Abstract
Chronic pain has profound effects on activity. Previous reports indicate chronic inflammatory conditions result in reduced activity which normalizes upon pain treatment. However, there is little systematic investigation of this process. Rheumatoid arthritis is an autoimmune disorder that causes significant joint pain. The K/BxN serum transfer mouse has been characterized as a model for rheumatoid arthritis and chronic pain. We investigated the activity of mice following K/BxN serum transfer vs. control serum and observed the activity changes following delivery of an NSAID, ketorolac. Previous studies have used running wheels and laser beams to monitor activity; we chose to validate a model using cost-effective infrared sensors on individual cages. Each mouse had its baseline activity obtained, which showed significant variation between individual C57Bl/6 mice. Arthritic mice had significantly decreased activity for only the first 11 nights. Conversely, previous work has shown that these animals display tactile allodynia that persists for at least 45 days. Mice were treated with ketorolac in their drinking water (10mg/kg, 15mg/kg, or 20mg/kg) for nights 6–8. The two highest doses showed significant normalization of activity levels. Four nights after ketorolac was stopped, treated animals were still significantly more active than control. The reversal of the reduced activity provides support that the depression relates to the arthritic pain state of the animal. These results indicate the efficacy of activity monitoring to better investigate behavior in persistent pain states. However, insofar as depressed activity reflects pain and disability, the present work raises questions as to the relevance of the tactile thresholds in defining behaviorally relevant pain states.
Keywords: Chronic pain, Arthritis, Ketorolac, Tactile allodynia
1. INTRODUCTION
Chronic pain can have profound effects upon activity and sleep wake cycles. In experiments assessing activity, chronic inflammatory conditions as produced by intra-articular Complete Freund's Adjuvant (CFA) or carrageenan results in reduced activity [1],[2]. The notion that the decline in normal function in these chronic states reflects upon a pain condition is suggested by the observation that spinal lesions reducing nociceptive transmission [3], treatment with NSAIDs [4], intra-articular botulinum toxin [5], or analgesic such as opiates [6] can normalize activity. This suggests that a chronic inflammatory state will reduce behavior and disrupt diurnal cyclicity. Drugs which can relieve pain should thus normalize behavior. However, there is little systematic study of this normalization in terms of activity. In fact, when examined, there have been interesting and unexpected findings such as early electroencephalography work which suggest differences between agents such as aspirin (acetylsalicylic acid) and acetaminophen [4].
The current literature on activity cycles and pain has typically involved models which have a relatively short time course (for intraplantar carrageenan the intervals are 1–2 days and CFA are 3–7 days). However, significant neurological remodeling appears to occur in chronic pain states (see below) which have not been reproduced in these less persistent models. Recent literature has focused on the K/BxN mouse serum transfer model of chronic arthritis [7]. The K/BxN mouse is a cross between KRN (expressing a KRN T cell receptor transgene) mice and NOD (non-obese diabetic) mice. These mice spontaneously make auto-antibodies against glucose-6-phosphate isomerase (GPI), which is present in the joints, and this leads to development of severe inflammatory arthritis [8]. Serum obtained from these mice can be used to initiate inflammatory arthritis in a wide range of mouse strains. After serum injection, this mouse develops severe joint inflammation through a period of up to 10–14 days and this is accompanied by a prominent tactile allodynia. Allodynia, defined as “pain in response to a non-nociceptive stimulus” by current taxonomic guidelines of the International Association for the Study of Pain, has often been used a measure of the pain state, especially in neuropathic pain studies [9]. In the K/BxN serum transfer model, tactile allodynia remains for at least 28 days, while inflammation resolves at 10–14 days. Such persistent pain states are often accompanied by a variety of trophic changes including upregulation of inflammatory cytokines such as IL-8 and peptides such as substance P in patients suffering from pain brought on from injury or surgical trauma[10]. Interestingly, data suggests that the tactile allodynia observed during the early phase is NSAID-sensitive while the later phase is not [7]. It is widely appreciated that the presence of persistent pain can have a prominent impact upon ongoing behavior. That is to say, ongoing pain will often cause a suppression of normal diurnal activity [11]. This is particularly true in arthritic pain states. Such information however has not been reported for chronic arthritic models such as the KBxN mouse.
The aim of this study therefore is to determine if there is a disruption in the activity cycle of the K/BxN mouse that correlates with the observed tactile allodynia. Based on the previously reported analgesic pharmacology of the KBxN tactile allodynia, we hypothesize that any disruption of diurnal activity would be reversed by treatment with an anti-inflammatory agent, such as ketorolac.
An additional aspect of this work is the ongoing validation of an activity model in mice with chronic joint pain. Previous work has used techniques such as the running wheel [5] and light beams to monitor the activity of mice. We developed a cost effective thermal sensing system that recognizes body movement in a restricted field (such as the home cage). This system has the advantage of being inexpensive, robust and applicable to large and small rodents alike.
2. MATERIALS AND METHODS
2.1 Animals
All experimental protocols were approved by the Institutional Animal Care committee. Male C57Bl/6 mice (20–25g) were purchased from Harlan (Indianapolis, IN). Mice were kept at room temperature on a 12 hour light/dark cycle with lights on at 7:00 am. Food and water was available ad libitum. Cage cleaning and allodynia was conducted during the light cycle. Cage cleaning was performed during the day and was kept at a minimum to minimize the disruption to the sleep/wake cycle of the animals.
2.2 Serum Transfer and Drug Treatment
Blood was collected from arthritic adult K/BxN mice and centrifuged at 10,000 rpm for 10 minutes, after which sera were pooled. Groups of 9 or 15 mice received 100µl of sera by intraperitoneal (i.p.) injection on day 0 and day 2 (total volume 200 µl).
Animals treated with ketorolac received it in their drinking water for days 6–9 (nights 6–8). Animals were estimated to drink 15 ml of water per 100g of body weight [12]. This allowed ketorolac to be delivered through drinking water in a concentration (mg/ml) in order to give each animal a dosage in mg/kg. Ketorolac was chosen because it is systemically bioavailable, well studied, and widely used in mice models. We also chose to administer it by mouth because we felt it was much less invasive than a continuous subcutaneous pump or daily injections which, while giving more precise dosages, would adversely affect mouse behavior. Directly measuring water would not have been satisfactory due to the loss of water from spillage when drinking and non-standardized water bottles with varying degrees of leakiness. Thus, it was felt that assumed water consumption as a function of body weight would be the best measure of drug delivery.
2.3 Assessment of activity
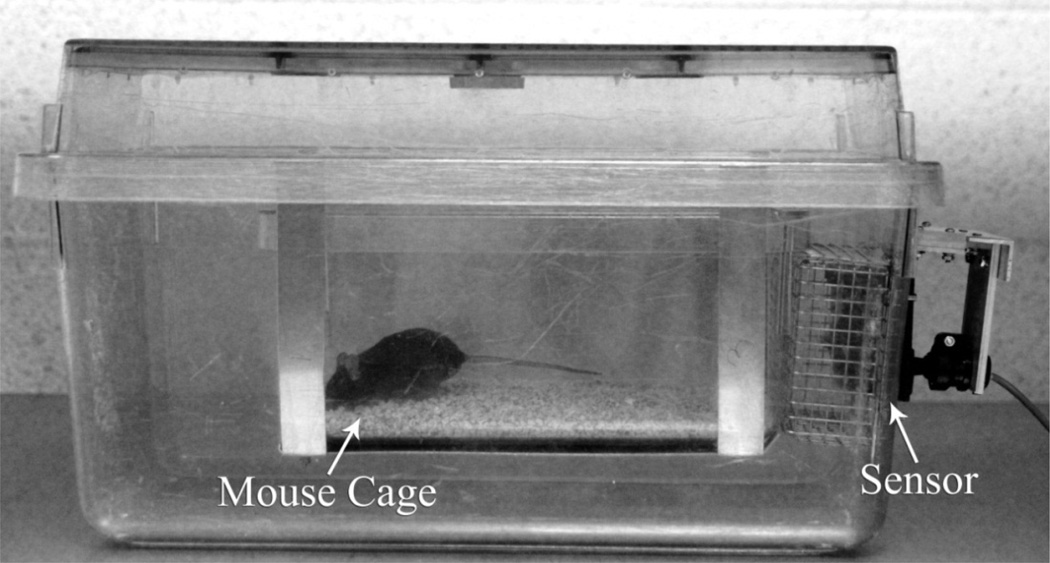
To investigate the effects of pain states on spontaneous behavior, animals were housed individually in UCSD ACP mouse cages each housed in a larger rat cage with an infrared motion sensing unit (Heath-Zenith Replacement Motion Sensor Model SL-5407) monitoring their activity through a wire mesh (Figure 1). This type of passive detector emits no energy and looks for a positional change in a body’s heat signature. The detector fits through a rectangular cut-out in one of the shorter cage sides and is shielded by a wire screen that also ensures cage integrity. Fifteen cages with their detectors are connected to a multi-channel switching box that powers the detectors and interfaces via USB with a dedicated PC computer. An additional detector is placed in the room to monitor human presence that could affect spontaneous animal behavior. Custom software running on the computer, DigSigMon, controls all motion detector triggering (input) and data collection (output). The current configuration records movement 15 times per minute continuously 24 hours a day without needing to be reset until the end of the experiment. Animals were first followed for an average of 7 days for baseline activity before beginning any treatment.
Fig. 1.
Specialized mouse cage.
2.4 Study paradigm
Current protocol starts with recording body weights prior to animals being placed individually in the activity monitoring cages. The following time line is then followed. Three cohorts of mice were tested. The first included arthritic (n=11) and control (n=4) mice which received K/BxN serum and wild type serum respectively. The second included 14 arthritic mice treated with Ketorolac (n=5 control, n=3 for each of three dosage levels). Ketorolac, an NSAID (10mg/kg, 15mg/kg, 20mg/kg; Sigma) was administered PO in drinking water for days 6–9 (which covered nights 6–8). The last cohort included wild type mice treated with ketorolac (n=5 control, n=3 for each of three dosage levels).
2.5 Study schedule
The schedule presented in Table 1 indicates treatments. Activity data are collected continuously on the 24 hr cycle though day 20.
Table 1.
Testing Schedule
| Day |
Cohort 1 (N=15) Arthritic vs. Control |
Cohort 2 (N=14) Arthritic: treated vs. untreated |
Cohort 3 (N=14) Wild type: treated vs. untreated |
| −10 | Mice placed in individual chambers; initiate baseline activity collection | ||
| 0 | First serum injection KBxN n=11, Control n=4; Tactile allodynia baseline | First serum injection KBxN serum n=14 | No Treatment |
| 2 | Second serum injection | No Treatment | |
| 6 | Tactile allodynia |
Drinking water with Ketorolac initiated 10mg/kg (n=3), 15mg/kg (n=3), 20mg/kg (n=3) |
|
| 9 | Ketorolac discontinued | ||
| 21 | Tactile allodynia | ||
3. RESULTS
3.1 Diurnal Patterns in the Mouse and the Effect of K/BxN-Induced Arthritis
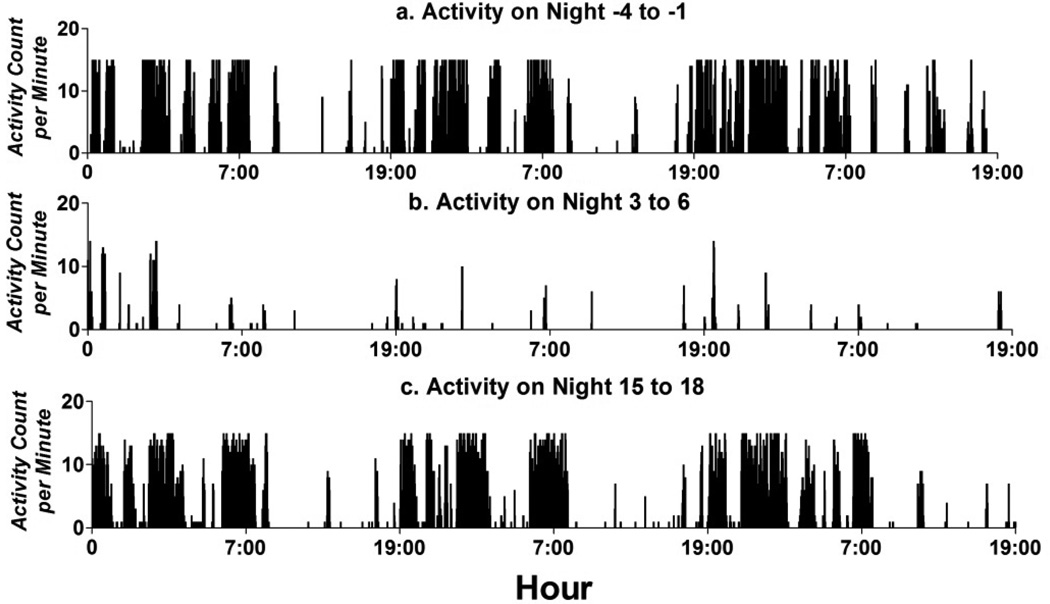
Diurnal patterns in mice show predominate activity during the nocturnal interval. with bouts of activity during the day time. When injected with K/BxN serum, this activity drops dramatically immediately after administration. Figure 2 shows the activity of one arthritic mouse pre-injection (2a), closely after injection (2b) and more than two weeks after injection (2c). The change in nighttime activity is much more pronounced than daytime activity simply due to the majority of the baseline activity occurring in the nighttime. By night 15, activity seems to be normalized.
Fig. 2.
Fig. 2a, b, c. Actogram of Diurnal Cyclicity in Mouse with K/BxN induced Arthritis. Mouse activity predominated at night, with small bouts of activity during the day. Mice had severely decreased activity following injection of the K/BxN serum which rebounded after approximately two weeks. During the arthritic state, activity continued to predominate during the night, but the difference in activity between day and night dropped when compared to baseline.
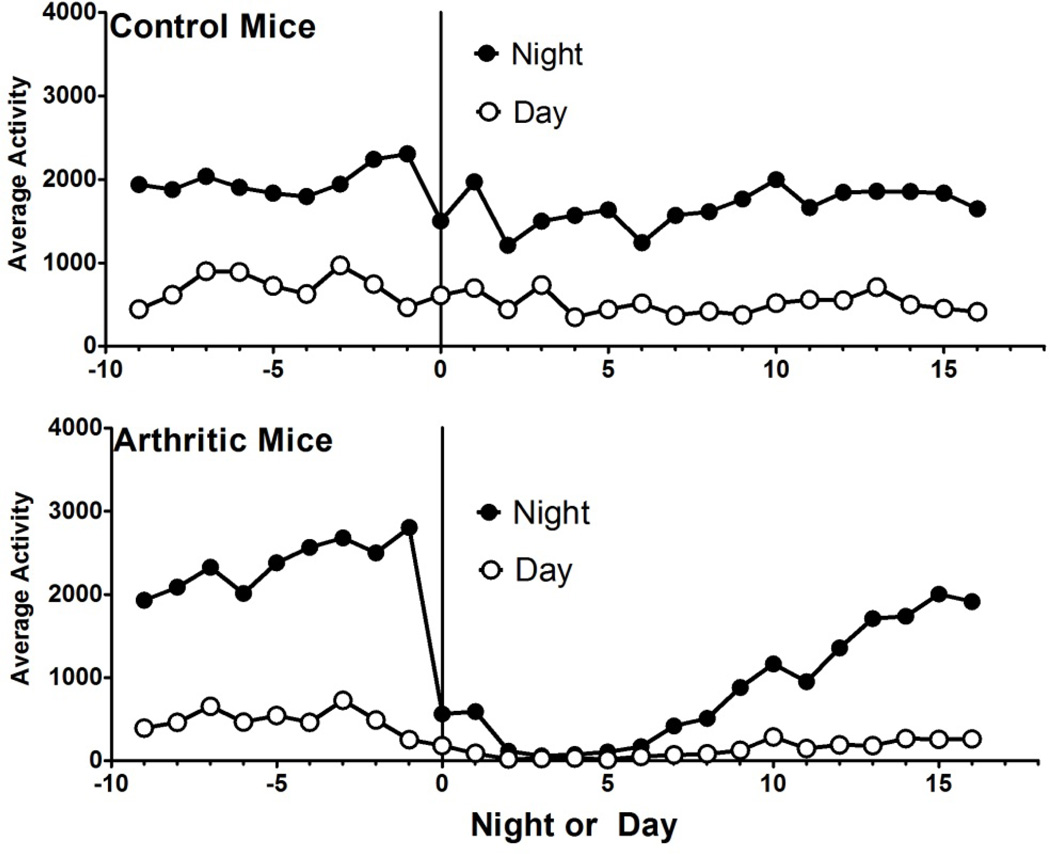
Daytime activity mirrored nighttime activity in peaks and troughs (Figure 3). Animals also displayed a decrease in activity anytime a human entered their room or handled them. This is visible on nights 0, 2, 6, and 11. On days 0 and 2, animals were injected with either control serum or K/BxN serum. The following nights, all animals showed a drop in activity. Animals who received K/BxN had a much more profound drop in activity which could be seen to normalize after about two weeks (statistical analysis below). Control animals rebounded the following night. Animals were tested for tactile allodynia on days 0, 6 and 21, and cages were cleaned on days −6, 0, 6 and 11. Dips corresponding to these days can be seen the night immediately following. For this reason, human room entry was minimized during future iterations.
Fig. 3.
Fig. 3a, b. Day and Night activity in Control and Arthritic Mice. Day activity seems to mirror night activity in the mouse, with nocturnal activity predominating. Mice were handled on days −6, 0, 6, and 11, which lead to a dip in both their day and night time activities in both control and arthritic mice. Arthritic mice show a profound decrease in activity which seems to rebound at two weeks.
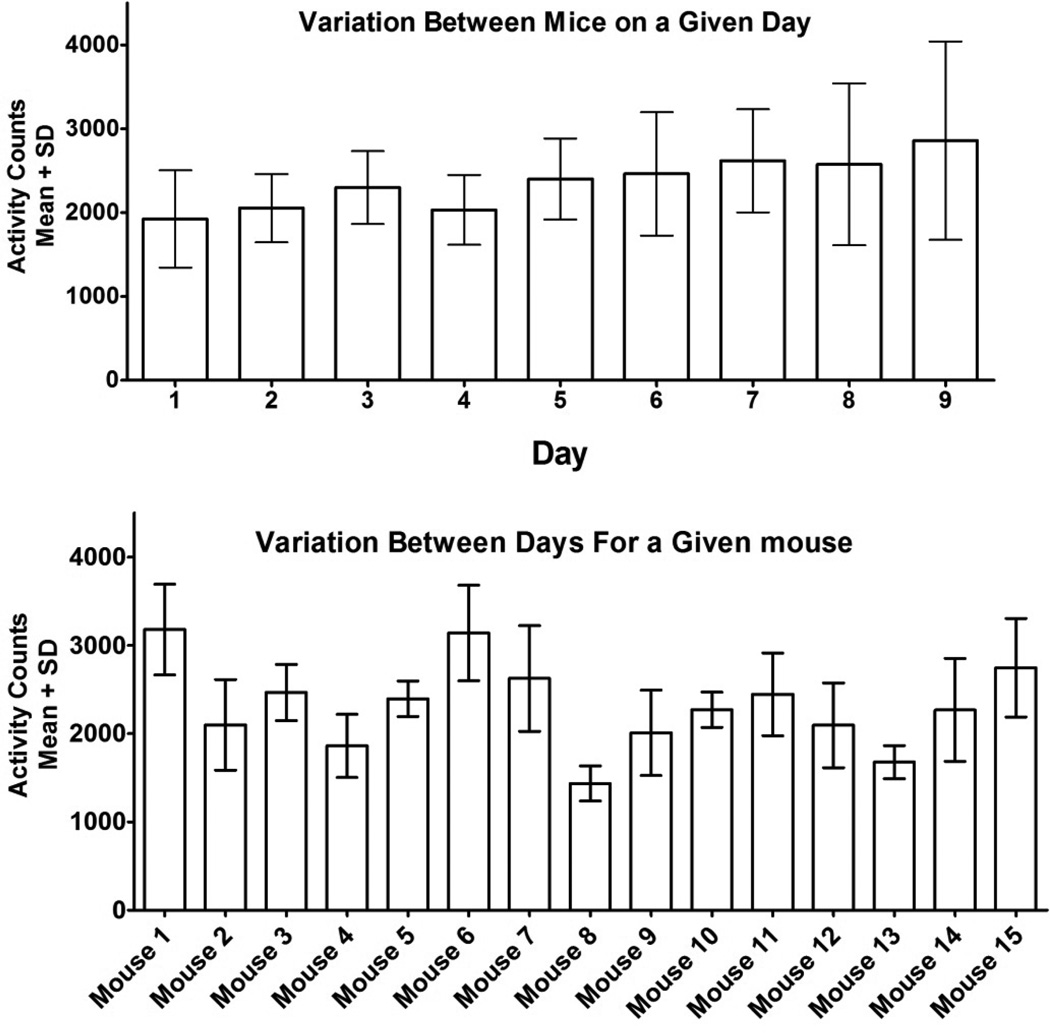
While the C57Bl6 strain is a homogenous strain, there was variability in the activity between individual animals. We compared variation between mice on any given day with variation between days for any given mouse (Figure 4a and b). The variance as a percentage of the mean was significantly lower for a given mouse over multiple days (95% CI: 14.64–21.0%) when compared to all mice on a given day (95% CI: 20.8–29.6%, p<.01). For this reason, we found it to be beneficial to correct raw activity data for a mouse by its baseline activity prior to statistical analysis of the data.
Fig. 4.
Fig. 4a. Variation between mice on a given day. The mean variation between mice as a percentage of the mean for any given day was 20.8–29.6% (95% CI). Variation seemed to increase the longer mice were separated from one another (this was done immediately preceding “day 1” in this figure). Fig. 4b. Variation between days for a given mouse. The mean variation between days as a percentage of the mean for any given mouse was 14.64–21.0% (95% CI).
Normalized data was obtained by observing animals for one week pre-injection and using this average to define normal activity for each mouse. Raw activity counts were then divided by this average, and these were termed our “normalized activity” data.
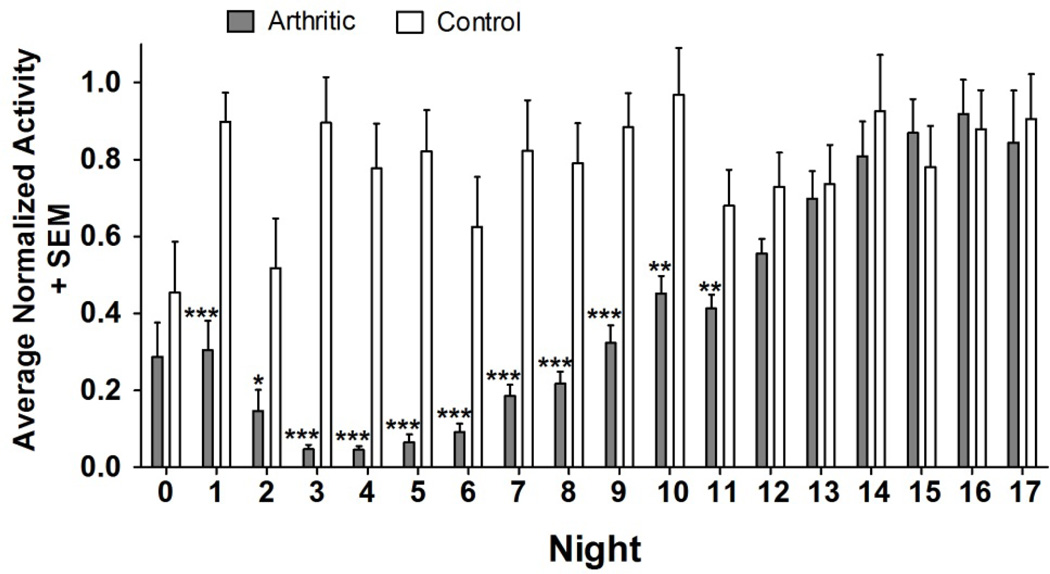
Normalized data was analyzed (Figure 5) and showed a significant drop in nocturnal activity in arthritic mice when compared to control mice from nights 1 to 11 (p<.05 for night 2, p<.01 for nights 10 and 11, and p<.001 for nights 1 and 3–9). After night 11, difference in activity between arthritic and control mice was not significant.
Fig. 5.
Arthritic vs. Control Mice. Average Nocturnal Activity (Normalized). Activity in arthritic mice showed a significant drop for nights 1–11, after which their activity returned to normal. Dips in control animals on nights 0 and 2 correspond to handling and injections on the days immediately preceding. Significance values were obtained from a one-tailed Mann Whitney U test (nonparametric t-test).
3.2 The Effect of Pain and Inflammation on Activity Depression Seen in K/BxN Serum Transfer Mice
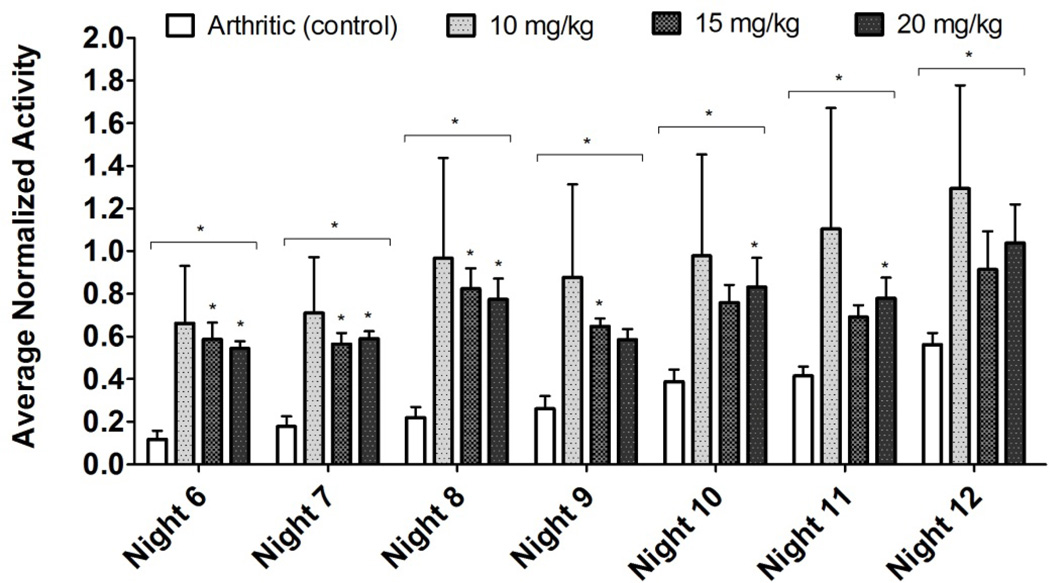
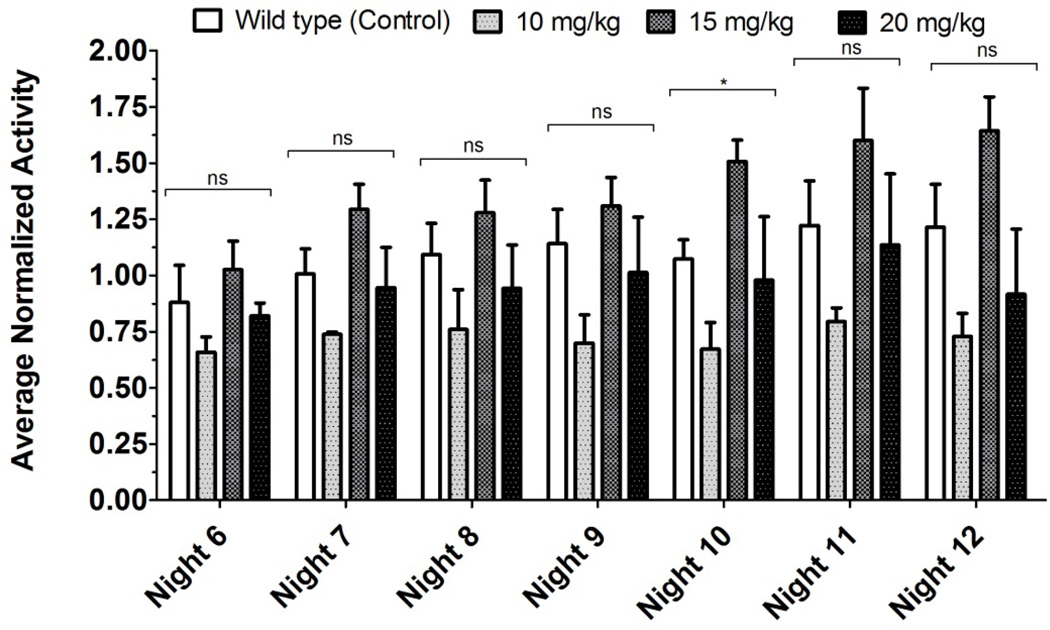
In order to assess the effect of pain on the decrease in activity due to the K/BxN serum transfer model, we assessed the effect of PO ketorolac on the activity of arthritic mice. Non-control arthritic animals received one of three dosage levels of ketorolac (10, 15, and 20 mg/kg per day) PO in their drinking water continuously for days 6–9 (nights 6–8). Our results show significant difference in activity through during administration of ketorolac which persists four nights after cessation of ketorolac administration when compared to control arthritic animals (Figure 6). Wild-type animals showed no significant change in activity upon administration of the same dosage levels of ketorolac when compared to wild-type controls, except for one night (night 10, Figure 7). Post-hoc analysis showed no difference between control and any treatment levels.
Fig. 6.
Arthritic vs. Arthritic Treated with Ketorolac: Nocturnal Activity (Normalized). Significance values above the bracket were obtained with a Kruskal-Wallis test (nonparametric ANOVA), significance values for each bar were obtained with a Dunn’s Multiple Comparison Test (controlled for test-wide error rate). Significant difference in means was observed for nights 6–12. Note that although drug administration was stopped on day 9 (night 8), there is significant difference in activity through night 12.
Fig. 7.
Wild Type vs. Wild Type Treated with Ketorolac: Nocturnal Activity (Normalized). Significance values above the bracket were obtained with a Kruskal-Wallis test (nonparametric ANOVA). Post-hoc testing with Dunn’s Multiple Comparison Test (which controlled for test-wide error rate) showed no significance for all nights. Significance was only noted on night 10 (between the means), with no significance found in post-hoc testing (any treatment vs. control).
4. DISCUSSION
Pain is considered to be an important modulator of ongoing activity. The activity model as assessed by thermal imaging sensors attached to cages is a measurement paradigm that can be applied to many pain models in which changes in activity secondary to the injury can be assessed. It should be emphasized that the thermal detector employed in these studies is representative of that obtainable from many different manufacturers throughout the world and can readily be found on online retailers. This makes it a very cost effective and convenient system to monitor large numbers of animals.
4.1 Heterogeneity of inbred strain activity
Differences between mouse strains in terms of diurnal cyclicity is well appreciated [13]. Of particular interest is that even in populations with homogenous genetics such as the C57Bl6 mice, variation between mice on a given day is greater than day-to-day variation for a given mouse. Accordingly, it is appropriate to use adapted animals with the treatment cohorts composed of animals assigned in a pseudo-random so that each cohort is composed as to have similar diurnal activity distributions. We note that such assignments are statistically and methodologically acceptable to reduce between groups variance, but raise the larger question as to whether these differences between animals reflect an intrinsic genetic drift in the particular (here the C57Bl/6) population. In any case, such assignments giving similar composition groups in regards to their baseline activity after adaptation is useful in reducing between-group variances, even when activity counts are normalized using baseline activity.
4.2 Effects of persistent arthritis on activity
Arthritic mice show a profound decrease in nocturnal activity when compared to control mice. This drop in activity is significant for nights 1–11 and normalizes by night 12. The drop in activity mostly affects night time activity. It thus also decreases the difference in night and day activity. This reduced activity is consistent with previous reports in mice and rats with inflammatory interventions that are typically of briefer duration [1],[2]. Previous work shows that K/BxN animals showed prominent clinical signs out to 10–14 days [7]. After this, the inflammatory markers (digit redness and swelling) resolve, but there was still evidence of significant joint remodeling. Accordingly, the present work suggests that the changes in activity cycle reflect mechanisms which correlate with the inflammatory state.
4.3 Role of pain in altering activity depression
An important issue is to determine if the depression of the diurnal activity is related to the pain state initiated secondarily to the inflammation. We had previously shown that an NSAID, ketorolac, was able to reverse the allodynia in the K/BxN animals. Our previous data showed that IP injection of ketorolac caused significantly decreased tactile allodynia within 60 minutes of administration which decreased to a non-significant difference within 4 hours. [7] Administration of PO ketorolac for nights 6–8 caused activity in arthritic mice to be significantly different for nights 6–12, which would suggest that the principal depressor of activity is inflammation. Interestingly, this activity was elevated beyond the administration of the drug itself. This suggests one of three possibilities: i) Ketorolac has a modest disease modifying effect on the K/BxN serum transfer model; ii) Ketorolac has a central mechanism which is causing a long-term change in behavioral response to some negative stimuli (pain, inflammation, etc.); iii) The inflammatory process requires a few days to recover from an NSAID. The last possibility appears unlikely due to the speed in which the inflammatory process initially took effect in arthritic animals (by night 1), but may represent a post-NSAID washout period for chronic inflammation to reappear.
4.4 Temporal disassociation between activity and tactile allodynia
Previous reports show that the K/BxN serum transfer model causes significant allodynia out to at least day 28[7]. However, as noted above, this study unexpectedly showed that the changes in activity cycle were gone well before this time period. It is understood that the sensitization to low-intensity stimuli that occurs in models of chronic inflammation reflects both peripheral sensitization as well as the activation of central facilitatory systems[14]. Peripheral sensitization has been attributed to remodeling of the neuron with higher levels of substance p and other chemotactic factors. The present temporal disassociation of allodynia and activity depression is interesting as it suggests that the change in sensitivity to light touch, the definition of allodynia, is not associated with effects upon spontaneous activity.
5.5 Conclusions
In summary, the diurnal activity model promises to be an important tool for assessing the behavioral response to pain and inflammatory states in mice. The work emphasizes that it is beneficial to correct for baseline activity levels. The K/BxN serum transfer model shows significant decrease in activity in a timeline which surprisingly is disassociated with the tactile allodynia. This implies the tactile allodynia, while a marker of many states evoked by persistent injury and inflammation does not appear to account for the events that lead to diminished behavioral activity. Importantly, the decrease in activity (as is the early phase tactile allodynia) is reversed by ketorolac, suggesting that pain or inflammation affecting a central pathway is at least partly responsible for the decrease in activity. The effect of ketorolac was noted to persist beyond its administration, suggesting a disease-modifying effect, a post-NSAID ramp up period, or an unexpected persistence of the oral agent.
ACKNOWLEDGEMENTS
This work was funded by NS16541 (TLY), and NIH Short-Term Research Training Grant T35HL04791 (MSAS). KRN T cell receptor (TCR) transgenic mice were a gift from Drs. D. Mathis and C. Benoist (Harvard Medical School, Boston, MA) and Institut de Génétique et de Biologie Moléculaire et Cellulaire (Strasbourg, France), and were maintained on a C57BL/6 background (K/B).
Biographies

Mohammed Suhail received his B.S. in General Biology from University of California San Diego, where he graduated cum laude. He is currently finishing his M.D. at University of California, San Diego.

Christina Christianson received her B.S. in Biochemistry from the University of Rochester and her Ph.D. in Molecular Pathology from the University of California San Diego. She is currently a postdoctoral researcher in Allergy and Immunology at National Jewish Health.

Fred Koehrn - BA (Occidental College 1974), MSc (San Diego State University 1980). Staff Research Associate for over 30 years with the University of California. Currently working in the UCSD School of Medicine, Department of Anesthesiology conducting pain research funded through NIH grants.

Shelle Malkmus worked for 15 years as a Registered Veterinary Technician doing clinical and emergency animal medicine, prior to switching to research. Since then, she has focused on mechanisms and pharmacology of acute and chronic nociception in several species; specializing in nerve injury and acute inflammatory models. She has taught numerous fellows, students and biotechnology researchers how to execute these models, collect tissue and conduct behavioral assays. Additionally, she has participated in developing automated pain assays. She holds a BS in Animal Physiology and Neuroscience from UC San Diego and has authored 23 peer reviewed articles, and three book chapters.

William M. Mitchell is Assistant Clinical Professor in Hematology / Oncology and Medical Director of The Doris A. Howell Palliative Care Service at the University of California San Diego. His primary interest is in pain and symptom management in patients with malignancies, including development of animal models for preclinical cancer pain investigation.

Maripat Corr received her B.S. from MIT and her M.D. from University of North Carolina, Chapel Hill. She is currently an Associate Professor of Medicine in the Department of Rheumatology at University of California San Diego, School of Medicine.

Tony L. Yaksh is Professor and Vice Chair in Anesthesiology at the University of California San Diego. His primary interest is in the biology of pain processing at the level of the primary afferent and spinal cord.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Calvino B, Crepon-Bernard MO, Le Bars D. Parallel clinical and behavioural studies of adjuvant-induced arthritis in the rat: possible relationship with 'chronic pain'. Behav.Brain Res. 1987;24:11–29. doi: 10.1016/0166-4328(87)90032-5. [DOI] [PubMed] [Google Scholar]
- 2.De Castro Costa M, De Sutter P, Gybels J, Van Hees J. Adjuvant-induced arthritis in rats: a possible animal model of chronic pain. Pain. 1981;10:173–185. doi: 10.1016/0304-3959(81)90193-7. [DOI] [PubMed] [Google Scholar]
- 3.Dardick SJ, Basbaum AI, Levine JD. The contribution of pain to disability in experimentally induced arthritis. Arthritis Rheum. 1986;29:1017–1022. doi: 10.1002/art.1780290811. [DOI] [PubMed] [Google Scholar]
- 4.Landis CA, Robinson CR, Helms C, Levine JD. Differential effects of acetylsalicylic acid and acetaminophen on sleep abnormalities in a rat chronic pain model. Brain Res. 1989;488:195–201. doi: 10.1016/0006-8993(89)90709-9. [DOI] [PubMed] [Google Scholar]
- 5.Krug HE, Frizelle S, McGarraugh P, Mahowald ML. Pain behavior measures to quantitate joint pain and response to neurotoxin treatment in murine models of arthritis. Pain Med. 2009;10:1218–1228. doi: 10.1111/j.1526-4637.2009.00711.x. [DOI] [PubMed] [Google Scholar]
- 6.Colpaert FC, Tarayre JP, Alliaga M, Bruins Slot LA, Attal N, Koek W. Opiate self-administration as a measure of chronic nociceptive pain in arthritic rats. Pain. 2001;91:33–45. doi: 10.1016/s0304-3959(00)00413-9. [DOI] [PubMed] [Google Scholar]
- 7.Christianson CA, Corr M, Firestein GS, Mobargha A, Yaksh TL, Svensson CI. Characterization of the acute and persistent pain state present in K/BxN serum transfer arthritis. Pain. 2010;151:394–403. doi: 10.1016/j.pain.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999;286:1732–1735. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- 9.Wallace MS, Rowbotham M, Bennett GJ, Jensen TS, Pladna R, Quessy S. A multicenter, double-blind, randomized, placebo-controlled crossover evaluation of a short course of 4030W92 in patients with chronic neuropathic pain. J.Pain. 2002;3:227–233. doi: 10.1054/jpai.2002.123650. [DOI] [PubMed] [Google Scholar]
- 10.Schinkel C, Gaertner A, Zaspel J, Zedler S, Faist E, Schuermann M. Inflammatory mediators are altered in the acute phase of posttraumatic complex regional pain syndrome. Clin.J.Pain. 2006;22:235–239. doi: 10.1097/01.ajp.0000169669.70523.f0. [DOI] [PubMed] [Google Scholar]
- 11.Brookoff D. Chronic pain: 1. A new disease? Hosp.Pract.(Minneap) 2000;35:45–52. 59. doi: 10.1080/21548331.2000.11444031. [DOI] [PubMed] [Google Scholar]
- 12.Harkness JE, Wagner JE. Biology and Medicine of Rabbits & Rodents. 3rd ed 1983. [Google Scholar]
- 13.Daszuta A, Gambarelli F, Ternaux JP. Sleep variations in C57BL and BALBc mice from 3 weeks to 14 weeks of age. Brain Res. 1983;283:87–96. doi: 10.1016/0165-3806(83)90084-6. [DOI] [PubMed] [Google Scholar]
- 14.Wasner G, Naleschinski D, Baron R. A role for peripheral afferents in the pathophysiology and treatment of at-level neuropathic pain in spinal cord injury? A case report. Pain. 2007;131:219–225. doi: 10.1016/j.pain.2007.03.005. [DOI] [PubMed] [Google Scholar]