Abstract
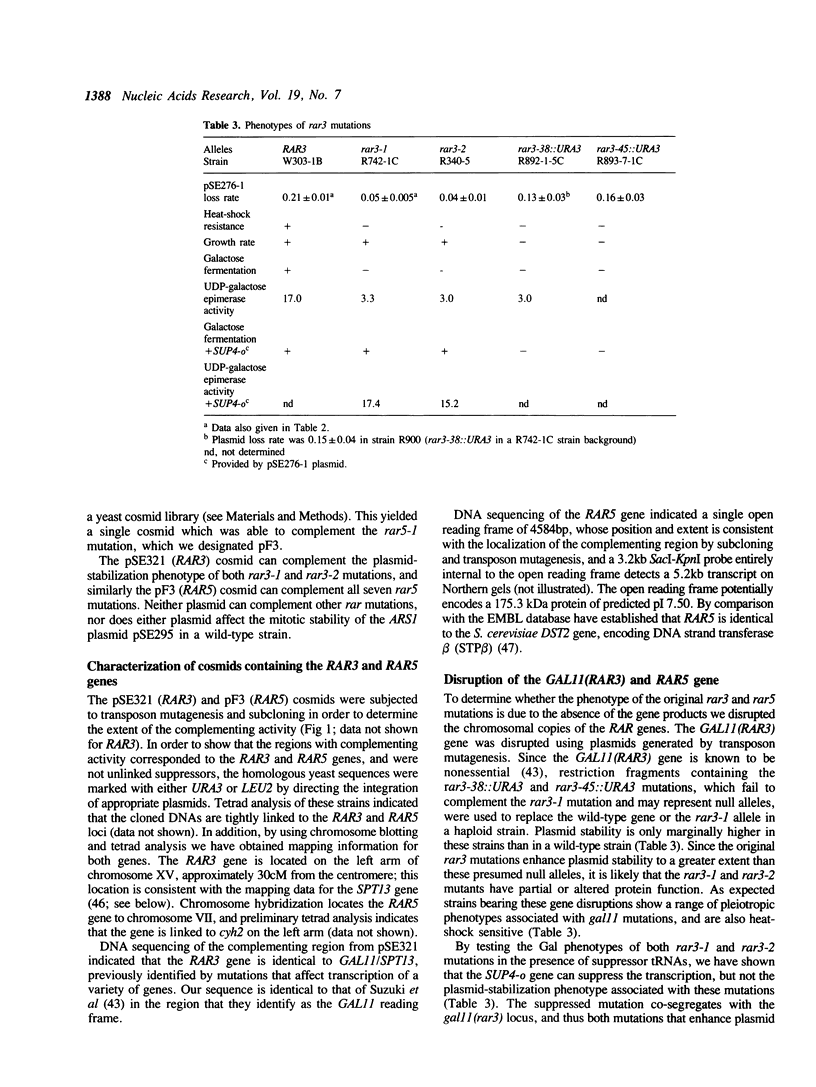
In an attempt to identify trans-acting factors involved in replication origin function, we have characterized the RAR3 and RAR5 genes, identified by mutations which increase the mitotic stability of artificial chromosomes whose replication is dependent on the activity of weak ARS elements. Sequence analysis has shown that the RAR3 gene is identical to GAL11/SPT13, which encodes a putative transcription factor involved in the expression of a wide range of genes. Change-of-function mutations that truncate the RAR3 protein appear to be required to enhance chromosome stability. In contrast, loss of the RAR5 protein results in enhanced chromosome stability, as if the protein is an inhibitor of ARS function. The RAR5 gene encodes the 175 kDa DNA strand transfer protein beta, an activity that can promote the transfer of a strand from a double-stranded DNA molecule to a complementary single strand. This observation implies that a presumed recombination activity can affect eukaryotic chromosomal replication.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankier A. T., Weston K. M., Barrell B. G. Random cloning and sequencing by the M13/dideoxynucleotide chain termination method. Methods Enzymol. 1987;155:51–93. doi: 10.1016/0076-6879(87)55009-1. [DOI] [PubMed] [Google Scholar]
- Bartlett R., Nurse P. Yeast as a model system for understanding the control of DNA replication in Eukaryotes. Bioessays. 1990 Oct;12(10):457–463. doi: 10.1002/bies.950121002. [DOI] [PubMed] [Google Scholar]
- Blow J. J., Nurse P. A cdc2-like protein is involved in the initiation of DNA replication in Xenopus egg extracts. Cell. 1990 Sep 7;62(5):855–862. doi: 10.1016/0092-8674(90)90261-c. [DOI] [PubMed] [Google Scholar]
- Borowiec J. A., Dean F. B., Bullock P. A., Hurwitz J. Binding and unwinding--how T antigen engages the SV40 origin of DNA replication. Cell. 1990 Jan 26;60(2):181–184. doi: 10.1016/0092-8674(90)90730-3. [DOI] [PubMed] [Google Scholar]
- Buchman A. R., Kimmerly W. J., Rine J., Kornberg R. D. Two DNA-binding factors recognize specific sequences at silencers, upstream activating sequences, autonomously replicating sequences, and telomeres in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Jan;8(1):210–225. doi: 10.1128/mcb.8.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A. R., Kornberg R. D. A yeast ARS-binding protein activates transcription synergistically in combination with other weak activating factors. Mol Cell Biol. 1990 Mar;10(3):887–897. doi: 10.1128/mcb.10.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A. R., Lue N. F., Kornberg R. D. Connections between transcriptional activators, silencers, and telomeres as revealed by functional analysis of a yeast DNA-binding protein. Mol Cell Biol. 1988 Dec;8(12):5086–5099. doi: 10.1128/mcb.8.12.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Animal virus DNA replication. Annu Rev Biochem. 1989;58:671–717. doi: 10.1146/annurev.bi.58.070189.003323. [DOI] [PubMed] [Google Scholar]
- Cheng L., Kelly T. J. Transcriptional activator nuclear factor I stimulates the replication of SV40 minichromosomes in vivo and in vitro. Cell. 1989 Nov 3;59(3):541–551. doi: 10.1016/0092-8674(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Cox M. M., Lehman I. R. Enzymes of general recombination. Annu Rev Biochem. 1987;56:229–262. doi: 10.1146/annurev.bi.56.070187.001305. [DOI] [PubMed] [Google Scholar]
- Diffley J. F., Stillman B. Purification of a yeast protein that binds to origins of DNA replication and a transcriptional silencer. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2120–2124. doi: 10.1073/pnas.85.7.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley J. F., Stillman B. Similarity between the transcriptional silencer binding proteins ABF1 and RAP1. Science. 1989 Nov 24;246(4933):1034–1038. doi: 10.1126/science.2511628. [DOI] [PubMed] [Google Scholar]
- Dykstra C. C., Hamatake R. K., Sugino A. DNA strand transfer protein beta from yeast mitotic cells differs from strand transfer protein alpha from meiotic cells. J Biol Chem. 1990 Jul 5;265(19):10968–10973. [PubMed] [Google Scholar]
- Eisen A., Camerini-Otero R. D. A recombinase from Drosophila melanogaster embryos. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7481–7485. doi: 10.1073/pnas.85.20.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler J. S., Winston F. The Saccharomyces cerevisiae SPT13/GAL11 gene has both positive and negative regulatory roles in transcription. Mol Cell Biol. 1989 Dec;9(12):5602–5609. doi: 10.1128/mcb.9.12.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francesconi S. C., Eisenberg S. Purification and characterization of OBF1: a Saccharomyces cerevisiae protein that binds to autonomously replicating sequences. Mol Cell Biol. 1989 Jul;9(7):2906–2913. doi: 10.1128/mcb.9.7.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H., Yonesaki T., Minagawa T. Sequence of the T4 recombination gene, uvsX, and its comparison with that of the recA gene of Escherichia coli. Nucleic Acids Res. 1985 Oct 25;13(20):7473–7481. doi: 10.1093/nar/13.20.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa T., Obonai K., Segawa T., Nogi Y. The enzymes of the galactose cluster in Saccharomyces cerevisiae. II. Purification and characterization of uridine diphosphoglucose 4-epimerase. J Biol Chem. 1980 Apr 10;255(7):2705–2707. [PubMed] [Google Scholar]
- Gibson S. I., Surosky R. T., Tye B. K. The phenotype of the minichromosome maintenance mutant mcm3 is characteristic of mutants defective in DNA replication. Mol Cell Biol. 1990 Nov;10(11):5707–5720. doi: 10.1128/mcb.10.11.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R. A., Stewart J. W., Sherman F. Amino acid replacements resulting from super-suppression of nonsense mutants of iso-1-cytochrome c from yeast. J Mol Biol. 1971 Oct 14;61(1):157–173. doi: 10.1016/0022-2836(71)90213-0. [DOI] [PubMed] [Google Scholar]
- Halfter H., Kavety B., Vandekerckhove J., Kiefer F., Gallwitz D. Sequence, expression and mutational analysis of BAF1, a transcriptional activator and ARS1-binding protein of the yeast Saccharomyces cerevisiae. EMBO J. 1989 Dec 20;8(13):4265–4272. doi: 10.1002/j.1460-2075.1989.tb08612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter H., Müller U., Winnacker E. L., Gallwitz D. Isolation and DNA-binding characteristics of a protein involved in transcription activation of two divergently transcribed, essential yeast genes. EMBO J. 1989 Oct;8(10):3029–3037. doi: 10.1002/j.1460-2075.1989.tb08453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieter P., Mann C., Snyder M., Davis R. W. Mitotic stability of yeast chromosomes: a colony color assay that measures nondisjunction and chromosome loss. Cell. 1985 Feb;40(2):381–392. doi: 10.1016/0092-8674(85)90152-7. [DOI] [PubMed] [Google Scholar]
- Himmelfarb H. J., Pearlberg J., Last D. H., Ptashne M. GAL11P: a yeast mutation that potentiates the effect of weak GAL4-derived activators. Cell. 1990 Dec 21;63(6):1299–1309. doi: 10.1016/0092-8674(90)90425-e. [DOI] [PubMed] [Google Scholar]
- Holden D. W., Spanos A., Banks G. R. Nucleotide sequence of the REC1 gene of Ustilago maydis. Nucleic Acids Res. 1989 Dec 25;17(24):10489–10489. doi: 10.1093/nar/17.24.10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P., Meyn M. S., Camerini-Otero R. D. Partial purification and characterization of a recombinase from human cells. Cell. 1986 Mar 28;44(6):885–894. doi: 10.1016/0092-8674(86)90011-5. [DOI] [PubMed] [Google Scholar]
- Huisman O., Raymond W., Froehlich K. U., Errada P., Kleckner N., Botstein D., Hoyt M. A. A Tn10-lacZ-kanR-URA3 gene fusion transposon for insertion mutagenesis and fusion analysis of yeast and bacterial genes. Genetics. 1987 Jun;116(2):191–199. doi: 10.1093/genetics/116.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis E. E., Clark K. L., Sprague G. F., Jr The yeast transcription activator PRTF, a homolog of the mammalian serum response factor, is encoded by the MCM1 gene. Genes Dev. 1989 Jul;3(7):936–945. doi: 10.1101/gad.3.7.936. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Kaguni J. M., Kornberg A. Topoisomerase I confers specificity in enzymatic replication of the Escherichia coli chromosomal origin. J Biol Chem. 1984 Jul 10;259(13):8578–8583. [PubMed] [Google Scholar]
- Kearsey S. E., Edwards J. Mutations that increase the mitotic stability of minichromosomes in yeast: characterization of RAR1. Mol Gen Genet. 1987 Dec;210(3):509–517. doi: 10.1007/BF00327205. [DOI] [PubMed] [Google Scholar]
- Kearsey S. Analysis of sequences conferring autonomous replication in baker's yeast. EMBO J. 1983;2(9):1571–1575. doi: 10.1002/j.1460-2075.1983.tb01626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearsey S. Structural requirements for the function of a yeast chromosomal replicator. Cell. 1984 May;37(1):299–307. doi: 10.1016/0092-8674(84)90326-x. [DOI] [PubMed] [Google Scholar]
- Kim J., Ljungdahl P. O., Fink G. R. kem mutations affect nuclear fusion in Saccharomyces cerevisiae. Genetics. 1990 Dec;126(4):799–812. doi: 10.1093/genetics/126.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerly W., Buchman A., Kornberg R., Rine J. Roles of two DNA-binding factors in replication, segregation and transcriptional repression mediated by a yeast silencer. EMBO J. 1988 Jul;7(7):2241–2253. doi: 10.1002/j.1460-2075.1988.tb03064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipling D., Kearsey S. E. Analysis of expression of hybrid yeast genes containing ARS elements. Mol Gen Genet. 1989 Sep;218(3):531–535. doi: 10.1007/BF00332420. [DOI] [PubMed] [Google Scholar]
- Kipling D., Kearsey S. E. Reversion of autonomously replicating sequence mutations in Saccharomyces cerevisiae: creation of a eucaryotic replication origin within procaryotic vector DNA. Mol Cell Biol. 1990 Jan;10(1):265–272. doi: 10.1128/mcb.10.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D., Kent J. C., Hartwell L. H. Genetic analysis of the mitotic transmission of minichromosomes. Cell. 1985 Feb;40(2):393–403. doi: 10.1016/0092-8674(85)90153-9. [DOI] [PubMed] [Google Scholar]
- Lemontt J. F., Fugit D. R., Mackay V. L. Pleiotropic Mutations at the TUP1 Locus That Affect the Expression of Mating-Type-Dependent Functions in SACCHAROMYCES CEREVISIAE. Genetics. 1980 Apr;94(4):899–920. doi: 10.1093/genetics/94.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louarn J., Bouché J. P., Patte J., Louarn J. M. Genetic inactivation of topoisomerase I suppresses a defect in initiation of chromosome replication in Escherichia coli. Mol Gen Genet. 1984;195(1-2):170–174. doi: 10.1007/BF00332741. [DOI] [PubMed] [Google Scholar]
- Lowenhaupt K., Sander M., Hauser C., Rich A. Drosophila melanogaster strand transferase. A protein that forms heteroduplex DNA in the absence of both ATP and single-strand DNA binding protein. J Biol Chem. 1989 Dec 5;264(34):20568–20575. [PubMed] [Google Scholar]
- Maine G. T., Sinha P., Tye B. K. Mutants of S. cerevisiae defective in the maintenance of minichromosomes. Genetics. 1984 Mar;106(3):365–385. doi: 10.1093/genetics/106.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermod N., O'Neill E. A., Kelly T. J., Tjian R. The proline-rich transcriptional activator of CTF/NF-I is distinct from the replication and DNA binding domain. Cell. 1989 Aug 25;58(4):741–753. doi: 10.1016/0092-8674(89)90108-6. [DOI] [PubMed] [Google Scholar]
- Morrison A., Araki H., Clark A. B., Hamatake R. K., Sugino A. A third essential DNA polymerase in S. cerevisiae. Cell. 1990 Sep 21;62(6):1143–1151. doi: 10.1016/0092-8674(90)90391-q. [DOI] [PubMed] [Google Scholar]
- Mosig G. The essential role of recombination in phage T4 growth. Annu Rev Genet. 1987;21:347–371. doi: 10.1146/annurev.ge.21.120187.002023. [DOI] [PubMed] [Google Scholar]
- Newlon C. S. Yeast chromosome replication and segregation. Microbiol Rev. 1988 Dec;52(4):568–601. doi: 10.1128/mr.52.4.568-601.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M., Suzuki Y., Nogi Y., Matsumoto K., Fukasawa T. Yeast Gal11 protein mediates the transcriptional activation signal of two different transacting factors, Gal4 and general regulatory factor I/repressor/activator site binding protein 1/translation upstream factor. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5373–5377. doi: 10.1073/pnas.87.14.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill E. A., Fletcher C., Burrow C. R., Heintz N., Roeder R. G., Kelly T. J. Transcription factor OTF-1 is functionally identical to the DNA replication factor NF-III. Science. 1988 Sep 2;241(4870):1210–1213. doi: 10.1126/science.3413485. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Pickett G. G., Kogoma T., Kornberg A. RNase H confers specificity in the dnaA-dependent initiation of replication at the unique origin of the Escherichia coli chromosome in vivo and in vitro. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1040–1044. doi: 10.1073/pnas.81.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijn J. M., van der Vliet P. C., Dathan N. A., Mattaj I. W. Anti-OTF-1 antibodies inhibit NFIII stimulation of in vitro adenovirus DNA replication. Nucleic Acids Res. 1989 Mar 11;17(5):1845–1863. doi: 10.1093/nar/17.5.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Shore D., Nasmyth K. Purification and cloning of a DNA binding protein from yeast that binds to both silencer and activator elements. Cell. 1987 Dec 4;51(5):721–732. doi: 10.1016/0092-8674(87)90095-x. [DOI] [PubMed] [Google Scholar]
- Sinha P., Chang V., Tye B. K. A mutant that affects the function of autonomously replicating sequences in yeast. J Mol Biol. 1986 Dec 20;192(4):805–814. doi: 10.1016/0022-2836(86)90030-6. [DOI] [PubMed] [Google Scholar]
- Snyder M., Buchman A. R., Davis R. W. Bent DNA at a yeast autonomously replicating sequence. Nature. 1986 Nov 6;324(6092):87–89. doi: 10.1038/324087a0. [DOI] [PubMed] [Google Scholar]
- Snyder M., Sapolsky R. J., Davis R. W. Transcription interferes with elements important for chromosome maintenance in Saccharomyces cerevisiae. Mol Cell Biol. 1988 May;8(5):2184–2194. doi: 10.1128/mcb.8.5.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srienc F., Bailey J. E., Campbell J. L. Effect of ARS1 mutations on chromosome stability in Saccharomyces cerevisiae. Mol Cell Biol. 1985 Jul;5(7):1676–1684. doi: 10.1128/mcb.5.7.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. The current status and portability of our sequence handling software. Nucleic Acids Res. 1986 Jan 10;14(1):217–231. doi: 10.1093/nar/14.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Stinchcomb D. T., Mann C., Davis R. W. Centromeric DNA from Saccharomyces cerevisiae. J Mol Biol. 1982 Jun 25;158(2):157–190. doi: 10.1016/0022-2836(82)90427-2. [DOI] [PubMed] [Google Scholar]
- Suzuki M. SPXX, a frequent sequence motif in gene regulatory proteins. J Mol Biol. 1989 May 5;207(1):61–84. doi: 10.1016/0022-2836(89)90441-5. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Nogi Y., Abe A., Fukasawa T. GAL11 protein, an auxiliary transcription activator for genes encoding galactose-metabolizing enzymes in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Nov;8(11):4991–4999. doi: 10.1128/mcb.8.11.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweder K. S., Rhode P. R., Campbell J. L. Purification and characterization of proteins that bind to yeast ARSs. J Biol Chem. 1988 Nov 25;263(33):17270–17277. [PubMed] [Google Scholar]
- Thrash-Bingham C., Fangman W. L. A yeast mutation that stabilizes a plasmid bearing a mutated ARS1 element. Mol Cell Biol. 1989 Feb;9(2):809–816. doi: 10.1128/mcb.9.2.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbly R. J. Isolation of Saccharomyces cerevisiae mutants constitutive for invertase synthesis. J Bacteriol. 1986 Jun;166(3):1123–1127. doi: 10.1128/jb.166.3.1123-1127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umek R. M., Linskens M. H., Kowalski D., Huberman J. A. New beginnings in studies of eukaryotic DNA replication origins. Biochim Biophys Acta. 1989 Jan 23;1007(1):1–14. doi: 10.1016/0167-4781(89)90123-1. [DOI] [PubMed] [Google Scholar]
- Verrijzer C. P., Kal A. J., Van der Vliet P. C. The DNA binding domain (POU domain) of transcription factor oct-1 suffices for stimulation of DNA replication. EMBO J. 1990 Jun;9(6):1883–1888. doi: 10.1002/j.1460-2075.1990.tb08314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S. S., Francesconi S. C., Tye B. K., Eisenberg S. The OBF1 protein and its DNA-binding site are important for the function of an autonomously replicating sequence in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Jul;9(7):2914–2921. doi: 10.1128/mcb.9.7.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]


