Abstract
Asthma leads to chronic airway inflammation that shares pathological features of chronic rejection after lung transplantation. Due to significant role of autoimmunity in chronic rejection, we hypothesized that immunity to self-antigens may also be present in asthma. The goal was to define immune responses to self-antigens in patients with asthma. Blood and clinical data were collected from 99 asthmatics and 60 controls. Serum was analyzed for antibodies (Abs) to Collagen V (ColV) by ELISA and correlated with disease severity. Asthmatics' sera were tested in human protein array to determine immune responses to other self-antigens. Asthmatics had higher concentration of Abs to ColV (predominantly IgG isotype) compared to control (p < 0.01). These Abs correlated with severe asthma (p<0.01) and corticosteroid use (p=0.032). Additionally, Abs to novel self-antigens epidermal group factor receptor (EGFr), activin A type 1 receptor, and alpha-catenin (α-catenin) were detected in asthmatics. We conclude that Abs to self-antigens (ColV, EGFr, Activin A type 1 receptor, and α-catenin) are present in asthmatics sera correlating with clinical disease. Epithelial damage from airway inflammation during asthma may result in exposure of cryptic self-antigens or their determinants resulting in immune response to self-antigens and these may contribute to pathogenesis of asthma.
Keywords: Asthma, Autoimmunity, Collagen V, epidermal growth factor receptor, Activin A type 1 receptor, alpha catenin
1. INTRODUCTION
Asthma is defined as “a complex common chronic disorder of the airways that is characterized by variable and recurring symptoms, airflow obstruction, bronchial hyper-responsiveness, and underlying inflammation” [1]. The airway inflammation leads to morphological changes that include epithelial shedding, basement membrane thickening, smooth muscle hypertrophy, mucosal hyperplasia and neovascularization. These encompass the chronic airway remodeling that is central to the pathogenesis of asthma.
Asthma was classified into – “extrinsic asthma” mediated predominantly by allergens; and “intrinsic asthma” without demonstrable exogenous allergens [2] which are now termed “allergic” and “non-allergic” asthma [3]. However, studies now suggest asthma to be a heterogenic condition with a variety of factors, both allergen and non-allergen, that contribute to its pathogenesis [4]. More recently studies have implicated the role for autoimmunity in the pathogenesis of asthma. Correlations of asthma with the prevalence of various autoimmune diseases such as Type I diabetes [5], rheumatoid arthritis [6], etc have been demonstrated. Additionally, there is evidence for a variety of auto-antibodies in patients with asthma such as anti-nuclear [7–9], anti-β2 –adrenergic receptor [10, 11], anti-bronchial epithelial cytokeratin 18 [12], anti-alpha enolase [13, 14], and antibodies (Abs) to IgE and the high affinity IgE receptor – FcεRI [15]. However the role of these Abs in asthma still remains unclear.
Studies from our laboratory, as well as others, have demonstrated the immunopathogenic role of immunity to extracellular matrix proteins (Collagens (Col)) in the development of chronic rejection following lung transplantation (LTx) [16–19]. Chronic lung rejection, clinically diagnosed as bronchiolitis obliterans syndrome (BOS), is an obstructive airway disease characterized by chronic airway inflammation similar to that seen in asthma. Mares et al first provided evidence of the role of autoimmunity to ColV in the development of BOS [20] and subsequent studies further established that induction of tolerance to ColV protected from development of BOS [21]. ColV is found in the perivascular, peribronchial tissues, and the alveolar interstitium [22]. Recent studies have elucidated the role of T helper cells including Th17 responses to ColV in the development of Abs to ColV and its possible role in the pathogenesis of BOS following LTx [17, 18, 23, 24]. In light of evidence that immunity to self–antigens including immune responses to extracellular matrix protein, ColV, is involved in the pathogenesis of obstructive airway disease following human LTx, we postulated a role of immunity to ColV in the development of inflammation and obstructive airway disease seen in asthma. Sera from asthma patients were analyzed for presence of Abs to ColV using ELISA and the isotype of Abs was determined. These were correlated with clinical disease severity and laboratory parameters such as blood counts, lung function tests and use of inhaled corticosteroids. Our results demonstrate that the prevalence and strength of immune responses to ColV correlates with severity of disease in patients with asthma. Further, our preliminary analysis demonstrated that asthma patients also have circulating Abs to other novel self-antigens including Epithelial Growth Factor receptor (EGFr), Activin A type 1 receptor and alpha-catenin (α-catenin). These results suggest a plausible role for immune responses to self-antigens in asthma.
2. Subjects and methods
2.1 Study participants
Asthmatic subjects attending the Pulmonary Clinic at Washington University/Barnes Jewish Hospital St. Louis, MO, were consecutively enrolled for this cross sectional study. Ninety-nine asthma patients were enrolled with criteria of: age between 18 to 60 years, confirmed diagnosis of asthma by a physician including a positive methacholine challenge test or ≥ 15% improvement in FEV1 (forced expiratory volume in 1 second) post brochodilation on spirometry, and use of asthma therapy for at least 1 year. Subjects with a history of smoking in the past 5 years or greater than 5-pack year smoking history and those with other concurrent lung disease were excluded from the study. Diagnosis of asthma and disease severity was determined by the American Thoracic Society (ATS) consensus workshop and NHLBI guidelines [25]. In addition to this, 60 non-asthmatic controls with no history of lung diseases or smoking were enrolled by convenience sampling.
Blood and sputum were obtained at the time of enrollment. Cell counts were determined at the clinical pathology laboratory at our center. Serum isolated from whole blood was stored at −70°C until future use. Spirometry and a methacholine challenge test was performed for all asthmatic subjects at the time of enrollment. Other demographic and clinical data were determined at the time of enrollment and retrospective review of patient clinical database. Subjects from whom blood was not obtained or those who did not undergo spirometry testing were not included in the study. In control subjects only blood was collected and sputum and pulmonary testing was not done.
This study was approved by the Institutional Review Board at Washington University in St. Louis School of Medicine and all subjects were enrolled after written informed consent.
2.2 Enzyme Linked Immunosorbent Assay (ELISA)
A standardized ELISA test for detecting human Abs to ColV was done as described previously [26]. In brief, ninety-six well ELISA plates were coated with 200ng/100 μl of human ColV (Sigma, St Louis, MO). Diluted serum (1:200) was tested for binding to ColV and Abs detected by secondary peroxidase conjugated mouse anti-human IgG (Jackson Immunoresearch, West Grove, PA). Plates were developed using tetramethylbenzidine and read at 450nm. Concentration of Abs was determined based on a standard curve using the binding of known concentration of standard rabbit anti-ColV (Abcam, Cambridge, MA) that was detected with mouse-anti-rabbit IgG secondary Ab (Santa Cruz Biotech). The assay was normalized to a known value of the Ab concentration of control serum from healthy individuals that is run in each assay plate. This assay is validated by our lab and has a linear range from 21ng/mL up to 2000ng/mL. All results were expressed in ng/mL. Our assay has a positive cut-off value of 125ng/mL as determined previously by normal human serum [26].
To determine the isotype specificity of anti-ColV we performed an ELISA similar to the above procedure on those sera using mouse anti-human IgG-HRP, IgA-HRP, IgM-HRP, or IgE-HRP secondary Abs (Jackson Immunoresearch). Concentration of individual isotypes was determined by a similar standard curve.
2.3 Identification of immunity to autoantigens in the sera of asthma patients by ProtoArray
Human protein microarray was performed on the pooled serum samples from asthmatics with Abs to ColV using the Human Protein ProtoArray kit (Invitrogen, Carlsbad, CA) to identify other Abs in sera of asthmatic subjects. These samples were used on the assumption that individuals with pre-existing humoral immunity to ColV were more likely to have Abs to other self-antigens as well. These were compared to pooled control serum.
Array was performed as per manufacturer's instructions. Briefly, microarrays were blocked with 1% bovine serum albumin for 1 hour at 4°C on an orbital shaker set at 50 rpm. Following incubation, array was washed and incubated with serum samples for 90 minutes at 4°C. Pooled sera from each group were tested on individual microarray plates. Secondary Alexa Fluor 647 goat anti-human Abs were used for detection and plates scanned using a microarray scanner. Images obtained were analyzed by GenePix Pro microarray data acquisition and ProtoArray Prospector software. From the list of proteins showing significant interactions with samples, those found in both asthma and control groups were excluded. Among the remaining targets, only those present in the lung were identified and reported.
2.4 Statistical analysis
Power calculation was done on the basis assumption of alpha = 0.05 and power of 0.8. Keeping the minimum detectable difference of our assay at 10ng/mL, led to calculation of a minimum number of 54 patients per group to maintain significance. Analysis was performed using GraphPad Prism (La Jolla, CA) and SPSS 19 (IBM Inc, Chicago) and data represented as mean ± standard deviation (SD). Shapiro Wilk's test was used to check for normality and non-normal data was log transformed. Concentration of Abs to ColV, and other clinical and demographic variables were compared using the ChiSquare test, Mann Whitney U test and Kruskal Wallis Test as appropriate. Pearson correlational analysis was performed to determine relationship to clinical variables. A two-sided level of significance was set at p<0.05.
3. RESULTS
3.1 Study Subjects
In total 159 individuals were included, that encompassed 99 asthmatics and 60 controls. Amongst those with asthma, 34 (34.3%) had mild intermittent or mild persistent asthma, 49 (49.4%) had moderate persistent asthma, and 16 (13.3%) had severe asthma. Clinical and demographic features are listed in Table 1. Although those with severe disease were significantly older compared to other groups (35.7±11.4 vs. 421.1±11.3 vs. 49.4±12.2 years, p=0.01) the mean age at the time of diagnosis of asthma was similar in all three groups (p=0.499, Table 1). Those with severe asthma disease had significantly lower FEV1 (81.5±13.0% vs. 76.4±12.6% vs. 67.4±13.3%, p=0.05). Similarly the inhaled corticosteroid dose was significantly higher in the severe asthmatics (in mg/day: 642.7±479 vs. 987.2±566 vs. 2567±2744, p=0.002).
Table 1.
Clinical and demographic characteristics of asthma subjects by severity of disease
| Variable | Mild (N=34) | Moderate (N=49) | Severe (N=16) | P* |
|---|---|---|---|---|
| Age, mean ± SD, years | ||||
| At time of study | 35.7 ± 11.4 | 42.1 ± 11.3 | 49.4 ± 12.2 | 0.017 |
| At diagnosis | 14.4 ± 15.4 | 18.3 ± 15.8 | 19.4 ± 14.0 | 0.499 |
| At symptom onset | 12.3 ± 13.3 | 14.9 ± 14.3 | 19.4 ± 14.0 | 0.404 |
| Gender, % male | 22.22 | 22.45 | 33.33 | 0.827 |
| Ethnicity, % | ||||
| Caucasian | 54.55 | 45.83 | 50.00 | 1.000 |
| Non-caucasian | 45.45 | 54.17 | 50.00 | |
| BMI, mean ± SD | 30.8 ± 7.1 | 33.8 ± 10.3 | 30.5 ± 5.3 | 0.471 |
| Asthma history, % | ||||
| Maternal | 36.36 | 46.15 | 0 | 0.373 |
| 1st degree family member | 63.64 | 69.23 | 25.00 | 0.379 |
| Skin atopy, % | ||||
| FEV1 mean ± SD, % | ||||
| Predicted, pre-BD | 81.5 ± 13.0 | 76.4 ± 12.6 | 67.4 ± 13.3 | 0.050 |
| Predicted, post-BD | 96.3 ± 13.5 | 86.8 ± 12.6 | 68.8 ± 11.1 | 0.020 |
| Change, post-BD | 9.9 ± 7.5 | 12.9 ± 11.3 | 10.3 ± 5.9 | 0.933 |
| Change ≥ 12, post-BD† | 36.36 | 37.50 | 50.00 | 1.000 |
| FVC, mean ± SD, % | ||||
| Predicted, pre-BD | 91.9 ± 13.0 | 86.8 ± 12.4 | 80.3 ± 13.2 | 0.097 |
| Predicted, post-BD | 102.7 ± 11.8 | 92.8 ± 8.9 | 83.4 ± 8.6 | 0.013 |
| Change, post-BD | 1.6 ± 6.1 | 5.3 ± 9.8 | 7.3 ± 5.1 | 0.203 |
| Change ≥ 12, post-BD† | 9.09 | 17.39 | 0.00 | 1.000 |
| FeNO, mean ± SD, ppm | 26.5 ± 14.6 | 34.8 ± 47.9 | 15.5 ± 2.8 | 0.712 |
| PC20, mean ± SD, mg | 2.5 ± 3.1 | 2.1 ± 1.7 | NA | 0.891 |
| Inhaled corticosteroid dose, mean ± SD, mg | 642.7 ± 479 | 987.2 ± 566 | 2567 ± 2744 | 0.002 |
| Inflammatory cells, % | ||||
| Eosinophils – circulating | 2.7 | 3.2 | 3.6 | 0.074 |
| Eosinophils – sputum | 2.7 | 2.9 | NA | NA |
| Neutrophils - sputum | 19.8 | 57.6 | 39.0 | NA |
percent of individuals. BD – bronchodilation. FEV1 – forced expiratory volume in one second. FVC – forced vital capacity. PC20 – provocative concentration of a substance (methacholine) capable of inducing a 20% decrease in FEV1. NA – no data available for analysis. Data represented as mean ± SD (where applicable) and compared by Kruskal Wallis Test.
The non-asthmatic control population had a male to female ratio of 6:3 with a mean age of 42 years (range 18–62 years). The control population had normal lung function with no history of lung disease or smoking.
3.2 Development of Abs to ColV in asthma patients and its correlation with severe asthma
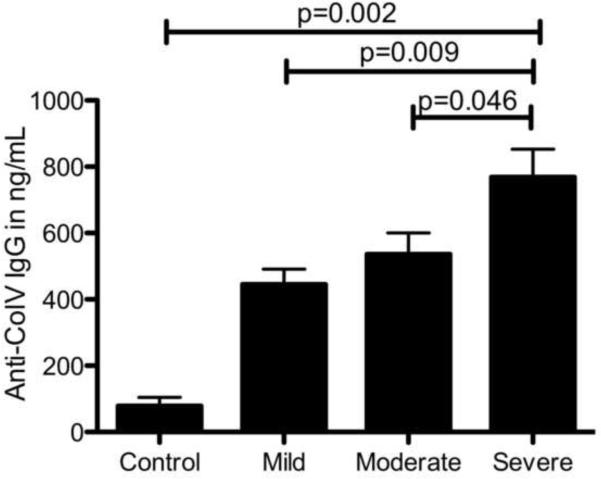
The serum concentration of Abs to ColV was determined by ELISA. Abs to ColV was significantly detected in all asthma cohorts (mild, moderate and severe disease) compared to the control (Figure 1). In addition, concentration of anti-ColV positively correlated with disease severity, with patients with severe asthma demonstrating the highest concentration of Abs in comparison to those with mild (p=0.009) or moderate disease (p=0.046) (in ng/mL – 445±45 vs. 536±64 vs. vs. 768±84) (Figure 1). The concentration of Abs in the control population was significantly lower than those with asthma (79±25.5 ng/mL, p=0.002). Among the mild asthmatics – 29 out of 34 patients were positive (>125ng/mL) based on our assay's cut-offs with an anti-ColV concentration ranging from 95ng/ml to 520ng/mL. All the patients in the moderate (anti-ColV range 226ng/mL to 630ng/mL) or severe asthma (anti-ColV range 514ng/mL to 874ng/mL) cohort were positive for anti-ColV. None of the control patients were positive for anti-ColV.
Figure 1.
Concentration of Abs to ColV (IgG) in asthma patients with mild, moderate and severe disease. Serum was analyzed by ELISA to detect Abs to ColV. Patients with severe asthma had significantly higher Abs to ColV compared to other groups. Data represented as mean ± SD in ng/mL and compared by Mann-Whitney U test
3.3 Abs to ColV in serum of asthma patients are predominantly of IgG isotype
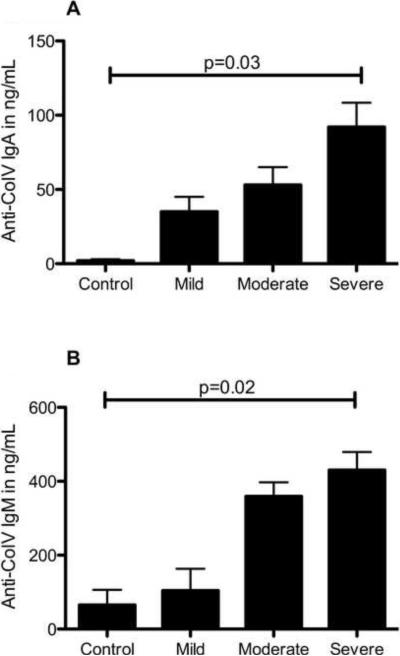
To determine the isotype specificity of ColV Abs in the sera of asthmatics, serum from patients positive for anti-ColV IgG (mild group n=29, moderate group n=49, severe group n=16) were tested additionally for IgA, IgM and IgE Abs to ColV. Patients with asthma develop IgG, IgA and IgM subtype of Abs to ColV that are significantly higher than among the control subjects (IgA: severe vs. moderate vs. mild vs. control: 92±16 vs. 53±12 vs. 24±10 vs. 2±1, p=0.03; IgM 430±49 vs. 259±38 vs. 104±59 vs. 65±41, p=0.02) (Figure 2). In addition, it was found that in all the three asthma groups, the percentage of IgG Abs to ColV was significantly higher in comparison to IgA and IgM subtypes (IgG:IgM:IgA: Mild– 76.1:17.8:6.1%, Moderate 63.1:30.5:6.4%, Severe 59.5:33.3:7.2%, Figure 3). IgM, and IgA isotype concentrations in the controls were within normal limits of our assay. Interestingly, IgE subtype of Abs was not detected either in controls or in the asthma subjects studied (data not shown).
Figure 2.
Isotype of Abs to ColV in patients with asthma compared to control Abs to ColV of isotypes IgA (Fig 2 A) and IgM (Fig 2 B) were detected using ELISA using specific secondary Abs. IgE isotype of Abs were not detected in both groups. Data represented as mean ± SD in ng/mL and compared by Kruskal Wallis test.
Figure 3.
Isotype distribution of anti-ColV in the sera of mild, moderate and severe asthma disease. In both mild/moderate asthmatic and severe asthmatics, Abs to ColV were predominantly of IgG isotype in the mild (76% of total anti-ColV), moderate (63%) and severe (59%) asthma patients. Data represented as mean in ng/mL
Hence, these results demonstrate that asthma patients develop Abs to ColV that correlate with disease severity. Abs formed are of IgG, IgA or IgM isotype amongst which, the IgG type of Abs to ColV are predominant in circulation.
3.4 Abs to ColV correlate with markers of asthma including lung function and dose of inhaled steroids
The relation between the concentration of Abs to ColV and demographic and spirometric variables was tested by Pearson correlational analysis (Table 2). Increasing concentration of Abs to ColV were strongly related to a less than 12% change in FVC following bronchodilation (r=22.78, p=0.006).
Table 2.
Correlational analyses of serum Abs to Colv and clinical characteristics of asthma patients
| Variable | All participants with asthma |
|
|---|---|---|
| Statistic | P | |
| Age | ||
| At time of study | −0.040 | 0.7205 |
| At diagnosis | 0.003 | 0.9794 |
| At symptom onset | 0.063 | 0.5821 |
| Gender | ||
| Male | 22.08 (19.173, 24.99) | 0.530 |
| Female | 20.94 (19.159, 22.727) | |
| Ethnicity | ||
| Caucasian | 19.82 (17.591, 22.05) | 0.055 |
| African American | 22.85 (20.672, 25.034) | |
| Other | ||
| BMI | −0.220 | 0.1131 |
| Asthma history, percent | ||
| Maternal | ||
| No | 22.27 (18.532, 26.014) | 0.609 |
| Yes | 20.87 (17.063, 24.689) | |
| 1st degree family member | ||
| No | 22.69 (17.169, 28.213) | 0.573 |
| Yes | 21.18 (18.196, 24.166) | |
| Skin atopy | ||
| No | 21.09 (15.567, 26.611) | 0.834 |
| Yes | 20.47 (18.312, 22.634) | |
| FEV1 | ||
| Predicted, pre-BD | −0.024 | 0.8351 |
| Predicted, post-BD | 0.010 | 0.9557 |
| Change, post-BD | −0.201 | 0.2323 |
| Change ≥ 12, post-BD | ||
| No | 21.74 (19.032, 24.456) | 0.705 |
| Yes | 20.87 (16.549, 25.191) | |
| FVC | ||
| Predicted, pre-BD | 0.028 | 0.8170 |
| Predicted, post-BD | −0.159 | 0.3622 |
| Change, post-BD | −0.277 | 0.1021 |
| Change ≥ 12, post-BD | ||
| No | 22.78 (20.545, 25.011) | 0.006 |
| Yes | 14.31 (7.1749, 21.451) | |
| FeNO | 0.072 | 0.6933 |
| PC20 | −0.426 | 0.0879 |
| Inhaled corticosteroid dose | 0.300 | 0.0322 |
| Inflammatory cells | ||
| Eosinophils – circulating | 0.347 | 0.0969 |
| Eosinophils – sputum | 0.239 | 0.2610 |
| Neutrophils - sputum | 0.265 | 0.1126 |
Pearson correlation coefficients are provided for associations between autoAb concentrations and continuous variables. Mean as well as upper and lower confidence limit intervals are provided for associations between autoAb concentrations and categorical variables. BD – bronchodilation. FEV1 – forced expiratory volume in one second. FVC – forced vital capacity. PC20 – provocative concentration of a substance (methacholine) capable of inducing a 20% decrease in FEV1. NA – analysis could not be performed
In addition, the Abs to ColV positively correlated with increasing daily corticosteroids dosage (r=0.3, p=0.032). It is important to note that severe asthmatics higher daily inhaled steroid usage (Table 1). Cinico-demographic variables including age, gender, BMI as well as parameters related to atopy such as eosinophil and neutrophil counts did not correlate with Abs to ColV (Table 2).
3.5 Abs to EGFr, Activin A receptor Type 1 and α-catenin alpha 1 in the sera from asthma patients
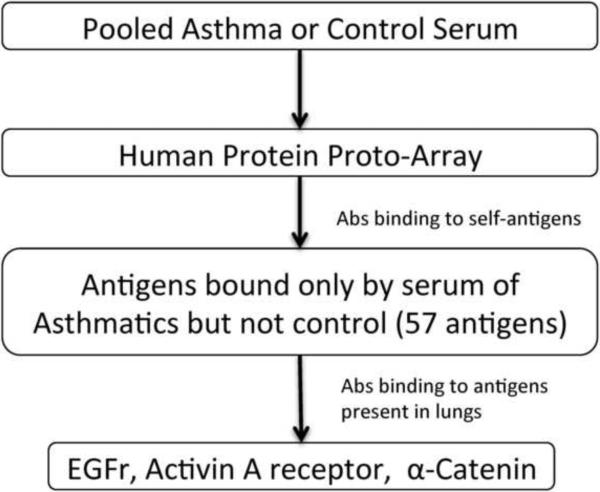
As a preliminary study to detect the presence of Abs to other self-antigens in asthma serum, we pooled 6 serum samples from each of the three asthma groups with the highest concentration of anti-ColV IgG and compared the results to pooled serum from control individuals. This was based on the assumption that if IgG Abs to ColV were detected, then it is likely that there maybe immune responses to other self-antigens. These asthmatic and control sera were tested on two separate human protein ProtoArray capable of detecting Abs to over 9000 proteins. Our analysis demonstrated that in the sera from asthmatics there were Abs which bound to 57 possible self-antigens compared to the control. We then analyzed for those antigens that are preferentially expressed in the lung (Figure 4). This led to identification of 3 antigens out of the 57 detected by the sera from asthmatics: 1) EGFr – modulates epidermal growth factor signaling; 2) Activin A receptor Type 1 – receptor for activin that modulates tissue development, inflammatory and repair responses; and 3) α-catenin – maintains cytoskeletal integrity as a component of adherence junctions.
Figure 4.
Flowchart of ProtoArray analysis of pooled asthma or control serum.
4. DISCUSSION
Asthma is mediated by a central chronic inflammatory process that results in characteristic airway remodeling. Various pathogenic mechanisms have been postulated in asthma. In this study we evaluated for the presence of immunity to self-antigens (ColV) in asthma subjects. Abs to ColV was detected in sera of asthmatics that significantly correlated with disease severity. These Abs, which were predominantly of the IgG isotype, were also positively associated with markers of asthma including lung function and steroid inhalation. In addition, preliminary protoarray based analysis of pooled serum from asthma patients demonstrated Abs to other self-antigens – EGFr, Activin A receptor and α-catenin that are expressed in the lung and are involved in growth factor signaling, tissue inflammation and repair and maintaining cytoskeletal integrity.
Although humoral immunity to self-antigens has been described in asthmatics [7–15], the role of these Abs in the pathogenesis and progression of asthma remains unclear. However, reports from our laboratory, as well as others, have shown an immunopathogenic role of Abs to ColV in chronic rejection (BOS) following LTx [16, 17, 26]. BOS is a chronic inflammatory airway obstructive disease that shares several histopathological features with airways in asthma, thus identification of Abs to ColV in asthmatics supports the hypothesis that these may be involved in the pathogenesis of asthma.
The concentration of Abs to ColV in patients with severe asthma was significantly higher as compared to the controls (p=0.002), as well as those with mild (p=0.009) and moderate asthma (p=0.046) (Figure 1). This demonstrates that Abs to ColV are seen in asthma and those patients affected the worst due to asthma display the highest amount of Abs to ColV. We hypothesize that airway inflammation in asthmatics leads to both inflammatory damage and remodeling of the airway epithelium which should correlate with the severity of the disease. This may result in increased exposure of cryptic self-antigens or its determinants that leads to a loss of peripheral tolerance and development of an immune response against these self-antigens. Although the pathological factors that may contribute to the development of such an immune response in asthma patients is currently not well understood, some likely etiological factors may include bacterial and viral respiratory infections, both of which have been postulated to bring about airway inflammation and exacerbate asthma [27–29]. Thus, the finding that such immune responses to ColV is found in the entire spectrum of asthma patients (Figure 1, 2, 3) highlights their association with the disease process. Also such an immune response self-antigens may not be limited to ColV alone but to other self-antigens in the lung, as suggested by our preliminary protoarray analysis using pooled sera from asthma patients.
The detection of IgA type of Abs to ColV as well as the absence of IgE type of Abs to ColV is interesting in the context of understanding the pathogenesis of asthma. IgA can interact with Fc-receptors on eosinophils and result in NADPH oxidase activation and eosinophil degranulation [30]. Thus IgA Abs to ColV may bind to damaged airway epithelial surfaces and exacerbate the inflammation by activation of eosinophils. In addition, IgE Abs to ColV were not seen and Abs to ColV did not correlate with makers of atopy including eosinophil counts (Table 2). This suggests that immunity to self-antigens (ColV) is present and may contribute to the pathogenesis of both allergen and non-allergen mediated asthma.
EGFr is expressed on bronchial epithelium, smooth muscle and basement membrane [31]. It is a tyrosine kinase receptor that is activated by many ligands including epidermal growth factor (EGF), transforming growth factor-β (TGF-β) and tumor necrosis factor α. This leads to downstream activation of MAPK, Akt and JNK that results in various cellular responses such as epithelial proliferation and differentiation. EGFr expression has been shown to be 2 to 3 fold higher in asthma patients compared to normal subjects [32] and also correlates with basement membrane thickening, goblet cell hyperplasia and airway mucous production [33, 34]. Additionally, EGFr activation increases IL-8 production that leads to neutrophil infiltration and lung inflammation [35]. Thus Abs to EGFr may act as its ligand resulting in sustained activation leading to both chronic airway inflammation and airway remodeling in asthma.
The activin A receptor modulates activin A, a protein involved in tissue development, inflammation and repair. Activin A levels have been shown to be increased in asthma both in the serum [36] as well as in the lung [37]. In addition it may induce allergen specific T cell response as well as airway remodeling mediated by TGF-β [36, 37]. Thus Abs to the activin A receptor may enhance immunity to allergens as well as the consequent inflammation, and remodeling in asthma.
α-Catenin, similar to E-cadherin and β-catenin, is a structural component of cell junctions and thus maintains inter-epithelial cell adherence. Reduced levels of α-catenin may contribute to a defective airway epithelial barrier allowing for eosinophil infiltration [38]. Thus, Abs to α-catenin may directly contribute and facilitate this process.
Due to the cross-sectional nature and retrospective acquisition of clinico-demographic data, this study is subject to limitations due to availability of data and selection bias. In addition, it is important to note that asthma itself is characterized by variable fluctuations in airway inflammation and the cross-sectional nature may account for patients that span the spectrum of symptomatic and asymptomatic patients. Due to the intrinsic design of the Protoarray along with the criteria employed for the selecting the Abs, it is unlikely that the results obtained are due to non-specific binding of serum proteins to the array plate. However, due to utilization of pooled asthma serum for our current analysis, future studies with individual patients sera and by the use of other methods such as immunoprecipitation and western blot analysis with purified proteins are necessary to confirm the novel Abs found and their potential role in the pathogenesis of asthma.
We acknowledge that this study determined only for the presence of Abs to self-antigens in asthma patients. We speculate that these immune responses may be pathogenic in the development of asthma based on the role of such immune responses in the pathogenesis of other chronic inflammatory diseases. Whether these Abs are indeed pathogenic in asthma or is it an epi-phenomenon of the ongoing airway inflammation will need to be determined in future studies on longitudinal cohorts of asthma patients.
In conclusion, this study provides evidence for the development of immunity to various lung associated self-antigens (ColV, EGFr, Activin A receptor and α-catenin) in patients with asthma. The development of humoral immune responses particularly to extra cellular matrix protein ColV directly correlated with the severity of asthma suggesting a possible pathogenic role for immune responses to self-antigens in the perpetuation of this disease. In addition, the predominance of an IgG type response with IgA and lack of IgE response to ColV as well as the presence of Abs to other self-antigens, also suggests that these immune responses to self-antigens may play a role in both allergen and non-allergen mediated asthma.
5. ACKNOWLEDGEMENT
This publication was made possible by an award from the NIH UL1 R024992, HL69149, SCOR HL56419, U19-AI070489 and American Lung Association Asthma Clinical Research Center grant (MC), and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. ML was supported by the Doris Duke Fellowship Grant. TM is supported by the BJC Foundation. The authors would like to thank Ms. Billie Glasscock for assistance in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- [2].Rackemann FM. A working classification of asthma. Am J Med. 1947;3:601. doi: 10.1016/0002-9343(47)90204-0. [DOI] [PubMed] [Google Scholar]
- [3].Romanet-Manent S, Charpin D, Magnan A, Lanteaume A, Vervloet D. Allergic vs nonallergic asthma: what makes the difference? Allergy. 2002;57:607. doi: 10.1034/j.1398-9995.2002.23504.x. [DOI] [PubMed] [Google Scholar]
- [4].Humbert M, Menz G, Ying S, Corrigan CJ, Robinson DS, Durham SR, et al. The immunopathology of extrinsic (atopic) and intrinsic (non-atopic) asthma: more similarities than differences. Immunol Today. 1999;20:528. doi: 10.1016/s0167-5699(99)01535-2. [DOI] [PubMed] [Google Scholar]
- [5].Stene LC, Nafstad P. Relation between occurrence of type 1 diabetes and asthma. Lancet. 2001;357:607. doi: 10.1016/S0140-6736(00)04067-8. [DOI] [PubMed] [Google Scholar]
- [6].Kero J, Gissler M, Hemminki E, Isolauri E. Could TH1 and TH2 diseases coexist? Evaluation of asthma incidence in children with coeliac disease, type 1 diabetes, or rheumatoid arthritis: a register study. J Allergy Clin Immunol. 2001;108:781. doi: 10.1067/mai.2001.119557. [DOI] [PubMed] [Google Scholar]
- [7].Leopold HC, Rynes S, Stoloff IL. Fluorescent Antibody Study for Antinuclear Antibodies in Bronchial Asthma. J Allergy Clin Immunol. 1965;36:175. doi: 10.1016/0021-8707(65)90165-6. [DOI] [PubMed] [Google Scholar]
- [8].Szczeklik A, Nizankowska E, Serafin A, Dyczek A, Duplaga M, Musial J. Autoimmune phenomena in bronchial asthma with special reference to aspirin intolerance. Am J Respir Crit Care Med. 1995;152:1753. doi: 10.1164/ajrccm.152.6.8520733. [DOI] [PubMed] [Google Scholar]
- [9].Comi AL, Tedeschi A, Lorini M, Miadonna A. Novel clinical and serological aspects in non-allergic asthma. Respir Med. 2007;101:2526. doi: 10.1016/j.rmed.2007.07.010. [DOI] [PubMed] [Google Scholar]
- [10].Lidor Y, Topilsky M, Spitzer SA, Yehoshua H. Autoimmune antibodies in intrinsic (nonatopic) asthma. Ann Allergy. 1980;44:296. [PubMed] [Google Scholar]
- [11].Harrison LC, Callaghan J, Venter JC, Fraser CM, Kaliner ML. Atopy, autonomic function and beta-adrenergic receptor autoantibodies. Ciba Found Symp. 1982:248. doi: 10.1002/9780470720721.ch14. [DOI] [PubMed] [Google Scholar]
- [12].Nahm DH, Lee YE, Yim EJ, Park HS, Yim H, Kang Y, et al. Identification of cytokeratin 18 as a bronchial epithelial autoantigen associated with nonallergic asthma. Am J Respir Crit Care Med. 2002;165:1536. doi: 10.1164/rccm.200201-009OC. [DOI] [PubMed] [Google Scholar]
- [13].Nahm DH, Lee KH, Shin JY, Ye YM, Kang Y, Park HS. Identification of alpha-enolase as an autoantigen associated with severe asthma. J Allergy Clin Immunol. 2006;118:376. doi: 10.1016/j.jaci.2006.04.002. [DOI] [PubMed] [Google Scholar]
- [14].Lee HA, Kwon B, Hur GY, Choi SJ, Nahm DH, Park HS. Isotype and IgG subclass distribution of autoantibody response to alpha-enolase protein in adult patients with severe asthma. Yonsei Med J. 2008;49:923. doi: 10.3349/ymj.2008.49.6.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sun RS, Chen XH, Sui JF, Liu RQ, Cheng TM, Ran XZ, et al. Detecting anti-FcepsilonRI autoantibodies in patients with asthma by flow cytometry. J Int Med Res. 2008;36:1214. doi: 10.1177/147323000803600607. [DOI] [PubMed] [Google Scholar]
- [16].Haque MA, Mizobuchi T, Yasufuku K, Fujisawa T, Brutkiewicz RR, Zheng Y, et al. Evidence for immune responses to a self-antigen in lung transplantation: role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection. J Immunol. 2002;169:1542. doi: 10.4049/jimmunol.169.3.1542. [DOI] [PubMed] [Google Scholar]
- [17].Wilkes DS, Heidler KM, Yasufuku K, Devito-Haynes L, Jankowska-Gan E, Meyer KC, et al. Cell-mediated immunity to collagen V in lung transplant recipients: correlation with collagen V release into BAL fluid. J Heart Lung Transplant. 2001;20:167. doi: 10.1016/s1053-2498(00)00308-9. [DOI] [PubMed] [Google Scholar]
- [18].Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T. CD4+25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human lung transplantation. Am J Transplant. 2006;6:1799. doi: 10.1111/j.1600-6143.2006.01383.x. [DOI] [PubMed] [Google Scholar]
- [19].Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180:4487. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mares DC, Heidler KM, Smith GN, Cummings OW, Harris ER, Foresman B, et al. Type V collagen modulates alloantigen-induced pathology and immunology in the lung. Am J Respir Cell Mol Biol. 2000;23:62. doi: 10.1165/ajrcmb.23.1.3924. [DOI] [PubMed] [Google Scholar]
- [21].Yasufuku K, Heidler KM, Woods KA, Smith GN, Jr., Cummings OW, Fujisawa T, et al. Prevention of bronchiolitis obliterans in rat lung allografts by type V collagen-induced oral tolerance. Transplantation. 2002;73:500. doi: 10.1097/00007890-200202270-00002. [DOI] [PubMed] [Google Scholar]
- [22].Madri JA, Furthmayr H. Collagen polymorphism in the lung. An immunochemical study of pulmonary fibrosis. Hum Pathol. 1980;11:353. doi: 10.1016/s0046-8177(80)80031-1. [DOI] [PubMed] [Google Scholar]
- [23].Bharat A, Fields RC, Trulock EP, Patterson GA, Mohanakumar T. Induction of IL-10 suppressors in lung transplant patients by CD4+25+ regulatory T cells through CTLA-4 signaling. J Immunol. 2006;177:5631. doi: 10.4049/jimmunol.177.8.5631. [DOI] [PubMed] [Google Scholar]
- [24].Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].National Asthma Education and Prevention Program Expert panel report 2: Guidelines for the diagnosis and management of asthma. 2006 [Google Scholar]
- [26].Bharat A, Saini D, Steward N, Hachem R, Trulock EP, Patterson GA, et al. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg. 2010;90:1094. doi: 10.1016/j.athoracsur.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Maffey AF, Barrero PR, Venialgo C, Fernandez F, Fuse VA, Saia M, et al. Viruses and atypical bacteria associated with asthma exacerbations in hospitalized children. Pediatric pulmonology. 2010;45:619. doi: 10.1002/ppul.21236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Horvat JC, Starkey MR, Kim RY, Phipps S, Gibson PG, Beagley KW, et al. Early-life chlamydial lung infection enhances allergic airways disease through age-dependent differences in immunopathology. The Journal of allergy and clinical immunology. 2010;125:617. doi: 10.1016/j.jaci.2009.10.018. [DOI] [PubMed] [Google Scholar]
- [29].Kloepfer KM, Gern JE. Virus/allergen interactions and exacerbations of asthma. Immunology and allergy clinics of North America. 2010;30:553. doi: 10.1016/j.iac.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pleass RJ, Lang ML, Kerr MA, Woof JM. IgA is a more potent inducer of NADPH oxidase activation and degranulation in blood eosinophils than IgE. Molecular immunology. 2007;44:1401. doi: 10.1016/j.molimm.2006.05.002. [DOI] [PubMed] [Google Scholar]
- [31].Amishima M, Munakata M, Nasuhara Y, Sato A, Takahashi T, Homma Y, et al. Expression of epidermal growth factor and epidermal growth factor receptor immunoreactivity in the asthmatic human airway. Am J Respir Crit Care Med. 1998;157:1907. doi: 10.1164/ajrccm.157.6.9609040. [DOI] [PubMed] [Google Scholar]
- [32].Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, et al. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J. 2000;14:1362. doi: 10.1096/fj.14.10.1362. [DOI] [PubMed] [Google Scholar]
- [33].Burgel PR, Escudier E, Coste A, Dao-Pick T, Ueki IF, Takeyama K, et al. Relation of epidermal growth factor receptor expression to goblet cell hyperplasia in nasal polyps. J Allergy Clin Immunol. 2000;106:705. doi: 10.1067/mai.2000.109823. [DOI] [PubMed] [Google Scholar]
- [34].Takeyama K, Fahy JV, Nadel JA. Relationship of epidermal growth factor receptors to goblet cell production in human bronchi. Am J Respir Crit Care Med. 2001;163:511. doi: 10.1164/ajrccm.163.2.2001038. [DOI] [PubMed] [Google Scholar]
- [35].Hamilton LM, Torres-Lozano C, Puddicombe SM, Richter A, Kimber I, Dearman RJ, et al. The role of the epidermal growth factor receptor in sustaining neutrophil inflammation in severe asthma. Clin Exp Allergy. 2003;33:233. doi: 10.1046/j.1365-2222.2003.01593.x. [DOI] [PubMed] [Google Scholar]
- [36].Karagiannidis C, Hense G, Martin C, Epstein M, Ruckert B, Mantel PY, et al. Activin A is an acute allergen-responsive cytokine and provides a link to TGF-beta-mediated airway remodeling in asthma. J Allergy Clin Immunol. 2006;117:111. doi: 10.1016/j.jaci.2005.09.017. [DOI] [PubMed] [Google Scholar]
- [37].Kariyawasam HH, Semitekolou M, Robinson DS, Xanthou G. Activin-A: a novel critical regulator of allergic asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2011 doi: 10.1111/j.1365-2222.2011.03784.x. [DOI] [PubMed] [Google Scholar]
- [38].de Boer WI, Sharma HS, Baelemans SM, Hoogsteden HC, Lambrecht BN, Braunstahl GJ. Altered expression of epithelial junctional proteins in atopic asthma: possible role in inflammation. Can J Physiol Pharmacol. 2008;86:105. doi: 10.1139/y08-004. [DOI] [PubMed] [Google Scholar]