Abstract
Symbionts are widespread and might have a substantial effect on the outcome of interactions between species, such as in host-parasitoid systems. Here, we studied the effects of symbionts on the outcome of host-parasitoid interactions in a four-partner system, consisting of the parasitoid wasp Leptopilina boulardi, its two hosts Drosophila melanogaster and D. simulans, the wasp virus LbFV, and the endosymbiotic bacterium Wolbachia. The virus is known to manipulate the superparasitism behavior of the parasitoid whereas some Wolbachia strains can reproductively manipulate and/or confer pathogen protection to Drosophila hosts. We used two nuclear backgrounds for both Drosophila species, infected with or cured of their respective Wolbachia strains, and offered them to L. boulardi of one nuclear background, either infected or uninfected by the virus. The main defence mechanism against parasitoids, i.e. encapsulation, and other important traits of the interaction were measured. The results showed that virus-infected parasitoids are less frequently encapsulated than uninfected ones. Further experiments showed that this viral effect involved both a direct protective effect against encapsulation and an indirect effect of superparasitism. Additionally, the Wolbachia strain wAu affected the encapsulation ability of its Drosophila host but the direction of this effect was strongly dependent on the presence/absence of LbFV. Our results confirmed the importance of heritable symbionts in the outcome of antagonistic interactions.
Introduction
Endosymbionts are extremely frequent in arthropods, especially in insects. By providing additional heritable genetic material, they may contribute to the adaptation of their insect host [1], [2]. A growing literature reports examples of beneficial effects provided by heritable endosymbionts to their hosts when the latter are engaged in antagonistic relationships with other species [3], [4], [5], [6], [7]. Host-parasitoid systems are therefore of great interest, as both protagonists may harbour symbiotic organisms influencing the outcome of their interaction, thus offering additional routes towards resistance or virulence besides host nuclear factors [8]. Indeed, it has been found that several bacteria protect their insect host from parasitoid-induced mortality in aphids [9], [10], [11] or in Drosophila hydei [12]. On the other hand, many insect parasitoids harbour viral symbionts allowing them to cope with the host's immune defenses, increasing the virulence of these parasitoids [13], [14], [15], [16]. These heritable viruses are injected into the parasitoid's host together with the eggs, suppressing, to varying degrees, the immune reaction of the parasitized host [17].
Whereas most studies have focused on the effect of a single symbiont either in the host or in the parasitoid on the outcome of the host-parasitoid interaction (but see [18]), we have investigated here the potential influence of two different symbionts, one infecting the host and the other infecting the parasitoid. This system involves the parasitoid wasp Leptopilina boulardi that is able to parasitize both Drosophila melanogaster and D. simulans larvae. Parasitization may lead to three different outcomes: (i) the parasitoid avoids the immune system of the Drosophila larva, reaches the adult stage and ultimately kills the Drosophila; (ii) the Drosophila succeeds in killing the parasitoid by a cascade of immune reactions leading to the encapsulation of the young wasp [19]; (iii) the interaction ends with the death of both protagonists.
Drosophila species are often infected by the maternally-transmitted bacterium Wolbachia. Different strains have been described, some inducing various reproductive manipulations in their hosts, such as cytoplasmic incompatibility, while others have unknown effect. This raises the question of the mechanism explaining their prevalence in natural populations [20], [21]. One hypothesis is that non-manipulating Wolbachia strains may increase the resistance of their Drosophila host to parasitoid attacks. It has been found that Wolbachia can confer resistance against various parasites such as RNA viruses [6], [22], [23], [24], [25], filarial nematodes and Plasmodium [26], [27], [28], [29]. Moreover, manipulating strains could combine the advantage of both a reproductive manipulation and a protective effect, improving even more their invasive potential. Counter-examples contrasting with protective effects found against pathogens were however also previously described. Wolbachia-infected D. simulans have, for instance, reduced ability to encapsulate parasitoids [18]. Similarly, Wolbachia in the isopod Armidillidium vulgare is able to infect host haemocytes [30], decreasing the immune-competence of its host, particularly by affecting the prophenoloxidase activity [31], a key pathway of the immune system in arthropods [32].
The parasitoid L. boulardi is often infected by a maternally-transmitted DNA virus called LbFV (Leptopilina boulardi Filamentous Virus), whose prevalence may exceed 90% in some locations [33]. This virus manipulates the behavior of adult females in a way that favours its own transmission [34]. Whereas virus-free females lay a single egg in encountered Drosophila larvae and usually avoid superparasitism, i.e. laying eggs in already parasitized larvae, virus-infected females readily lay eggs in previously parasitized host larvae. Infected offspring are consequently exposed to strong competition, as only one parasitoid is able to fully develop inside a single host larva. Superparasitism is adaptive for the virus as it enables its horizontal transmission among the parasitoid larvae competing within the same fly larva. Theoretical work has shown that the virus is selected for increasing the natural superparasitism tendency of the parasitoid because it allows infection of new parasitoid matrilines [35]. Additionally, both the vertical and the horizontal transmission of the virus may be facilitated by increased virulence of the parasitoid against Drosophila's immune response.
In this paper, we tested the combined effect of LbFV (infecting the parasitoid) and of different strains of Wolbachia (infecting the Drosophila host) on the outcome of the host-parasitoid interaction using two genetic backgrounds of D. melanogaster and D. simulans and one genetic background of L. boulardi. We measured the successful encapsulation rate by counting adult flies that survived parasitoid attack. In a second experiment, we also controlled for the occurrence and effect of superparasitism by measuring encapsulation in Drosophila larvae. The results showed that symbionts indeed influence the final outcome in this host-parasitoid interaction.
Methods
Insect lines and rearing conditions
Two nuclear backgrounds of each of Drosophila melanogaster (YW-BNE and w 1118) and D. simulans (CO and DSR) were used, either infected with or cured of different Wolbachia strains, leading to eight inbred lines as described in Table 1. All flies were reared under a 12L∶12D photoperiod at 21°C and fed with a standard diet [36]. Cured lines were obtained by mixing in each fly vial, 0.5 mL of a 100 µg/mL rifampicin antibiotic solution to the 10 mL/vial fly food, for three generations. To eliminate any potential direct effect of the antibiotics, Drosophila lines were then reared on antibiotic-free food for several generations before the start of the experiments. Their Wolbachia infection status was checked by PCR detection using the 81F-691R wsp primers specific to Wolbachia [37].
Table 1. Description of the Drosophila lines with their respective Wolbachia strain, and the L. boulardi lines.
| Insect species | Nuclear background | Origin | Symbiont strain | Reference |
| D. melanogaster | YW-BNE | Toowong, Brisbane, Australia | wMel | [55] |
| Cured | This study | |||
| w 1118 | Pasadena, California, USA | wMelPop | [56] | |
| Cured | This study | |||
| D. simulans | CO | Coffs Harbour, Australia | wAu | [20] |
| Cured | This study | |||
| DSR | Riverside, California, USA | wRi | [57] | |
| Cured | This study | |||
| L. boulardi | Sienna9 | Sienna, Italy | LbFV particles from a French population | [38] |
| Uninfected | [38] |
Two reference lines of L. boulardi, designated NSref and Sref, with the same nuclear genetic background but a different virus-infection status were used (Table 1). NSref is an inbred uninfected line (with an estimated homozygosity greater than 82%) originating from Sienna (Italy), that lay only one egg per Drosophila larva on average [38]. Sref is LbFV-infected and is derived from the NSref line, which was infected with viral particles originating from the south of France (Gotheron) via natural horizontal transfer, after a superparasitism event. This newly infected line proved stable over generations for virus infection and susceptible to the behavioral manipulation exerted by LbFV (increase in superparasitism tendency). Before the start of our experiments, parasitoids were maintained under a 12L∶12D photoperiod at 26°C, on a laboratory Wolbachia-free D.melanogaster line originating from Lyon (France). Both NSref and Sref have been shown to be Wolbachia-free in a previous study [38]. Viral infection status of these two L. boulardi lines was determined by diagnostic PCR using the primers 500-R/102F designed for specific detection of LbFV [39]. LbFV has, to date, never been found in Drosophila hosts [39].
Experiment 1: Contribution of LbFV and Wolbachia to the host-parasitoid interaction
In experiment 1, we addressed the question of the contribution of LbFV and Wolbachia on several key traits of the Drosophila-parasitoid interaction. For each line, one hundred eggs were deposited into rearing vials (n = 40 per Drosophila nuclear background per Wolbachia infection status combination, 320 vials in total). Twenty-four hours later, a single female parasitoid, either LbFV-infected or not, was introduced into each vial (n = 15 for each parasitoid infection status) and removed 24 hours later. Ten control vials for each Drosophila line (Wolbachia-infected and Wolbachia-free lines) were kept without parasitoid. Experiments were carried out in large incubators at 26°C under 12L∶12D photoperiod and 70% relative humidity.
From day 7, Drosophila flies that were not parasitized, or were parasitized but eliminated the parasitoid, started to emerge and were collected daily and counted at the end of the experiment. In response to parasitism, Drosophila larvae can initiate a protective immune reaction, which can lead to the encapsulation of the parasitoid egg or larva [19]. Successful encapsulations are easily detected in the adult flies' abdomens, under a stereomicroscope, by crushing the entire individual between two glass slides. The number of flies containing capsules was recorded. Parasitoids started to emerge 12 days after the emergence of the first flies (day 19), were removed from the vials and counted at the end of the experiment. For technical reasons, the experiment was split into two temporal blocks, half of the vials of each treatment being launched on one day and the other half on the following day.
Fitness-related traits involved in the Drosophila-parasitoid interaction
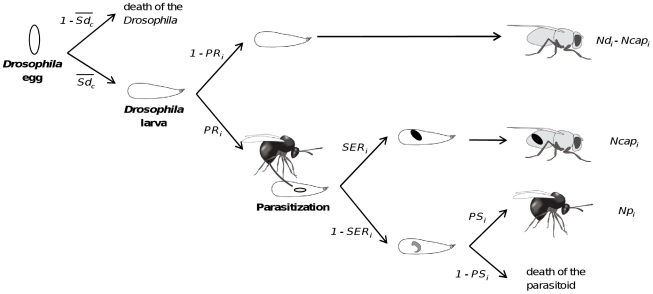
Different key life-history traits influencing the outcome of the Drosophila-parasitoid interaction were measured (Figure 1). The parasitism rate (PRi), or the proportion of Drosophila larvae parasitized by a single female parasitoid in a given vial i, was estimated by comparing the number of emerged flies in the treatment vial i (Ndi) to the mean number of flies in the control vials (Nc) of each Drosophila line as follows:
with Ncapi being the number of adult flies containing capsules in vial i.
Figure 1. Temporal sequence of the Drosophila-parasitoid interaction and key life-history traits.
Drosophila natural mortality (not due to parasitism) was assumed to occur early, before introduction of the parasitoid. (Sdc): mean Drosophila survival in control vials, (PRi): parasitism rate in vial i, (SERi): successful encapsulation rate in i, (PSi): parasitoid developmental success in i, (Ncapi): number of flies with successful encapsulation in i and (Npi): number of emerging parasitoids in i.
From this estimator, the successful encapsulation rate (SERi), defined as the proportion of parasitized Drosophila larvae that survived up to the adult stage, was calculated by dividing the number of flies containing capsules by the estimated number of parasitized larvae:
The parasitoid developmental success (PSi), defined as the proportion of parasitoids that survived up to the adult stage after successfully avoiding encapsulation, was calculated as follows:
with Npi being the number of adult parasitoid offspring in vial i.
Overall fitness
We used the number of adult parasitoid offspring Npi as the best approximation of the female parasitoid's overall fitness. To take into account variation in intrinsic mortality among the Drosophila nuclear backgrounds, we calculated an index of the Drosophila's fitness relative to their natural mortality, i.e. in the absence of parasitoid. Drosophila fitness (Sdrel) exposed to parasitoids was thus defined as the fly survival in vial i (Sdi) relative to their respective survival in control vials (Sdc):
Experiment 2: Direct and indirect effect of the virus on encapsulation
Virus-infected and uninfected parasitoids display contrasting egg-laying strategies (frequent superparasitism for Sref and rare for NSref). The virus' effects on the outcome of host-parasitoid interactions may therefore either result from a direct effect of the virus or from an indirect effect through the occurrence of superparasitism. In order to distinguish between these effects, we performed a second experiment using only the cured DSR line. We chose this particular line for its successful encapsulation rate, clearly dependent on the parasitoid infection status. Forty vials were prepared for both uninfected and virus-infected parasitoid lines. Half of these contained 100 Drosophila eggs deposited on day 1 (experiment 2.1), and the other half contained 125 eggs deposited on day 2 (experiment 2.2). We used two Drosophila densities (100 eggs or 125 eggs) in order to vary the host/parasitoid ratio and possibly the frequency of superparasitism. For each larval density, ten additional vials without parasitoid were used as controls. From each treatment vial, ten randomly chosen Drosophila pupae were dissected under a stereomicroscope. We recorded the number of parasitoid eggs, parasitoid larvae and the number of capsules found in each pupa. The larval encapsulation rate (LERi) is the proportion of fly larvae that encapsulated all parasitoids. For this analysis, we only considered fly larvae containing either one or two parasitoids since larvae containing more than two parasitoids were too rare to support strong statistical analyses. The encapsulation rate at adult stage (SERi) was measured as previously described in this paper except that dissected larvae were taken into account by subtracting 10 flies from the mean number of flies in the control vials (Nc).
Statistical analyses
All data sets were analysed with the R software (version 2.11.1) (R Development Core Team, 2005). Except for the larval encapsulation rate, all life-history traits were analysed using linear models after adequate transformation to reach the assumptions of normality and homoscedasticity. Some experimental vials in which parasitoids did not lay any eggs were disregarded from the analyses. Linear models were constructed by putting first the temporal block effect in order to remove the potential stochastic effects before testing the parameters of interest, i.e. the effects of the two symbionts. The larval encapsulation rate was analysed using a generalized linear model with a binomial error structure (logit link function) given the binary nature of the data (encapsulation of all parasitoids or not).
Results
Experiment 1: Contribution of LbFV and Wolbachia to the host-parasitoid interaction
In the global analysis of experiment 1, there was a contribution of the temporal block (either on its own or in complex interaction with other factors, Table 2) suggesting that some environmental parameters that were not controlled for in this experiment significantly influenced the outcome of the Drosophila-parasitoid interaction. Moreover, the Drosophila nuclear background was always significant showing that the outcome of host-parasitoid interaction was highly dependent on the host genotype (Table 2). Particularly, analyses per Drosophila nuclear background showed that the complex patterns of statistical interactions observed in the global analysis were mostly due to CO flies (Table S1). In the next sections, we will focus on symbiont effects and their interactions, consistently with our main goal.
Table 2. Analysis of variance of life-history traits of the host-parasitoid interaction in experiment 1.
| Parameters | df | Successful encapsulation rate (square root-transformed) | Parasitism rate (arcsine square root-transformed) | Parasitoid developmental success (square root-transformed) | Number of parasitoid offspring (square root-transformed) | Drosophila relative survival (log-transformed) | |||||
| F | P | F | P | F | P | F | P | F | P | ||
| Block (1) | 1 | 11.25 | 0.001* | 2.04 | 0.15 | 3.55 | 0.06 | 1.74 | 0.19 | 10.65 | 0.001* |
| Drosophila nuclear background (2) | 3 | 4.08 | 0.008* | 5.71 | 0.0009* | 15.89 | <0.0001* | 36.15 | <0.0001* | 8.36 | <0.0001* |
| Virus (3) | 1 | 46.55 | <0.0001* | 3.65 | 0.06 | 8.47 | 0.004* | 0.88 | 0.35 | 31.93 | <0.0001* |
| Wolbachia (4) | 1 | 1.15 | 0.28 | 0.0001 | 0.99 | 5.99 | 0.02* | 3.73 | 0.054 | 0.21 | 0.65 |
| interactions | |||||||||||
| (1)×(2) | 3 | 2.38 | 0.07 | 1.05 | 0.37 | 1.39 | 0.25 | 0.84 | 0.48 | 0.31 | 0.82 |
| (1)×(3) | 1 | 0.2 | 0.65 | 4.47 | 0.04* | 0.27 | 0.6 | 0.002 | 0.97 | 5.68 | 0.02* |
| (2)×(3) | 3 | 4.11 | 0.007* | 0.2 | 0.89 | 2.68 | 0.05* | 1.97 | 0.12 | 1.5 | 0.22 |
| (1)×(4) | 1 | 0.17 | 0.68 | 10.68 | 0.001* | 0.37 | 0.54 | 1.71 | 0.19 | 15.7 | 0.0001* |
| (2)×(4) | 3 | 0.08 | 0.97 | 4.95 | 0.002* | 4.13 | 0.007* | 5.39 | 0.001* | 3.92 | 0.009* |
| (3)×(4) | 1 | 3.52 | 0.06 | 5.98 | 0.02* | 0.02 | 0.89 | 3.09 | 0.08 | 7.2 | 0.008* |
| (1)×(2)×(3) | 3 | 0.35 | 0.79 | 2.99 | 0.03* | 6.5 | 0.0003* | 6.74 | 0.0002* | 4.06 | 0.008* |
| (1)×(2)×(4) | 3 | 0.69 | 0.55 | 0.35 | 0.79 | 2.45 | 0.06 | 0.66 | 0.57 | 1.06 | 0.37 |
| (1)×(3)×(4) | 1 | 7.21 | 0.008* | 3.45 | 0.06 | 0.32 | 0.57 | 0.004 | 0.94 | 11.33 | 0.0009* |
| (2)×(3)×(4) | 3 | 8.69 | <0.0001* | 1.38 | 0.25 | 1.27 | 0.29 | 0.58 | 0.63 | 4.76 | 0.003* |
| (1)×(2)×(3)×(4) | 3 | 4.2 | 0.007* | 4.08 | 0.008* | 0.58 | 0.63 | 0.63 | 0.59 | 6.65 | 0.0003* |
| residuals | 191 | ||||||||||
significant effect. Level of significance is α = 5%.
Effect of LbFV
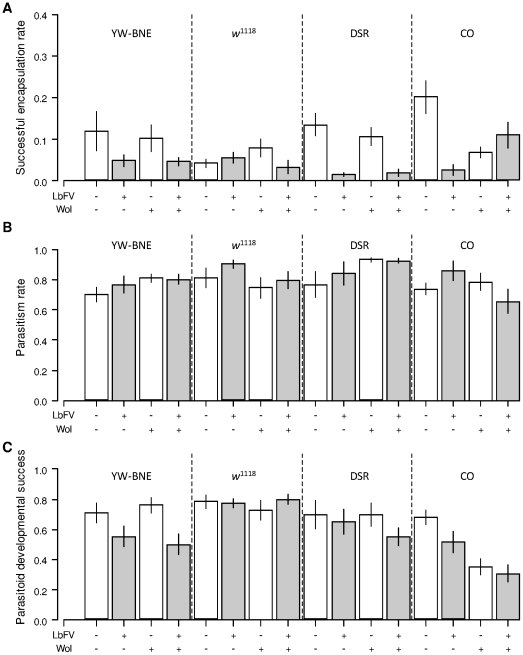
Successful encapsulation rates were relatively low: on average 7.5% of parasitized fly larvae successfully encapsulated the parasitoid(s) and survived to the adult stage (Figure 2A). Despite this low range in encapsulation level, significant differences were found according to the virus infection status. Virus-infected parasitoids were less frequently encapsulated than their uninfected counterparts (4.4% versus 10.6%; Figure 2A; Table 2). Analysis of the data per Drosophila nuclear background indicated that this difference was significant in both D. simulans nuclear backgrounds (DSR: F1,41 = 47.52, P<0.0001; CO: F1,48 = 13.26, P = 0.0007; Table S1). In D. melanogaster nuclear backgrounds, a similar trend was observed but was only marginally significant when corrected for multiple comparisons (Level of significance: 0.0125; YW-BNE: F1,52 = 5.3, P = 0.03; w 1118: F1,50 = 2.98, P = 0.09; Table S1). Importantly, the virus effect was independent of the block effect (Table 2, Table S1).
Figure 2. Fitness-related traits in experiment 1.
(A) Successful encapsulation rate; (B) Parasitism rate; (C) Parasitoid developmental success ; (−) not infected; (+) infected; (Wol) Wolbachia. White and grey: virus-free and virus-infected parasitoids respectively. Bars are standard errors.
Virus-infected parasitoids tended to show a slightly higher parasitism rate but this difference was not significant (Figure 2B ; Table 2). The viral infection had, on average, a negative effect on parasitoid developmental success (Figure 2C ; Table 2), even if the decrease was only significant in YW-BNE flies (F1,52 = 12.98; P<0.0007; Table S1).
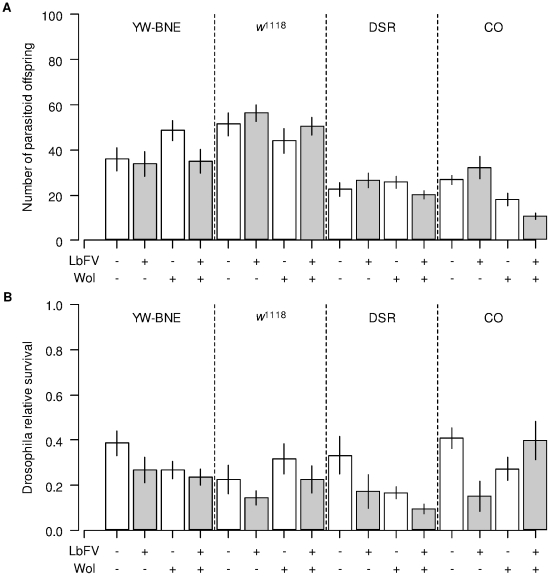
Both virus-infected and virus-free parasitoids produced a similar number of offspring (Figure 3A ; Table 2). On the host side, the presence of the virus decreased Drosophila relative survival, consistent with the lower successful encapsulation rate of virus-infected parasitoids (Figure 3B ; Table 2). This negative effect of the virus on Drosophila fitness was significant in both D. simulans nuclear backgrounds (DSR: F1,41 = 9.15, P = 0.004; CO: F1,48 = 21.52, P<0.0001; Table S1) and involved an interaction with the block effect for CO background. A similar trend, marginally significant, was observed in D. melanogaster (YW-BNE: F1,52 = 3.95, P = 0.052; w 1118: F1,50 = 3.83, P = 0.06; Table S1).
Figure 3. Overall fitness of parasitoids and Drosophila hosts in experiment 1.
(A) Number of parasitoid offspring ; (B) Drosophila relative survival; (−) not infected; (+) infected; (Wol) Wolbachia. White and grey: virus-free and virus-infected parasitoids respectively. Bars are standard errors.
Effect of Wolbachia
Overall, Wolbachia did not impact the ability of flies to escape parasitism (no effect on parasitism rate), or their ability to successfully encapsulate parasitoids (Figure 2A; Table 2). However, Wolbachia presence correlated with a slight reduction in parasitoid developmental success (Figure 2C; Table 2) but this effect was only significant in CO flies (F1,48 = 15.62; P = 0.0003; Table S1). Consistently, there was a tendency for a decrease in the number of parasitoids in the presence of Wolbachia (Table 2) but this effect was again only significant in CO flies (F1,48 = 17.08; P<0.0001; Table S1).
LbFV-by-Wolbachia interaction
There was a marginally significant LbFV-by-Wolbachia interaction for successful encapsulation rate and significant LbFV-by-Wolbachia interactions for parasitism rate as well as for Drosophila relative survival (Figure 2A, 2B & 3B; Table 2). However, the analysis per nuclear background revealed that these virus-by-Wolbachia interactions for these traits were only significant within CO background (Successful encapsulation rate: F1,48 = 23.93, P<0.0001; Parasitism rate: F1,48 = 7.68, P = 0.008; Drosophila relative survival: F1,48 = 26.5; P<0.0001; Table S1).
Within this background, Wolbachia (wAu) infection was correlated with a reduction in the successful encapsulation rate of virus-free parasitoids (Tukey's honest significance test, P = 0.01), but with a slight increase in the encapsulation rate of virus-infected parasitoids (Tukey's honest significance test, P = 0.02). Also, within this background, Wolbachia (wAu) infection was correlated with a significant reduction in parasitism rate when Drosophila were attacked by infected parasitoids (Tukey's honest significance test, P = 0.03) but was not correlated with parasitism rate when Drosophila were attacked by uninfected parasitoids (Tukey's honest significance test, P = 0.91). Finally, Wolbachia (wAu) infection had no effect on Drosophila relative survival when CO flies were exposed to virus-free parasitoids (Tukey's honest significance test, P = 0.61) whereas wAu-free flies had a lower survival than wAu-infected flies when exposed to virus-infected parasitoids (Tukey's honest significance test, P = 0.001).
We must stress that these virus-by-Wolbachia interactions should be interpreted with care since they were all highly dependent on the temporal block, according to the significant interactions of third order (Successful encapsulation rate: F1,48 = 17.78; P<0.0001; Parasitism rate: F1,48 = 39.64; P<0.0001; Drosophila relative survival: F1,48 = 11.44; P<0.001; Table S1).
Experiment 2: Direct and indirect effects of LbFV on encapsulation
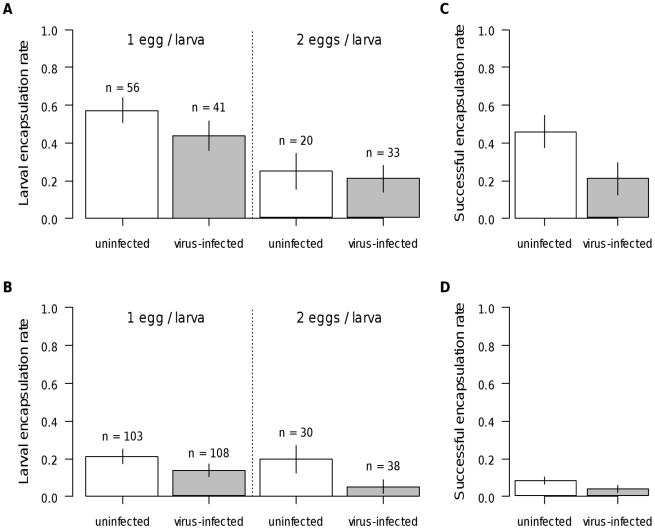
The most important effect detected in experiment 1 was the decrease in encapsulation rate when parasitoids were infected by LbFV. As the virus modifies the way females distribute their eggs among Drosophila larvae, we tried to separate out a direct effect of the virus from a potential indirect effects of superparasitism on encapsulation. To this end, we used the Wolbachia-cured DSR Drosophila line in a second experiment since, in this line, the virus effect previously observed was strong. In this second experiment, measures on adult flies confirmed the result from experiment 1: virus-infected parasitoids are less often successfully encapsulated than virus-free parasitoids (Figure 4C & D; F1,75 = 15.3; P<0.0001). There was also a high variability between experiments 2.1 (low larval density) and 2.2 (high larval density) with a significantly lower successful encapsulation rate in experiment 2.2 (F1,75 = 38.18; P<0.0001).
Figure 4. Encapsulation rates in cured DSR flies in experiment 2.
Top: experiment 2.1 (low larval density). Bottom: experiment 2.2 (high larval density). (A & B) Larval encapsulation rate. (C & D) Successful encapsulation rate with “n” giving the number of dissected larvae. White and grey: virus-free and virus-infected parasitoids respectively. Bars are standard errors.
In both experiment 2.1 and 2.2, substantial superparasitism rates (proportion of superparasitized Drosophila larvae among parasitized ones) were observed with both virus-infected (≈61% and 30% for low and high larval density respectively) and uninfected parasitoids (≈30% and 26% for low and high larval density respectively).
The dissections of larvae confirmed the previous finding obtained on adults that infected parasitoids were less frequently encapsulated than uninfected parasitoids (Figure 4A & B; Table 3). This effect involved both a direct and an indirect effect of the virus. Analysis of monoparasitized Drosophila larvae demonstrated a direct effect of the virus: LbFV-infected parasitoids had a 11.8% reduction in the chance of being encapsulated compared with uninfected parasitoids (Dev = 5.34; df = 1; P = 0.02). Additionally, superparasitized larvae showed a 11.7% decrease in successful encapsulation events (encapsulation of all developing parasitoids) compared with monoparasitized larvae (Figure 4A &B; Table 3). Since LbFV is associated with an increase in the superparasitism tendency of females, this effect constitutes an indirect effect of the virus on encapsulation.
Table 3. Analysis of the larval encapsulation rate in cured DSR flies in experiments 2.1 (low larval density) and 2.2 (high larval density).
| df | Deviance | P | |
| Experiment | 1 | 31.99 | <0.0001* |
| Virus | 1 | 7.99 | 0.005* |
| Superparasitism | 1 | 9.74 | 0.002* |
| Experiment×virus | 1 | 0.18 | 0.67 |
| Experiment×superparasitism | 1 | 1.77 | 0.18 |
| Virus×superparasitism | 1 | 0.18 | 0.67 |
| Experiment×virus×superparasitism | 1 | 1.18 | 0.28 |
significant effect in the generalized linear model. Level of significance is α = 5%.
Discussion
Drosophila hosts can suffer high mortality rates due to parasitoid attacks [33], [40]. As a consequence, resistance against parasitoids should be strongly selected for, and encapsulation is one very common host defense strategy [41]. The expression of resistance is however affected by various factors such as host genotype-by-parasitoid genotype interactions [42], [43] or trade-offs with other traits [44]. The influence of bacterial symbionts on encapsulation was only recently investigated [12], [18]. Here, we tested the effect of two symbionts on the outcome of the interaction between Drosophila of several nuclear backgrounds and the parasitoid Leptopilina boulardi. We demonstrated that the behavior-manipulating virus LbFV of the wasp can interplay with the immune reaction of the Drosophila host by increasing the virulence of the parasitoid. Additionally, the Wolbachia strain wAu affected the encapsulation rate in CO flies, however, the direction of this effect depended on the parasitoid's infection status and it was not observed with any of the other Wolbachia strains tested.
In a first experiment, we tested the effect of LbFV and Wolbachia across different Drosophila nuclear backgrounds. Drosophila parasitized by virus-infected parasitoids had a lower successful encapsulation rate. This trend was consistent for all four Drosophila nuclear backgrounds tested but only significant in the D. simulans CO and DSR backgrounds. The non-significant trend in YW-BNE and w 1118 possibly results from a low statistical power due to the overall low encapsulation rate rather than a true absence of virus effect.
In a second set of experiments using Wolbachia-cured DSR flies as hosts, we tested whether this virus effect is caused by a direct effect on encapsulation or by an indirect effect of the increased tendency to superparasitize of virus-infected females. Considering that encapsulation is a costly physiological process, we should expect that flies would not be able to encapsulate more than a few parasitoids. Thus, the higher the superparasitism rate is, the lower the encapsulation rate should be. Dissections of larvae showed that the successful encapsulation rate variation measured on adult flies was indeed partly explained by the occurrence of superparasitism. Superparasitized larvae often failed to encapsulate all parasitoids whereas monoparasitized larvae succeeded more frequently, a result that is consistent with earlier studies on other host-parasitoid systems [45], [46]. For instance, in Spodoptera littoralis exposed to superparasitism by Microplitis rufiventris, a decrease in both cellular (encapsulation) and humoral response efficiencies was demonstrated [45].
In addition to this indirect effect of the virus on encapsulation rate through the induction of superparasitism, we also demonstrated a significant direct effect of the virus. In monoparasitized larvae, the presence of LbFV was associated with a decrease in larval encapsulation rate. This effect may arise either because the Drosophila immune response is depressed by the presence of the virus, or because infected-parasitoids have an increased virulence ability. The mechanism responsible for this protection, yet unknown, could involve either a virus-driven immune suppression as observed with polydnaviruses [47] or an evasion of the immune system [48], [49].
Whereas the direct protective effect of the virus is clearly advantageous for the parasitoid, the fitness reward from the indirect effect of superparasitism is unclear. In our experiment, one single female was put in each treatment vial, and could therefore directly benefit from self-superparasitism. In nature, however, conspecific-superparasitism is likely to be much more frequent than self-superparasitism. In such conditions, it is unknown if the superparasitizing female would benefit from the protective effect offered by superparasitism since this would depend on the outcome of the within-Drosophila competition between parasitoid larvae.
Besides encapsulation, the virus also negatively affected parasitoid developmental success. This virus effect could be either a direct effect of the physiological cost of infection, or an indirect effect of the increased superparasitism in infected parasitoids, as suggested by a previous study [50]. Indeed, virus-infected parasitoids are expected to develop more frequently in superparasitized larvae and must cope with intense competition. Despite this cost on developmental success, virus-infected and uninfected parasitoids produced similar numbers of offspring in all tested host-parasitoid combinations suggesting that this cost is compensated by the virus-mediated decrease in successful encapsulation.
Except for wAu, Wolbachia did not affect any of the tested traits. A positive effect of Wolbachia on the successful encapsulation rate was expected, at least for wMel and wMelPop since these strains have previously been shown to increase hemolymph melanization, a key reaction involved in encapsulation, in both D. melanogaster and D. simulans [51]. No effect was however detected for wMel, wMelPop nor wRi. Fytrou et al. (2006) found that wRi-infected DSR flies were less efficient in encapsulating the parasitoid Leptopilina heterotoma. Their results differ from our findings on the similar DSR Drosophila line, and indicate that the final outcome of host-parasitoid interactions also depends on the parasitoid species.
A surprising result was the complex interaction observed between viral infection in the parasitoid and infection by Wolbachia in Drosophila that was only observed with the strain wAu in D. melanogaster. Overall, Wolbachia-free CO flies suffered more from virus-infected parasitoid attacks than wAu-infected flies did. The slight increase in the encapsulation rate of virus-infected parasitoids suggests that a wAu-mediated protection might be activated in presence of LbFV. This is consistent with the strong antiviral protection of wAu in CO flies, allowing resistance against the RNA virus DCV [23]. However, the effect on encapsulation was not detected for the other Wolbachia strains tested, although they were also found to have an antiviral activity in previous studies [6], [22], [23]. In addition, virus-infected parasitoids exhibited higher parasitism rates on Wolbachia-free than on Wolbachia-infected larvae, whereas virus-free parasitoids displayed a similar parasitism rate whatever the infection status of CO flies. This result suggests that wAu might either influence the ability of infected parasitoids to locate Drosophila larvae, modify their egg-laying preferences or that wAu-infected Drosophila larvae might be better at avoiding parasitoid attacks when the parasitoid is infected by the virus. We must however be cautious as all these interaction effects strongly depended on the temporal block and thus on unknown environmental parameters. Further investigations should be carried out before concluding that wAu can be beneficial to its host, and to determine by which way wAu interacts with LbFV.
In conclusion, our data confirm that symbionts in hosts and parasitoids contribute to variation in extremely important phenotypes such as resistance and virulence, in addition to classical nuclear factors [42], [52]. Results also encourage a reconsideration of the cost-benefit balance of LbFV infection for L. boulardi. A virus-induced increase in L. boulardi's virulence might depict an ongoing evolution towards a mutualistic association between the virus and the parasitoid, similar to what is believed to have occurred between ancestral polydnaviruses and their wasp carriers [15]. From the host side, we again demonstrated, but only for wAu strain, that Wolbachia might not only be a reproductive parasite in arthropods, but may as well contribute to variation of traits involved in host-parasitoid interactions. Because symbionts benefit from vertical transmission, they produce heritable variation on which natural selection can act and directly contribute to the adaptation of their host. As such, there is a crucial need to view infections by so-called parasites in a broader ecological context by considering several life-history traits of their hosts and their interactions with other species within the community [53]. More generally, we should also take symbionts into account as a potential force shaping this community [54].
Supporting Information
Analysis of variance of life-history per Drosophila nuclear background in experiment 1. * Significant effect; Level of significance: α = 0.0125 (Bonferroni correction for multiple comparisons). Successful encapsulation rate: square root-transformed data; Parasitism rate: arcsine square root-transformed data; Parasitoid developmental success: square root-transformed data; Number of parasitoid offspring: square root-transformed data; Drosophila relative survival: log-transformed data.
(DOC)
Acknowledgments
We thank Dr. I. Iturbe-Ormaetxe, Dr. J. Brownlie and Dr. E.A. McGraw for constructive discussions and Yi San Leong for help with the establishment of the cured Drosophila lines. Finally, we thank the two anonymous reviewers for their helpful comments.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Prof. O’Neill’s Australian Research Council grant (DP0772992), Centre National de la Recherche Scientifique (UMR CNRS 5558). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Moran N. Symbiosis as an adaptive process and source of phenotypic complexity. Proc Natl Acad Sci USA. 2007;104:8627–8633. doi: 10.1073/pnas.0611659104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbert SF, McDonald E, Boyle N, Buttino N, Gyi L, et al. Symbiosis as a source of selectable epigenetic variation: taking the heat for the big guy. Philos T Roy Soc B. 2010;365:671–678. doi: 10.1098/rstb.2009.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scarborough CL, Ferrari J, Godfray HCJ. Aphid protected from pathogen by endosymbiont. Science. 2005;310:1781–1781. doi: 10.1126/science.1120180. [DOI] [PubMed] [Google Scholar]
- 4.Oliver K, Russell J, Moran N, Hunter M. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc Natl Acad Sci USA. 2003;100:1803–1807. doi: 10.1073/pnas.0335320100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodrich-Blair H, Clarke DJ. Mutualism and pathogenesis in Xenorhabdus and Photorhabdus: two roads to the same destination. Mol Microbiol. 2007;64:260–268. doi: 10.1111/j.1365-2958.2007.05671.x. [DOI] [PubMed] [Google Scholar]
- 6.Teixeira L, Ferreira A, Ashburner M. The Bacterial Symbiont Wolbachia Induces Resistance to RNA Viral Infections in Drosophila melanogaster. Plos Biol. 2008;6:2753–2763. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ. Adaptation via Symbiosis: Recent Spread of a Drosophila Defensive Symbiont. Science. 2010;329:212–215. doi: 10.1126/science.1188235. [DOI] [PubMed] [Google Scholar]
- 8.Schneider DS, Chambers MC. Rogue Insect Immunity. Science. 2008;322:1199–1200. doi: 10.1126/science.1167450. [DOI] [PubMed] [Google Scholar]
- 9.Oliver KM, Degnan PH, Burke GR, Moran NA. Facultative Symbionts in Aphids and the Horizontal Transfer of Ecologically Important Traits. Annu Rev Entomol. 2010;55:247–266. doi: 10.1146/annurev-ento-112408-085305. [DOI] [PubMed] [Google Scholar]
- 10.Vorburger C, Sandrock C, Gouskov A, Castaneda LE, Ferrari J. Genotypic variation and the role of defensive endosymbionts in an all-parthenogenetic host-parasitoid interaction. Evolution. 2009;63:1439–1450. doi: 10.1111/j.1558-5646.2009.00660.x. [DOI] [PubMed] [Google Scholar]
- 11.Vorburger C, Gehrer L, Rodriguez P. A strain of the bacterial symbiont Regiella insecticola protects aphids against parasitoids. Biol Letters. 2010;6:109–111. doi: 10.1098/rsbl.2009.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie JL, Vilchez I, Mateos M. Spiroplasma Bacteria Enhance Survival of Drosophila hydei Attacked by the Parasitic Wasp Leptopilina heterotoma. Plos One. 2010;5:7. doi: 10.1371/journal.pone.0012149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stasiak K, Renault S, Federici BA, Bigot Y. Characteristics of pathogenic and mutualistic relationships of ascoviruses in field populations of parasitoid wasps. J Insect Physiol. 2005;51:103–115. doi: 10.1016/j.jinsphys.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Renault S, Stasiak K, Federici B, Bigot Y. Commensal and mutualistic relationships of reoviruses with their parasitoid wasp hosts. J Insect Physiol. 2005;51:137–148. doi: 10.1016/j.jinsphys.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bezier A, Annaheim M, Herbiniere J, Wetterwald C, Gyapay G, et al. Polydnaviruses of Braconid Wasps Derive from an Ancestral Nudivirus. Science. 2009;323:926–930. doi: 10.1126/science.1166788. [DOI] [PubMed] [Google Scholar]
- 16.Volkoff AN, Jouan V, Urbach S, Samain S, Bergoin M, et al. Analysis of Virion Structural Components Reveals Vestiges of the Ancestral Ichnovirus Genome. Plos Pathog. 2010;6:10. doi: 10.1371/journal.ppat.1000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drezen JM, Bézier A, Lesobre J, Huguet E, Dupuy C. Virulence genes of parasitoid wasps encoded by symbiotic viruses. ICOPA XI: Proceedings of the 11th International Congress of Parasitology. 40128 Bologna: Medimond S R L; 2006. pp. 59–64. [Google Scholar]
- 18.Fytrou A, Schofield PG, Kraaijeveld AR, Hubbard SF. Wolbachia infection suppresses both host defence and parasitoid counter-defence. P Roy Soc Lond B Bio. 2006;273:791–796. doi: 10.1098/rspb.2005.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carton Y, Nappi AJ. Drosophila cellular immunity against parasitoids. Parasitol Today. 1997;13:218–227. doi: 10.1016/s0169-4758(97)01058-2. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann AA, Clancy D, Duncan J. Naturally-occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibility. Heredity. 1996;76:1–8. doi: 10.1038/hdy.1996.1. [DOI] [PubMed] [Google Scholar]
- 21.Merçot H, Charlat S. Wolbachia infections in Drosophila melanogaster and D. simulans: polymorphism and levels of cytoplasmic incompatibility. Genetica. 2004;120:51–59. doi: 10.1023/b:gene.0000017629.31383.8f. [DOI] [PubMed] [Google Scholar]
- 22.Hedges L, Brownlie J, O'Neill S, Johnson K. Wolbachia and Virus Protection in Insects. Science. 2008;322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- 23.Osborne SE, Leong YS, O'Neill SL, Johnson KN. Variation in Antiviral Protection Mediated by Different Wolbachia Strains in Drosophila simulans. Plos Pathog. 2009;5:9. doi: 10.1371/journal.ppat.1000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frentiu FD, Robinson J, Young PR, McGraw EA, O'Neill SL. Wolbachia-Mediated Resistance to Dengue Virus Infection and Death at the Cellular Level. Plos One. 2010;5:8. doi: 10.1371/journal.pone.0013398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 26.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu GJ, Pyke AT, et al. A Wolbachia Symbiont in Aedes aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 27.Kambris Z, Cook PE, Phuc HK, Sinkins SP. Immune Activation by Life-Shortening Wolbachia and Reduced Filarial Competence in Mosquitoes. Science. 2009;326:134–136. doi: 10.1126/science.1177531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes GL, Koga R, Xue P, Fukatsu T, Rasgon JL. Wolbachia Infections Are Virulent and Inhibit the Human Malaria Parasite Plasmodium Falciparum in Anopheles Gambiae. Plos Pathog. 2011;7:8. doi: 10.1371/journal.ppat.1002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glaser RL, Meola MA. The Native Wolbachia Endosymbionts of Drosophila melanogaster and Culex quinquefasciatus Increase Host Resistance to West Nile Virus Infection. Plos One. 2010;5 doi: 10.1371/journal.pone.0011977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chevalier F, Herbinière-Gaboreau J, Bertaux J, Raimond M, Morel F, et al. The Immune Cellular Effectors of Terrestrial Isopod Armadillidium vulgare: Meeting with Their Invaders, Wolbachia. Plos One. 2010;6:e18531. doi: 10.1371/journal.pone.0018531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sicard M, Chevalier F, De Vlechouver M, Bouchon D, Greve P, et al. Variations of immune parameters in terrestrial isopods: a matter of gender, aging and Wolbachia. Naturwissenschaften. 2010;97:819–826. doi: 10.1007/s00114-010-0699-2. [DOI] [PubMed] [Google Scholar]
- 32.Cerenius L, Soderhall K. The prophenoloxidase-activating system in invertebrates. Immunol Rev. 2004;198:116–126. doi: 10.1111/j.0105-2896.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 33.Patot S, Martinez J, Allemand R, Gandon S, Varaldi J, et al. Prevalence of a virus inducing behavioural manipulation near species range border. Mol Ecol. 2010;19:2995–3007. doi: 10.1111/j.1365-294X.2010.04686.x. [DOI] [PubMed] [Google Scholar]
- 34.Varaldi J, Fouillet P, Ravallec M, Lopez-Ferber M, Boulétreau M, et al. Infectious Behavior in a Parasitoid. Science. 2003;302:1930. doi: 10.1126/science.1088798. [DOI] [PubMed] [Google Scholar]
- 35.Gandon S, Rivero A, Varaldi J. Superparasitism evolution: Adaptation or manipulation? Am Nat. 2006;167:E1–E22. doi: 10.1086/498398. [DOI] [PubMed] [Google Scholar]
- 36.David J. A new medium for rearing Drosophila in axenic condition. Drosophila Information Service. 1962;36:128. [Google Scholar]
- 37.Zhou WG, Rousset F, O'Neill S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. P Roy Soc Lond B Bio. 1998;265:509–515. doi: 10.1098/rspb.1998.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varaldi J, Ravallec M, Labrosse C, Lopez-Ferber M, Boulétreau M, et al. Artifical transfer and morphological description of virus particles associated with superparasitism behaviour in a parasitoid wasp. J Insect Physiol. 2006;52:1202–1212. doi: 10.1016/j.jinsphys.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Patot S, Lepetit D, Charif D, Varaldi J, Fleury F. Molecular Detection, Penetrance, and Transmission of an Inherited Virus Responsible for Behavioral Manipulation of an Insect Parasitoid. Appl Environ Microb. 2009;75:703–710. doi: 10.1128/AEM.01778-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fleury F, Ris N, Allemand R, Fouillet P, Carton Y, et al. Ecological and genetic interactions in Drosophila–parasitoids communities: a case study with D. melanogaster, D. simulans and their common Leptopilina parasitoids in south-eastern France. Genetica. 2004;120:181–194. doi: 10.1023/b:gene.0000017640.78087.9e. [DOI] [PubMed] [Google Scholar]
- 41.Carton Y, Nappi AJ. Immunogenetic aspects of the cellular immune response of Drosophila against parasitoids. Immunogenetics. 2001;52:157–164. doi: 10.1007/s002510000272. [DOI] [PubMed] [Google Scholar]
- 42.Dupas S, Carton Y, Poirie M. Genetic dimension of the coevolution of virulence-resistance in Drosophila - parasitoid wasp relationships. Heredity. 2003;90:84–89. doi: 10.1038/sj.hdy.6800182. [DOI] [PubMed] [Google Scholar]
- 43.Dubuffet A, Dupas S, Frey F, Drezen JM, Poirié M, et al. Genetic interactions between the parasitoid wasp Leptopilina boulardi and its Drosophila hosts. Heredity. 2007;98:21–27. doi: 10.1038/sj.hdy.6800893. [DOI] [PubMed] [Google Scholar]
- 44.Kraaijeveld AR, Limentani EC, Godfray HCJ. Basis of the trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. P Royal Soc Lond B Bio. 2001;268:259–261. doi: 10.1098/rspb.2000.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hegazi E, Khafagi W. The effects of host age and superparasitism by the parasitoid, Microplitis rufiventris on the cellular and humoral immune response of Spodoptera littoralis larvae. J Invertebr Pathol. 2008;98:79–84. doi: 10.1016/j.jip.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Giordanengo P, Nenon JP. Melanization and encapsulation of eggs and larvae of Epidinocarsis lopezi by its host Phenacoccus manihoti - Effects of superparasitism and egg-laying patterns. Entomol Exp Appl. 1990;56:155–163. [Google Scholar]
- 47.Beckage NE. Modulation of immune responses to parasitoids by polydnaviruses. Parasitology. 1998;116:S57–S64. doi: 10.1017/s0031182000084948. [DOI] [PubMed] [Google Scholar]
- 48.Asgari S, Theopold U, Wellby C, Schmidt O. A protein with protective properties against the cellular defense reactions in insects. Proc Natl Acad Sci USA. 1998;95:3690–3695. doi: 10.1073/pnas.95.7.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kinuthia W, Li DM, Schmidt O, Theopold U. Is the surface of endoparasitic wasp eggs and larvae covered by a limited coagulation reaction? J Insect Physiol. 1999;45:501–506. doi: 10.1016/s0022-1910(98)00164-4. [DOI] [PubMed] [Google Scholar]
- 50.Varaldi J, Boulétreau M, Fleury F. Cost induced by viral particles manipulating superparasitism behaviour in the parasitoid Leptopilina boulardi. Parasitology. 2005;131:1–8. doi: 10.1017/s0031182005007602. [DOI] [PubMed] [Google Scholar]
- 51.Thomas P, Kenny N, Eyles D, Moreira LA, O'Neill SL, et al. Infection with the wMel and wMelPop strains of Wolbachia leads to higher levels of melanization in the hemolymph of Drosophila melanogaster, Drosophila simulans and Aedes aegypti. Dev Comp Immunol. 2011;35:360–365. doi: 10.1016/j.dci.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Dupas S, Frey F, Carton Y. A single parasitoid segregating factor controls immune suppression in Drosophila. J Hered. 1998;89:306–311. doi: 10.1093/jhered/89.4.306. [DOI] [PubMed] [Google Scholar]
- 53.Sternberg ED, Lefèvre T, Rawstern AH, de Roode JC. A virulent parasite can provide protection against a lethal parasitoid. Infection, Genetics and Evolution. 2011;11:399–406. doi: 10.1016/j.meegid.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 54.Ferrari J, Vavre F. Bacterial symbionts in insects or the story of communities affecting communities. Philos T Roy Soc B. 2011;366:1389–1400. doi: 10.1098/rstb.2010.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamada R, Floate KD, Riegler M, O'Nein SL. Male development time influences the strength of Wolbachia-Induced cytoplasmic incompatibility expression in Drosophila melanogaster. Genetics. 2007;177:801–808. doi: 10.1534/genetics.106.068486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Min KT, Benzer S. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc Natl Acad Sci USA. 1997;94:10792–10796. doi: 10.1073/pnas.94.20.10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoffmann AA, Turelli M, Simmons GM. Unidirectional incompatibility between populations of Drosophila simulans. Evolution. 1986;40:692–701. doi: 10.1111/j.1558-5646.1986.tb00531.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Analysis of variance of life-history per Drosophila nuclear background in experiment 1. * Significant effect; Level of significance: α = 0.0125 (Bonferroni correction for multiple comparisons). Successful encapsulation rate: square root-transformed data; Parasitism rate: arcsine square root-transformed data; Parasitoid developmental success: square root-transformed data; Number of parasitoid offspring: square root-transformed data; Drosophila relative survival: log-transformed data.
(DOC)