assertion that the Paxillosida were primitive. However both Wada et al. [171] and Lafay et al. [172] included relatively few taxa and used conveniently sampled, local species as avatars for large, highly diverse groups (such as the highly diverse Valvatacea). Many of their sampled species, including Astropecten and Luidia, have since been shown to occur on highly derived branches [69], [83]. Gale [111] has continued to argue Mortensen's perspective of a “primitive” Paxillosida in spite of phylogenetic evidence to the contrary from morphology [4], [61] and recent evidence from several molecular studies [69], [173], [174] that have shown the Paxillosida in derived positions.
Knott and Wray [175] presented one of the first, well-sampled phylogenetic analyses of the Asteroidea from COI, mtRNA and previously collected ribosomal gene sequences. Janies [176] presented a combined evidence tree of the Echinodermata, which supported the Asteroidea as monophyletic, but did not recover any consistently monophyletic groupings.
Matsubara et al., [177] determined the Solasteridae as the sister group to the Asterinidae and subsequently revisited the phylogenetic relationship of the Forcipulatida to other asteroids [174]. Waters et al., [178] addressed molecular relationships within the Asterinidae. Yasuda et al. [179] reported complete mitochondrial genome sequences for the Crown-of-thorns starfish Acanthaster, and provided a COI phylogeny showing Acanthaster+Oreaster in addition to other asterinids on a valvatidan clade as the sister group to two paxillosidans (Astropecten and Luidia) rooted against a forcipulate (Pisaster), an echinoid and a holothurian. Foltz et al. [180] supported the monophyly of the Forcipulatacea using combined mitochondrial and nuclear sequences.
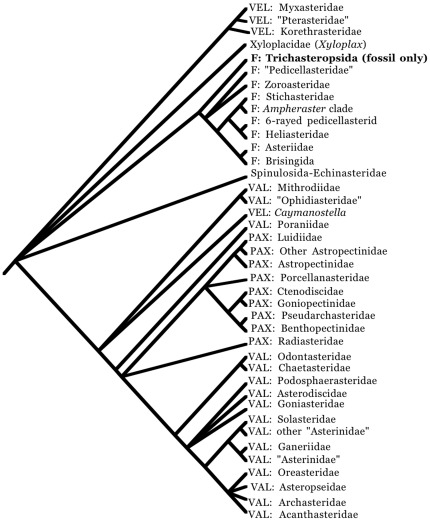
Mah and Foltz [69] reconstructed a comprehensively sampled phylogeny of the Valvatacea which supported the sister group relationship between the Asterinidae and Solasteridae as determined by Matsubara et al. [177] as well as supporting stemward relationships for the Poraniidae and the Velatida (Pterasteridae, Myxasteridae, Korethrasteridae). Although basal relationships were not well supported, the Paxillosida was not supported among basal taxa within the Valvatacea relative to a Forcipulatacean outgroup (Fig. 1) [69]. A subsequent phylogenetic analysis of the Forcipulatacea [181] further supported forcipulate monophyly, re-established the Stichasteridae, and clarified relationships among groups within the Asteriidae and among the Forcipulatacea.
Diversity among the Living Asteroidea
All living asteroids, termed Neoasteroidea by Gale [5], are phylogenetically distinct from those in the Paleozoic [5], [6]. Gale [5] named the Post-Paleozoic Asteroidea as the Neoasteroidea. Based on construction of the ambulacral column, Blake and Hagdorn [6] recognized the Neoasteroidea at the infraclass level within a subclass Ambuloasteroidea
Figure 2 summarizes phylogenetic perspectives from Foltz and Mah [69], [181], Blake [4], and Janies et al. [173]. Polytomies are present where phylogenetic data is incomplete or ambiguous but the diagram assumes a monophyletic Neoasteroidea. Groupings used below reflect discrete phylogenetic lineages rather than traditional taxonomic units. The Velatida has not found full support as a member of the Spinulosacea and, except for Caymanostella, is retained separately.
Figure 2. Summary diagram of phylogenetic tree.
Topology from combined trees of Mah and Foltz [69], [181], Janies et al [173], and Blake [4]. “Asterinidae” refers to paraphyletic clades as outlined by Mah and Foltz [69].
Mah and Foltz [69], [181] presented a 3-gene phylogeny that has further clarified relationships and classification in the Forcipulatacea and the Valvatacea. These include the paraphyly of the Asterinidae along with several proposed taxonomic changes, namely the assignment of the Solasteridae to the Valvatida and placement of some ophidiasterids in the Goniasteridae, the new position of the Poraniide, the paraphyly of the Pedicellasteridae and others, which are outlined in discussions below. Gale [111] has proposed the Forcipulatida as rooted among several valvatidan taxa as the “Tripedicellaria.” This is a classification with no precedent in the historical literature from morphology [4], [148], [154] and it has found no support with other recent molecular data [69], [173], [177], [179]; it therefore is not followed herein.
The Forcipulatacea
The Forcipulatacea is a diverse, primarily cold-water (some temperate and tropical members are known) lineage of modern asteroids that occur in all of the world's oceans from the intertidal to the deepest abyssal depths (>6000 m). The Forcipulatacea includes 393 species in 77 genera (Table 1) [182], which ranks them as among the most diverse of the Asteroidea. Forcipulataceans are most diverse at high-latitudes with rich faunas in the Arctic and especially in the Antarctic.
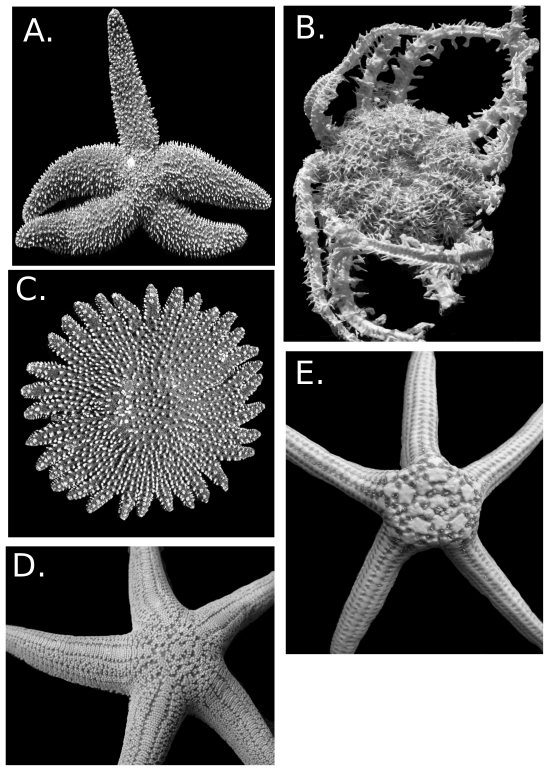
Although the Forcipulatacea display a wide range of morphologies (Fig. 3), taxonomists traditionally have found them to be readily separated from the remainder of the crown group. Characters helping to characterize forcipulataceans but not found in all members include the presence of distinct 3-part “forcipulate” pedicellariae (although pedicellariae vary among taxa), four rows of tube feet; foreshortened (or “compressed”) ambulacral and adambulacral ossicles, the latter alternating in furrow profile in taxa with four rows of tube feet; a reticulated dorsal skeleton; a well-developed adoral carina (abutted adambulacral plates adjacent to the mouth, the proximal skeleton recessed to form a so-called actinostome); small mouth-angle ossicles; the longest actinal series adjacent to the marginals rather than adjacent to the adambulacrals; and a small disk with thick, tapering arms.
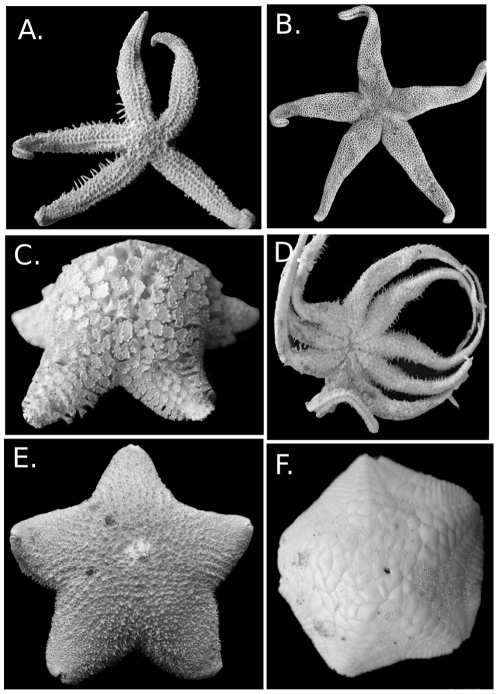
Figure 3. Forcipulatacean diversity.
A. Asterias forbesi (Asteriidae) USNM 43197 B. Odinella nutrix (Brisingida) USNM E13561. C. Heliaster cumingii No number. D. Stichaster striatus (Stichasteridae) USNM 1085979. E. Doraster constellatus (Zoroasteridae) USNM E23145.
Most historical accounts [113], [158] have set apart the Forcipulatacea or “forcipulate” asteroids (i.e., the Forcipulatida+Brisingida) from the other members of the Asteroidea. This is a position that has been further supported by modern phylogenetic treatments of morphology [1], [4], [173], [174] and is reflected in Fig. 2. Gale [111] has placed forcipulates in a derived position within taxa historically regarded as members of the Valvatida. This position has not found historical agreement and is not followed by the treatment herein.
Monophyly of the Forcipulatacea itself has been relatively uncontroversial with support from traditional taxonomy [101], [183], morphology-based phylogenetic studies [4], [5] and molecules [173], [174], [177], [180], [181]. Subgroupings within the Forcipulatacea have encountered more difficulty, especially those associated with the Asteriidae, such as the Labidiasteridae [101], [113], [183], [184]. Mah and Foltz [181] provided the taxonomic foundation for the summary below.
The Forcipulatacea, particularly the Asteriidae (e.g., Fig. 3A), includes some of the most heavily studied and most familiar of marine invertebrates in ecology and environmental biology. Relevant taxa include Pisaster ochraceus, which has become an iconic representative of the keystone species concept as outlined by Paine [32], [33], [34] and Asterias amurensis, which has been introduced to southern Australia as a pest species that threatens endemic shellfish [41],[42],[43],[44]. The Atlantic Asterias rubens and Asterias forbesi have been among the most familiar of ecological subjects in marine biology studies [185], [186]. As important ecological members, asteriids such as the European Asterias rubens, the North Pacific Evasterias troscheli, and the temperate South Pacific Coscinasterias muricata have also been used as subjects in several oil pollution studies [50], [51], [187 respectively].
Diversity Within the Forcipulatacea
Mah and Foltz [181] supported six primary lineages within the Forcipulatacea. This includes the Asteriidae (e.g., Fig. 3A) [188] the Brisingida (e.g., Fig. 3B) [189], a modified Heliasteridae (Fig. 3C) [190], the Stichasteridae (Fig. 3D) [191], the Zoroasteridae (Fig. 3E) [192] and a paraphyletic “Pedicellasteridae” [193]. Many of the traditional asteriid subgroupings outlined by Fisher [101], [183], which were raised to family-level by Clark and Downey [2] and Clark and Mah [66], were not supported as monophyletic, although some of the Northern/Southern Hemisphere taxonomic divisions in his identification keys were observed. Some groups, such as the Labidiasteridae, are artificial and have been dismantled [181], [184]. Basal relationships among forcipulatacean lineages were not well supported, but higher-level groups were recovered from the analysis.
The Brisingida (Fig. 3B) [189] is a clade of exclusively deep-sea asteroids possessing a small disk with tightly articulated plates and six to 20 elongate arms, which are extended into the surrounding water column and used for feeding [194]. Brisingids are suspension feeders that utilize needle-like spines with dense coverings of pedicellariae to capture tiny crustaceans and other food particles [195], [196]. They are found between 100–6000 m and have been reported from all oceans, except the Arctic. The Brisingida have been repeatedly supported as monophyletic by morphology [4], [181], [184], [197] and DNA [180], [181], and include 110 species in 17 genera [189]. Within the Brisingida, the monophyletic Freyellidae (47 species in seven genera) [198] occupy a much deeper bathymetric range than non-freyellids [197]. The Brisingidae (63 species in ten genera) [199] itself is likely paraphyletic and includes Brisingaster, Novodinia, and Odinella, which are likely basal within the overall brisingid clade relative to Brisinga or other non-freyellids [200]. The Zoroasteridae (Fig. 3E, 7 genera, 36 species) [192] and the “Pedicellasteridae” (7 genera, 32 species) [193] both occur only in the deep-sea (bathyal to abyssal depths) and are phylogenetically basal among extant forcipulataceans. The basal location of these taxa was consistent with Blake [1] who supported Jurassic “asteriid” fossils as closely related to zoroasterids and pedicellasterids. Zoroasterids possess a single row of marginals, a character present in Paleozoic and early transitional asteroid fossils from the Triassic [4], [6]. Pedicellasterids display numerous plesiomorphic characters, such as biserial tube foot rows, an absent or reduced adoral carina and a weakly developed abactinal skeleton. Mah and Foltz [181] did not recover the Pedicellasteridae as a monophyletic group, instead finding support for multiple basal lineages within the Forcipulatacea, suggesting that the term “pedicellasterid” is best applied as a grade within forcipulates, rather than a monophyletic family. A phylogeny of the Zoroasteridae [92] separated the more imbricate zoroasterids, such as Zoroaster and Cnemidaster, which occur from bathyal to abyssal depths, from zoroasterids with reticulate skeletons, such as Myxoderma, which occur at shelf to bathyal depths. This suggested diversification of the more derived imbricate taxa, such as Zoroaster, into the deep-sea.
The Heliasteridae (Fig. 3C, nine species in two genera) [190] includes the tropical shallow-water Heliaster, which occurs throughout the Pacific coast of Mexico and South America and Labidiaster, which occurs in the South Atlantic and in the adjacent Southern Ocean. Heliaster comprises a species complex in the East Pacific region [166] with some ecological importance [32]. Pliocene fossils from Florida have indicated that this complex at one time occurred over a much larger region [201]. Labidiaster annulatus in the Southern Ocean is a benthopelagic predator [202], [203]. Mah and Foltz [181] recovered a sister-group relationship between Heliaster and Labidiaster, which provided the basis for synonymy of the artificial and paraphyletic Labidiasteridae within the Heliasteridae. Mah [184], Foltz et al. [180], and Mah and Foltz [181] dismantled the Labidiasteridae, showing that each of its members was assignable to phylogenetically distant lineages.
Two of the most ecologically important and diverse groups within the Forcipulatacea, are the Asteriidae (with most species in the Northern Hemisphere) (Fig. 3A, 35 genera, 178 species) [188] and the mostly Southern Hemisphere Stichasteridae (Fig. 3D, 9 genera, 28 species) [191]. In spite of being phylogenetically distant from one another, the Asteriidae and Stichasteridae include taxa that apparently occupy similar if not convergent ecological niches in intertidal and shallow-water marine ecoystems [32], [204], [205], [206], including “keystone” positions as predators of bivalves and other mollusks.
Multiple lineages are present within the Asteriidae and the Stichasteridae. Four major lineages are present in the Asteriidae including the genus Sclerasterias a Boreal clade, which contains Northern Hemisphere cold-temperate water taxa, such as the Pacific-Arctic-Atlantic Leptasterias species complex [104], [105], [106], and two sister clades, the Pan Tropical and Antarctic asteriids. The Pan Tropical asteriid clade is composed of taxa such as Coscinasterias, Meyenaster, and Astrometis, which occur at low-latitudes in tropical (non-reef) to temperate settings. Antarctic asteriids occur at high latitudes in the Southern Ocean and adjacent regions and are the most diverse of the Antarctic asteroid fauna. High-latitude asteriids include brooding taxa, such as Diplasterias, Lysasterias, and Anasterias [108].
The Stichasteridae occur on two major lineages. One primarily shallow-water cluster, including Stichaster, Cosmasterias, Smilasterias, and Allostichaster which occur in an austral distribution in South America, South Africa, and Australia/New Zealand and its sister lineage which is composed primarily of deep to cold-water taxa with widespread distributions, such as Neomorphaster.
The Paxillosida and “Notomyotida”
The Paxillosida (Fig. 4), including the Benthopectinidae, occurs at depths ranging from littoral habitats (e.g., Astropecten in the Astropectinidae occurs at 0–2 m in some settings) to the deepest abyss (>5000 m) (e.g., the Porcellanasteridae). Most of the Paxillosida are primarily cold-water and are well represented in the deep-sea as well as at high latitudes (Arctic and Antarctic) but include diverse, shallow-water tropical to temperate water taxa as well (e.g., Astropecten, Luidia). The review herein follows the phylogeny of Mah and Foltz [69] and includes the Benthopectinidae and the Pseudarchasteridae as members of the Paxillosida.
Figure 4. Paxillosida (including Benthopectinidae) diversity.
A. Ctenodiscus australis, abactinal surface USNM 37148 B. Same specimen, showing actinal surface and fasciolar grooves. C. Dytaster grandis USNM E15959 D. Luidia clathrata USNM 8507 E. Pseudarchaster parelii USNM 1085998 F. Luidiaster antarcticus USNM 1121741.
The primary life mode of taxa within the Paxillosida, with the exception of the Benthopectinidae, involves burial or ploughing through unconsolidated sediment [61], [207]. Examples of characters that have been considered adaptations to life in sediment and simultaneously synapomorphies for many members of the Paxillosida include paxillate plates (abactinal, marginal and actinal), pointed tube feet, superambulacral plates, cribiform organs, the presence of an anal cone, and actinolateral fasciolar channels.
The Paxillosida includes both detritivores and predators of mollusks and other invertebrates [14], [15], and many spend part or most of their lives buried. Paxillosidan life modes are associated with poorly consolidated sediment bottoms. Some groups, such as the goniopectinids, ctenodiscids, and porcellanasterids are detritivores that live buried in or under mud [208] whereas others live buried under surface sediments but are predatory on mollusks and other invertebrates [14]. Ecology in most of the Paxillosida is poorly understood, but observations of Astropecten, Ludia, and other paxillosidans suggest complexity and ecological importance [209], [210], [211], [212], [213]. Although Pseudarchaster appears to be more phylogenetically distant from the other Paxillosida, it shows a generalized detritivore/predatory feeding life mode similar to astropectinids [14].
Little is known regarding the biology of the Benthopectinidae. Jangoux summarizes stomach contents from four taxa, which suggests they are either predators/sediment feeders/detritivores. Blake [214] and Clark and Downey [2] have speculated that benthopectinids used muscles to hold up their arms in the water column for suspension feeding and interpreted their well-developed arm spines as defensive adaptations to predators that have limited them to deep-water. Available images of benthopectinids do not suggest burrowing or show arms extended into the water column.
Diversity Within the Paxillosida (and “Notomyotida”)
Mah and Foltz [69] used three genes (12S, 16S, and histone H3) to reconstruct the phylogeny of the Valvatacea, and recovered a Paxillosida that was composed of traditional members (e.g., Astropectinidae, Goniopectinidae, Luidiidae, etc.) but also several groups displaying intermediate morphology. This included the Benthopectinidae and the Pseudarchasteridae as sister taxa to a clade containing the Goniopectinidae and the Ctenodiscidae. The Luidiidae was recovered as the sister lineage to one containing multiple astropectinids, including Macroptychaster, Lonchotaster, Leptychaster, Dipsacaster and the radiasterid, Mimastrella. Although the Porcellanasteridae was not sampled in Mah and Foltz's [69] analysis, it was supported as one by Blake [4] and is considered as a member of the Paxillosida herein.
The Porcellanasteridae (12 genera, 30 species) [215], Goniopectinidae (3 genera, 10 species) [216], Ctenodiscidae (Fig. 4A,B, 1 genus, 5 species) [217] as well as most members of the Astropectinidae (Fig. 4C, 26 genera in 243 species) [72] all occur primarily in deep-sea settings (∼100–4000 m). Common to all of these families are genera that have a cosmopolitan (or nearly so) distribution. For example, the porcellanasterid, Porcellanaster ceruleus displays a cosmopolitan distribution [63], [89]. Multiple genera within the Astropectinidae possess widespread, deep-sea distributions at bathyal to abyssal depths, including Dytaster, Leptychaster, Lonchotaster, Persephonaster, Plutonaster, and Psilaster. Ctenodiscus, the sole member of the Ctenodiscidae is present throughout the world's ocean basins, occurring from the Arctic to the deep-sea tropics to the subAntarctic. Many of these taxa display few characters or characters that differ only gradually across their range.
In contrast to the deep-sea Paxillosida, there are two genera, Astropecten and Luidia (Fig. 4D) with large numbers of species that occur in temperate and tropical settings. Although both genera occur across a wide range, most taxa are primarily shallow-water and live in relatively coarse sediments compared to other deeper-water Paxillosida, which occur in finer, deep-sea muddy bottoms. Döderlein produced a taxonomic overview of both genera [164], [165]. Zulliger and Lessios [83] analyzed 117 specimens of Astropecten belonging to 40 species from around the world, using 12S, 16S and COI genes, and identified three main clades in the Indo-Pacific, the Neotropics, and the eastern Atlantic and Mediterranean, which displayed morphological convergence and several species complexes, such as the A. polyacanthus complex in the Indo-Pacific.
The Benthopectinidae (Fig. 4F) and the Pseudarchasteridae (Fig. 4E) were supported by Mah and Foltz [69] as sister taxa and both have shown close morphological resemblance/affinities to the Goniasteridae [4]. The Pseudarchasteridae (e.g., Pseudarchaster, Paragonaster) includes 29 species in four genera [218], whereas the Benthopectinidae (e.g., Benthopecten, Nearchaster) includes 69 species in eight genera [219]. Both families occur primarily in deep-sea (shelf to abyssal) or high-latitude/polar settings and include many widely distributed taxa.
The Poraniidae (Sister clade to Valvatida+Paxillosida)
Mah and Foltz's [69] work placed the Poraniidae (Fig. 5E), which had historically been a member of the Valvatida, as the sister clade to a Valvatida+Paxillosida dichotomy, thus removing it from the Valvatida [4]. This is consistent with the morphology-based phylogeny of Blake and Hagdorn [6] that showed a Poraniidae+Noriaster clade as sister to solasterids, asterinids, echinasterids, paxillosidans, and goniasterids.
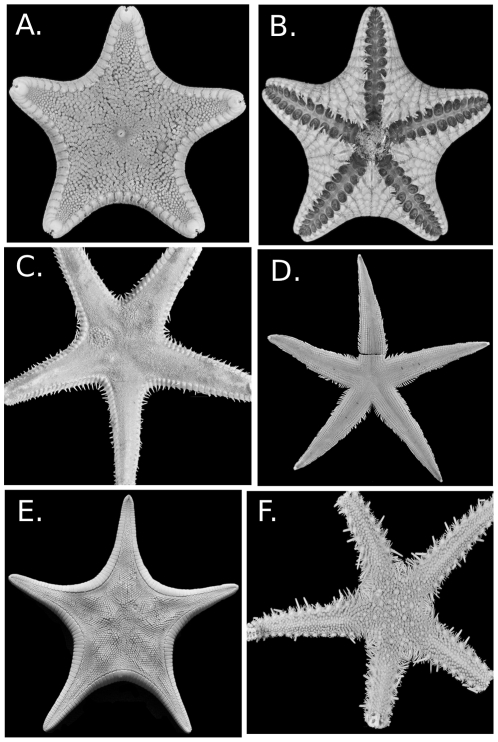
Figure 5. Diversity within the Valvatacea.
A. Pentagonaster pulchellus (Goniasteridae) USNM E9756 B. Pentaster obtusatus (Oreasteridae) USNM C. Tremaster mirabilis (Asterinidae) USNM E46295 D. Nardoa tuberculata (Ophidiasteridae) E16509 E. Porania pulvillus (Poraniidae) USNM 11035 F. Crossaster campbellicus USNM 1122950.
The Poraniidae includes 22 species in seven genera [220], which are distributed in cold-water settings throughout the world, including high-latitude/polar regions and the deep-sea. Poraniids inhabit primarily cold-water settings, primarily at high latitudes or in the deep-sea [221] and are distinctive asteroids with a typically thickened fleshy body wall that has obscured the endoskeleton and made classification of the group difficult [222]. Our understanding of poraniid biology is largely based on information derived from two polar species, Porania antarctica and Porania pulvillus and the temperate water Poraniopsis spp.
Feeding in known poraniids [14] suggests that most are detritivores or predators. Bowden et al. [223] shows Porania antarctica feeding on stalked crinoids in the Antarctic. Ericsson and Hansson [224] observed P. pulvillus feed on octocorals, a brachiopod, and several ascidian species. Dearborn [202] observed P. antarctica feed on detritus, but sometimes preying on sea urchins. Gemmill [225] described ciliary suspension feeding in P. pulvillus, although further confirmation of this behavior has not been observed.
The Valvatida
In terms of numbers of taxa at all levels, including families, genera, and species, the Valvatida (Fig. 5) is the most taxonomically numerous within the Asteroidea and as such, life modes and ecology are diverse. Mah and Foltz's [69] analysis found that the Solasteridae (Fig. 5F), which have historically been assigned to the Spinulosida [158] were nested within the clade containing the Asterinidae, which has further extended the limit of diversity within the Valvatida.
Life modes in the Solasteridae are different from other Valvatida. Jangoux [14] outlined feeding of multiple solasterid taxa, including Solaster and Lophaster. Most solasterids are primarily predators of other mobile or otherwise active invertebrate taxa, including gastropods, cnidarians, and other echinoderms, such as holothurians and asteroids [226], [227]. Blake [1] has interpreted the decalcified skeletons, and wider, more open tube foot grooves as associated with active predation, but also as a more vulnerable body form, which may limit solasterids from tropical regions
Non-solasterid valvatidans possess a generalized life mode, feeding primarily on sessile prey items. Jangoux [14] summarized various benthic prey including encrusting algae, organic biofilm, foraminiferans, sponges, bryozoans, hydroids, corals, gorgonians, multiple anthozoans, ascidians, and various detrital food sources (e.g., fecal pellets, dead fish, urchins, etc.).
Different valvatidan taxa are involved in complex ecological interactions, especially with cnidarians. Acanthaster planci, the Indo-Pacific Crown-of-Thorns Starfish is an important predator of scleractinian reef corals [47]. Goniasterids are important predators of shallow-water pennatulaceans [228] as well as deep-sea corals [100], [229].
Blake [1], [4], [71] argued that the success of tropical-shallow-water valvatidans, such as the Oreasteridae, Ophidiasteridae, Acanthasteridae and others was related to multiple characters, such as spines, narrow tube foot furrows, thick granulated epidermis, and well-developed body skeletons, that provided defenses against predators. Many of these tropical shallow-water taxa are abundant and are significant members of the ecological communities of these regions [230]. The growth and biology of several several tropical valvatidans (e.g., oreasterids, archasterids) has become of increasing concern [231], [232], [233], [234] as many of these species are taken for tourist and aquarium/pet industries [235]. Linckia laevigata, a brilliant blue ophidiasterid is among one of the most heavily trafficked species in pet and tourist trades [236], [237].
Several high-latitude valvatidans, such as those that occur in the Antarctic, including odontasterids, ganeriids, and solasterids, are predators on sessile prey, such as sponges but also on other echinoderms [202], [238]. Several Antarctic valvatidans, such as the odontasterid Odontaster validus, Perknaster fuscus, and Acodontaster conspicuus are ecologically important [239]. Odontaster validus, probably is the most intensively studied of Antarctic asteroids [39], [109], [110], [238], [239], [240].
The Asterinidae have served as model organisms in developmental and reproductive biology as well as in ecology and conservation studies. Patiria miniata, the Pacific Northwest bat star, common along the west coast of North America, has become one of the primary model organisms in developmental gene studies [241], [242]. Building on this research, other taxa of asterinids have been heavily used in a wide variety of studies, including life history evolution [243], gene expression [244], and the evolution of reproduction and larval development [245], [246]. Many asterinids occupy intertidal and nearshore habitats and are important subjects in the study of marine ecosystems [247], [248] especially in the context of their reproductive biology [249].
Based on observations of feeding in most shallow-water to temperate species, most asterinids appear to be detritivores or omnivores that feed on encrusting organisms, algae, decaying corpses, and other detritus [14]. At least one asterinid, the New Zealand Stegnaster inflatus, has developed elaborate ambush methods for capturing mobile prey [250].
Diversity Within the Valvatida
The Valvatida is a diverse lineage that includes some of the most taxon-rich families within the Asteroidea. Most members of the Valvatida possess a well-defined marginal plate series that frequently outlines the periphery of the body. In addition, boundaries between plates are relatively well-defined and the disk is large with well-defined actinal regions, and a relatively heavily calcified or otherwise modified skeleton. Valvatidan taxa include the Acanthasteridae, Archasteridae, Asterodiscididae, Asteropseidae, Goniasteridae (Fig. 5A), Oreasteridae (Fig. 5B), Ophidiasteridae (Fig. 5D), and the Odontasteridae (Fig. 5D and see Table 1). Other taxa supported as valvatidans display substantial departure from this overall body plan, including the Asterinidae (Fig. 5C), Ganeriidae, and Solasteridae (Fig. 5F). No published molecular data is available for the enigmatic Podosphaerasteridae, but morphological studies [4], [251] have consistently placed it among the Valvatida.
Several members of the Valvatida are important members of tropical shallow-water settings, such as reefs, mangroves, and sandy bottoms [230]. Valvatidans typically found in these regions include Culcita (Oreasteridae), Acanthaster (Acanthasteridae), Protoreaster (Oreasteridae) and Archaster (Archasteridae). Many are widely distributed throughout the Indo-Pacific. For example, Acanthaster planci is present from the coast of Baja California, north to Hawaii and Japan, and is present west to the east coast of Africa in the Indian Ocean [47]. Although groups such as the Oreasteridae (Fig. 5B) and the Ophidiasteridae (Fig. 5D) are known primarily from tropical shallow-water habitats [3], [252], [253], many individual members of these groups occur in deeper water. Mah [254] and H.E.S. Clark [255] describe deep-water oreasterid taxa (Astrosarkus and Acheronaster, respectively). Deep-sea ophidiasterids, such as Tamaria are well documented [2], [99] but poorly understood.
Cold-water valvatidans are highly diverse (Table 2). The Goniasteridae (Fig. 5A) [73] includes the greatest number of genera (n = 65) and species (n = 256) within the living Asteroidea. Most goniasterids occur in cold-water settings, primarily the deep-sea (e.g., Litonotaster, Nymphaster), but also in Antarctic and subAntarctic settings (e.g., Pergamaster) [256] in cold to temperate water intertidal zones (e.g., Tosia). Some goniasterids (e.g., Fromia, Anchitosia) are also widely distributed in tropical habitats [229], [257], [258]. Although the Goniasteridae includes more taxa than almost any other family of asteroids, relatively few comprehensive reviews are available [99], [259], [260], [261].
The Odontasteridae [262] and Ganeriidae [263] occur mainly in the Antarctic and subAntarctic as well as in the deep-sea. Odontasterids were supported as basal to the clade containing all of the Valvatida and possess several characters, such as paxillate abactinal and marginal plates, that suggest shared, possibly plesiomorphic characters with the Paxillosida. Ganeriids are more derived and show close relationship to asterinids and solasterids.
Mah and Foltz [69] supported the Asterinidae as a member of the Valvatida and presented a potentially significant shift in asteroid classification by showing the traditional Asterinidae as a paraphyletic assemblage. This has changed the perception of the Asterinidae, from that of a traditionally derived, monophyletic grouping to a plesiomorphic grade relative to the more derived morphology in the Solasteridae and Ganeriidae. Some asterinids are shown as sister taxa to ganeriid and solasterid clades whereas others are present on more stemward positions on the Valatida clade.
The Asterinidae (Fig. 5E) [264] and Solasteridae (Fig. 5F) [265] are morphologically significantly different from the other Valvatida. The Solasteridae have historically been considered members of either the Spinulosida or the Velatida [4]. Many solasterids, including Solaster and Crossaster, possess anywhere from six to 15 arms and possess reticulated, lightly calcified skeletons compared to other valvatidans. Most solasterids occur in cold to temperate water settings, but one genus, Seriaster, occurs in the tropical shallow water settings of New Caledonia [266].
The Asterinidae are highly diverse, occupying different habitats and displaying a diverse, but consistent, series of body forms. Asterinids are morphologically distinctive with flattened bodies that range from swollen and thickened (e.g., Patiriella) to nearly parchment-like in thickness (e.g., Anseropoda) with body forms that range from pentagonal (e.g., Meridiastra, Tremaster) to more stellate (e.g., Nepanthia) and can have five to nine arms. Abactinal plates are either flat, scalar, and overlapping or are more crescentic-in shape approaching an appearance of chain-mail armor. O'Loughlin and Waters [267] summarized a full range of asterinid body forms. Most asterinid diversity is known from shallow tropical to temperate-water settings (e.g., Aquilonastra, Asterina, Parvulastra) with relatively small adult size (diameter = 0.5 to 2.0 cm). Temperate to cold-water forms, such as Patiria, Patiriella, and Stegnaster are larger in size (from eight to 15 cm in diameter). Cold-water asterinids, such as Tremaster (Fig. 6C) and Anseropoda show the largest sizes among the Asterinidae and are widely distributed in deep-sea settings, showing nearly global distributions with occurrence in Antarctica, the Indian Ocean, the central Pacific, Hawaii, and in the North Atlantic [267].
Figure 6. Forcipulatacea, Spinulosidan, Velatidan Diversity.
A. Ampheraster marianus (“Pedicellasteridae”-Forcipulatacea) USNM E16024. B. Henricia obesa (Echinasteridae) USNM 1120449. C. Remaster gourdoni (Korethrasteridae) USNM E 47646. D. Myxaster sol (Myxasteridae) Yale Peabody Museum 36040 E. Diplopteraster multipes (Pterasteridae) USNM 5530. F. Caymanostella spinimarginata (Caymanostellidae) USNM E 27575.
The Podosphaerasteridae includes the sole genus Podosphaeraster, which has been recorded from the deep-sea in the Atlantic and Pacific Oceans. Podosphaeraster is unique among asteroids in having a highly divergent, round, sphaere-like body shape, resulting in ongoing interest regarding plate homologies [251],[268]. A.M. Clark [269] originally assigned Podosphaeraster to the Mesozoic Sphaerasteridae. Blake [4], [270] disagreed with this assignment. Fujita and Rowe [251] later re-classified Podosphaeraster in a new monotypic family, the Podosphaerasteridae. Both Blake [4], [270] and Fujita and Rowe [251] outlined close affinities between Podosphaeraster and the Goniasteridae, but this relationship has not been fully tested with molecular data and although its classification is stable, many phylogenetic questions remain.
The Caymanostellidae (Fig. 6F) are dorsoventrally flattened with scalar plates and are known primarily from deep-sea wood substrates [271], [272] and superficially appear similar to concentricycloids. Little to nothing is known regarding caymanostellid biology or ecology. Given the unusual morphology of caymanostellids, especially given their resemblance to concentricycloids, determination of the phylogenetic position and classification of caymanostellids has been an active field of study.
Morphological evidence from fossils and modern forms have argued for an affinity with Tremaster and related tremasterines within the Asterinidae [272], [273], which suggested placement within the Valvatida. Caymanostellids were absent from Gale [5], [111] but were supported among the Velatida by Blake [4]. Caymanostella is supported among the Valvatida as the sister taxon to Archaster in the molecular tree of Janies et al [173]. Morphological and molecular evidence appears to support the Caymanostellidae as members of the Valvatida by several of the published studies. However, given uncertainties regarding taxon sampling, this relationship is expressed in Figure 2 as part of a valvatacean polytomy and among the Valvatacea in Table 1.
The Spinulosida
Phylogenetic efforts have changed the taxonomic composition of the Spinulosida in the 20th Century from the more inclusive definition outlined in Fisher [158] to the more restricted monotypic Spinulosida, which included only the Echinasteridae [4]. The Echinasteridae contains a large number of species (n = 133) assigned to a relatively small number of genera (n = 8) (Table 1) [70]. The largest genera are the tropical, shallow water Echinaster, which includes 27 species [274] distributed in the Atlantic, Indian, and Pacific Oceans and the globally distributed cold-temperate water Henricia, which includes 91 species [275]. Henricia is found at high-latitudes and in deep-sea settings.
Echinasterids generally possess a small disk with narrow, elongate arms and body wall plates that are similar in appearance, forming a reticulated mesh. Variably sized spinelets are found on every plate, these vary in shape from conical and thorny to fine and more nearly cylindrical.
Feeding in echinasterids varies, but a survey of known species of Henricia and Echinaster suggest that they consume microalgae, biofilms, and encrusting invertebrates, such as sponges and tunicates. Anderson [276] provided an important account of feeding and the digestive system in Henricia.
Diversity Within the Spinulosida
Henricia (Fig. 6B) includes 68% (91/133) of the total number of echinasterid species [275], a total strikingly disproportionate as compared to totals for other genera assigned to the family. Henricia is present in cold-water settings, such as in the deep-sea (to >1000 m) and in polar or subpolar regions [2], [65], [158], [275]. Many species of Henricia intergrade morphologically such that clearly defined boundaries are difficult to recognize [2], [158], [277]. Molecular and reproductive approaches to the systematics of Henrica have led to the discovery of new cryptic species, such as Henricia pumila from the well-studied intertidal regions of the Pacific Northwest [278].
Echinaster displays an issue similar to the one observed in Henricia. It is a wide-ranging species that shows intergradation and problematic species boundaries. Other echinasterid genera, such as Metrodira, Plectaster and Rhopiella, include far fewer species that have more restricted range distributions.
The Velatida
Based on the molecular phylogeny of Mah and Foltz [181], three families –the Pterasteridae (Fig. 6E) [74], Myxasteridae (Fig. 6D) [279], and Korethrasteridae (Fig. 6C) [280] are upheld as members of the Velatida, a classification that differs from Blake [4] who placed the Solasteridae and the Caymanostellidae within the Velatida.
The molecular phylogenies of Mah and Foltz [69], [181] support a monophyletic Velatida occupying a position separated from the Forcipulatacea and Valvatacea. Taxon sampling from within the Velatida was incomplete [181], but monophyly for the Korethrasteridae was supported. Asthenactis, a myxasterid was upheld within the Pterasteridae, but full taxon sampling remains ongoing. Morphology-supported phylogenies [4], [5], [111] have placed the Velatida in derived positions with the velatida embedded or closely related among taxa within other clades. The molecular phylogeny of Janies et al. [173] supported Xyloplax along with Pteraster and Hymenaster on a sister clade to the other living Asteroidea.
Pterasterids, korethrasterids, and myxasterids occur almost exclusively in cold-water settings, with most present in bathyal to abyssal or high-latitude habitats. The former three families possess paxillae covering the body surface. Oral plates are prominent, marginal plates weakly developed or absent, and pedicellariae are absent.
A unique, canopy-like secondary dorsal covering, a so-called “supradorsal membrane,” is found in the Pterasteridae. The supradorsal membrane is supported at the tips of highly elongate paxillae, and it encloses a so-called “nidamental cavity” between the membrane and the dorsal surface of the body. The nidamental cavity is open to the sea along the margins of the body and also through an opening or so-called osculum at the center of the dorsal disk. Muscles move water through the nidamental cavity, bringing fresh water to the respiratory papulae in the dorsal body wall. The supradorsal membrane is relatively sturdy, even canvas-like, in shallower-water Pteraster but more delicate and almost gelatinous in deeper-water Hymenaster. Pterasterids also have the ability to secrete copious amounts of apparently protective mucus [281], [282].
Reproductive biology in pterasterids is atypical and includes brooding [283], [284] and pelagic direct development [285], .
Food items of korethrasterids and myxasterids have yet to be recorded, but observations of Pteraster spp. show that they feed primarily on sponges [14], [227]. Gut contents of the deep-sea pterasterid Hymenaster suggest that they consume sediment and other detritus [14].
Diversity Within the Velatida
Nearly all velatidans are found in deep-water and polar habitats. Many species assigned to individual genera are similar in overall appearance and are geographically widely distributed.
The Myxasteridae (example in Fig. 6D) is composed of 9 species in 3 genera [279] and possess five to ten arms, a weakly calcified skeleton, and occur at bathyal/abyssal depths (750–3800 m depths) in the Atlantic and Pacific oceans. They are rarely encountered animals with fewer then fifteen specimens known for the family in collections throughout the world. The Korethrasteridae (Fig. 6C) occurs in Arctic, Antarctic and deep-sea regions, and includes only 7 species assigned to 3 genera [280]. Although korethrasterids are not as rare as myxasterids, biology of the group, including feeding and reproduction remain poorly understood. Korethrasterids consistently possess five rays with paxillar plates covering the body surface
Taxonomically, the Pterasteridae (Fig. 6E) is the most diverse within the Velatida including 116 species in 8 genera [74]. Nearly all pterasterids occur in either cold or temperate water habitats, especially in the deep-sea or at high-latitudes in Arctic and Antarctic regions. One exception is the widely distributed Euretaster, which occurs in tropical, shallow-water settings throughout the Indo-Pacific.
The Concentricycloidea
The Concentricycloidea, initially included the South Pacific Xyloplax medusiformis [7] and later came to include the tropical Atlantic X. turneri [8]. The original authors perceived the Concentricycloidea as morphologically distinct enough to warrant recognition at the class level.
Rowe et al [8] hypothesized that Xyloplax was “derived from asteroid asterozoans, possibly from a common ancestor of certain valvatids…” but clarified that “…the degree of developmental and morphological shift is such that it cannot be defined as a member of the class Asteroidea.” Work on spermatozoon morphology, spermiogenesis and microstructure [17], [287] were used to further argue the distinctiveness of Concentricycloidea as a separate class.
Following these initial reports, subsequent studies of Xyloplax classification emphasized phylogenetics, using cladistics to analyze synapomorphies, i.e., unique characters or molecular data that support a clade. Smith [288] placed concentricycloids within the Asteroidea, proposing shared synapomorphies between Xyloplax and the caymanostellid, Caymanostella. Pearse and Pearse [289] were the first to perform a phylogenetic analysis of Xyloplax along with other Echinodermata. Their results were equivocal, but they were unable to support submerging Xyloplax within the Asteroidea as proposed by Smith [288].
Janies and Mooi [290] and Janies [176] provided the first molecular/combined data analyses to include Xyloplax. Janies' tree supported Xyloplax as a derived branch, on the same branch as the asteriid Rathbunaster, within the Asteroidea using 18S and 28S rDNA sequences. Janies et al [173] later presented a molecular phylogeny, including data from seven loci (18S rRNA, 28S rRNA, histone H3 from the nucleus, 16S rRNA, 12S rRNA, cytochrome c oxidase subunit I, tRNA-Ala, tRNA-Leu, and tRNA-Pro of the mitochondrion), which placed Xyloplax as a sister taxon to a branch containing Hymenaster and Pteraster. Janies et al. [173] and Janies and McEdward [291], [292] argued that concentricycloids were progenetic velatid asteroids based on studies of larval asteroid morphology.
Mah [293] described a third species, Xyloplax janetae and presented a position intermediate between retaining Xyloplax as a separate class [7], [8] and inclusion within the Asteroidea [173], [288] by placing Concentricycloidea within the asteroid lineage, but as a sister-group to the Neoasteroidea, the group including all living asteroids. This placement is consistent with the hypothesis of an evolutionary bottleneck at the Permian-Triassic transition [168], which may have resulted in the extinction of Xyloplax's closest sister taxa.
Mah [293] does not necessarily disagree with new phylogenetic data. Separation of the Velatida from other asteroid groups and its possible position as sister taxon to the other asteroid groups on the tree is a new one. However, members of the Velatida possess several autapomorphies, such as the absence of a clear marginal series, the absence of pedicellariae, and the lack of actinal plates, that set the group apart from other neoasteroids. These have historically been interpreted as highly derived [4], [5] but taken in the context of Janies et al., [173] and the phylogenetic trees presented by Mah and Foltz [69], [181] the Velatida display prominence as a distinct group within the Neoasteroidea, separate from the Forcipulatacea and the Valvatacea. Janies et al. [173] supported Xyloplax as the sister group to other living velatidans. If the Velatida were to be supported as the sister-group to the remaining Neoasteroidea then Mah's placement of Xyloplax (including the Velatida) would be consistent with the basal position of Xyloplax as presented by Janies et al. [173] but not necessarily as the sister group to the Neoasteroidea. However, identification of the definitive sister group to modern asteroids from fossil morphology [4], [5], [111] remains unresolved and in need of continuing efforts. Definitive sister-group rooting for asteroid phylogeny using molecular data is premature with many obstacles, including taxon sampling and identification of long-branches that have yet to be overcome [294].
Extinct Groups
Most of the larger extant families of asteroids have been recognized in the fossil record, and although a few extinct families have been recognized, these are not large and do not differ greatly from those that do survive. Although fossil asteroids can be found all over the world, fossil deposits from the Mesozoic, especially the Cretaceous of Europe are among the most heavily studied and the best known. Accounts below are limited to extinct higher taxa with no surviving members.
Perhaps largely reflecting their modern occurrences and robust construction, most fossil taxa have been assigned to either Valvatida or Paxillosida. Included among extinct families is the Pycinasteridae is a small family, occuring primarily in the Mesozoic and early Cenozoic [94] that shows affinities with the Goniasteridae. The Stauranderasteridae has recently been reviewed by Villier et al. [295] and displays some morphological features that are reminiscent of the Oreasteridae. Paleobiology of stauranderasterids is poorly known, but at least some taxa have been collected from Jurassic tropical, shallow-water sediments [93]. The Mesozoic Sphaerasteridae was considered convergent with living Podosphaeraster by Blake [270] and were formally separated by Fujita and Rowe [251]. Relatively few recent accounts of fossil sphaerasterids [296], [297] are available.
Although the Goniasteridae is extant, a significant number of goniasterid genera occur only as fossil. A total of 102 living and extant goniasterid genera are recognized. Goniasterids can be broken down into three groups: 57 are known only from the extant, 8 are known from both living and fossil, and 37 are fossil-only genera. No other post-Paleozoic asteroids have such a significant number of taxa contributing to the overall diversity.
Among the non-valvatidan fossil groups within the Valvatacea is the Paleobenthopectininae [214], whose members were supported as the sister group to the extant Benthopectinidae within the Notomyotida as reconstructed by Blake [4]. Mah and Foltz [69] placed the benthopectinids as a lineage within the Paxillosida. Villier et al. [95] allied the Paleobenthopectininae as members of the Velatida with members showing affinities with the Myxasteridae. Blake et al. [169] described Noriaster, an early member of the Poraniidae from the Triassic of Northern Italy.
Within the Velatida is the monotypic Jurassic Tropidasteridae which Blake [298] supported as phylogenetically near velatidans, such as the Myxasteridae, Korethrasteridae and the Pterasteridae.
The Trichasteropsida is a member of the Forcipulatacea [4], [6] and occupies a basal position relative to other forcipulataceans, both owing to its phylogenetic position and its Triassic fossil occurrence, which places its two members, Trichasteropsis and Berckhermeraster among the earliest of post-Paleozoic fossil asteroids [6]. Gale [111] established the monotypic Terminasteridae within the Forcipulatida.
Conclusions and Future Research
Asteroid biodiversity and systematics remains an active area of research that has brought additional depth to our understanding of echinoderm evolution and historical changes in the marine setting.
The use of molecular tools to infer asteroid phylogeny and classification is still comparatively new, nevertheless significant changes have already emerged and this trend can be expected to continue at all taxonomic levels. For example, classification within the Asteriidae had been problematic since Fisher's [101], [183] revision of the Forcipulata. The recent revision of the Forcipulatacea by Mah and Foltz [181] shows strongly supported lineages within the Asteriidae that are not immediately obvious from external morphology. Zulliger and Lessios [83] presented a molecular phylogeny of the species-rich Astropecten, including taxa collected from throughout its range. Their work identified multiple species complexes and recognized morphological and ecological convergence among taxa present throughout Astropecten's global distribution.
Historically, interpretations of phylogeny have been based primarily on morphology, although early ontogeny has also played a significant role. Molecular phylogenetics circumvents the circularity of using morphology for interpretation of both phylogenetic history and functional phylogenetic changes. For example, taxa such as Pseudarchaster possess morphological adaptations that suggest living on unconsolidated sediment (e. g., presence of paxillae, well-developed fasciolar channels, etc.). However, emphasis on certain characters (e.g., suckered tube feet) has historically placed Pseudarchaster (and other pseudarchasterines) within the Valvatida precluding their inclusion within the Paxillosida, which has been historically defined by the presence of pointed tube feet. The molecular phylogeny of the Valvatacea by Mah and Foltz [69] supported Pseudarchaster as a member of the Paxillosida, running contrary to its traditional taxonomic position.
New collections of specimens from marine exploration continue to provide further data for our understanding of biodiversity in shallow-water and deep-sea settings. Additional sampling has not only added to our discovery of undescribed biodiversity [200], [229], [254], [257], [293] but has also provided us with new measures of zonation and abundance [299]. The availability of video has also brought an unprecedented wealth of ecological data from high resolution, in situ observations [100].
The fossil record is meager, but field and museum research continues to reveal important discoveries about the earlier history of asteroids, and can be expected to continue to do so.
In spite of the considerable progress, which has been summarized herein, several topics remain crucial for future research.
Basal phylogenetic relationships. In spite of comprehensive phylogenetic efforts, such as those of Mah and Foltz [69], [181] basal relationships among major lineages of asteroids remains a contentious subject. Support for early divergence of asteroid lineages has been elusive, pending discovery of more conserved genetic markers that will permit inference of basal relationships. Also important to understanding the early diversification of modern asteroids are fossils from the early Mesozoic/late Paleozoic that provide further evidence for early diversification of the crown-group.
Problematic groups. Xyloplax and Podosphaeraster. Current data from molecular phylogenies has not settled the phylogenetic questions regarding these enigmatic taxa and little is known regarding the biology and development of these highly unusual asteroids. These questions are, in part, tied to development of a well-supported phylogeny of the Asteroidea, which is concern #1 (above).
Undiscovered Biodiversity. A large potential exists for undiscovered asteroid taxa. This includes the potential for cryptic species that will likely be discovered in widely occurring deep-sea taxa. Museum collections of taxa from improved and increased expeditions, as well as living and fossil collections will also become important as unidentified material is processed.
Acknowledgments
The authors are greatly indebted to Sabine Stohr who invited Mah's participation in the project and Mark Costello who facilitated Mah's contributions to WoRMS. Jen Hammock, formerly of the U.S. Antarctic Research Program (and now with Encyclopedia of Life) is thanked for her assistance with image permissions and protocols. Adrian Testa provided photographs of specimens from the USARP. This manuscript benefited from unpublished data provided by Loic Villier, at the Universite de Provence in Marseille, France. David W. Foltz is thanked for his editorial support. John Pearse and an unknown reviewer provided useful editorial reviews of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This publication has been supported by National Science Foundation 1036358 Assembling the Echinoderm Tree of Life grant to Louisiana State University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Blake DB. Adaptive zones of the class Asteroidea (Echinodermata). Bull Mar Sci. 1990;46(3):701–718. [Google Scholar]
- 2.Clark AM, Downey ME. Starfishes of the Atlantic. 1992. 794 Chapman and Hall, London.
- 3.Clark AM, Rowe FWE. Monograph of Shallow-water Indo-West Pacific Echinoderms. Br Mus (Nat Hist) Publ. 1971;690:ix, 1–238, 31 pls. [Google Scholar]
- 4.Blake DB. Classification and phylogeny of post-Paleozoic sea stars (Asteroidea: Echinodermata). J Nat Hist. 1987;21:481–528. [Google Scholar]
- 5.Gale AS. Phylogeny and classification of the Asteroidea (Echinodermata). Zool J Linn Soc. 1987;89:107–132. [Google Scholar]
- 6.Blake DB, Hagdorn H. The Asteroidea (Echinodermata) of the Muschelkalk (Middle Triassic of German). Paläont Zeitschr. 2003;77(1):23–58. [Google Scholar]
- 7.Baker AN, Rowe FWE, Clark HES. A new class of Echinodermata from New Zealand. Nature. 1986;321:862–864. [Google Scholar]
- 8.Rowe FWE, Baker AN, Clark HES. The morphology, development and taxonomic status of Xyloplax Baker, Rowe and Clark, 1986 (Echinodermata: Concentricycloidea) with the description of a new species. Proc R Soc Lond B. 1988;233:431–459. [Google Scholar]
- 10.Hyman LH. The Invertebrates, 4, Echinodermata. 1955. 763 McGraw-Hill Book Company, New York.
- 11.Boolootian R. Physiology of Echinodermata. New York, London, Sydney: Interscience Publishers; 1966. 846 [Google Scholar]
- 12.Clark AM. Starfishes and related echinoderms, 3rd ed. British Museum (Natural History); 1977. 160 [Google Scholar]
- 13.Lawrence JM. A Functional Biology of Echinoderms. 1987. 340 John Hopkins University Press, Baltimore.
- 14.Jangoux M. Food and feeding mechanisms: Asteroidea. pp. 117–159. In: Jangoux M, Lawrence JM, A.A., editors. Echinoderm Nutrition. Balkema Rotterdam; 1982. 653 [Google Scholar]
- 15.Sloan NA. Aspects of the feeding biology of asteroids. Oceanogr Mar Biol Ann Rev. 1980;18:57–124. [Google Scholar]
- 16.Chia FS, Koss R. Ch. 4 Asteroidea in Microscopic Anatomy of Invertebrates: Echinodermata. 1994;vol. 14:169–245. [Google Scholar]
- 17.Rowe FWE, Healy JM, Anderson DT. Ch. 3 Concentricycloidea in Microscopic Anatomy of Invertebrates. 1994;vol. 14:149–167. [Google Scholar]
- 18.Lawrence JM. Function of eponymous structures in echinoderms: a review. Canadian Journal of Zoology. 2001:1251–1264. [Google Scholar]
- 19.Flammang P. Fine structure of the podia in three species of paxillosid asteroids of the genus Luidia (Echinodermata). Belg J Zool. 1995;125(1):125–134. [Google Scholar]
- 20.Flammang P. Adhesion in echinoderms. Echinoderm Stud. 1996;5:1–60. [Google Scholar]
- 21.Flammang P, Demeulenaere S, Jangoux M. The role of podial secretions in two species of sea stars (Echinodermata). Biol Bull. 1994;187:35–47. doi: 10.2307/1542163. [DOI] [PubMed] [Google Scholar]
- 22.Flammang P, Michel A, van Cauwenberge A, Alexandre H, Jangoux M. A study of the temporary adhesion of the podia in the sea star Asterias rubens (Echinodermata, Asteroidea) through their footprints. J Exp Biol. 1998;201:2383–2395. doi: 10.1242/jeb.201.16.2383. [DOI] [PubMed] [Google Scholar]
- 23.Santos R, Haesaerts D, Jangoux M, Flammang P. Comparative histological and immunohistochemical study of sea star tube feet (Echinodermata, Asteroidea). J Morph. 2005;263:259–269. doi: 10.1002/jmor.10187. [DOI] [PubMed] [Google Scholar]
- 24.Valentincic T. Innate and learned responses to external stimuli in asteroids. Echinoderm Stud. 1983;1:111–138. [Google Scholar]
- 25.Chia FS, Walker CW. Ch. 5. Echinodermata: Asteroidea, pp. 301–353. In: Giese AC, Pearse JS, Pearse VB, editors. Reproduction of Marine Invertebrates Vol. VI-Echinoderms and Lophophorates. Boxwood Press; 1991. 808 [Google Scholar]
- 26.McEdward LR, Miner BG. Larval and life-cycle patterns in echinoderms. Can J Zool. 2001;79:1125–1170. [Google Scholar]
- 27.Menge BA. Competition for food between two intertidal starfish species and its effect on body size and feeding. Ecology. 1972;53:635–644. [Google Scholar]
- 28.Menge BA. The effect of wave action and competition on brooding and competition on brooding and reproductive effort in the seastar Leptasterias hexactis. Ecology. 1974;55:84–93. [Google Scholar]
- 29.Menge BA. Brood or broadcast? The adaptive significance of different reproductive strategies in the two intertidal sea stars Leptasterias hexactis and Pisaster ochraceus. Mar Biol. 1975;31:87–100. [Google Scholar]
- 30.Menge BA, Daley BA, Lubchenco J, Sanford E, Dahlhoff E, et al. Top-down and bottom-up regulation of New Zealand rocky intertidal communities. Ecol Monogr. 1999;69(3):297–330. [Google Scholar]
- 31.Menge JL, Menge BA. Role of resource allocation, aggression, and spatial heterogeneity in coexistence of two competing intertidal starfish. Ecol Monogr. 1974;44:189–209. [Google Scholar]
- 32.Paine RT. Food web complexity and species diversity. Am Nat. 1966;100:65–75. [Google Scholar]
- 33.Paine RT. The Pisaster-Tegula interaction: prey patches, predator food preference and intertidal community structure. Ecology. 1969;50:950–961. [Google Scholar]
- 34.Paine RT. Intertidal Community Structure: Experimental studies on the relationship between a dominant competitor and its principal predator. Oecologia. 1974;15:93–120. doi: 10.1007/BF00345739. [DOI] [PubMed] [Google Scholar]
- 35.Pearse JS, Eernisse DJ. Photoperiodic regulation of gametogenesis and gonadal growth in the sea star Pisaster ochraceus. Mar Biol. 1982;67:121–125. [Google Scholar]
- 36.Pearse JS, Eernesse DJ, Pearse VB, Beauchamp KA. Photoperiodic regulation of gametogenesis in sea stars, with evidence for an annual calender independent of fixed daylength. Am Zool. 1986;26:417–431. [Google Scholar]
- 37.Pearse JS, Beauchamp KA. Photoperiodic regulation of feeding and reproduction in a brooding sea star from Central California. Int J Invertebr Reprod Dev. 1986;9:289–297. [Google Scholar]
- 38.Pearse JS, Walker CS. Photoperiodic regulation of gametogenesis in a North Atlantic sea star, Asterias vulgaris. Int J Invertebr Reprod Dev. 1986;9:71–77. [Google Scholar]
- 39.Pearse JS, Bosch I. Photoperiodic regulation of gametogenesis in the Antarctic sea star Odontaster validus Koehler: Evidence for a circannual rhythm modulated by light. Invert Reprod Develop. 2002;41(1–3):73–81. [Google Scholar]
- 40.Ortiz M, Jesse S, Stotz W, Wolff M. Feeding behavior of the asteroid Meyenaster gelatinosus in response to changes in abundance of the scallop Argopecten purpuratus in northern Chile. Arch Hydrobiol. 2003;157(2):213–225. [Google Scholar]
- 41.Ross DJ, Johnson CR, Hewitt CL. Impact of introduced seastars Asterias amurensis on survivorship of juvenile commercial bivalves Fulvia tenuicostata. Mar Ecol Prog Ser. 2002;241:99–112. [Google Scholar]
- 42.Ross DJ, Johnson CR, Hewitt CL. Variability in the impact of an introduced predator (Asterias amurensis: Asteroidea) on soft-sediment assemblages. J Exp Mar Biol Ecol. 2003;288:257–278. [Google Scholar]
- 43.Ross DJ, Johnson CR, Hewitt CL, Ruiz GM. Interaction and impacts of two introduced species on a soft-sediment marine assemblage in SE Tasmania. Mar Biol. 2004;144:747–756. [Google Scholar]
- 44.Ross DJ, Johnson CR, Hewitt CL. Abundance of the introduced seastar, Asterias amurensis, and spatial variability in soft sediment assemblages in SE Tasmania: Clear correlations but complex interpretation. Est Coas Shelf Sci. 2006;67:695–707. [Google Scholar]
- 45.Chesher RH. Destruction of Pacific corals by the sea star Acanthaster planci. Science. 1969;165(3890):280. doi: 10.1126/science.165.3890.280. [DOI] [PubMed] [Google Scholar]
- 46.Brodie J, Fabricius K, De'ath G, Okaji K. Are increased nutrient inputs responsible for more outbreaks of crown of thorns starfish? An appraisal of the evidence. Mar Pollut Bull. 2005;51(1–4):266–278. doi: 10.1016/j.marpolbul.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 47.Birkeland C, Lucas JS. Acanthaster planci: Major Management Problem of Coral Reefs. CRC Press; 1990. 257 [Google Scholar]
- 48.Coteur G, Gosselin P, Wantier P, Chambost-Manciet Y, Danis B, et al. Echinoderms as bioindicators, bioassays, and impact assessment tools of sediment- associated metals and PCBs in the North Sea. Arch Environ Contam Toxicol. 2003;45(2):190–202. doi: 10.1007/s00244-003-0199-x. [DOI] [PubMed] [Google Scholar]
- 49.Danis B, Cotret O, Teyssie JL, Fowler SW, Bustamante P, et al. Delineation of PCB uptake pathways in a benthic sea star using a radiolabelled congener. Mar Ecol Prog Ser. 2003;253:155–163. [Google Scholar]
- 50.O'Clair CE, Rice SD. Depression of feeding and growth rates of the seastar Evasterias troschelii during long-term exposure to the water-soluble fraction of crude oil. Mar Biol. 1985;84(3):331–340. [Google Scholar]
- 51.Georgiades ET, Danis B, Gillan DC, Dubois Ph, Temara A, et al. Effect of crude oil contaminated sediment exposure on cytochrome P450 enzymes in the Australian asteroid Coscinasterias muricata. Chemosphere. 2006;65(10):1869–1877. doi: 10.1016/j.chemosphere.2006.03.071. [DOI] [PubMed] [Google Scholar]
- 52.Cerra A, Byrne M. Evolution of development in the sea star genus Patiriella: clade-specific alterations in cleavage. Evol Dev. 2004;6(2):105–113. doi: 10.1111/j.1525-142x.2004.04015.x. [DOI] [PubMed] [Google Scholar]
- 53.Kitajima A, Hamaguchi Y. Determination of first cleavage plane: the relationships between the orientation of the mitotic apparatus for first cleavage and the position of meiotic division-related structures in starfish eggs. Dev Biol. 2005;280(1):48–58. doi: 10.1016/j.ydbio.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 54.Melarange R, Elphick MR. Comparative analysis of nitric oxide and SALMFamide neuropeptides as general muscle relaxants in starfish. J Exp Biol. 2003;206(5):893–899. doi: 10.1242/jeb.00197. [DOI] [PubMed] [Google Scholar]
- 55.Gollub M, Shaw L. Isolation and characterization of cytidine-5′-monophosphate-N-acetylneuraminate hydroxylase from the starfish Asterias rubens. Comp Biochem Physiol B, Biochem Mol Biol. 2003;134B(1):89–101. doi: 10.1016/s1096-4959(02)00190-2. [DOI] [PubMed] [Google Scholar]
- 56.Koseoglu M, Eroglu A, Toner M, Sadler KC. Starfish oocytes form intracellular ice at unusually high temperatures. Cryobiol. 2001;43(3):248–259. doi: 10.1006/cryo.2001.2348. [DOI] [PubMed] [Google Scholar]
- 57.Stickle WB, Weidner EH, Kozloff EN. Parasitism of Leptasterias spp. (Echinodermata: Asteroidea) by the ciliated protozoan Orchitophrya stellarum (Scuticociliata). Invertebr Biol. 2001;120(1):88–95. [Google Scholar]
- 58.McClintock JB, Amsler MO, Angus RA, Challenger RC, Schram JB, et al. The Mg-Calcite composition of Antarctic echinoderms: Important implications for predicting the impacts of ocean acidification. J Geol. 2011;119(5):457–466. [Google Scholar]
- 59.Gooding RA, Harley CDG, Tang E. Elevated water temperature and carbon dioxide concentration increase the growth of a keystone echinoderm. Proc Nat Acad Sci. 2009;106(23):9316–9321. doi: 10.1073/pnas.0811143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szathmary PL, Helmuth B, Wethey DS. Climate change in the rocky intertidal zone: predicting and measuring the body temperature of a keystone predator. Mar Ecol Prog Ser. 2009;374:43–56. [Google Scholar]
- 61.Blake DB. Asteroidea: Functional morphology, classification and phylogeny. Echinoderm Studies. 1989;3:179–223. [Google Scholar]
- 62.Mah CL. World Asteroidea Database. 2009. Available: http://www.marinespecies.org/asteroidea. Accessed 2011 March 28.
- 63.Clark AM. An index of names of recent Asteroidea, Part 1: Paxillosida and Notomyotida. Echinoderm Stud. 1989;3:225–347. [Google Scholar]
- 64.Clark AM. An index of names of recent Asteroidea, Part 2: Valvatida. Echinoderm Stud. 1993;4:187–366. [Google Scholar]
- 65.Clark AM. An index of names of recent Asteroidea-Part 3: Velatida and Spinulosida. Echinoderm Stud. 1996;5:183–250. [Google Scholar]
- 66.Clark AM, Mah C. An index of names of recent Asteroidea - Part 4: Forcipulatida and Brisingida. Echinoderm Stud. 2001;6:229–347. [Google Scholar]
- 67.Lemaitre R, Harasewych MG, Hammock J, editors. ANTIZ v 1.07: A Database of Antarctic and Subantarctic Marine Invertebrates. 2009. National Museum of Natural History, Smithsonian Institution. World Wide Web electronic publication. URL http://invertebrates.si.edu/ANTIZ.
- 68.Stöhr S, O'Hara T. World Ophiuroidea Database. 2007. Available: http://www.marinespecies.org/ophiuroidea. Accessed 2011 October 08.
- 69.Mah CL, Foltz DW. Molecular Phylogeny of the Valvatacea (Asteroidea, Echinodermata). Zool J Linn Soc. 2011;161:769–788. [Google Scholar]
- 70.Mah C, Hansson H. Echinasteridae. In: Mah, C.L. (2011) World Asteroidea Database. Accessed through: Mah, C.L. (2011). 2011. World Asteroidea Database at http://www.marinespecies.org/Asteroidea/aphia.php?p=taxdetailsandid=123132 on 2011-10-27.
- 71.Blake DB. Some biological controls on the distribution of shallow-water sea stars (Asteroidea; Echinodermata). Bull Mar Sci. 1983;33(3):703–712. [Google Scholar]
- 72.Mah C, Hansson H. Astropectinidae. In: Mah CL (2011) World Asteroidea Database. Accessed through: Mah CL (2011). 2011. World Asteroidea Database at http://www.marinespecies.org/asteroidea/aphia.php?p=taxdetailsandid=123127 on 2011-10-04.
- 73.Mah C, Hansson H. Goniasteridae. In: Mah CL (2011). World Asteroidea Database. Accessed through: Mah CL (2011). 2011. World Asteroidea Database at http://www.marinespecies.org/Asteroidea/aphia.php?p=taxdetailsandid=123135 on 2011-10-10.
- 74.Mah C, Hansson H. Pterasteridae. In: Mah CL (2011). World Asteroidea Database. Accessed through: Mah CL (2011). 2011. World Asteroidea Database at http://www.marinespecies.org/Asteroidea/aphia.php?p=taxdetailsandid=123142 on 2011-10-10.
- 75.Duxbury AC, Duxbury AB, Sverdrup KA. An Introduction to the World's Oceans 10th edition. McGraw Hill Science/Education/Math Publishers; 2010. 528 [Google Scholar]
- 76.Fontanella FM, Hopkins TM. Preliminary phylogeny of Echinaster (Othilia) from the Gulf of Mexico based on morphological characters (Echinodermata: Asteroidea). 2001. 337 Pp. 91–95 in Echinoderm Research 2001 (Banyuls-sur-Mer), Féral and David (eds)
- 77.Kochzius M, Seidel C, Hauschild J, Kirchhoff S, Mester P, et al. Genetic population structures of the blue starfish Linckia laevigata and its gastropod ectoparasite Thyca crystalline. Mar Ecol Prog Ser. 2009;396:211–219. [Google Scholar]
- 78.Williams ST. Species boundaries in the starfish genus Linckia. Mar Biol. 2000;136:137–148. [Google Scholar]
- 79.Williams ST, Benzie JAH. Indo-West Pacific patterns of genetic differentiation in the high-dispersal starfish Linckia laevigata. Mol Ecol. 1997;6:559–573. [Google Scholar]
- 80.Williams ST, Benzie JAH. Evidence of a biogeographic break between populations of a high dispersal starfish: congruent regions within the Indo-West Pacific defined by color morphs, mt DNA, and allozyme data. Evolution. 1998;52(1):87–99. doi: 10.1111/j.1558-5646.1998.tb05141.x. [DOI] [PubMed] [Google Scholar]
- 81.Vogler C, Benzie J, Lessios H, Barber P, Wörheide G. A threat to coral reefs multiplied? Four species of crown-of-thorns starfish. Biol Lett. 2008;4:696–699. doi: 10.1098/rsbl.2008.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gérard K, Roby C, Chevalier N, Thomassin B, Chenuil A, et al. Assessment of three mitochondrial loci variability for the crown-of-thorns starfish: A first insight into Acanthaster phylogeography. Compt Rend Biol. 2008;331:137–143. doi: 10.1016/j.crvi.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 83.Zulliger DE, Lessios HA. Phylogenetic relationships in the genus Astropecten Gray (Paxillosida: Asteropectinidae) on a global scale: molecular evidence for morphological convergence, species-complexes and possible cryptic speciation. Zootaxa. 2010;2504:1–19. [Google Scholar]
- 84.Waters JM, Roy MS. Global phylogeography of the fissiparous sea-star genus Coscinasterias. Mar Biol. 2003;142:185–191. [Google Scholar]
- 85.Waters JM, Roy MS. Marine biogeography of south Australia: phylogeographical structure in a temperate sea-star. Journal of Biogeography. 2003;30:1787–1796. [Google Scholar]
- 86.Ayers KL, Waters JM. Marine biogeographic disjunction in central New Zealand. Marine Biology. 2005;147:1045–1052. [Google Scholar]
- 87.Naughton KM, O'Hara TD. A new brooding species of the biscuit star Tosia (Echinodermata: Asteroidea : Goniasteridae), distinguished by molecular, morphological and larval characters. Invertebr Syst. 2009;23(4):348–366. [Google Scholar]
- 88.Alton MS. Bathymetric distribution of sea stars (Asteroidea) off the northern Oregon coast. J Fish Res Board Can. 1966;23(11):1673–1714. [Google Scholar]
- 89.Madsen FJ. The Porcellanasteridae: A monographic revision of an abyssal group of sea-stars. Galathea Rep. 1961;4:33–174, 13 plates. [Google Scholar]
- 90.Downey ME. Revision of the Atlantic Brisingida (Echinodermata: Asteroidea), with description of a new genus and family. Smtihson Contrib Zool. 1986;435:1–57. [Google Scholar]
- 91.Blake DB, Zinsmeister WJ. Two early Cenozoic sea stars (Class Asteroidea) from Seymour Island, Antarctic Peninsula. J Paleont. 1979;53(5):1145–1154. [Google Scholar]
- 92.Mah CL. Phylogeny of the Zoroasteridae (Zorocallina; Forcipulatida): Evolutionary Events in Deep-Sea Asteroidea displaying Paleozoic Features. Zool J Linn Soc. 2007;150:177–210. [Google Scholar]
- 93.Villier L, Blake DB, Jagt JWM, Kutscher M. A preliminary phylogeny of the Pterasteridae (Echinodermata, Asteroidea) and the first fossil record: Late Cretaceous of Germany and Belgium. Paläontol Z. 2004;78(2):281–299. [Google Scholar]
- 94.Jagt JWM. Late Cretaceous-Early Palaeogene echinoderms and the K/T boundary in the southeast Netherlands and northeast Belgium-Part 5: Asteroids. Scr Geol. 2000;121:377–503. [Google Scholar]
- 95.Villier L, Charbonnier S, Riou B. Sea stars from Middle Jurassic Lagerstätte of La Volute sur Rhône (Ardèche, France). J Paleont. 2009;83(3):389–398. [Google Scholar]
- 96.Yamaoka M. Fossil asteroids from the Miocene Morozaki group, Aichi Prefecture, Central Japan. Kaseki No Tomo. 1987;31:5–23. (in Japanese) [Google Scholar]
- 97.Downey ME. Zorocallida, new order, and Doraster constellatus, new genus and species, with notes on the Zoroasteridae (Echinodermata: Asteroidea). Smithson Contrib Zool. 1970;64:1–18. [Google Scholar]
- 98.Howell KL, Rogers AD, Tyler PA, Billett DSM. Reproductive isolation among morphotypes of the Atlantic sea star species Zoroaster fulgens (Asteroidea: Echinodermata). Mar Biol. 2004;144:977–984. [Google Scholar]
- 99.Clark HES, McKnight DG. The Marine Fauna of New Zealand: Echinodermata: Asteroidea (sea-stars). NIWA Biodiv Mem. 2001;117:1–269. [Google Scholar]
- 100.Mah CL, Nizinski M, Lundsten L. Phylogenetic Revision of the Hippasterinae (Goniasteridae; Asteroidea): Systematics of Deep Sea Corallivores, including one new genus and three new species. Zool J Linn Soc. 2010;160:266–301. [Google Scholar]
- 101.Fisher WK. Asteroidea of the North Pacific and adjacent waters. Part 3. Forcipulata (concluded). Bull US Nat Mus. 1930;76(3):1–356. [Google Scholar]
- 102.Grainger EH. Sea Stars (Echinodermata: Asteroidea) of Arctic North America. Fisheries Research Board of Canada Bulletin. 1966;152:1–70. [Google Scholar]
- 103.Djakonov AM. Starfish of the USSR Seas. Israel Program for scientific translations, Ltd. Jerusalem 183 pp. (translated from the Russian Djakonov, 1950 Starfish of the Soviet Union, Tabl Anal Faune URSS. 1968;34:1–203). [Google Scholar]
- 104.Foltz DW, Nguyen AT, Kiger JR, Mah CL. Pleistocene speciation of sister taxa in a North Pacific clade of brooding sea stars (Leptasterias). Mar Biol. 2008;154:593–602. [Google Scholar]
- 105.Flowers JM, Foltz DW. Reconciling molecular systematics and traditional taxonomy in a species-rich clade of sea stars (Leptasterias subgenus Hexasterias). Mar Biol. 2001;139:475–483. [Google Scholar]
- 106.Hrincevich AW, Rocha-Olivares R, Foltz DW. Phylogenetic analysis of molecular lineages in a species-rich subgenus of sea stars (Leptasterias subgenus Hexasterias). Am Zool. 2000;40(3):365–374. [Google Scholar]
- 107.Wares J. Biogeography of Asterias: North Atlantic climate change and speciation. Biol Bull. 2001;201:95–103. doi: 10.2307/1543530. [DOI] [PubMed] [Google Scholar]
- 108.Clark AM. Asteroidea. BANZ Antarctic Research Expedition 1929–1931. 1962;B9:68–70. [Google Scholar]
- 109.Janosik AM, Halanych KM. Unrecognized Antarctic Biodiversity: A case study of the genus Odontaster (Odontasteridae; Asteroidea). Integ Comp Biol. 2010;50:981–992. doi: 10.1093/icb/icq119. [DOI] [PubMed] [Google Scholar]
- 110.Janosik AM, Mahon AR, Halanych KM. Evolutionary history of Southern Ocean Odontaster sea star species (Odontasteridae; Asteroidea). Polar Biol. 2011;34:575–586. [Google Scholar]
- 111.Gale AS. The phylogeny of post-Paleozoic Asteroidea (Neoasteroidea, Echinodermata). Spec Pap Palaeontol. 2011;85:1–112. [Google Scholar]
- 112.Spencer WK. Early Palaeozoic starfish. Phil Trans R Soc, London, ser B. 1951;235:87–129. doi: 10.1098/rstb.1951.0001. [DOI] [PubMed] [Google Scholar]
- 113.Spencer WK, Wright CW. Asterozoans, Part U: Echinodermata. In: Moore RC, editor. Treatise on Invertebrate Paleontology. 1. Vol. 3. Lawrence: University of Kansas Press; 1966. pp. U4–U107. [Google Scholar]
- 114.Shackleton JD. Skeletal homologies, phylogeny and classification of the earliest asterozoan echinoderms. J Syst Palaeontol. 2005;3:29–114. [Google Scholar]
- 115.Dean J. What makes an ophiuroid? A morphological study of the problematic Ordovician stelleroid Stenaster and the palaeobiology of the earliest asteroids and ophiuroids. Zool J Linn Soc. 1999;126:225–250. [Google Scholar]
- 116.Blake DB. A new Ordovician asteroid (Echinodermata) with somasteroid-like skeletal elements. J Paleont. 2008;82(4):645–656. [Google Scholar]
- 117.Fell HB. The phylogeny of sea-stars. Phil Trans Royal Soc London, Ser B. 1963;246:381–435. [Google Scholar]
- 118.Lehmann WM. Die Asterozoen in den Dachschiefern des rheinischen Unterdevons. Abh Hess Landesamtes Bodenforsch. 1957;21:1–160. [Google Scholar]
- 119.LeClair E. Effects of anatomy and environment on the relative preservability of asteroids: a biomechanical comparison. Palaios. 1993;8:233–243. [Google Scholar]
- 120.Villier L. Reconstitution du squelette d'astérides fossils à partir d'ossicules isolés: intérêt taxinomique et phylogénétique. CR Acad Sci. 1999;328:353–358. [Google Scholar]
- 121.Schuchert C. Revision of Paleozoic Stelleroidea with special reference to North American Asteroidea. Bull US Nat Mus. 1915;88:1–311. [Google Scholar]
- 122.Ubaghs G. Piveteau, editor. Classe des Stelléroides (Stelleroidea). Pp. 774–842. Traité de Paléontologie III. 1953. 1063 Masson et Cie Editeurs, Paris.
- 123.Bartels C, Briggs DEG, Brassel G. The Fossils of the Hunsrück Slate: Marine Life in the Devonian. 1998. Cambridge Paleobiology Series 3, 309 p., Cambridge (Cambridge University Press)
- 124.Blake DB. Re-evaluation of the Devonian family Helianthasteridae Gregory, 1899 (Asteroidea: Echinodermata). Paläont Zeitschr. 2009;83:293–308. [Google Scholar]
- 125.Smith AB, Jell AB. Cambrian edrioasteroids from Australia and the origin of starfishes. Mem Queensl Mus. 1990;28(2):715–778. [Google Scholar]
- 126.Zhao Y, Sumrall CD, Parsley RL, Peng J. Kailidiscus, a new plesiomorphic edrioasteroid from the basal Middle Cambrian Kaili Biota of Guizhou Province, China. J Paleont. 2010;84(4):688–680. [Google Scholar]
- 127.Fell HB. The evolution of the echinoderms. Ann Rep Board of Regents Smithson Inst, Publ. 1963;4518:457–490. [Google Scholar]
- 128.Philip GM. Ancestry of Sea Stars. Nature. 1965;206:766–768. [Google Scholar]
- 129.Guensburg T, Sprinkle J. The oldest known crinoids and a new look at crinoid origins. Am Paleont. 2003;11(3):3–5. [Google Scholar]
- 130.Guensburg TE, Sprinkle J. Solving the mystery of crinoid ancestry: New fossil evidence of arm origin and development. J Paleont. 2009;83:350–364. [Google Scholar]
- 131.Mooi R. Not all written in stone: interdisciplinary syntheses in echinoderm paleontology. Can J Zool. 2001;79:1209–1231. [Google Scholar]
- 132.Spencer WK. British Palaeozoic Asterozoa. 1914–1940. 540 Pt. 1–10. Palaeontogr Soc Monogr (Lond)
- 133.Fell HB. A surviving somasteroid from the eastern Pacific Ocean. Science. 1962a;136:633–636. doi: 10.1126/science.136.3516.633. [DOI] [PubMed] [Google Scholar]
- 134.Fell HB. A living somasteroid, Platasterias latiradiata Gray. Univ Kans Paleontol Contrib, Art. 1962b;6:1–16. [Google Scholar]
- 135.Madsen FJ. The Recent Sea-star Platasterias and the fossil Somasteroidea. Nature. 1966;209:1367. [Google Scholar]
- 136.Blake DB. Sea Star Platasterias: Ossicle morphology and taxonomic position. Science. 1972;175:306–307. doi: 10.1126/science.176.4032.306. [DOI] [PubMed] [Google Scholar]
- 137.Müller AH. Lehrbuch der Paläozoologie, v. 2, Invertebraten, pt. 3, Arthropoda 2 – Stomochorda. 1963. 698 Veb Gustav Fischer Verlag, Jena.
- 138.Kesling RV. Notes on Protopalaeaster narrawayi Hudson. J Paleontol. 1962;36:933–942. [Google Scholar]
- 139.Kesling RV. A drastic reappraisal of “Lepidasterella babcocki Schuchert” as Helianthaster gyalinus Clarke a streptophiuran auluroid. Contrib Mus Paleont Univ Mich. 1964;19:115–133. [Google Scholar]
- 140.Kesling RV. Neopalaeaster enigmaticus, new starfish from Upper Mississippian Paint Creek Formation in Illinois. Contrib Mus Paleont Univ Mich. 1967;21:73–85. [Google Scholar]
- 141.Kesling RV. Silicaster, a new genus of Devonian starfish. Contrib Mus Paleont Univ Mich. 1969;22:249–261. [Google Scholar]
- 142.Kesling RV. Three Permian starfish from Western Australia and their bearing on revision of the Asteroidea. Contrib Mus Paleont Univ Mich. 1969;22:361–376. [Google Scholar]
- 143.Kesling RV. Drepanaster wrighti, a new species of brittle star from the Middle Devonian Arkona Shale of Ontario. Contrib Mus Paleont Univ Mich. 1970;23(4):73–79. [Google Scholar]
- 144.Kesling RV. Antiquaster magrumi, a new unusual brittle-star from the Middle Devonian Silica Formation of northwestern Ohio. Contrib Mus Paleont Univ Mich. 1971;23:181–191. [Google Scholar]
- 145.Kesling RV, Strimple HL. Calliasterella americana, a new starfish from the Pennsylvanian of Illinois. J Paleont. 1966;40:1157–1166. [Google Scholar]
- 146.McKnight DG. Classification of somasteroids and asteroids (Asterozoa: Echinodermata). J Roy Soc New Zeal. 1975;5:13–19. [Google Scholar]
- 147.Schuchert C. Fossilium Catalogus, I: Animalia, pars 3, Stelleroidea Palaeozoica. W. Junk (Berlin); 1914. 53 [Google Scholar]
- 148.Sladen WP. Report on the Asteroidea. Report on the Scientific Results of the Voyage of H.M.S. Challenger during the years 1873–1876 . Zoology. 1889;30(51):xlii+893 pages 118 plates. [Google Scholar]
- 149.Schöndorf F. Ueber einige “Ophiuriden und Asteriden” des englischen Silur und ihre Bedeutung für die Systematik paläozoischer Seesterne. Jahrb. Nassauischen Vereins Naturk. 1910;63:206–256. [Google Scholar]
- 150.Blake DB. Compsaster formosus Worthen and Miller (Asteroidea; Echinodermata): A Carboniferous homeomorph of the post-Paleozoic Asteriidae. Palaeont Zeitschr. 2002;76:357–367. [Google Scholar]
- 151.Blake DB, Guensburg TE. Predation by the Ordovician asteroid Promopalaeaster on a pelecypod. Lethaia. 1994;27:235–239. [Google Scholar]
- 152.Herringshaw LG, Smith MP, Thomas AT. Evolutionary and ecological significance of Lepidaster grayi, the earliest multiradiate starfish. Zool J Linn Soc. 2007;150:743–754. [Google Scholar]
- 153.Blake DB, Rozhnov S. Aspects of life mode among Ordovician asteroids: Implications of new specimens from Baltica. Acta Palaeontol Pol. 2007;53:519–533. [Google Scholar]
- 154.Cuénot L. Grassé, editor. Anatomie, Ethologie et Systématique des Echinodermes. Pp. 3–363. Traité de Zoologie-Anatomie, Systématique, Biologie. 1966. 1077 Masson et Cie Editeurs, Paris.
- 155.Perrier E. Mémoire sur les étoiles de mer recueillis dans la Mer des Antilles et la Golfe de Mexique Durant les expeditions de dragage faites sous la direction de M. Alexandre Agassiz. Nouvelles Arch Mus Hist Nat, Paris. 1884;6(2):127–276. [Google Scholar]
- 156.Viguier C. Classification des Stellérides. Compt Rend Acad Sci, Paris. 1878;84:681–683. [Google Scholar]
- 157.Perrier E. Stellérides. Expéd Sci Travailleur-Talismann. 1894;3:1–431. [Google Scholar]
- 158.Fisher WK. Asteroidea of the North Pacific and adjacent waters. 1. Phanerozonia and Spinulosida. Bull US Nat Mus. 1911;76:xiii+420: 1–122 pls. [Google Scholar]
- 159.Mortensen T. Echinoderm larvae and their bearing on classification (reply). Nature. 1922;110:806–807. [Google Scholar]
- 160.Mortensen T. Echinoderm larvae and their bearing on classification (reply). Nature. 1923;111:322–323. [Google Scholar]
- 161.MacBride EW. Echinoderm larvae and their bearing on evolution. Nature. 1921;108:529–530. [Google Scholar]
- 162.MacBride EW. Echinoderm larvae and their bearing on classification (reply). Nature. 1923;111:47. [Google Scholar]
- 163.MacBride EW. Echinoderm larvae and their bearing on classification (reply). Nature. 1923;111:323–324. [Google Scholar]
- 164.Döderlein LH. Die Asteriden der Siboga-Exped. 1. Die Gattung Astropecten und ihre Stammesgeschicte. Siboga Exped. 1917;46a:1–190. [Google Scholar]
- 165.Döderlein LH. Die Asteriden der Siboga-Exped. 2. Die Gattung Luidia und ihre Stammesgeschicte. Siboga-Exped. 1920;46b:193–291. [Google Scholar]
- 166.Clark HL. The starfishes of the genus Heliaster. Bull Mus Comp Zool. 1920;51(2):25–76. [Google Scholar]
- 167.Madsen FJ. On the zoogeography and origin of the abyssal fauna in view of the knowledge of the Porcellanasteridae. Galathea Rep. 1961;4:177–218. [Google Scholar]
- 168.Twitchett RJ, Oji T. Early Triassic recovery of echinoderms. C R Palevol. 2005;4:531–542. [Google Scholar]
- 169.Blake DB, Tintori A, Hagdorn H. A new, early crown-group asteroid (Echinodermata) from the Norian (Triassic) of Northern Italy. Riv Ital Paleontol S. 2000;106(2):141–156. [Google Scholar]
- 170.Blake DB, Bielert F, Bielert U. New crown-group asteroids (Echinodermata; Triassic of Germany). Paläont Zeitschr. 2006;80/3:284–295. [Google Scholar]
- 171.Wada H, Komatsu M, Satoh N. Mitochondrial rDNA phylogeny of the Asteroidea suggests the primitiveness of the Paxillosida. Mol Phylogenet Evol. 1996;6(1):97–106. doi: 10.1006/mpev.1996.0062. [DOI] [PubMed] [Google Scholar]
- 172.Lafay B, Smith AB, Christen R. A combined morphological, and molecular approach to the phylogeny of asteroids. Syst Biol. 1995;44(2):190–208. [Google Scholar]
- 173.Janies D, Voight J, Daly M. Echinoderm Phylogeny Including Xyloplax, a Progenetic Asteroid. Sys Biol. 2011;60(4):420–438. doi: 10.1093/sysbio/syr044. [DOI] [PubMed] [Google Scholar]
- 174.Matsubara M, Komatsu M, Araki T, Asakawa S, Yokobori S, et al. The phylogenetic status of Paxillosida (Asteroidea) based on complete mitochondrial DNA sequences. Mol Phylogenet Evol. 2005;36:598–605. doi: 10.1016/j.ympev.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 175.Knott KE, Wray GE. Controversy and consensus in asteroid systematics: new insights to ordinal and familial relationships. Am Zool. 2000;40(3):382–392. [Google Scholar]
- 176.Janies D. Phylogenetic relationships of extant echinoderm classes. Can J Zool. 2001;79:1232–1250. [Google Scholar]
- 177.Matsubara M, Komatsu M, Wada H. Close relationship between Asterina and Solasteridae (Asteroidea) supported by both nuclear and mitochondrial gene molecular phylogenies. Zool Sci. 2004;21:785–793. doi: 10.2108/zsj.21.785. [DOI] [PubMed] [Google Scholar]
- 178.Waters JM, O'Loughlin PM, Roy MS. Molecular systematics of some Indo-Pacific asterinids (Echinodermata, Asteroidea): does taxonomy reflect phylogeny. Molecular Phylogenetics and Evolution. 2004;30:872–878. doi: 10.1016/j.ympev.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 179.Yasuda N, Nagai S, Hamaguchi M, Okaji K, Gérard K, et al. Gene flow of Acanthaster planci (L.) in relation to ocean currents revealed by microsatellite analysis. Mol Ecol. 2009;18:1574–1590. doi: 10.1111/j.1365-294X.2009.04133.x. [DOI] [PubMed] [Google Scholar]
- 180.Foltz DW, Bolton MT, Kelley SP, Kelley BD, Nguyen AT. Combined mitochondrial and nuclear sequences support the monophyly of forcipulatacean sea stars. Mol Phylogenet Evol. 2007;43:627–634. doi: 10.1016/j.ympev.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 181.Mah CL, Foltz DW. Molecular Phylogeny of the Forcipulatacea (Asteroidea: Echinodermata): Systematics and Biogeography. Zool J Linn Soc. 2011;162:646–660. [Google Scholar]
- 182.Mah C. Forcipulatacea. Accessed through: Mah, C.L. (2012). 2012. World Asteroidea database at http://www.marinespecies.org/Asteroidea/aphia.php?p=taxdetails&id=582527 on 2012-03-04.
- 183.Fisher WK. Asteroidea of the North Pacific and adjacent waters. Part 2. Forcipulata (part). Bull US Nat Mus. 1928;76(2):1–245. [Google Scholar]
- 184.Mah C. Preliminary phylogeny of the forcipulatacean Asteroidea. Am Zool. 2000;40(3):375–381. [Google Scholar]
- 185.Gaymer CF, Himmelman JH, Johnson LE. Distribution and feeding ecology of the seastars Leptasterias polaris and Asterias vulgaris in the northern Gulf of St Lawrence, Canada. J Mar Biol Assoc UK. 2001;81(5):827–843. [Google Scholar]
- 186.Wong MC, Barbeau MA. Prey selection and the functional response of sea stars (Asterias vulgaris Verrill) and rock crabs (Cancer irroratus Say) preying on juvenile sea scallops (Placopecten magellanicus (Gmelin)) and blue mussels (Mytilus edulis Linnaeus). J Exp Mar Biol Ecol. 2005;327(1):1–21. [Google Scholar]
- 187.Joly-Turquin G, Dubois P, Coteur G, Danis B, Leyzour S, et al. Effects of the Erika Oil Spill on the Common Starfish Asterias rubens, Evaluated by Field and Laboratory Studies. Arch Environ Contam Toxicol. 2009;56(2):209–220. doi: 10.1007/s00244-008-9176-8. [DOI] [PubMed] [Google Scholar]
- 188.Mah C, Danis B, Hansson H. Asteriidae. Accessed through: Mah CL (2011). 2011. World Asteroidea Database at http://www.marinespecies.org/asteroidea/aphia.php?p=taxdetailsandid=123121 on 2011-10-05.
- 189.Mah C, Hansson H. Brisingida. In: Mah, CL (2011) World Asteroidea Database. Accessed through: Mah, CL (2011). 2011. World Asteroidea database at http://www.marinespecies.org/asteroidea/aphia.php?p=taxdetailsandid=123085 on 2011-10-24.
- 190.Mah C. Heliasteridae. In: Mah CL (2011). World Asteroidea Database. Accessed through: Mah CL (2011). 2011. World Asteroidea Database at http://www.marinespecies.org/asteroidea/aphia.php?p=taxdetailsandid=255069 on 2011-10-24.
- 191.Mah C. Stichasteridae. In: Mah, CL (2011). World Asteroidea Database. Accessed through: Mah CL (2011). 2011. World Asteroidea Database at http://www.marinespecies.org/Asteroidea/aphia.php?p=taxdetailsandid=152515 on 2011-10-05.
- 192.Mah C, Hansson H. Zoroasteridae. In: Mah CL (2010). World Asteroidea Database. Accessed through: Mah CL (2010). 2010. World Asteroidea Database at http://www.marinespecies.org/Asteroidea/aphia.php?p=taxdetailsandid=123125 on 2011-10-05.
- 193.Mah C, Hansson H. Pedicellasteridae. In: Mah CL (2011). World Asteroidea Database. Accessed through: Mah CL (2011). 2011. World Asteroidea Database at http://www.marinespecies.org/Asteroidea/aphia.php?p=taxdetailsandid=123124 on 2011-10-24.
- 194.Pawson DL. Some aspects of the biology of deep-sea echinoderms. Thalassia Jugosl. 1976;12(1):287–293. [Google Scholar]
- 195.Galkin SV, Korovchinsky NM. Vertical and geographical spread of the sea stars of the genus Freyella and notes about their ecology and origin. Tr Inst Okeanol Akad Nauk SSSR. 1984;119:164–178 (in Russian). [Google Scholar]
- 196.Emson RH, Young CM. Feeding mechanism of the brisingid starfish Novodinia antillensis. Mar Biol. 1994;118:433–442. [Google Scholar]
- 197.Mah C. Preliminary phylogeny and taxonomic revision of the Brisingida (Asteroidea: Forcipulatacea). In: Mooi R, Telford M, editors. Proc 9th Int Echinoderm Conf. San Francisco: Ballkema; 1998. pp. 273–277. [Google Scholar]
- 198.Mah C, Hansson H. Freyellidae. In: Mah, C.L. (2010). World Asteroidea Database. Accessed through: Mah, C.L. (2010). 2010. World Asteroidea Database at http://www.marinespecies.org/asteroidea/aphia.php?p=taxdetailsandid=123120 on 2011-10-28.
- 199.Mah C, Hansson H. Brisingidae. In: Mah, C.L. (2011). World Asteroidea Database. Accessed through: Mah, C.L. (2011). 2011. World Asteroidea Database at http://www.marinespecies.org/asteroidea/aphia.php?p=taxdetailsandid=123119 on 2011-10-28.
- 200.Mah C. Redescription and taxonomic notes on the South Pacific brisingidan Brisingaster robillardi (Asteroidea) with new ontogenetic and phylogenetic information. Zoosystema. 1999;21(3):535–546. [Google Scholar]
- 201.Jones DS, Portell RW. Occurrence and biogeographic significance of Heliaster (Echinodermata: Asteroidea) from the Pliocene of Southwest Florida. J Paleont. 1988;62(1):126–132. [Google Scholar]
- 202.Dearborn JH. Adaptations within Antarctic Ecosystems, Proc. Of the 3rd SCAR Symposium on Antarcctic Biology, GA Llano (ed) Gulf Publishing Company, Houston; 1977. foods and feeding characteristics of Antarctic asteroids and ophiuroids. Pp. 293–326.1252 [Google Scholar]
- 203.Dearborn JH, Edwards KC, Fratt DB. Diet, feeding behavior, and surface morphology of the multi-armed Antarctic sea star Labidiaster annulatus (Echinodermata: Asteroidea). Mar Ecol Prog Ser. 1991;77:65–84. [Google Scholar]
- 204.Paine RT. A short –term experimental investigation of resource partitioning in a New Zealand rocky intertidal habitat. Ecology. 1971;52(6):1096–1106. [Google Scholar]
- 205.Barker M. Observations on the settlement of the brachiolaria larvae of Stichaster australis (Verrill) and Coscinasterias calamaria (Gray) (Echinodermata: Asteroidea) in the laboratory and on the shore. J Exp Mar Biol Ecol. 1977;30:95–108. [Google Scholar]
- 206.Barker M. Breeding and recruitment in a population of the New Zealand starfish Stichaster australis. J Exp Mar Biol Ecol. 1979;41(3):195–211. [Google Scholar]
- 207.Blake DB. Burke, et al., editors. Paxillosidans are not primitive asteroids: a hypothesis based on functional considerations. Echinoderm Biology. 1988. pp. 309–314. Balkema, Rotterdam.
- 208.Shick JM, Edwards KC, Dearborn JH. Physiological ecology of the deposit-feeding sea star Ctenodiscus crispatus: ciliated surfaces and animal-sediment interactions. Mar Ecol Prog Ser. 1981;5:165–184. [Google Scholar]
- 209.Schmid PH, Schaerer R. Predator-prey interaction between two competing sea star species of the genus Astropecten. PSZNI Mar Ecol. 1981;2(3):207–214. [Google Scholar]
- 210.Sloan NA, Robinson SMC. Winter feeding by asteroids on a subtidal sandbed in British Columbia. Ophelia. 1983;22(2):125–140. [Google Scholar]
- 211.McClintock JB, Lawrence JM. Characteristics of foraging in the soft-bottom benthic starfish Luidia clathrata (echinodermata: Asteroidea): prey selectivity, switching behavior, functional responses and movement patterns. Oecologia. 1985;66(2):291–298. doi: 10.1007/BF00379867. [DOI] [PubMed] [Google Scholar]
- 212.Beddingfield SD, McClintock JB. Feeding behavior of the sea star Astropecten articulatus (Echinodermata: Asteroidea): an evaluation of energy-efficient foraging in a soft-bottom predator. Mar Biol. 1993;115(4):669–676. [Google Scholar]
- 213.Ventura CRR, Fernandes FDC. Bathymetric distribution and population size structure of paxillosid seastars (Echinodermata) in the Cabo Frio Upwelling ecosystem of Brazil. Bull Mar Sci. 1995;56(1):268–282. [Google Scholar]
- 214.Blake DB. The Benthopectinidae (Asteroidea: Echinodermata) of the Jurassic of Switzerland. Ecol Geolog Helvet. 1984;77:631–647. [Google Scholar]
- 215.Mah C, Danis B, Hansson H. Porcellanasteridae. In: Mah CL (2010). World Asteroidea database. Accessed through: Mah CL (2010). 2010. World Asteroidea Database at http://www.marinespecies.org/Asteroidea/aphia.php?p=taxdetailsandid=123130 on 2011-10-05.
- 216.Mah C. Goniopectinidae. In: Mah, C.L. (2010). World Asteroidea Database. Accessed through: Mah, C.L. (2010). 2010. World Asteroidea Database at http://www.marinespecies.org/asteroidea/aphia.php?p=taxdetailsandid=152509 on 2011-10-28.
- 217.Mah C, Hansson H. Ctenodiscidae. In: Mah, C.L. (2011). World Asteroidea Database. Accessed through: Mah, C.L. (2011). 2011. World Asteroidea Database at http://www.marinespecies.org/Asteroidea/aphia.php?p=taxdetailsandid=123128 on 2011-10-28.
- 218.Mah C. Pseudarchasteridae. In: Mah CL (2011). World Asteroidea Database. Accessed through: Mah, CL (2011). 2011. World Asteroidea Database at http://www.marinespecies.org/Asteroidea/aphia.php?p=taxdetailsandid=292826 on 2011-10-05.
- 219.Mah C, Danis B, Hansson H. Benthopectinidae. In: Mah CL (2011). World Asteroidea Database. Accessed through: Mah CL (2011). 2011 World Asteroidea Database at http://www.marinespecies.org/asteroidea/aphia.php?p=taxdetailsandid=123126 on 2011-10-05. [Google Scholar]
- 220.Mah C, Hansson H. Poraniidae. In: Mah, C.L. (2011). World Asteroidea database. Accessed through: Mah, C.L. (2011). 2011. World Asteroidea database at http://www.marinespecies.org/Asteroidea/aphia.php?p=taxdetailsandid=123138 on 2012-01-11.
- 221.Clark AM. Notes on Atlantic and other Asteroidea. 4. Families Poraniidae and Asteropseidae. Bull Br Mus Nat Hist (Zool) 1984;47(1):19–51. [Google Scholar]
- 222.Hotchkiss FHC, Clark AM. Restriction of the family Poraniidae sensu Spencer and Wright, 1966 (Echinodermata: Asteroidea). Bull Br Mus (Nat Hist), Zool. 1976;30(6):263–268. [Google Scholar]
- 223.Bowden DA, Schiaparelli S, Clark, MR, Rickard GJ. A lost world? Archaic crinoid-dominated assemblages on an Antarctic seamount. Deep-sea Res II. 2010;58(1–2):119–127. [Google Scholar]
- 224.Ericsson J, Hansson HG. Observations on the feeding biology of Porania pulvilus (O.F. Müller) (Asteroidea) from the Swedish West Coast. Ophelia. 1973;12:53–58. [Google Scholar]
- 225.Gemmill JF. 1. On the ciliation of asteroids and on the question of ciliary nutrition in certain species. Proc Zool Soc Lond. 1915;1:1–19. [Google Scholar]
- 226.Van Veldhuizen HD, Oakes VJ. Behavioral responses of seven species of asteroids to the asteroid predator Solaster dawsoni. Oecologia. 1981;48:214–220. doi: 10.1007/BF00347967. [DOI] [PubMed] [Google Scholar]
- 227.Mauzey KP, Birkeland C, Dayton PK. Feeding behavior of asteroids and escape responses of their prey in the Puget Sound region. Ecology. 1968;49:603–619. [Google Scholar]
- 228.Birkeland C. Interactions between a sea pen and seven of its predators. Ecol Monogr. 1974;44:211–232. [Google Scholar]
- 229.Mah CL. Phylogenetic analysis and biogeography of the deep-sea goniasterid, Circeaster (Echinodermata: Asteroidea) including descriptions of six new species. Zoosystema. 2006;28:917–954. [Google Scholar]
- 230.Yamaguchi M. Coral reef asteroids of Guam. Biotropica. 1975;7:12–23. [Google Scholar]
- 231.Bos AR, Gumanao GS, Alipoyo JCE, Cardona LT. Population dynamics, reproduction and growth of the Indo-Pacific horned sea star, Protoreaster nodosus. Mar Biol. 2008;156(1):55–63. [Google Scholar]
- 232.Bos AR, Gumanao GS, van Katwijk MM, Mueller B, Saceda MM, et al. Ontogenetic habitat shift, population growth, and burrowing behavior of the Indo-Pacific beach star Archaster typicus (Echinodermata; Asteroidea). Mar Biol. 2010;158(3):639–648. doi: 10.1007/s00227-010-1588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Scheibling RE, Metaxas A. Abundance, spatial distribution, and size structure of the sea star Protoreaster nodosus in Palau, with notes on feeding and reproduction. Bull Mar Sci. 2008;82(2):221–235. [Google Scholar]
- 234.Gasparini JL, Floeter SR, Ferreira CEL, Sazima I. Marine ornamental trade in Brazil. Biodiv Conserv. 2005;14:2883–2899. [Google Scholar]
- 235.Micael J, Alves MJ, Costa AC, Jones MB. Exploitation and conservation of echinoderms. Oceanogr Mar Biol Annu Rev. 2009;47:191–208. [Google Scholar]
- 236.Green E. Cato JC, Brown CL, editors. Ch. 3. International Trade in Marine Aquarium Species: Using the Global Marine Aquarium Database. Pp. 31–47. Marine Ornamental Species: Collection, Culture and Conservation. 2003. 395 Iowa State Press, Ames, Iowa.
- 237.Wabnitz C, Taylor M, Green E, Razak T. From Ocean to Aquarium: the global trade in marine ornamental species. 2003. 65 UNEP_WCMC, Cambridge, UK.
- 238.McClintock JB. Trophic biology of antarctic shallow-water echinoderms. Mar Ecol Prog Ser. 1994;111:191–202. [Google Scholar]
- 239.Dayton PK, Robillard GA, Paine RT, Dayton LB. Biological accommodation in the benthic community at McMurdo Sound, Antarctica. Ecol Monographs. 1974;44(1):105–128. [Google Scholar]
- 240.McClintock JB, Pearse JS, Bosch I. Population structure and energetics of the shallow-water antarctic sea-star Odontaster validus in contrasting habitats. Mar Biol. 1988;99:235–246. [Google Scholar]
- 241.Hinman VF, Davidson EH. Evolutionary plasticity of developmental gene regulatory network architecture. Proc Nat Acad Sci (USA) 2007;104(49):19404–19409. doi: 10.1073/pnas.0709994104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Otim O, Hinman VF, Davidson E. Expression of AmHNF6, a sea star orthologue of a transcription factor with multiple distinct roles in sea urchin development. Gene Expr Patterns. 2004;5(3):381–386. doi: 10.1016/j.modgep.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 243.Byrne M. Life history diversity and evolution in the Asterinidae. Integr Comp Biol. 2006;46(3):243–254. doi: 10.1093/icb/icj033. [DOI] [PubMed] [Google Scholar]
- 244.Byrne M, Cisternas P, Elia L, Relf B. Engrailed is expressed in larval development and in the radial nervous system of Patiriella sea stars. Dev Genes Evol. 2005;215:608–617. doi: 10.1007/s00427-005-0018-7. [DOI] [PubMed] [Google Scholar]
- 245.Byrne M. Changes in larval morphology in the evolution of benthic development by Patiriella exigua (Asteroidea: Asterinidae), a comparison with the larvae of Patiriella species with planktonic development. Biol Bull. 1995;188:293–305. doi: 10.2307/1542306. [DOI] [PubMed] [Google Scholar]
- 246.Byrne M, Hart MW, Cerra A, Cisternas P. Reproduction and larval morphology of broadcasting and viviparious species in the Cryptasterina species complex. Biol Bull. 2003;205-285-294 doi: 10.2307/1543292. [DOI] [PubMed] [Google Scholar]
- 247.Jackson AC, Murphy RJ, Underwood AJ. Patiriella exigua: grazing by a starfish in an overgrazed intertidal system. Mar Ecol Progr Ser. 2009;376:153–163. [Google Scholar]
- 248.Pillay D, Branch GM, Steyn A. Unexpected effects of starfish grazing on sandflat communities following an outbreak. Mar Ecol Progr Ser. 2010;398:173–182. [Google Scholar]
- 249.Byrne M, Walker SJ. Distribution and reproduction of intertidal species of Aquilonastra and Cryptasterina (Asterinidae) from One Tree Reef, Southern Great Barrier Reef. Bull Mar Sci. 2007;81(2):209–218. [Google Scholar]
- 250.Grace RV. Feeding behaviour of Stegnaster inflatus Hutton (Asteroidea; Asterinidae). Tane New Zealand. 1974;20:162–165. [Google Scholar]
- 251.Fujita T, Rowe FWE. Podosphaerasteridae fam. Nov. (Echinodermata: Asteroidea: Valvatida), with a new speices, Podosphaeraster toyoshiomaruae, from Southern Japan. Species Div. 2002;7:317–332. [Google Scholar]
- 252.Döderlein LH. Die Asteriden der Siboga-Exped. III. Oreasteridae. Siboga-Exped. 1935;46:71–110, pls. 15–20. [Google Scholar]
- 253.Clark HL. The echinoderm fauna of Torres Strait: Its composition and its origin. Carnegie Instit Wash Publ. 1921;214:1–223. [Google Scholar]
- 254.Mah CL. Astrosarkus idipi, a new Indo-Pacific genus and species of Oreasteridae (Valvatida; Asteroidea) displaying extreme skeletal reduction. Bull Mar Sci. 2003;73(3):685–698. [Google Scholar]
- 255.Clark HES. A new genus and two new species of sea-stars from North of New Zealand, with notes on Rosaster species (Echinodermata: Asteroidea). Nat Mus NZ Rec. 1982;2(5):35–42. [Google Scholar]
- 256.Mah C. Revision and Key to High-Latitude (Antarctic and subAntarctic) Goniasteridae. Zootaxa. 2011;2759:1–48. [Google Scholar]
- 257.Mah CL. A phylogeny of Iconaster and Glyphodiscus (Goniasteridae; Valvatida; Asteroidea) with descriptions of four new species. Zoosystema. 2005;27:131–167. [Google Scholar]
- 258.Mah CL. Systematics, Phylogeny, and Historical Biogeography of the Pentagonaster clade (Goniasteridae, Valvatida, Asteroidea). Invertebr Syst. 2007;21:311–339. [Google Scholar]
- 259.Döderlein LH. Die Asteriden der Siboga-Exped. II. Pentagonasteridae. Siboga Exped. 1924;46 [Google Scholar]
- 260.Döderlein LH. Die Asteriden der Siboga-Exped. III. Die unterfamile Oreasterinae. Siboga Exped. 1936;46:295–369, pls. 21–32. [Google Scholar]
- 261.Halpern JA. Goniasteridae (Echinodermata: Asteroidea) of the Straits of Florida. Bull Mar Sci. 1970;20(1):193–286. [Google Scholar]
- 262.Mah C, Hansson H. Odontasteridae. In: Mah CL (2010). World Asteroidea Database. Accessed through: Mah, CL (2010). 2010. World Asteroidea Database at http://www.marinespecies.org/asteroidea/aphia.php?p=taxdetailsandid=123136 on 2011-10-10.
- 263.Mah C. Ganeriidae. In: Mah CL (2011). World Asteroidea Database. Accessed through: Mah CL (2011). 2011. World Asteroidea Database at http://www.marinespecies.org/asteroidea/aphia.php?p=taxdetailsandid=149878 on 2011-10-10.
- 264.Mah C, Hansson H. Asterinidae. In: Mah CL (2011). World Asteroidea Database. Accessed through: Mah CL (2011). 2011. World Asteroidea Database at http://www.marinespecies.org/asteroidea/aphia.php?p=taxdetailsandid=123133 on 2011-10-04.
- 265.Mah C, Hansson H. Solasteridae. In: Mah, C.L. (2011). World Asteroidea Database. Accessed through: Mah, C.L. (2011). 2011. World Asteroidea Database at http://www.marinespecies.org/Asteroidea/aphia.php?p=taxdetailsandid=123143 on 2011-10-28.
- 266.Jangoux M. Les asterides littoraux de Nouvelle-Caledonie. Bull Mus Nat Hist Natl. 1984;4 6A(2):279–293. [Google Scholar]
- 267.O'Loughlin PM, Waters JM. A molecular and morphological revision of genera of Asterinidae (Echinodermata: Asteroidea). Mem Mus Vic. 2004;61(1):1–40. [Google Scholar]
- 268.Rowe FWE, Nichols D, Jangoux M. Anatomy of the spherical, valvatid starfish Podosphaeraster (Echinodermata; Asteroidea) with comments on the affinities of the genus. Micronesica. 1982;18(1):89–93. [Google Scholar]
- 269.Clark AM. A new genus and species of recent starfishes belonging to the aberrant family Sphaerasteridae, with notes on the possible origin and affinities of the family. Annals of the Magazine of Natural History. 1962;13(5):243–251. [Google Scholar]
- 270.Blake DB. Constructional Morphology and Life Habits of the Jurassic Sea Star Sphaeraster Quenstedt. Neues Jahrbuech Geologie u Palaeontologie Abh. 1984;169(1):74–101. [Google Scholar]
- 271.Fujita T, Stampanato S, Jangoux M. Belyaevostella hyugaensis, a new species of deep-sea asteroid (Asteroidea, Caymanostellidae) found on a sunken wood from off Southern Japan. Bull Nat Sci Mus Tokyo, ser A. 1994;20(4):183–188. [Google Scholar]
- 272.Rowe FWE. A review of the family Caymanostellidae (Echinodermata: Asteroidea) with the description of a new species of Caymanostella Belyaev and a new genus. Proc Linn Soc NSW. 1989;111(4):293–307. [Google Scholar]
- 273.Smith AB, Tranter TH. Protremaster, a new Lower Jurassic genus of asteroid from Antarctica. Geol Mag. 1985;122:351–359. [Google Scholar]
- 274.Mah C, Hansson H. Echinaster Müller and Troschel, 1840. In: Mah CL (2011). World Asteroidea database. Accessed through: Mah CL (2011). 2011. World Asteroidea database at http://www.marinespecies.org/asteroidea/aphia.php?p=taxdetailsandid=123275 on 2011-10-10.
- 275.Mah C, Hansson H. Henricia Gray, 1840. In: Mah CL (2011). World Asteroidea Database. Accessed through: Mah CL (2011). 2011. World Asteroidea database at http://www.marinespecies.org/asteroidea/aphia.php?p=taxdetailsandid=123276 on 2011-10-05.
- 276.Anderson JM. Histological studies on the digestive system of a starfish, Henricia, with notes on Tiedemann's pouches in starfishes. Biol Bull. 1960;119:371–398. [Google Scholar]
- 277.Madsen FJ. The Henricia sanguinolenta complex (Echinodermata, Asteroidea) of the Norwegian Sea and adjacent waters. A re-evaluation, with notes on related species. Steenstrupia. 1987;13(5):201–268. [Google Scholar]
- 278.Eernisse DJ, Strathmann MF, Strathmann RR. Henricia pumila sp. nov.: a brooding sea star (Asteroidea)from the coastal northeastern Pacific. Zootaxa. 2010;2329:22–36. [Google Scholar]
- 279.Mah C. Myxasteridae. In: Mah, C.L. (2010). World Asteroidea Database. Accessed through: Mah CL (2010). 2010. World Asteroidea Database at http://www.marinespecies.org/asteroidea/aphia.php?p=taxdetailsandid=123141 on 2011-10-10.
- 280.Mah C, Hansson H. Korethrasteridae. In: Mah CL (2011). World Asteroidea Database. Accessed through: Mah CL (2011). 2011. World Asteroidea database at http://www.marinespecies.org/asteroidea/aphia.php?p=taxdetailsandid=123140 on 2011-10-10.
- 281.Rodenhouse IZ, Guberlet JE. The morphology and behavior of the cushion star, Pteraster tesselatus Ives. Univ Wash Publ Biol. 1946;12:21–48. [Google Scholar]
- 282.Nance JM, Braithwaite LF. The function of mucous secretions in the cushion star Pteraster tesselatus Ives. J Exp Mar Biol Ecol. 1979;40:259–266. [Google Scholar]
- 283.McClary DJ, Mladenov PV. Reproductive pattern in the brooding and broadcasting sea star Pteraster militaris. Mar Biol. 1989;103:531–540. [Google Scholar]
- 284.McClary DJ, Mladenov PV. Brooding biology of the sea star Pteraster militaris (O.F. Müller): energetic and histological evidence for nutrient translocation to brooded juveniles. J Exp Mar Biol. 1990;142:183–199. [Google Scholar]
- 285.McEdward LR. Morphology and development of a unique type of pelagic larva in the starfish Pteraster tesselatus (Echinodermata: Asteroidea). Biol Bull. 1992;182:177–187. doi: 10.2307/1542111. [DOI] [PubMed] [Google Scholar]
- 286.McEdward LR. Evolution of pelagic direct development in the starfish Pteraster tesselatus (Asteroidea: Velatida). Biol J Linn Soc. 1995;54:299–327. [Google Scholar]
- 287.Healy JM, Rowe FWE, Anderson DT. Spermatozoa and spermiogenesis in Xyloplax (Class Concentricycloidea): a new type of spermatozoon in the Echinodermata. Zool Scripta. 1988;17(3):297–310. [Google Scholar]
- 288.Smith AB. Burke RD, et al., editors. To group or not to group: The taxonomic position of Xyloplax. Echinoderm Biology. 1988. 818 pp. 17–23, AA. Balkema, Rotterdam.
- 289.Pearse VB, Pearse JS. David, Guille, Féral, Roux, editors. Echinoderm phylogeny and the place of the concentricycloids. Pp. 121–126. Echinoderms Through Time (Echinoderms-Dijon) 1994. 940 Balkema, Rotterdam.
- 290.Janies D, Mooi R. Carnevalii C, Bonsoro F, editors. Xyloplax is an asteroid. Echinoderm Research, 1998. 1999. pp. 311–316. Balkema Rotterdam.
- 291.Janies DA, McEdward LR. Wilson WH, Stricker SA, Shinn GL, editors. A hypothesis for the evolution of the concentricycloid water-vascular system. Pp. 246–257. Reproduction and Development of Marine Invertebrates. 1994. 325 J. Hopkins Press, Baltimore.
- 292.Janies DA, McEdward LR. David B, Guille A, Feral J-P, Roux M, editors. Heterotopy, pelagic direct development, and new body plans in velatid asteroids. Pp. 319–324. Echinoderms Through Time (Echinoderms, Dijon) 1994. 940 Balkema, Rotterdam.
- 293.Mah CL. A new species of Xyloplax from the Northeast Pacific: comparative morphology and a reassessment of phylogeny. Invert Biol. 2006;125(2):136–153. [Google Scholar]
- 294.Perseke M, Bernhard D, Fritzsch G, Brümmer F, Sadler PF, et al. Mitochondrial genome evolution in Ophiuroidea, Echinoidea, and Holothuroidea: Insights in phylogenetic relationships of Echinodermata. Mol Phylo Evol. 2010;56:201–211. doi: 10.1016/j.ympev.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 295.Villier L, Kutscher KM, Mah CL. Systematics and paleontology of middle Toarcian Asteroidea (Echinodermata) from the ‘Seuil du Poitou’, Western France. Geobios. 2004;37(6):807–825. [Google Scholar]
- 296.Breton G. Valettaster? Sphaerasteridae Mesozoique. Bull Trim Soc Géol Normandie et Amis Muséum du Havre. 1985;72(1and2):91–99. [Google Scholar]
- 297.Néraudeau D, Breton G. Astérides du Cénomanien de Charente-Maritime (SW France) [Cenomanian Asteroids from Charente-Maritime (SW France)]. Geobios. 1993;26(1):105–120. [Google Scholar]
- 298.Blake DB. Redescription and interpretation of the asteroid species Tropidaster pectinatus from the Jurassic of England. Palaeontology. 1996;39(1):179–188. [Google Scholar]
- 299.Howell KL, Billett DSM, Tyler PA. Depth-related distribution and abundance of seastars (Echinodermata: Asteroidea) in the Porcupine Seabight and Porcupine Abyssal Plain, N.E. Atlantic. Deep-Sea Res I. 2002;49:1901–1920. [Google Scholar]