Abstract
The disease chytridiomycosis is responsible for declines and extirpations of amphibians worldwide. Chytridiomycosis is caused by a fungal pathogen (Batrachochytrium dendrobatidis) that infects amphibian skin. Although we have a basic understanding of the pathophysiology from laboratory experiments, many mechanistic details remain unresolved and it is unknown if disease development is similar in wild amphibian populations. To gain a better understanding of chytridiomycosis pathophysiology in wild amphibian populations, we collected blood biochemistry measurements during an outbreak in mountain yellow-legged frogs (Rana muscosa) in the Sierra Nevada Mountains of California. We found that pathogen load is associated with disruptions in fluid and electrolyte balance, yet is not associated with fluctuations acid-base balance. These findings enhance our knowledge of the pathophysiology of this disease and indicate that disease development is consistent across multiple species and in both laboratory and natural conditions. We recommend integrating an understanding of chytridiomycosis pathophysiology with mitigation practices to improve amphibian conservation.
Introduction
Fungi have not traditionally been regarded as highly virulent pathogens in terrestrial vertebrates [1]–[2]. Yet the fungal pathogen Batrachochytrium dendrobatidis (Bd), which causes a skin disease known as chytridiomycosis, is implicated in global amphibian declines and extinctions [3]–[5]. Resolving the mechanisms of pathogenesis is an imperative step in understanding how Bd can cause devastating declines [6]. The pathogen was described over a decade ago [7] and it has taken many years to gain a basic understanding of the physiologic effects of chytridiomycosis.
Insights into the pathophysiology of chytridiomycosis have come from controlled laboratory infection experiments, but many of the mechanistic details of pathogenesis remain unresolved [8]. Recent studies indicate that normal skin functioning is impaired in frogs with Bd infections [9]–[10]. These pathophysiological changes are particularly problematic for infected hosts because the skin of healthy amphibians tightly regulates osmotic balance (i.e. regulation of electrolyte concentrations relative to water, [11]–[12]). In frogs with severe cases of chytridiomycosis, the breakdown of skin transport processes is associated with reductions in blood plasma electrolyte concentrations [9]. Specifically, hyponatremia (low sodium) and hypokalemia (low potassium) have been reported in amphibians that develop clinical chytridiomycosis [9], [13]–[14]. These conditions are thought to cause asystolic cardiac arrest and death [9]. However, other causes of cardiac abnormalities (such as hypoxia or low blood oxygen) have not been conclusively ruled out as contributing factors. Thus, the details of Bd pathogenesis require further investigation [8].
It is unknown if the effects observed in laboratory experiments accurately characterize disease development in natural populations because to date no study has provided physiological data from Bd-infected amphibians in the wild. One natural system has provided the opportunity to investigate the pathophysiology of chytridiomycosis in greater detail, the Sierra Nevada of California. The population-level dynamics of chytridiomycosis have been studied intensively in this system [15] and have added to our understanding of Bd in host species both in enzootic [16] and epizootic [17] disease states.
In this system, Bd was initially detected in a small number of individuals and then spread within and between populations in a wave-like pattern across the landscape, causing mass die-offs in mountain yellow-legged frogs (a species complex consisting of Rana muscosa and Rana sierrae, [16]–[17]). Bd was detected in populations using a non-invasive, quantitative diagnostic technique that allows an estimate of the infection intensity in individual hosts [18]–[19]. In individual frogs, disease development and mortality were linked with intensity of infection; the Bd-load (number of infectious zoospores) increased until the infection intensity exceeded a threshold value (∼10,000 zoospore equivalents, [17]). Mortality and rapid population decline consistently occurred beyond this fungal load level, which established the quantitative diagnostic of Bd-load as a reliable indicator of severe disease [17]. Similar patterns of disease spread have been documented in other regions including other frog and salamander populations [20]–[22], but few systems have been studied in such fine detail as in the Sierra Nevada.
The comprehensive and integrated approach to studying the host-pathogen dynamics in populations of mountain yellow-legged frogs provided an opportunity to assess the pathophysiological responses to infection during a chytridiomycosis outbreak. We collected morphological and blood biochemistry measurements during an outbreak in order to 1) better understand the pathophysiology of chytridiomycosis and 2) determine if the physiological effects observed in laboratory inoculation experiments are analogous to disease development in wild amphibians. With this information we can identify the best treatments regimes for intervention during a chytridiomycosis outbreak.
Methods
Ethics statement
Sequoia-Kings Canyon National Park, University of California, Berkeley, San Francisco State University, and University of California, Santa Barbara Institutional Animal Care and Use Committees approved the scientific research and ethics permits for this project.
Study area for animal collections
Milestone Basin (36.647816° north, −118.454484°west; Figure 1 top row) and Sixty Lake Basin (37.180999°, −118.444582°; Figure 1 bottom row) are located in Sequoia–Kings Canyon National Park in subalpine-alpine zones (elevation 3,000–3,500 m). We sampled adult mountain yellow-legged frogs (Rana muscosa) on four occasions from July to October in 2004. Each sampling trip lasted approximately 3 weeks and during each trip we sampled 40–70 frogs at different lakes within the basins. We encountered frogs at the perimeters of water bodies in the lake basins and collected adult frogs with clean dip nets. We handled each individual using new latex gloves and recorded each animal's mass (measured to the nearest 0.01 g), snout-to-vent length (SVL).
Figure 1. Maps of the two study basins where frog populations were monitored during chytridiomycosis outbreaks.
Sequence of maps shows the spread of Batrachochytrium dendrobatidis (Bd) through Milestone Basin (A–E) and Sixty Lakes Basin (F–J). Lake color (green for Bd-negative populations, yellow for Bd-infected populations, and black for extinct frog populations) shows Bd infection and frog population status, and the light gray shaded region surrounds the area where frog populations were Bd-positive in each year. Open circle indicates the pond where Bd was first detected and where moribund frogs were collected for blood samples in 2004. Modified from [17].
Diagnostics
We collected non-invasive skin swab samples from each individual by rubbing a sterile, synthetic-cotton swab over the ventral surfaces and digits [17], [19]. Skin swabs were air-dried in the field for five minutes and stored in a cool location until they could be transported to the laboratory. In the laboratory, we stored swabs 4°C until analysis, which occurred a four-month period. We used a quantitative measure of Bd genomic equivalents using a Taqman real-time polymerase chain reaction (PCR) assay [18]–[19]. We used a standard protocol [18] for our extraction methods and universal Bd standards (0.1–100 zoospore equivalents run in triplicate) provided by A. Hyatt.
Blood biochemistry
We collected blood samples (∼5–10% of body weight) via cardiac puncture [12] using 50 cc syringes. We centrifuged blood samples (11,000 RPM, ZipoCrit centrifuge) immediately in the field and used an i-STAT portable clinical analyzer (Heska Corporation, Loveland, CO, USA) to assess blood parameters in the field. In some cases the limited blood volume (e.g. from sub-adult frogs) restricted the number of analyses that could be accurately performed. In those cases, we omitted unreliable results from our analysis. We used the following plasma biochemical parameters for statistical analysis: hematocrit, total protein, blood urea nitrogen content, pH, total carbon dioxide, partial pressure carbon dioxide, partial pressure oxygen, bicarbonate, base excess extracellular fluid, anion gap, glucose, sodium, potassium and chloride.
Statistics
We analyzed the data with R, version 2.7.1 (www.r-project.org). We calculated the body condition index (BCI) by taking the residuals of the linear regression of body mass on snout-to-vent length [23]. We tested for normality (Q-Q plots and Shapiro-Wilk's test) and used a log transformation where violations of normality assumptions occurred.
Due to the high number of dependent variables, we first analyzed for dimension reduction with Principle Components Analysis (PCA) using the NIPALS algorithm [24]. We used Cattell's scree test to decide number of components and included eigan values >1 [25]. We used factors with loadings of 0.3 or greater for interpretation and multiple regression analysis to determine if relationships existed between Bd-load (genetic equivalents with a log transformation, log10 (n+1)) and the principle components. We then ran correlation tests on parameters that loaded highly (>0.3) on principle components that were significantly associated with Bd-load. Lastly, we grouped amphibians into categories of uninfected, low (1–10,000) and high (over 10,000) zoospore equivalents to determine if there were clear differences among groups of amphibians based on the 10,000 zoospore threshold value that is thought to be critical for amphibian mortality in these species [17].
Results
We detected Bd from diagnostic skin swabs in Milestone Basin in July 2004. By October of 2004, Bd had spread throughout the entire basin (Figure 1) and frogs showed clinical signs of chytridiomycosis (Figure 2). Over the course of four sampling trips, we collected and analyzed blood biochemistry measurements from adult frogs (n = 121).
Figure 2. Appearance and behavior of mountain yellow-legged frogs (Rana muscosa) and during a chytridiomycosis outbreak in Sixty Lakes Basin, Sierra Nevada Mountains, California.
A) A frog showing clinical signs of severe chytridiomycosis including abnormal posture. B) Dead frogs following a chytridiomycosis outbreak in Milestone Basin.
Two principle components accounted for 55% of the variance in the data and were selected based on the criteria described above. A pattern matrix (Table 1) shows the component loadings for each factor including BCI and blood biochemical parameters. PCA analysis indicated that discrete biochemical parameters could be grouped to represent more general physiological factors. Factor names for each of the two principle components (“Acid-base balance” and “Fluid and electrolyte balance”) were selected based on the parameters with high loadings (>0.3) and on general knowledge of biological functions of each parameter.
Table 1. Component loadings of blood biochemistry parameters measured in mountain yellow-legged frogs infected with Batrachochytrium dendrobatidis.
| Variable | PC1 | PC2 |
| Acid-base balance | Fluid and electrolyte balance | |
| Body Condition Index | 0.06 | 0.34 |
| Hematocrit | 0.01 | −0.24 |
| Protein | 0.08 | −0.34 |
| Blood Urea Nitrogen | −0.01 | −0.29 |
| pH | 0.45 | −0.01 |
| Total Carbon Dioxide | 0.52 | 0.04 |
| Partial Carbon Dioxide | 0.28 | 0.16 |
| Partial Oxygen | −0.02 | 0.02 |
| Bicarbonate | 0.39 | −0.05 |
| Base Excess Ext. Fluid | 0.54 | 0.00 |
| Anion Gap | 0.00 | −0.09 |
| Glucose | 0.03 | −0.48 |
| Sodium | 0.02 | 0.38 |
| Potassium | −0.03 | −0.29 |
| Chloride | −0.02 | 0.36 |
| Eigenvalue | 1.60 | 1.23 |
Interpretable factor loadings (>0.3) are in boldface.
Acid-base balance is the maintenance of proper pH levels in the blood [26]. Parameters that loaded highly (>0.3) for acid-base balance included pH, total carbon dioxide, bicarbonate and base excess extracellular fluid. When assessed for a relationship with Bd-load, there was no statistically significant correlation (Pearson standard parametric correlation: r = −0.12, df = 119, P-value = 0.20).
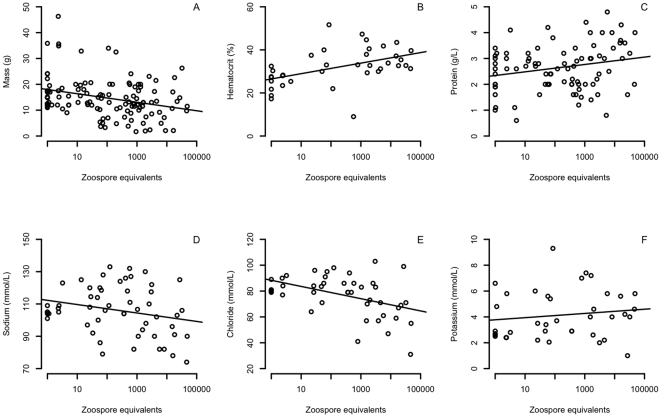
Fluid and electrolyte balance is characterized by the correct ratios of solutes to fluids (i.e. water) and generally indicates osmotic equilibrium or hydration status. Parameters that loaded highly (>0.3) for fluid and electrolyte balance included BCI, protein, glucose, sodium, potassium, and chloride. This principle component was significantly correlated with Bd-load (Pearson standard parametric correlation: r = 0.28, df = 119, P-value = 0.002). Of the parameters that loaded highly on the fluid and electrolyte balance principle component, all except BCI and plasma potassium concentrations were significantly correlated with Bd-load (Table 2). We also assessed hematocrit and mass as individual parameters because they are strong indicators of hydration status. We found positive correlations between Bd-load and hematocrit (Pearson standard parametric correlation: r = 0.54, df = 32, P-value<0.001; Figure 3B), and a negative correlation between Bd-load and mass (Pearson standard parametric correlation: r = −0.25, df = 98, P-value = 0.009; Figure 3A).
Table 2. Pearson correlation between Bd-load (genomic equivalents determined by qPCR analysis) and parameters that loaded highly on the Fluid and electrolyte balance component.
| Parameter | r | df | P-value |
| Body Condition Index | −0.02 | 119 | 0.75 |
| Protein | 0.3 | 73 | 0.02* |
| Glucose | 0.37 | 66 | 0.001* |
| Sodium | −0.24 | 61 | 0.05* |
| Potassium | 0.03 | 45 | 0.79 |
| Chloride | −0.44 | 42 | 0.002* |
Figure 3. Mass and blood biochemistry parameters collected from mountain yellow-legged frogs during a chytridiomycosis outbreak.
A–C) Mass, hematocrit and protein values suggest dehydration in frogs with high loads of Batrachochytrium dendrobatidis (Bd). D–F) Plasma electrolyte concentrations (sodium and chloride) indicate abnormalities in fluid and electrolyte balance in frogs with high Bd-loads.
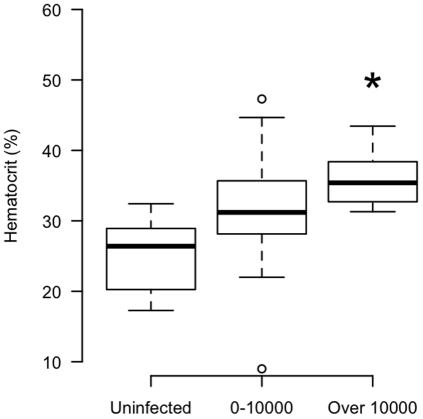
When also grouped amphibians into categories of uninfected, low (1–10,000 zoospores) and high (over 10,000 zoospores) infection intensities, our results suggested possible differences among groups for each physiological parameter. However, with the exception of hematocrit (ANOVA, P<0.01, Tukey HSD, P = 0.02 for control and over 10,000 zoospore equivalent groups; Figure 4), these differences were not significant, probably due to the variability among individuals.
Figure 4. Hematocrit in yellow-legged frogs grouped by intensity of infection with Batrachochytrium dendrobatidis.
The group of frogs with over 10,000 zoospore equivalents had significantly different hematocrit percentages compared with the group that was uninfected (Tukey HSD, P = 0.02).
Discussion
The well-studied die-offs in mountain yellow-legged frogs (Rana muscosa) have characterized the nature of chytridiomycosis-related amphibian declines occurring around the world; Bd spreads rapidly into naïve amphibian populations and causes high levels of mortality. The frog populations of the Sierra Nevada were screened for Bd-infection from 1996–2008 and, following the initial detection of Bd in 2004, were reduced by >75% (Figure 1, [17]). Quantification of Bd-load over a relatively short sampling period (months before and after the detection of Bd) demonstrated a rapid increase in infection intensity in the dead and dying individuals from these populations. Thus, mortality in these natural populations was inextricably linked with the spread of Bd and the subsequent increase in infection intensities [17]. However, no mechanistic explanation for mortality in wild amphibian die-offs was previously available. Our study provides new pathophysiological data collected from natural populations during a chytridiomycosis outbreak and adds new information to understand the mechanisms of mortality in chytridiomycosis. Based on these results, we suggest a more informed approach to treating diseased amphibians during chytridiomycosis outbreaks.
Our results indicate that increases Bd-load, which were documented in great detail during this outbreak, were significantly associated with indicators of fluid and electrolyte balance. Specifically, we found that increasing Bd-load is associated reductions in plasma electrolyte concentrations, and we also found some evidence of dehydration. Taken together, these two findings suggest that frogs with severe chytridiomycosis have a greater loss of solutes from circulation than could be determined with electrolyte concentration measurements alone.
Even moderate dehydration with concomitant reductions in solute concentrations suggests that frogs with chytridiomycosis have a more pronounced loss of electrolytes than previously estimated. Fluid and electrolyte balance is normally evaluated by measures of blood solute concentrations relative to body condition index (BCI), which is determined by measures of body mass relative to body size (snout-to-vent length, SVL). An increase in blood solute concentrations, and especially in hematocrit, accompanied by a decrease in body mass would point toward dehydration. In contrast, over-hydration is indicated by a decrease in blood solute concentrations with an increase in body mass. We found negative correlations between Bd-load and body mass, and positive correlations between Bd-load and protein and hematocrit concentrations (Figure 3 A, B, C). However, there were negative relationships between Bd-load and other solute concentrations, such as sodium and chloride (Figure 3 D, E, Table 2). Other laboratory studies have shown similar dramatic decreases in electrolyte concentrations, but with only weak evidence of dehydration [9], [13]–[14]. However, these studies are not directly comparable because the environment in the laboratory does not simulate natural conditions, sample sizes were considerably smaller compared to the present study and, in some laboratory tests, electrolyte concentrations were below the detection limit of the available equipment [9]. Thus, our results support the hypothesis that fluid and electrolyte imbalance is a critical component of the pathophysiology of chytridiomycosis, but they also suggest that electrolyte loss is even greater than previously thought in diseased frogs.
Our results also indicate that acid-base balance is not considerably altered in amphibians with severe chytridiomycosis. Acid-base balance, which requires multi-system regulation [26], is one facet of Bd pathophysiology that has been difficult to study. Blood pH levels are determined metabolic (e.g. bicarbonate generation in the kidney, known as the bicarbonate buffering system) and respiratory (e.g. carbon dioxide production in various tissues) mechanisms, both of which act in concert to correct for perturbations in pH that result in acidosis or alkalosis [26]. Carver et al. [10] tested oxygen consumption and carbon dioxide production using flow-through respirometry; they found no evidence of altered metabolic rates in infected frogs. Voyles et al. [14] measured partial pressure carbon dioxide in blood samples and oxygen saturation (using pulse oximeter; [9]) and did not find significant changes in respiratory gases in severely diseased frogs. However, these previous studies were limited in their ability to assess blood pH and bicarbonate. Our study provides information on these parameters (Table 1), and suggests there is little evidence of changes in acid-base balance in diseased frogs.
Electrolyte depletion has been the most consistent finding in studies that have aimed to determine the physiological effects of chytridiomycosis, and our study documents these pathophysiological changes in free-living amphibian populations during an outbreak. These results indicate that pathophysiological responses to an increase in Bd-load in amphibian epidermis are consistent across multiple species and between captive and wild amphibian populations. Therefore, optimized treatments that correct pathophysiological abnormalities could be invaluable where wild amphibian populations experience chytridiomycosis outbreaks and Bd-induced declines.
Treatment regimes that aim to decrease fungal load in wild amphibian populations may be the best strategy to mitigate outbreaks of chytridiomycosis [27], and the success of field treatments will be considerably enhanced by a detailed understanding of disease development and pathogenesis. Many different strategies to reduce fungal loads of amphibians in a natural setting have been suggested [28], but few have been attempted during chytridiomycosis outbreaks in the wild. One approach that holds promise is treating individual amphibian hosts with antifungal solutions in the wild (e.g. using fungicidal chemicals or anti-Bd “probiotic” treatments, [28]), which is now being trialed for Rana muscosa and Rana sierrae (V. Vredenburg, unpublished work).
The success of implementing treatment strategies in wild amphibians will hinge on the timing of treatment, and also on the effectiveness of treatments in reducing Bd-load and correcting pathophysiological abnormalities. The timing of treatments is important because the damage may be irreparable in the final stages of disease progression. The effectiveness of anti-Bd agents is critical to reduce Bd-loads and suppress the advancement of disease. We suggest that electrolyte supplementation should be used in conjunction with antifungal treatments to reduce the risk of mortality during outbreaks when frogs are showing clinical signs of disease. Berger et al. [27] reviewed multiple clinical trials designed to optimize the use of both antifungal treatments and isotonic electrolyte supplementation. This approach has been attempted with some success in clinical settings (S. Young, unpublished work). The optimal combination for a particular species may require additional background work to determine the best treatments accounting for host life-stage, behavioral and/or ecological characteristics [27]. Nonetheless, the application of well-informed treatment practices in wild amphibian populations is an attainable goal for amphibian conservation biologists. Treatment regimes for individual hosts that incorporate an understanding disease progression may prove to be an indispensible tool for mitigating chytridiomycosis outbreaks, facilitating population recovery and confronting the conservation challenges associated with chytridiomycosis and amphibian loss.
Acknowledgments
We thank the National Park Service and especially R. Knapp H. Werner, D. Boiano, D. Graber and the SEKI helicopter crew at Ash Mountain. We also thank A. Hyatt for providing Bd standards and W.F. Voyles, J. Eastman and P. Jorgensen for analytical and editorial assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by National Institutes of Health Grant R01ES12067 and National Science Foundation Grant EF-0723563. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Schaechter M, Medoff G, Schlessinger D, Popoff CM. Mechanisms of microbial disease: Williams & Wilkins Baltimore; 1993. [Google Scholar]
- 2.Mueller GM, Bills GF, Foster MS. Biodiversity of Fungi: Inventory and Monitoring Methods: Academic Press; 2004. [Google Scholar]
- 3.Berger L, Speare R, Daszak P, Green DE, Cunningham AA, et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci U S A. 1998;95:9031–9036. doi: 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schloegel LM, Hero JM, Berger L, Speare R, McDonald K, et al. The decline of the sharp-snouted day frog (Taudactylus acutirostris): The first documented case of extinction by infection in a free-ranging wildlife species? EcoHealth. 2006;3:35–40. [Google Scholar]
- 5.Wake DB, Vredenburg VT. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci U S A. 2008;105:11466. doi: 10.1073/pnas.0801921105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daszak P, Cunningham AA, Hyatt AD. Infectious disease and amphibian population declines. Divers Distrib. 2003;9:141–150. doi: 10.3201/eid0506.990601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longcore JE, Pessier AP, Nichols DK. Batrachochytrium dendrobatidis gen et sp nov, a chytrid pathogenic to amphibians. Mycologia. 1999;91:219–227. [Google Scholar]
- 8.Voyles J, Rosenblum EB, Berger L. Interactions between Batrachochytrium dendrobatidis and its amphibian hosts: a review of pathogenesis and immunity. Microbes Infect. 2011;13:25–32. doi: 10.1016/j.micinf.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Voyles J, Young S, Berger L, Campbell C, Voyles WF, et al. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science. 2009;326:582. doi: 10.1126/science.1176765. [DOI] [PubMed] [Google Scholar]
- 10.Carver S, Bell BD, Waldman B. Does Chytridiomycosis Disrupt Amphibian Skin Function? Copeia. 2010;2010:487–495. [Google Scholar]
- 11.Boutilier RG, Stiffler DF, Toews DP. Exchange of respiratory gases, ions, and water in amphibious and aquatic amphibians. Environmental physiology of the amphibians. 1992:81–124. [Google Scholar]
- 12.Wright KM, Whitaker BR. Amphibian Medicine and Captive Husbandry: Malabar FL: Krieger Publishing Company; 2001. [Google Scholar]
- 13.Marcum RD, St-Hilaire S, Murphy PJ, Rodnick KJ. Effects of Batrachochytrium dendrobatidis infection on ion concentrations in the boreal toad Anaxyrus (Bufo) boreas boreas. Dis Aquat Org. 2010;91:17–21. doi: 10.3354/dao02235. [DOI] [PubMed] [Google Scholar]
- 14.Voyles J, Berger L, Young S, Speare R, Webb R, et al. Electrolyte depletion and osmotic imbalance in amphibians with chytridiomycosis. Dis Aquat Org. 2007;77:113–118. doi: 10.3354/dao01838. [DOI] [PubMed] [Google Scholar]
- 15.Briggs CJ, Vredenburg VT, Knapp RA, Rachowicz LJ. Investigating the population-level effects of chytridiomycosis: an emerging infectious disease of amphibians. Ecology. 2005;86:3149–3159. [Google Scholar]
- 16.Briggs CJ, Knapp RA, Vredenburg VT. Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proc Natl Acad Sci U S A. 2010;107:9695–9700. doi: 10.1073/pnas.0912886107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc Natl Acad Sci U S A. 2010;107:9689–9694. doi: 10.1073/pnas.0914111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Org. 2004;60:141–148. doi: 10.3354/dao060141. [DOI] [PubMed] [Google Scholar]
- 19.Hyatt AD, Boyle DG, Olsen V, Boyle DB, Berger L, et al. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis Aquat Org. 2007;73:175–192. doi: 10.3354/dao073175. [DOI] [PubMed] [Google Scholar]
- 20.Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, et al. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc Natl Acad Sci U S A. 2006;103:3165–3170. doi: 10.1073/pnas.0506889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinney VC, Heemeyer JL, Pessier AP, Lannoo MJ. Seasonal pattern of Batrachochytrium dendrobatidis infection and mortality in Lithobates areolatus: Affirmation of Vredenburg's “10,000 Zoospore Rule”. PLoS One. 2011;6:e16708. doi: 10.1371/journal.pone.0016708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng TL, Rovito SM, Wake DB, Vredenburg VT. Coincident mass extirpation of neotropical amphibians with the emergence of the infectious fungal pathogen Batrachochytrium dendrobatidis. Proc Nat Acad Sci. 2011;108:9502–9507. doi: 10.1073/pnas.1105538108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bancila RI, Hartel T, Plaiasu R, Smets J, Cogalniceanu D. Comparing three body condition indices in amphibians: a case study of yellow-bellied toad Bombina variegata. Amphibia Reptilia. 2010;31:558–562. [Google Scholar]
- 24.Geladi P, Kowalski BR. Partial least-squares regression: a tutorial. Anal chim acta. 1986;185:1–17. [Google Scholar]
- 25.Budaev SV. Using principal components and factor analysis in animal behaviour research: caveats and guidelines. Ethology. 2010;116:472–480. [Google Scholar]
- 26.Rose BD, Post TW. Clinical physiology of acid-base and electrolyte disorders: McGraw-Hill Professional; 2001. [Google Scholar]
- 27.Berger L, Speare R, Pessier A, Voyles J, Skerratt LF. Treatment of chytridiomycosis requires urgent clinical trials. Dis Aquat Org. 2010;92:165–174. doi: 10.3354/dao02238. [DOI] [PubMed] [Google Scholar]
- 28.Woodhams DC, Bosch J, Briggs CJ, Cashins S, Davis LR, et al. Mitigating amphibian disease: strategies to maintain wild populations and control chytridiomycosis. Front Zool. 2011;8:8–31. doi: 10.1186/1742-9994-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]