Abstract
Emerging data suggest that environmental endocrine disrupting chemicals (EDCs) may contribute to the pathophysiology of obesity and diabetes. In prior work, the phenylsulfamide fungicide tolylfluanid (TF) was shown to augment adipocyte differentiation, yet its effects on mature adipocyte metabolism remain unknown. Because of the central role of adipose tissue in global energy regulation, the present study tested the hypothesis that TF modulates insulin action in primary rodent and human adipocytes. Alterations in insulin signaling in primary mammalian adipocytes were determined by the phosphorylation of Akt, a critical insulin signaling intermediate. Treatment of primary murine adipose tissue in vitro with 100 nM TF for 48 h markedly attenuated acute insulin-stimulated Akt phosphorylation in a strain- and species-independent fashion. Perigonadal, perirenal, and mesenteric fat were all sensitive to TF-induced insulin resistance. A similar TF-induced reduction in insulin-stimulated Akt phosphorylation was observed in primary human subcutaneous adipose tissue. TF-treatment led to a potent and specific reduction in insulin receptor substrate-1 (IRS-1) mRNA and protein levels, a key upstream mediator of insulin’s diverse metabolic effects. In contrast, insulin receptor-β, phosphatidylinositol 3-kinase, and Akt expression were unchanged, indicating a specific abrogation of insulin signaling. Additionally, TF-treated adipocytes exhibited altered endocrine function with a reduction in both basal and insulin-stimulated leptin secretion. These studies demonstrate that TF induces cellular insulin resistance in primary murine and human adipocytes through a reduction of IRS-1 expression and protein stability, raising concern about the potential for this fungicide to disrupt metabolism and thereby contribute to the pathogenesis of diabetes.
Keywords: adipocyte, insulin resistance, environmental endocrine disruption
1. Introduction
Over the last several decades, diabetes rates in the United States have correlated closely with synthetic organic chemical production [1]. Given the close connection between obesity and the development of type 2 diabetes [2], disruption of adipose tissue function may mediate global changes in whole-body energy metabolism brought about by chemical exposure. Several fundamental properties of body fat make it the perfect metabolic target of endocrine disrupting chemicals (EDCs).1 Many putative EDCs are highly lipophilic with the capacity to bioaccumulate in the triglyceride stores of the adipocyte resulting in high local concentrations in fat pads. Thus, even short-term EDC exposure could lead to long-term effects through slow leaching of chemicals from lipid droplets. Also, adipocyte differentiation and metabolism are regulated by multiple nuclear hormone receptors that control gene transcription. Since many of these transcription factors have lipophilic compounds as their endogenous ligands, EDCs could interfere with their physiological regulation. Indeed, EDCs affect estrogen, androgen and thyroid hormone nuclear receptor activities [3–5], leading some to propose that modulation of nuclear hormone receptors may result in metabolic disruption [6].
Underscoring the potential role of the adipocyte as an EDC target, the environmental obesogen hypothesis posits a causal link between synthetic chemicals and the obesity epidemic [7, 8]. Epidemiological data support a link between particular pollutants and waist-to-hip ratio as well as body mass index [9]. Furthermore, experimental evidence has demonstrated the potential for environmental pollutants to inappropriately modulate adipocyte development [8, 10–12]. Less is known, however, about the capacity of EDCs to modulate insulin action and adipocyte metabolism, critical measures of an EDC’s potential to contribute to metabolic diseases such as obesity and diabetes. Population-based studies have correlated exposure to some environmental contaminants with the development of insulin resistance or diabetes [13, 14]. Most experimental studies have been restricted to cell lines, which report both an augmentation of insulin-stimulated metabolism [15] as well as an inhibition of insulin action [16, 17], depending on the EDC studied. More recent evidence, however, suggests that some pollutants possess the capacity to inhibit global insulin action in animal models [18]. However, the molecular mechanisms responsible for environmental disruption of insulin signaling remain largely undefined.
Adipose tissue performs a central role in energy regulation because of its ability to store excess calories in times of surfeit while mobilizing those stores in times of deficit. The balance between storage and mobilization is regulated by several hormones, chief among these are insulin and catecholamines. Working through its surface receptor, insulin stimulates a series of intracellular signaling events, including the autophosphorylation of the insulin receptor, tyrosine phosphorylation of insulin receptor substrate-1 (IRS-1), activation of phosphatidylinositol 3-kinase (PI3K), and elevation of PIP3 levels. These events lead to the translocation and activation of Akt via phosphorylation on serine/threonine residues. Akt plays a critical role in insulin stimulated glucose and lipid metabolism in adipose tissue. The end result of insulin action on the adipocyte is a shift in the metabolic balance from energy mobilization to storage and a concomitant release of the adipokine leptin that regulates feeding behavior. Biochemical insulin resistance can therefore be defined as a relative reduction in insulin’s ability to trigger its intracellular signaling cascade. A failure of insulin signaling in adipose tissue prevents the safe removal and storage of free fatty acids from the circulation, which subsequently can induce insulin resistance in the liver and skeletal muscle [19]. Thus, an initiating defect in adipocyte insulin action could be the foundational disturbance upon which global energy regulation is disrupted.
Tolylfluanid (TF, Euparen® Multi) is a member of the phenylsulfamide family of fungicides used in antifouling paints as well as on fruit crops in agricultural areas outside of the United States including Europe, Australia, and New Zealand [20, 21]. TF has the capacity to augment preadipocyte-to-adipocyte differentiation in the 3T3-L1 cell line [12] and to bind to the glucocorticoid receptor [22]; however, little is known about the ability of TF to alter adipocytic insulin signaling and metabolism. Studies were therefore undertaken to determine whether this EDC can induce adipocytic insulin resistance and thereby potentially contribute to the metabolic derangements underlying the development of diabetes.
2. Materials and Methods
2.1. Chemicals and Reagents
TF was purchased from Fluka (St. Louis, MO). Type II collagenase (Clostridium histolyticum), puromycin (Streptomyces alboniger), and all inorganic chemicals were obtained from Sigma (St. Louis, MO). Fetal bovine serum (FBS) as well as phenol red-free Dulbecco’s Modified Eagle’s Medium (DMEM) were obtained from HyClone (Logan, UT), while DMEM containing phenol red was obtained from Cellgro (Manassas, VA). Lactate Dehydrogenase (LDH) Cytotoxicity Assay Kit was obtained from Cayman Chemicals (Ann Arbor, MI). Protein A Dynabeads were acquired from Invitrogen (Carlsbad, CA). Anti-phospho-Akt (Ser473), anti-Akt, anti-IRS-1, and anti-PI3K p85 subunit (PI3K-p85) monoclonal antibodies were purchased from Cell Signaling Technology (Danvers, MA). Anti-insulin receptor-β (IR-β) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phosphotyrosine antibody was purchased from Millipore (Billerica, MA). E.N.Z.A. Total RNA Kit II was obtained from Omega Bio-Tek (Norcross, GA). The iScript cDNA Synthesis Kit was acquired from Bio-Rad (Hercules, CA). PerfeCTa SYBR Green FastMix was purchased from Quanta BioSciences (Gaithersburg, MD), while the Mouse Leptin ELISA Kit was obtained from Crystal Chem (Downers Grove, IL).
2.2. Adipocyte Cell Culture
Primary adipocytes were obtained from male mice (approximately 8 weeks of age) and rats (approximately 12 weeks of age) sacrificed according to Institutional Animal Care and Use Committee-approved protocols. Fat pads were harvested by sterile dissection with careful attention to removal of the testes and epididymis from the perigonadal fat. Human adipose tissue from subcutaneous abdominal fat was obtained from female patients undergoing surgery for breast reconstruction (n = 3) or cosmetic liposuction (n = 1) after obtaining informed consent per an Institutional Review Board-approved protocol (10-474-A). The dissected fat was placed in DMEM containing 10% FBS and coarsely minced into chunks weighing approximately 100–300 mg; these chunks were then equally apportioned into either vehicle- (ethanol) or 100 nM TF-supplemented DMEM containing 10% FBS and incubated for 48 h at 37°C in 5% CO2; the total ethanol concentration was ≤0.1%. Primary adipocytes were then isolated from fat pads by collagenase digestion and flotation centrifugation as previously described [23].
2.3. Lactate Dehydrogenase Assay
TF cytotoxicity was assessed using the Lactate Dehydrogenase (LDH) Cytotoxicity Assay Kit per the manufacturer’s instructions (Cayman). After incubating primary adipocytes for 48 h in the presence of vehicle or 100 nM TF, aliquots of the culture medium were taken; total cell LDH content was measured after incubating adipocytes in 0.1% Triton X-100 for 1 h. LDH was reported as percent released into the medium relative to total cellular LDH content.
2.4. Insulin Signaling Assays
Isolated adipocytes were equally apportioned into 1.5 ml microfuge tubes (approximately 100–300 μl of packed cells per tube). Insulin stimulation was initiated by addition of an equal volume of Krebs-Ringer buffer (pH 7.4) supplemented with 1% bovine serum albumin (BSA), and 3 mM glucose (KRB*) −/+ indicated concentrations of insulin followed by incubation in a water bath at 37°C for 10 min with gentle vortexing every 2 min. Insulin action was terminated by addition of ice-cold KRB* and placing the cells on ice. The adipocytes were then washed twice with ice-cold KRB (lacking BSA) to remove the excess BSA. Samples for immunoblotting were prepared by the addition of an equal volume of 2XHB buffer (prepared from adding equal parts of homogenization buffer [23] and Laemmli 4X buffer [167 mM Tris, 8 mM EDTA, 27% glycerol, 1.3% β-mercaptoethanol, 416 mM sodium dodecyl sulfate, and 0.3 mM bromophenol blue]) and vigorous vortexing. Samples were then centrifuged at 9,300 × g at room temperature for 10 min, and the infranatant layer (between the pelleted cell debris and floating lipid layer) was removed and heated at 95°C for 5 min.
2.5. Immunoprecipitation
Protein lysates from cultured primary adipocytes were prepared as outlined above except after washing the cultured adipocytes, homogenization buffer alone was added before centrifuging and removing the infranatant layer. Immunoprecipitation (IP) of IRS-1 or IR-β was performed using Protein A Dynabeads per the manufacturer’s instructions. Briefly, antibodies were conjugated to Protein A Dynabeads for 10 min followed by incubation of the protein lysate with the bead-antibody complex for an additional 10 min. The supernatant was removed via magnetic separation and stored for immunoblotting. The protein-bead complex was then washed multiple times with PBS, and the isolated proteins were eluted via the addition of Laemmli Sample Buffer and heating at 95°C for 5 min. The IP samples underwent immunoblotting for IRS-1 and the co-IPed protein PI3K-p85, the regulatory subunit of PI3K. Alternatively, immunoblotting was performed for IR-β and phospho-tyrosine.
2.6. SDS-PAGE and Immunoblotting
All samples were resolved on 10% sodium dodecyl sulfate polyacrylamide gels and transferred to polyvinylidene fluoride after preconditioning the membranes in methanol. Western blots were probed as described [24]. Blots were then incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Bio-Rad) and developed using Amersham ECL Advance (GE Healthcare). Densitometry was performed for immunoblots using ImageJ version 1.44 (National Institutes of Health) with the insulin effect determined by calculating the ratio of the total areas for the bands corresponding to pAkt and total Akt.
2.7. Quantitative Real Time PCR (qRT-PCR)
RNA was isolated from cultured primary adipose tissue using the E.N.Z.A. Total RNA Kit II (Omega). The purity and concentration of the isolated RNA was assessed using a Nanadrop 2000; 260/280 ratios were ~2.0. The Bio-Rad iScript Kit was used to synthesize cDNA. Quantitiative RT-PCR was performed using SYBR green on a Bio-Rad MyiQ RT-PCR detection system. Primers were from Integrated DNA Technologies and as follows: 18S rRNA forward 5′-CGGCTACCACATCCAAGGA-3′, reverse 5′-GCTGGAATTACCGCGGCT-3′, IRS-1 forward 5′-GCCAGAGGATCGTCAATAGC-3′, reverse 5′-GAGGAAGACGTGAGGTCCTG-3′. Primers were designed using Primer3 and assessed for specificity by melting curve analysis. Gene expression levels were evaluated by the delta-delta Ct method after confirmation that amplification efficiency was between 90% – 110% for all primer pairs; 18S rRNA was used as a reference gene to control for total mRNA recovery.
2.8. Protein Stability Assay
Perigonadal adipose tissue from male C57BL/6 mice was harvested and cultured in phenol red-free DMEM with 10% FBS in the presence of 100 nM TF or vehicle (ethanol). After 12 h, the protein translation inhibitor puromycin was added to a final concentration of 100 μM, and the fat was incubated for a further 2 h. Cellular levels of IRS-1 were then determined by immunoblotting after IP as described above.
2.9. Leptin Secretion Assay
Perigonadal fat pads from male C57BL/6 mice were harvested and cultured in phenol red-free DMEM with 10% FBS. The tissue samples were then treated with vehicle or 100 nM TF for 48 h. After 24 h in culture, insulin was added to a subset of each treatment at a final concentration of 2.5 nM for the final 24 h of TF treatment. Media samples from the cultured tissues were obtained, and secreted leptin was measured using the Mouse Leptin ELISA Kit per the manufacturer’s instructions (Crystal Chem).
2.10. Statistical Analyses
Significance was determined using a two-tailed Student’s T-test for comparisons between two conditions and by analysis of variance (ANOVA) with Tukey’s post hoc test for comparisons of more than two conditions using GraphPad Prism version 5.04 (La Jolla, CA).
3. Results
3.1. Effect of Tolylfluanid on Insulin Signaling in Primary Rodent Adipocytes
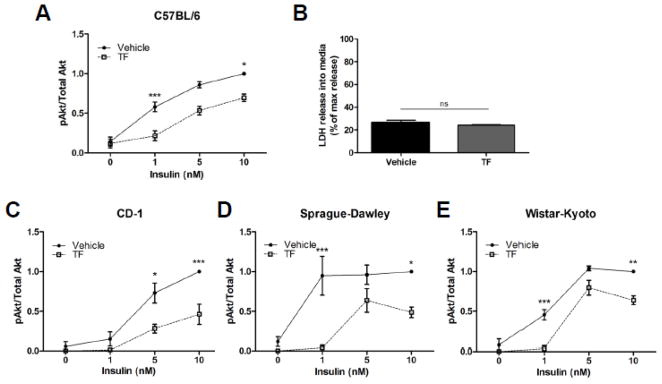
To study the impact of TF pretreatment on insulin signaling in primary mammalian adipocytes, gonadal fat pads were sterilely removed from C57BL/6 male mice, coarsely minced and incubated for 48 h in media in the absence and presence of 100 nM TF, an intermediate concentration shown in prior studies to promote adipocyte differentiation in the 3T3-L1 cell line [12]. Tissue pieces were washed and then stimulated for 10 min with the indicated concentration of insulin. The relative phosphorylation of Akt under insulin stimulation was studied by phospho-specific immunoblotting with total Akt levels serving as a protein loading control. Exposure of perigonadal fat pads from inbred C57BL/6 mice to 100 nM TF greatly attenuated Akt phosphorylation upon stimulation with 1 and 10 nM insulin (63% and 30% reduction, respectively) (Figure 1A). These results were not a consequence of non-specific, TF-induced cellular toxicity as there was no detectable increase in LDH release into the media during the 48 h treatment (Figure 1B). Additionally, total Akt levels were unchanged indicating that impairment of insulin signal transduction rather than a reduction of Akt expression was responsible for the results obtained. The C57BL/6 mouse strain is known to be particularly prone to insulin resistance [25], so the ability of TF to modulate insulin action was also tested in fat obtained from the outbred CD-1 mouse strain. Again, pretreatment with 100 nM TF reduced Akt phosphorylation by 61% at 5 nM insulin and by 54% at 10 nM insulin relative to vehicle-treated controls (Figure 1C). To ensure that this was not a species-specific effect, adipocytes from two strains of rat were subjected to similar analysis. Exposure of adipocytes to 100 nM TF from Sprague-Dawley rats (Figure 1D) as well as Wistar-Kyoto rats (Figure 1E) inhibited insulin-stimulated Akt phosphorylation. For Sprague-Dawley rats, TF reduced Akt phosphorylation by 96% and 51% at 1 nM and 10 nM insulin, respectively; in adipocytes from Wistar-Kyoto rats, Akt phosphorylation was reduced by 92% and 36% at 1 nM and 10 nM insulin, respectively.
Figure 1.
Effect of TF on insulin-stimulated Akt phosphorylation in primary rodent adipocytes. Perigonadal depots were sterilely harvested, coarsely minced and incubated with 100 nM TF for 48 h. Cells were then stimulated for 10 min with the indicated concentration of insulin. Lysates were prepared and subjected to anti-phospho-Akt and – total Akt immunoblotting. The phosphorylated-to-total Akt ratio was used to determine insulin action in perigonadal fat pads from male C57BL/6 mice (Panel A; n = 8 except for 5 nM insulin where n = 4), CD-1 mice (Panel C; n = 6 except for 5 nM insulin where n = 4), Sprague-Dawley rats (Panel D; n = 6 except for 5 nM insulin where n = 5), or Wistar-Kyoto rats (Panel E; n = 3). Data are presented as means ± S.E.M. normalized to vehicle-treated adipocytes exposed to 10 nM insulin. Panel B shows LDH release into a media relative to total cellular LDH as a measure of cytotoxicity. * p < 0.05; ** p < 0.01; *** p < 0.001.
3.2. Effects of Tolylfluanid on Insulin Signaling in Specific Fat Depots
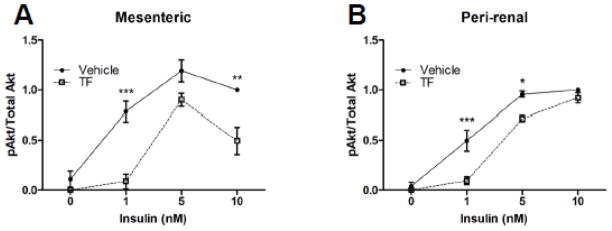
Adipose tissue from different fat depots varies in its metabolic behavior, with visceral fat being particularly deleterious to health [26]. To determine whether fat from various depots differ in their sensitivity to TF, insulin signaling after TF treatment was analyzed in mesenteric and perirenal fat in CD-1 mice since these depots are most similar to human visceral fat. Under these conditions, TF reduced insulin-stimulated Akt phosphorylation in adipocytes from the mesenteric fat pad by 89% and 50% at 1 nM and 10 nM insulin, respectively (Figure 2A). Akt phosphorylation in perirenal fat cells was reduced by 81% and 25% at 1 nM and 5 nM insulin, respectively (Figure 2B).
Figure 2.
Depot-specific sensitivity of adipose tissue to endocrine disruption by TF. Adipose tissue depots were harvested, treated, and analyzed as in Figure 1. The phosphorylated-to-total Akt ratio was used to determine insulin action in mesenteric (Panel A; n = 4 except for 5 nM insulin where n = 3) and perirenal (Panel B; n = 5 except for 5 nM insulin where n = 4) adipose depots from male CD-1 mice. Data are presented as means ± S.E.M. normalized to vehicle-treated adipocytes exposed to 10 nM insulin. * p < 0.05; ** p < 0.01; *** p < 0.001.
3.3. Effect of Tolylfluanid on Insulin Signaling in Primary Human Adipocytes
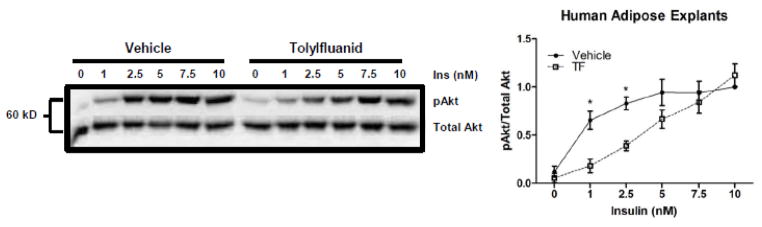
While TF consistently reduced insulin-stimulated Akt phosphorylation in rodent adipocytes, the ultimate question related to the potential diabetogenic properties of this chemical pertains to its ability to antagonize insulin action in human adipocytes. To test this possibility, human subcutaneous abdominal adipose tissue was obtained from volunteer female patients undergoing breast reconstruction or cosmetic liposuction. Data obtained for subcutaneous adipose tissue from women undergoing the two procedures were similar; therefore, the data was pooled. Analysis of the combined data revealed that treatment with TF significantly reduced insulin-stimulated Akt phosphorylation by 72% and 53% at 1 nM and 2.5 nM insulin, respectively (Figure 3). Thus, TF treatment induced insulin resistance in primary adipose tissue from three mammalian species, including humans.
Figure 3.
Effect of TF on insulin-stimulated Akt phosphorylation in human adipocytes. Human adipose tissue was pretreated for 48 hr with 100 nM TF, and then insulin dose response curves were performed. A representative immunoblot of insulin-stimulated Akt phosphorylation is shown (Panel A). The phosphorylated-to-total Akt ratio was used to determine insulin action in human fat obtained from surgical patients (n = 4 except for 5 nM insulin where n = 3) (Panel B). Data were normalized to vehicle-treated adipocytes exposed to 10 nM insulin. * p < 0.05.
3.4. Mechanism of Tolylfluanid-Induced Insulin Resistance
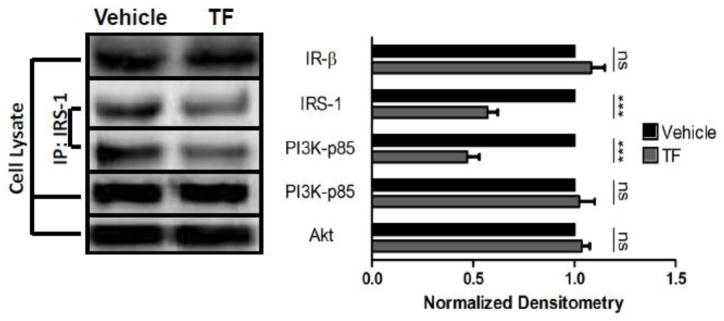
To investigate the molecular mechanisms of TF-induced insulin resistance, the mediators of insulin signal transduction upstream of Akt phosphorylation were interrogated in vehicle- and TF-treated perigonadal adipose tissue from C56BL/6 mice (Figure 4). Levels of IR-β and p85 regulatory PI3K subunit were unchanged after TF-treatment. Likewise, autophosphorylation of IR-β after insulin stimulation was unaltered by TF treatment (data not shown). In contrast, IRS-1 levels were reduced compared to vehicle-treated controls by 43%, and in parallel, IRS-1 tyrosine phosphorylation was comparably reduced after TF treatment (data not shown). Additionally, while total cellular levels of PI3K were unchanged, there was a 53% reduction in PI3K association with IRS-1, a critical step in the activation of the lipid kinase. Concordant with these studies in primary murine fat, preliminary experiments demonstrate similar reductions in IRS-1 levels in human adipose tissue (data not shown).
Figure 4.
Mechanism of TF-induced insulin resistance. Perigonadal fat from male C57/BL6 mice was treated as in Figure 1. Levels of IR-β, PI3K, and total Akt were measured in cell lysates, while IRS-1 and IRS-1-associated PI3K were assessed after immunoprecipitation. Representative immunoblots are shown (Left) as well as cumulative densitometry for each protein as means ± S.E.M. normalized to vehicle-treated controls (n = 4–6). IRS-1 densitometry is represented by IRS-1/Akt and IP PI3K-p85 densitometry is represented by IP PI3K-P85/supernatant PI3K-P85, each independently normalized to EtOH. *** p < 0.001.
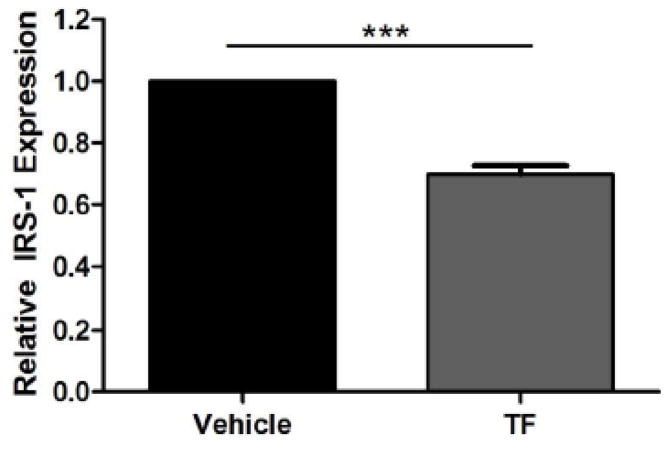
Cellular levels of IRS-1 are controlled by several mechanisms [27, 28]. Using qRT-PCR, the ability of TF to modulate IRS-1 transcription was investigated. Treatment of perigonadal fat from C57BL/6 mice with 100 nM TF for 48 h resulted in a 30% reduction in IRS-1 mRNA levels (Figure 5). Cumulatively, these results indicate TF specifically reduced cellular IRS-1 levels, likely through a transcriptional mechanism, which in turn disrupted propagation of insulin signal transduction and activation of Akt.
Figure 5.
Effect of TF on IRS-1 gene transcription. RNA was isolated from cultured C57BL/6 perigonadal fat pads after 48 h incubation with 100 nM tolylfluanid and reverse transcribed. Quantitative RT-PCR was used to assess fold change differences between IRS-1 expression in vehicle- versus TF-treated adipocytes. Data are presented as means ± S.E.M. of the fold induction of IRS-1 relative to 18S rRNA normalized to vehicle-treated controls (n = 6). *** p < 0.001.
3.5. Time- and Concentration-Dependence of Tolyfluanid-Mediated IRS-1 Downregulation
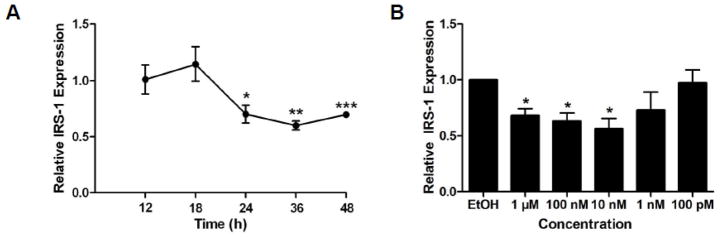
To ascertain the duration of treatment necessary for TF-mediated downregulation of IRS-1 transcription, gonadal fat from C57BL/6 mice was treated with vehicle (ethanol) or 100 nM TF for 12–48 h. Relative IRS-1 transcript levels, matched to vehicle-treated controls exposed for the same duration, were reduced by 30% at 24 h and remained significantly depressed at later time points (Figure 6, Panel A). The dose-response relationship of TF exposure on IRS-1 transcription was determined by exposing gonadal fat from C57BL/6 mice to either vehicle or 100 pM to 1 μM TF for 48 h. Relative IRS-1 transcription was significantly reduced from 10 nM to 1 μM with a peak reduction of 44% at 10 nM (Figure 6, Panel B). Collectively, these results suggest that TF has the capacity to impair insulin signal transduction at lower concentrations and after shorter durations of exposure than initially investigated.
Figure 6.
Time- and Concentration-Dependence of Tolyfluanid-Mediated IRS-1 Downregulation. For time-dependence (Panel A), RNA was isolated from cultured C57BL/6 perigonadal fat pads after 12–48 h incubation with 100 nM tolylfluanid or vehicle (ethanol, EtOH). For concentration-dependence (Panel B), RNA was similarly isolated after exposure to vehicle or TF at concentrations ranging from 100 pM to 1μM. Isolated RNA was reverse transcribed, and qRT-PCR was used to assess fold change differences between vehicle- versus TF-treated adipocytes. Data are presented as means ± S.E.M. of the fold induction of IRS-1 relative to 18S rRNA normalized to vehicle-treated controls (n = 3–5 for time-dependence; n = 4 for concentration-dependence). * p < 0.05; ** p < 0.01; *** p < 0.001.
3.6. Effect of Tolylfluanid on IRS-1 Protein Stability
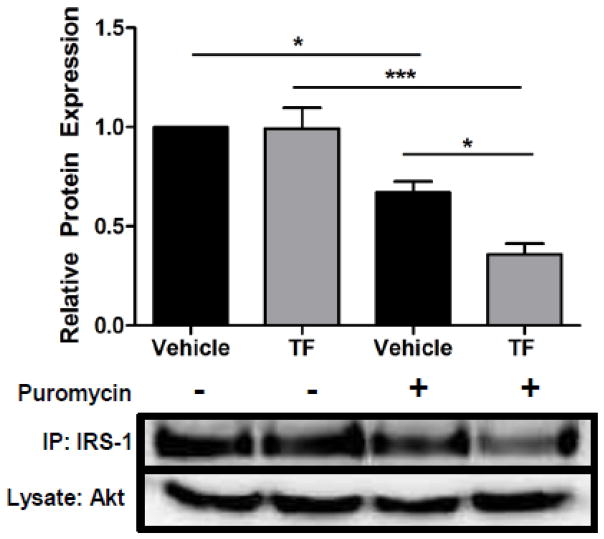
To determine whether transcriptional downregulation of IRS-1 was solely responsible for reductions of IRS-1 protein levels, the effect of TF on protein stability was measured. IRS-1 ubiquitination, an important regulator of proteasomal degradation of IRS-1, was unchanged by TF treatment (data not shown), suggesting that this pathway was not the principal mechanism by which TF induces insulin resistance. However, protein stability is governed by processes beyond ubiquitination; therefore, the effects of TF on IRS-1 stability were directly assessed. Primary adipose tissue from male C57BL/6 mice were treated with either vehicle (ethanol) or 100 nM TF for 12 h, a duration of treatment during which IRS-1 protein levels remain unchanged. The protein translation inhibitor puromycin was then added to a final concentration of 100 μM for an additional 2 h. Relative to untreated controls, puromycin treatment resulted in a 33% reduction in IRS-1 levels in vehicle-treated adipocytes (Figure 7). After pretreatment with TF, puromycin reduced IRS-1 protein levels by 64%. Thus, compared to puromycin-treated controls, TF increased the degradation of IRS-1 protein by 54%. In contrast, Akt levels were unchanged demonstrating that the puromycin was not exerting a toxic effect on the cells.
Figure 7.
Effect of TF on IRS-1 Protein Stability. Perigonadal fat pads from C57BL/6 mice were treated with vehicle or 100 nM TF for 12 h followed by incubation with the protein translation inhibitor puromycin at a final concentration of 100 μM for an additional 2 h. Levels of Akt were measured in cell lysates, while IRS-1 protein levels were determined after immunoprecipitation. Representative immunoblots (Bottom) are shown as well as cumulative densitometry for IRS-1 as means ± S.E.M. normalized to vehicle-treated controls (n = 3). IRS-1 densitometry is expressed as IRS-1/Akt normalized to vehicle (ethanol, EtOH). * p < 0.05; *** p < 0.001.
3.7. Effect of Tolylfuanid on Adipocytic Leptin Secretion
To investigate the physiological consequences of TF-mediated disruption of insulin signaling on adipocyte physiology, studies were performed to interrogate the effects of this novel endocrine disruptor on leptin production. Treatment of primary adipocytes from C57BL/6 mice with 100 nM TF resulted in a 17% reduction in basal leptin secretion over 48 h (Figure 8, Panel A). Treatment of adipocytes with 2.5 nM insulin for 24 h augmented leptin secretion by 73.6%; however, TF treatment reduced insulin-stimulated leptin secretion by 14.6% (Figure 8, Panel B). Because of the central role of leptin in regulating global energy metabolism [29], these results suggest that disruption of insulin signaling by TF results in a phenotypic change in the adipocyte that could promote alterations in global energy regulation that favor weight gain.
Figure 8.
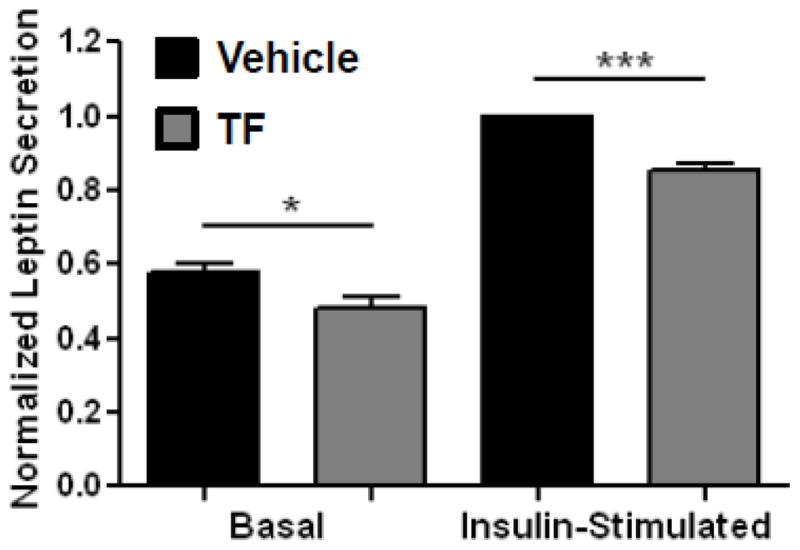
Effect of TF on Leptin Secretion. Perigonadal fat pads from C57BL/6 mice were treated with TF for 48 h. A subset of samples were also treated for the final 24 h of the 48 h incubation period with 2.5 nM insulin. Aliquots of media were then analyzed for leptin secretion. Data are presented as means ± S.E.M (n = 7) normalized to the vehicle-treated, insulin-stimulated condition. * p < 0.05; *** p < 0.001.
4. Discussion
The phenylsulfamide class of fungicides includes TF and dichlofluanid, a structural analogue of TF that differs solely by the absence of a phenolic methyl group. Both of these fungicides are used in agriculture as well as in the shipping industry as booster biocides in antifouling paints where they are used to limit adherence of organisms to ships’ hulls. Tissue levels of phenylsulfamides in humans have not been reported; however, there is ample evidence of human exposure. Approximately 20% of apples tested in the Czech Republic [30] and 46% analyzed in Slovenia were shown to be TF-positive [31]. In fact, TF was among the most common pesticides detected in fruits and vegetables in southeastern Poland at concentrations up to 1.44 mg/kg [32]. Greenhouse-grown strawberries in Norway had TF levels of approximately 13 mg/kg four days after the most recent application [33]. Importantly, washing did not significantly reduce TF residues on apples [30]. Marine concentrations of phenylsulfamides are a consequence of both agricultural run-off as well as leaching from anti-fouling paints, with levels varying depending upon local agricultural and shipping activities. Reported dichlofluanid concentrations in marine waters are as high as 1.8 nM (reviewed in [34]), with worst case predictions up to 17.4 nM [35]. Importantly, recent bans on organotin-based antifouling paints are predicted to increase use of alternative booster biocides, including phenylsulfamide fungicides, with an expected rise in environmental levels [35]. In addition to exposure among the general population, some individuals are at heightened risk through occupational exposure to phenylsulfamides, including farm workers during harvesting activities (average hand exposure to dichlofluanid of 1.14 mg/m2/hr with maximum wrist exposure of 4.87 mg/m2/hr) [36] and shipyard workers applying marine paints (potential dermal exposure to dichlofluanid of 277 mg/hr (for hands) and 267 mg/hr (for total body less hands) [37]. Precise extrapolation of these environmental levels and occupational exposures to in vivo concentrations are difficult in the absence of direct measurements; however, it is expected that bioaccumulation of phenylsulfamides in adipose tissue would be significant given their highly hydrophobic character (e.g. log partition coefficient of octanol-to-water of 3.9 for TF) [20]. Current evidence suggesting that TF has the capacity to augment adipocyte differentiation [12] and impair insulin action should prompt efforts to measure phenylsulfamide levels in exposed populations in order to assess their role in the pathogenesis of obesity and diabetes.
Despite evidence for human exposure, the metabolic effects of TF have not been previously studied. Because of the importance of adipocytes in the control of global energy homeostasis, studies were undertaken to determine the effect of TF on adipocytic insulin signaling. Under insulin stimulation, a series of intracellular signaling events takes place including the phosphorylation of Akt, a critical regulator of glucose and lipid metabolism and gene transcription [38]. Initial analysis revealed that TF treatment of adipocytes from the perigonadal fat pad of two strains of mice (with differing propensities to develop diabetes), as well as two strains of rat, resulted in a dramatic attenuation of insulin-stimulated Akt phosphorylation. Similar studies were also performed using human adipose tissue obtained from surgical patients. Consistent with the effects observed with rodent adipocytes, TF pretreatment decreased subsequent insulin signaling in human fat, suggesting that humans may be sensitive to the deleterious metabolic effects of this fungicide.
It is now widely recognized that the distribution of adipose tissue has a much greater impact on global insulin sensitivity than the total amount of body fat. An increase in the waist:hip ratio in humans, reflective of higher amounts of visceral fat, correlates with a more severe insulin resistance; conversely, accumulation of adipose mass below the waist in humans (the “pear shape” versus the “apple shape”) has a much less deleterious effect [26]. To ascertain whether TF reduced insulin sensitivity in the visceral fat compartment, mesenteric and perirenal, adipose depots were pretreated with TF to determine their sensitivity to this EDC. Consistent with data from the perigonadal fat pad, TF inhibited insulin action in both the mesenteric and perirenal fat pads. Since murine mesenteric and perirenal fat pads are homologous to human visceral fat, the anti-insulin effect of TF in these depots suggests that the adverse effects of this metabolic disruptor may compound the additional mechanisms of insulin resistance inherent to visceral adipose tissue. Unfortunately, this supposition could not be directly tested using human visceral fat due to the requirement of surgical intervention to obtain tissue from this depot.
Although a growing body of epidemiological data has linked EDC exposure to metabolic derangements in humans, the precise molecular mechanisms by which these chemicals exert their effects remain poorly understood. The present study provides evidence that TF disrupts the insulin-mediated activation of Akt specifically through a reduction in IRS-1 levels (Figure 9). The expression of all other components of the insulin signaling pathway between the insulin receptor and Akt was unaffected. IRS-1 is a large protein (180 kDa) that is phosphorylated on tyrosine residues by the insulin receptor, resulting in the recruitment of various proteins that further transduce the signal. IRS-1 has many tyrosine as well as serine/threonine residues that are subject to phosphorylation by a number of kinases from various signaling cascades. In general, tyrosine phosphorylation of IRS-1 augments insulin signaling whereas serine/threonine phosphorylation diminishes insulin action. Thus, through its phosphorylation state as well as its overall levels, IRS-1 integrates signals from multiple signaling cascades to establish the cell’s overall responsiveness to insulin [28].
Figure 9.
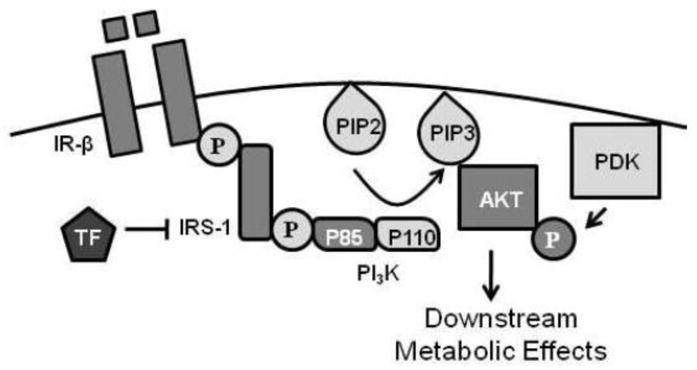
Overview of effects of TF on insulin signal transduction cascade in adipose tissue. Insulin binding to its cell surface receptor (IR) results in auto-transphosphorylation of the two β-subunits and activation of the insulin receptor’s intrinsic tyrosine kinase activity. This leads to tyrosine phosphorylation of IRS-1, which recruits and activates PI3K through action on the regulatory subunit (p85) leading to the generation of higher ordered lipids (PIP3). Generation of PIP3 recruits Akt to the cell membrane where it is activated by serine/threonine phosphorylation prior to conducting its downstream effects. TF reduces cellular insulin sensitivity by reducing IRS-1 levels, partially through reduction in IRS-1 gene transcription.
The TF-induced reduction of IRS-1 protein levels corresponded with a decrease in mRNA expression as well as protein stability for the insulin signaling intermediate. The transcriptional regulation of IRS-1 is complex and not fully defined, and the molecular intermediaries responsible for the reduction of IRS-1 mRNA levels in adipose tissue by TF are currently under investigation. IRS-1 protein levels are signaled for degradation at least partially via ubiquitination [39]; however, TF does not appear to alter this process (data not shown). The precise mechanism by which TF destabilizes the IRS-1 protein is actively under study. Interestingly, the sensitivity of human adipocytes to TF were overcome by high concentrations of insulin, whereas rodent adipocytes continued to show TF-mediated inhibition of insulin-stimulated Akt phosphorylation under similar conditions. This apparent discrepancy may reflect differences between humans and rodents in the degree to which they downregulate expression of the insulin receptor in response to high to supraphysiologic concentrations of insulin; however, this may also reflect species variability in either the extent or the mechanism (transcriptional vs. translational) by which TF downregulates IRS-1.
In addition to attenuating the insulin responsiveness of adipocytes, TF alters the endocrine function of fat cells. During TF treatment, leptin secretion into the media is reduced, and while insulin treatment augments leptin release, treatment with this metabolic disruptor reduces insulin-stimulated leptin release relative to vehicle-treated controls. Leptin plays a critical role in global energy balance with low levels resulting in both a reduction in energy expenditure as well as an increase in energy intake [29]. Collectively, these changes promote an increase in the deposition of body fat. Thus, it is possible that exposure to phenylsulfamide pesticides such as TF could promote fat accumulation directly through the induction of adipocyte differentiation [12] as well as indirectly through a modulation of adipokine production that promotes positive energy balance. Furthermore, adipose tissue accumulation under the influence of TF may be particularly deleterious since fat cells exposed to TF are resistant to insulin action, thus favoring a diabetic phenotype.
The described investigations demonstrate a consistent TF-induced attenuation of insulin action at 100 nM that is strain- and species-independent. This concentration was selected since it was an intermediate dose that promoted adipocyte differentiation in the 3T3-L1 cell line [12], and these studies serve as proof-of-principle that TF can function as a metabolic disruptor in adipose tissue. It is important to note the short duration of exposure (48 h) at which an effect on insulin action was observed in these studies. Since the relevant human physiological state likely reflects chronic exposure over months or even years, it is possible that lower doses over a longer period may unmask similar anti-insulin actions of TF. Furthermore, bioaccumulation of TF is likely given the chemical’s highly hydrophobic character [20]. The capacity of TF to enrich in the hydrophobic environment of the adipocyte is particularly important to recognize given that the limited measured levels of TF in the environment are lower than the treatment doses used in the present study. Interestingly, the enrichment of EDCs in fat may partially explain the stronger correlations between POPs and diabetes among obese individuals in some epidemiological studies [14]. While this could reflect synergistic action between EDCs and an underlying predisposition to metabolic disruption, it is possible that bioaccumulation in the adipocyte lipid droplet enhances an EDC’s capacity to inhibit insulin action, especially given the critical importance of adipose tissue in regulating global energy metabolism.
The present study shows that the fungicide and putative endocrine disruptor TF impairs adipocytic insulin action, yet, several open questions remain. While TF consistently decreased insulin action in multiple strains of two rodent species, these studies were restricted to male rodents. Full extrapolation is complicated by the known influence of sex steroids on insulin action [40]. Because similar effects were observed in human adipocytes obtained from female patients, it is expected that TF would have similar effects in female rodents; however, the unknown menstrual status of these patients leaves open the possibility of effect modification by sex steroids. An additional limitation of the current work is the ex vivo analysis of TF action on insulin signaling. Global energy balance is a consequence of multiple tissues (e.g. adipose tissue, liver, muscle, and brain) interacting to maintain metabolic homeostasis. Given the significant reductions in adipocytic insulin action mediated by TF as well as the effects of this chemical on leptin secretion, carefully controlled in vivo studies examining the effects of this novel EDC on whole-body energy metabolism are warranted. Such experiments will also be able to address the open question of whether metabolism of TF itself influences its ability to modulate insulin action. Collectively, these analyses are critically important for understanding the magnitude of the health threat posed by this novel metabolic disruptor with the capacity to adversely affect insulin action in adipose tissue.
Highlights.
Insulin signaling in primary rodent and human adipocytes was impaired by the fungicide tolylfluanid.
Tolylfluanid specifically reduced the mRNA and protein expression of insulin receptor substrate-1.
Reduction of insulin signaling resulted in decreased leptin release from primary adipocytes.
Acknowledgments
The project was supported by the National Institutes of Health [K08-ES019176 and F32-ES017397 to R.M.S.; T32-HL007237 supporting B.A.N.; T35-DK062719 supporting A.T.H. and D.A.C.] and by the Diabetes Research and Training Center [P60-DK020595]. C.O.B. is a Howard Hughes Medical Institute Research Training Fellow.
Footnotes
Disclosure
The authors declare no conflict of interest.
EDCs, endocrine disrupting chemicals; TF, tolylfluanid
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neel BA, Sargis RM. The paradox of progress: environmental disruption of metabolism and the diabetes epidemic. Diabetes. 2011;60:1838–1848. doi: 10.2337/db11-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steyn NP, Mann J, Bennett PH, Temple N, Zimmet P, Tuomilehto J, Lindstrom J, Louheranta A. Diet, nutrition and the prevention of type 2 diabetes. Public Health Nutr. 2004;7:147–165. doi: 10.1079/phn2003586. [DOI] [PubMed] [Google Scholar]
- 3.Jugan ML, Levi Y, Blondeau JP. Endocrine disruptors and thyroid hormone physiology. Biochem Pharmacol. 2010;79:939–947. doi: 10.1016/j.bcp.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Urbatzka R, van Cauwenberge A, Maggioni S, Vigano L, Mandich A, Benfenati E, Lutz I, Kloas W. Androgenic and antiandrogenic activities in water and sediment samples from the river Lambro, Italy, detected by yeast androgen screen and chemical analyses. Chemosphere. 2007;67:1080–1087. doi: 10.1016/j.chemosphere.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 5.Dickerson SM, Gore AC. Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dysfunction across the life cycle. Rev Endocr Metab Disord. 2007;8:143–159. doi: 10.1007/s11154-007-9048-y. [DOI] [PubMed] [Google Scholar]
- 6.Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol. 2011;73:135–162. doi: 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- 7.Baillie-Hamilton PF. Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med. 2002;8:185–192. doi: 10.1089/107555302317371479. [DOI] [PubMed] [Google Scholar]
- 8.Grun F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147:S50–55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 9.Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, Webster TF. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ Health. 2008;7:27. doi: 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirchner S, Kieu T, Chow C, Casey S, Blumberg B. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol Endocrinol. 2010;24:526–539. doi: 10.1210/me.2009-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feige JN, Gelman L, Rossi D, Zoete V, Metivier R, Tudor C, Anghel SI, Grosdidier A, Lathion C, Engelborghs Y, Michielin O, Wahli W, Desvergne B. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis. J Biol Chem. 2007;282:19152–19166. doi: 10.1074/jbc.M702724200. [DOI] [PubMed] [Google Scholar]
- 12.Sargis RM, Johnson DN, Choudhury RA, Brady MJ. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity (Silver Spring) 2010;18:1283–1288. doi: 10.1038/oby.2009.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DH, Lee IK, Jin SH, Steffes M, Jacobs DR., Jr Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2007;30:622–628. doi: 10.2337/dc06-2190. [DOI] [PubMed] [Google Scholar]
- 14.Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker BA, Jacobs DR., Jr A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diabetes Care. 2006;29:1638–1644. doi: 10.2337/dc06-0543. [DOI] [PubMed] [Google Scholar]
- 15.Sakurai K, Kawazuma M, Adachi T, Harigaya T, Saito Y, Hashimoto N, Mori C. Bisphenol A affects glucose transport in mouse 3T3-F442A adipocytes. Br J Pharmacol. 2004;141:209–214. doi: 10.1038/sj.bjp.0705520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu HF, Tsou TC, Chao HR, Kuo YT, Tsai FY, Yeh SC. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on adipogenic differentiation and insulin-induced glucose uptake in 3T3-L1 cells. J Hazard Mater. 2010;182:649–655. doi: 10.1016/j.jhazmat.2010.06.081. [DOI] [PubMed] [Google Scholar]
- 17.Nishiumi S, Yoshida M, Azuma T, Yoshida K, Ashida H. 2,3,7,8-tetrachlorodibenzo-p-dioxin impairs an insulin signaling pathway through the induction of tumor necrosis factor-alpha in adipocytes. Toxicol Sci. 2010;115:482–491. doi: 10.1093/toxsci/kfq052. [DOI] [PubMed] [Google Scholar]
- 18.Ruzzin J, Petersen R, Meugnier E, Madsen L, Lock EJ, Lillefosse H, Ma T, Pesenti S, Sonne SB, Marstrand TT, Malde MK, Du ZY, Chavey C, Fajas L, Lundebye AK, Brand CL, Vidal H, Kristiansen K, Froyland L. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ Health Perspect. 2010;118:465–471. doi: 10.1289/ehp.0901321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab. 2000;11:351–356. doi: 10.1016/s1043-2760(00)00323-4. [DOI] [PubMed] [Google Scholar]
- 20.EPA; U.S.E.P. Agency. Pesticide Fact Sheet: Tolylfluanid. Washington, DC: 2002. [Google Scholar]
- 21.Chemwatch . Global Chemicals Management. Vol. 2011. Chemwatch, Glen Huntly; Victoria, Australia: 2011. [Google Scholar]
- 22.Johansson M, Johansson N, Lund BO. Xenobiotics and the glucocorticoid receptor: additive antagonistic effects on tyrosine aminotransferase activity in rat hepatoma cells. Basic Clin Pharmacol Toxicol. 2005;96:309–315. doi: 10.1111/j.1742-7843.2005.pto960406.x. [DOI] [PubMed] [Google Scholar]
- 23.Jurczak MJ, Danos AM, Rehrmann VR, Allison MB, Greenberg CC, Brady MJ. Transgenic overexpression of protein targeting to glycogen markedly increases adipocytic glycogen storage in mice. Am J Physiol Endocrinol Metab. 2007;292:E952–963. doi: 10.1152/ajpendo.00559.2006. [DOI] [PubMed] [Google Scholar]
- 24.Lazar DF, Wiese RJ, Brady MJ, Mastick CC, Waters SB, Yamauchi K, Pessin JE, Cuatrecasas P, Saltiel AR. Mitogen-activated protein kinase kinase inhibition does not block the stimulation of glucose utilization by insulin. J Biol Chem. 1995;270:20801–20807. doi: 10.1074/jbc.270.35.20801. [DOI] [PubMed] [Google Scholar]
- 25.Schreyer SA, Wilson DL, LeBoeuf RC. C57BL/6 mice fed high fat diets as models for diabetes-accelerated atherosclerosis. Atherosclerosis. 1998;136:17–24. doi: 10.1016/s0021-9150(97)00165-2. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 27.Meng X, Kondo M, Morino K, Fuke T, Obata T, Yoshizaki T, Ugi S, Nishio Y, Maeda S, Araki E, Kashiwagi A, Maegawa H. Transcription factor AP-2beta: a negative regulator of IRS-1 gene expression. Biochem Biophys Res Commun. 2010;392:526–532. doi: 10.1016/j.bbrc.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 28.White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002;283:E413–422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- 29.Leibel RL. The role of leptin in the control of body weight. Nutr Rev. 2002;60:S15–19. doi: 10.1301/002966402320634788. discussion S68–84, 85–17. [DOI] [PubMed] [Google Scholar]
- 30.Stepan R, Ticha J, Hajslova J, Kovalczuk T, Kocourek V. Baby food production chain: pesticide residues in fresh apples and products. Food Addit Contam. 2005;22:1231–1242. doi: 10.1080/02652030500239623. [DOI] [PubMed] [Google Scholar]
- 31.Cesnik HB, Gregorcic A, Bolta SV, Kmecl V. Monitoring of pesticide residues in apples, lettuce and potato of the Slovene origin, 2001–04. Food Addit Contam. 2006;23:164–173. doi: 10.1080/02652030500401199. [DOI] [PubMed] [Google Scholar]
- 32.Sadlo SS, Jazwa E, Zawislak AA. Pesticide Residues in Fruit and Vegetables from Southeastern Poland, 2004–05. Polish J of Environ Stud. 2007;16:313–219. [Google Scholar]
- 33.Stensvand A, Christiansen A. Investigation on fungicide residues in greenhouse-grown strawberries. J Agric Food Chem. 2000;48:917–920. doi: 10.1021/jf990418k. [DOI] [PubMed] [Google Scholar]
- 34.Konstantinou IK, Albanis TA. Worldwide occurrence and effects of antifouling paint booster biocides in the aquatic environment: a review. Environ Int. 2004;30:235–248. doi: 10.1016/S0160-4120(03)00176-4. [DOI] [PubMed] [Google Scholar]
- 35.Bellas J. Comparative toxicity of alternative antifouling biocides on embryos and larvae of marine invertebrates. Sci Total Environ. 2006;367:573–585. doi: 10.1016/j.scitotenv.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 36.Tielemans E, Louwerse E, de Cock J, Brouwer D, Zielhuis G, Heederik D. Exposure to fungicides in fruit growing: re-entry time as a predictor for dermal exposure. Am Ind Hyg Assoc J. 1999;60:789–793. doi: 10.1080/00028899908984503. [DOI] [PubMed] [Google Scholar]
- 37.Links I, Van Der Jagt KE, Christopher Y, Lurvink M, Schinkel J, Tielemans E, Van Hemmen JJ. Occupational exposure during application and removal of antifouling paints. Ann Occup Hyg. 2007;51:207–218. doi: 10.1093/annhyg/mel074. [DOI] [PubMed] [Google Scholar]
- 38.Chang L, Chiang SH, Saltiel AR. Insulin signaling and the regulation of glucose transport. Mol Med. 2004;10:65–71. doi: 10.2119/2005-00029.Saltiel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun XJ, Goldberg JL, Qiao LY, Mitchell JJ. Insulin-induced insulin receptor substrate-1 degradation is mediated by the proteasome degradation pathway. Diabetes. 1999;48:1359–1364. doi: 10.2337/diabetes.48.7.1359. [DOI] [PubMed] [Google Scholar]
- 40.Yeung EH, Zhang C, Mumford SL, Ye A, Trevisan M, Chen L, Browne RW, Wactawski-Wende J, Schisterman EF. Longitudinal study of insulin resistance and sex hormones over the menstrual cycle: the BioCycle Study. J Clin Endocrinol Metab. 2010;95:5435–5442. doi: 10.1210/jc.2010-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]