Abstract
Gait freezing is an episodic arrest of locomotion due to an inability to take normal steps. Pedunculopontine nucleus stimulation is an emerging therapy proposed to improve gait freezing, even where refractory to medication. However, the efficacy and precise effects of pedunculopontine nucleus stimulation on Parkinsonian gait disturbance are not established. The clinical application of this new therapy is controversial and it is unknown if bilateral stimulation is more effective than unilateral. Here, in a double-blinded study using objective spatiotemporal gait analysis, we assessed the impact of unilateral and bilateral pedunculopontine nucleus stimulation on triggered episodes of gait freezing and on background deficits of unconstrained gait in Parkinson’s disease. Under experimental conditions, while OFF medication, Parkinsonian patients with severe gait freezing implanted with pedunculopontine nucleus stimulators below the pontomesencephalic junction were assessed during three conditions; off stimulation, unilateral stimulation and bilateral stimulation. Results were compared to Parkinsonian patients without gait freezing matched for disease severity and healthy controls. Pedunculopontine nucleus stimulation improved objective measures of gait freezing, with bilateral stimulation more effective than unilateral. During unconstrained walking, Parkinsonian patients who experience gait freezing had reduced step length and increased step length variability compared to patients without gait freezing; however, these deficits were unchanged by pedunculopontine nucleus stimulation. Chronic pedunculopontine nucleus stimulation improved Freezing of Gait Questionnaire scores, reflecting a reduction of the freezing encountered in patients’ usual environments and medication states. This study provides objective, double-blinded evidence that in a specific subgroup of Parkinsonian patients, stimulation of a caudal pedunculopontine nucleus region selectively improves gait freezing but not background deficits in step length. Bilateral stimulation was more effective than unilateral.
Keywords: Parkinson’s disease, gait freezing, deep brain stimulation, pedunculopontine nucleus
Introduction
Gait freezing is an episodic arrest of forward progress in locomotion due to an inability to take normal steps (Giladi and Nieuwboer, 2008). It is a common, intrusive feature of Parkinsonian disorders, which causes falls and diminishes quality of life (Giladi et al., 2001; Moore et al., 2007; Kerr et al., 2010). Gait freezing is only partially and often poorly responsive to levodopa and subthalamic nucleus stimulation (Bloem et al., 2004; Ferraye et al., 2008). Pedunculopontine nucleus stimulation is proposed to improve gait freezing, even when resistant to medication (Mazzone et al., 2005; Plaha and Gill, 2005). However, the precise effects of pedunculopontine nucleus stimulation on Parkinsonian gait disturbance are not yet established (Peppe et al., 2010). The clinical application of this new treatment is controversial and basic questions remain regarding patient selection, targeting and whether bilateral stimulation is better than unilateral (Stefani et al., 2007; Zrinzo et al., 2007; Ferraye et al., 2009; Moro et al., 2010; Thevathasan et al., 2011a).
In this double-blinded study, we assessed spatiotemporal aspects of gait in Parkinsonian patients with severe gait freezing implanted with pedunculopontine nucleus stimulators and compared results to those of Parkinsonian patients without gait freezing and healthy controls. We assessed the impact of unilateral and bilateral pedunculopontine nucleus stimulation on triggered episodes of gait freezing as well as on background deficits of gait.
Subjects and methods
Subjects and clinical assessments
Three subject groups were assessed: (i) seven patients with Parkinson’s disease complicated by severe freezing of gait, chronically implanted with bilateral pedunculopontine nucleus stimulators (PD-FOG group); (ii) eight patients with Parkinson’s disease of akinetic/rigid subtype without significant gait freezing (Parkinson’s disease control group); and (iii) nine age-matched healthy controls. For patients in the PD-FOG group, an inclusion criterion was the presence of clinically evident gait freezing at baseline during experiments, so that freezing related deficits could be accurately captured and to avoid the introduction of floor effects (see Supplementary Material and ‘Discussion’ section). In Parkinson’s disease controls, gait freezing was considered absent based on a screening history, corroborated by the ‘never freezing’ response on the Freezing of Gait Questionnaire and finally, by a lack of clinically evident freezing during experiments. Parkinson’s disease controls were considered akinetic/rigid in subtype based on a predominance of bradykinetic features and absent or only mild tremor, consistent with previous criteria (Selikhova et al., 2009). Patients with Parkinson’s disease were matched for age, disease duration, motor severity and cognitive status. Subjects were recruited from centres in Oxford, England and Brisbane, Australia. Ethics committee approval was obtained from both centres and participants gave written informed consent.
Seventeen patients with Parkinson’s disease had received pedunculopontine nucleus stimulators from the study centres at the time of experiments. Patients with Parkinson’s disease were selected for pedunculopontine nucleus stimulation because of severe gait freezing and postural instability persisting even ON medication, causing frequent falls. The persistence of these deficits despite adequate dopaminergic medication was determined clinically, including by examination in a practically defined ON medication state. This was the dominant symptomatic issue at surgery and motor fluctuations, if present, were not severe. In Parkinson’s disease, gait freezing becomes more common and less medication responsive with disease progression (Giladi et al., 2001; Bloem et al., 2004). The overall prevalence of gait freezing in Parkinson’s disease is ∼50% (Macht et al., 2007). However, severe ON medication gait freezing as the predominant issue is unusual in Parkinson’s disease (Factor, 2008; Jankovic, 2008). As there is no definitive test for Parkinson’s disease in life, we stress that the diagnosis of Parkinson’s disease in this study is presumptive.
Of the 17 patients implanted with pedunculopontine nucleus stimulators at the study centres, six were not recruited due to death (one patient), living overseas or out of state (two patients), unilateral stimulation (as analyses employed within-subject comparisons) (one patient), stimulation still under titration (one patient), deep brain stimulation system explanted due to therapeutic failure (one patient). Eleven remaining patients implanted with pedunculopontine nucleus stimulators were recruited, four of whom were later excluded; two were unable to perform experimental tasks when OFF medication (either off or on pedunculopontine nucleus stimulation) due to severe akinesia and two in whom gait freezing could not be provoked OFF medication and off stimulation. Of the seven patients in the PD-FOG group ultimately assessed, clinical outcomes (using unblinded rating scales) of four and reaction times of six are previously reported (Thevathasan et al., 2011a, b) Nine control patients with Parkinson’s disease were recruited and one excluded when freezing unexpectedly emerged when OFF medication during experiments.
Patients in the PD-FOG group were receiving bilateral stimulation to the caudal pedunculopontine nucleus region. One patient was also receiving subthalamic nucleus stimulation (switched off for experiments). No other patients had received surgery to any other brain target. Surgical implantation of the pedunculopontine nucleus from both centres is described previously (Pereira et al., 2008; Thevathasan et al., 2011a). Figure 1 demonstrates the stimulation locations (midpoint between active contacts for bipolar stimulation and cathodes for monopolar). Contacts were identified on postoperative computerized tomography fused with preoperative MRI and transformed onto Montreal Neurological Institute space using the fMRIB Software Library (Smith et al., 2004). Using local landmarks as described previously (Ferraye et al., 2009), coordinates were calculated as follows; laterality from midline (mean 7.1 mm, range 4.6–9 mm), ventrodorsal distance (d) from floor of the fourth ventricle (mean 5.8 mm, range 4.1–7.4 mm) and rostro-caudal distance (h) from a pontomesencephalic line connecting the pontomesencephalic junction to the inferior colliculi caudal margin (mean −5.3 mm, range −2.2 to −8.0 mm). In Montreal Neurological Institute space, the coordinates relative to the anterior commissure for the average stimulation location, were as follows: X = 7.1 mm, Y = −32 mm, Z = −22 mm. The relative location/extent of the pedunculopontine nucleus has been outlined, based on choline-acetyltransferase immunohistochemical (ChAT5) staining in the human (Mesulam et al., 1989; Manaye et al., 1999). Stimulation parameters were as follows: frequency 35 Hz (except one patient, 40 Hz), voltage range 2.2–4.3 V and pulse width 60 µs.
Figure 1.
Localization of stimulation locations (coloured dots) represented in Montreal Neurological Institute (MNI) space (sagittal and coronal views). The relative location/extent of the pedunculopontine nucleus has been outlined on the sagittal view, based on choline-acetyltransferase immunohistochemical (ChAT5) staining in the human. Coordinates were calculated in millimetres from midline (laterality), ventrodorsal distance (d) from floor of the fourth ventricle and rostro-caudal distance (h) from a pontomesencephalic line connecting the pontomesencephalic junction to the inferior colliculi caudal margin, as described previously (Ferraye et al., 2009). The mean (ranges) of these stimulation site coordinates were as follows: laterality 7.1 mm (4.6–9 mm), ventrodorsal distance (d) 5.8 mm (4.1–7.4 mm), rostro-caudal distance (h) −5.3 mm (−2.2 to −8.0 mm). In Montreal Neurological Institute space, the coordinates relative to the anterior commissure for the average stimulation location, was as follows; X = 7.1 mm, Y = −32 mm, Z = −22 mm. PM = ponto-mesencephalic line connecting the pontomesencephalic junction to the caudal end of the inferior colliculi; SC = superior colliculus; IC = inferior colliculus.
Clinical assessments included the motor subsection of the Unified Parkinson’s Disease Rating Scale (UPDRS, score/108), rated unblinded by the same neurologist specialized in movement disorders (W.T.) at both centres. UPDRS was segmented into items 27–30 (IT27/30, score/16) assessing posture, gait and balance and residual items 1–26 (R-UPDRS, score/92) assessing bradykinesia, rigidity and tremor. Patients prospectively completed the Gait and Falls Questionnaire (score/64), which assesses Parkinsonian freezing, festination and falls (Giladi et al., 2000). The Freezing of Gait Questionnaire (score/24) and Falls Question (score/4) are components of the Gait and Falls Questionnaire (Giladi et al., 2000, 2009). These questionnaires were administered 1 day prior to surgery and on the day of experiments and reflected function in patients’ usual environments and medication states in the preceding weeks. Cognition was assessed with the Mini-Mental State Examination (score/30).
Clinical details of the study participants are shown in Tables 1 and 2. PD-FOG, Parkinson’s disease control and healthy control groups were not significantly different in age [F(2,21) = 0.317, P = 0.732]. PD-FOG and Parkinson’s disease control patients did not differ with respect to disease duration [t(13) = −0.053, P = 0.958], R-UPDRS subscore [t(12) = 0.570, P = 0.579] or Mini-Mental State Examination [t(11) = −0.416, P = 0.686]. Patients in the PD-FOG group had higher scores in IT27/30 [t(12) = −5.543, P < 0.001], Gait and Falls Questionnaire [t(13) = −9.212, P < 0.001], Freezing of Gait Questionnaire [t(12) = −10.240, P = 0.001] and Falls Question [t(12) = −10.223, P < 0.001].
Table 1.
Baseline characteristics, mean (SD)
| Age (years) | Sex | Parkinson’s disease duration (years) | MMSE | R-UPDRS OFF meds/stim | IT27-30 OFF meds/stim | GFQ | FOGQ | FallsQ | |
|---|---|---|---|---|---|---|---|---|---|
| Healthy controls | 67.3 (8.3) | 7M, 2F | |||||||
| Parkinson’s disease controls | 64.4 (6.1) | 5M, 3F | 11.9 (3.4) | 29.2 (1.0) | 29.4 (9.5) | 2.9 (1.6) | 3.8 (3.7) | 1.9 (1.9) | 0.4 (0.7) |
| PD FOG | 66.9 (9.6) | 5M, 2F | 12.0 (5.5) | 28.9 (1.6) | 26.7 (8.3) | 8.3 (2.1)* | 44.9 (12.0)* | 19.7 (4.5)* | 3.8 (0.4)* |
Questionnaire scores for PD FOG patients are preoperative. *Different from Parkinson's disease controls, P ≤ 0.001. R-UPDRS = items 1–26 of Unified Parkinson’s disease rating scale part III, assessing akinesia, rigidity and tremor (score/92). IT27-30 = items 27–30 of Unified Parkinson’s disease rating scale part III, assessing gait, posture and balance (score/16). For all motor scales, higher scores indicate worse function. MMSE = Mini-Mental State Examination (score/30), with lower scores indicating worse function. For one patient with PD FOG, preoperative FOGQ scores were missing (see Table 2). For Parkinson’s disease controls, UPDRS in one patient and MMSE in two patients were not tested.
GFQ = Gait and Falls Questionnaire (score/64); FOGQ = Freezing of Gait Questionnaire (score/24); FallsQ = Falls Question (score/4); MMSE = Mini-Mental State Examination (score/30).
Table 2.
Patients in the PD FOG group
| Patient | Age/sex | Parkinson’s disease duration (years) | Post-op duration (years, months) | Levodopa dose equivalent (mg/day) | UPDRS III OFF/ON meds (off stim) | IT27-30 off/on stim (OFF meds) | GFQ pre/ post-op | FOGQ pre/ post-op | FallsQ pre/ post-op | Supportive for UK brain bank criteriaa |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 61F | 10 | 2 | 800 | 40/23 | 10/9 | 61/36 | 24/16 | 4/3 | D, A, P |
| 2 | 72M | 18 | 2, 5 | 2500 | 25/17 | 6/6 | 30/16 | 14/11 | 4/2 | D, A, T, P |
| 3 | 76M | 6 | 2 | 600 | 26/14 | 6/4 | 51/18 | 22/7 | 3/3 | A, P |
| 4 | 72F | 10 | 2 | 950 | 38/22 | 11/8 | 48/26 | 22/13 | 4/2 | D, A, T, P |
| 5 | 77M | 6 | 0, 6 | 1400 | 31/17 | 10/10 | 31/14 | ^/6 | ^/2 | A, P |
| 6 | 55M | 20 | 1 | 850 | 51/19 | 8/6 | 38/40 | 14/15 | 4/4 | D, A, T, P |
| 7 | 55M | 14 | 0, 2 | 1650 | 34/24 | 7/4 | 55/37 | 22/16 | 4/4 | A, P |
Postoperative clinical assessments were performed on the same day as gait analysis. Patients 6 and 7 were from Oxford, other patients from Brisbane. Patient 6 also had subthalamic nucleus stimulators, which were turned off during experiments.
a Additional to disease duration and levodopa response as documented elsewhere in the table. Reaction time data of Patients 1–6 and 2-year clinical scores of Patients 1–4 have been reported previously.
^ = not known; Key to UK Brain bank criteria: D = dyskinesias; A = asymmetry persistent; T = tremor at rest; P = progressive disease course; UPDRS III = part III (motor) Unified Parkinson’s disease rating scale (score/108); IT27-30 = items 27–30 of Unified Parkinson’s disease rating scale, assessing gait, posture and balance (score/16); GFQ = Gait and Falls Questionnaire (score/64); FOGQ = Freezing of Gait Questionnaire (score/24); FallsQ = Falls Question (score/4).
Experiments
Assessments were performed after overnight withdrawal of dopaminergic medication and after 12 h pedunculopontine nucleus stimulation washout.
PD-FOG patients were assessed during four conditions, presented in counterbalanced order (using the Latin square method): off pedunculopontine nucleus stimulation, bilateral pedunculopontine nucleus stimulation, left pedunculopontine nucleus stimulation and right pedunculopontine nucleus stimulation. Patients were blinded to condition. The mean of left and right unilateral stimulation results was used in analyses. Choice of contacts and stimulation parameters were as employed for chronic therapy. After changing stimulation, a 30-min wash-in period was enforced between conditions. On questioning at the conclusion of the wash-in period, patients were unable to detect the condition of stimulation better than chance.
Data were acquired with an 8.3 -m long electronic walkway (GAITRite, CIR Systems Inc.), which detected footsteps through embedded pressure sensors (Bilney et al., 2003). GAITRite has been validated to assess spatiotemporal parameters of gait in health and Parkinson’s disease (Bilney et al., 2003; Chien et al., 2006).
All participants performed two tasks, presented in counterbalanced order:
Turn task: this aimed to capture gait freezing, known to be precipitated by turning and tight spaces (Okuma, 2006; Almeida and Lebold, 2010). Subjects walked to a central marker placed on the surface two-thirds down the walkway, turned 180° around this marker and returned to the starting position. In sequential trials, patients alternately turned left and right. Patients were confined to a turning arc limited by the width of the electronic walkway (70 cm), which was placed in a narrow corridor 1.4 m wide (either pre-existing or created by a movable screen).
Straight task: this aimed to capture background deficits of gait. Subjects walked at self-selected speed down the centre of the walkway. Distractions were minimized and subjects were requested not to talk. The walkway was positioned to record established walking and not gait initiation or slowing down towards destination.
Patients performed four trials per task and the mean result used in analyses. Trials with falls were discarded and repeated. During experiments, one researcher (W.T.) supervised proceedings, observed for the presence or absence of gait freezing in patients with Parkinson’s disease, monitored patient safety (including following discretely behind patients during trials in case of falls) and altered stimulation. A second, blinded researcher operated the GAITRite system and tagged the data according to the order of condition. Offline, blinded researchers computed the parameters, including manually deriving the primary end-points (see below). Conditions of stimulation were then revealed to permit statistical analysis.
Parameters and data analysis
The primary outcome measure was gait freezing severity as quantified by task duration (s) and cadence (steps/min) during turning. Freezing was not clinically scored during tasks. The turn task parameters were assessed manually by researchers, blinded to condition, as follows. The 180° arc of the turn was selected for assessment by the appearance of footsteps at the marker region. Foot-strike was visually identified, frame by frame, so that task duration and cadence could be derived for every trial. This method could not detect any high frequency attempts at stepping that did not alter foot position as reported previously in gait freezing (Hausdorff et al., 2003a; Spildooren et al., 2010). Here cadence pertained to successful stepping and reflected a fundamental feature of gait freezing—a deficiency in steps that alter position (Giladi and Nieuwboer, 2008). Turn task duration was considered a global measure of functional limitation from freezing when compared to control subjects. For the straight task, mean cadence, mean step length and step length standard deviation (SD) were computed automatically by GAITRite software. Step length coefficients of variation were then calculated.
Statistics
The Kolmogorov–Smirnov Test demonstrated that parameters during turning were unlikely to be normally distributed. Log transformed data of all parameters were normally distributed and used in analyses. Level of significance was P < 0.05.
Differences between subject groups were assessed with ANOVA and post hoc independent samples t-tests. Two such ANOVAs were performed, one with PD-FOG patients off stimulation and one with PD-FOG patients on bilateral stimulation. In the PD-FOG group, differences between stimulation conditions were assessed with repeated measures ANOVA and post hoc paired t-tests. Post hoc tests were corrected for multiple comparisons using the False Discovery Rate Procedure (Bejamini and Hochberg, 1995)
Results
Patients in the PD-FOG group experienced clinically visible gait freezing episodes only when turning and not during the straight walking task.
Primary outcome: gait freezing during turning
Turn task duration
Turn task duration (Fig. 2A) differed between PD-FOG patients off stimulation, Parkinson’s disease controls and healthy subjects [F(2,21) = 61.213, P < 0.001]. Post hoc tests revealed that turn task duration was greater in PD-FOG patients off stimulation than in Parkinson’s disease controls [mean 31.1 s PD-FOG versus 2.7 s Parkinson’s disease controls, t(13) = 7.223, P < 0.001] and healthy controls [2.3 s, t(14) = 7.627, P < 0.001]. Pedunculopontine nucleus stimulation did not return this measure to normal, so that turn task duration in PD-FOG patients on bilateral stimulation (mean 11 s), although improved, remained different to the other subject groups [F(2,21) = 29.066, P < 0.001].
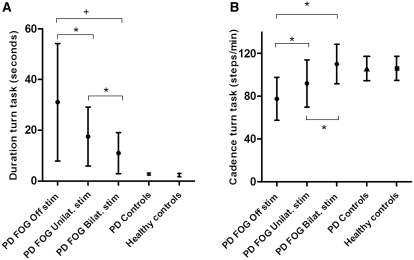
Figure 2.
Gait analysis parameters (means ± SD) recorded when turning in a tight space, a task that precipitated visible gait freezing in PD-FOG patients but not in Parkinson’s disease controls or healthy controls. Results in PD-FOG patients are grouped according to stimulation condition: off stimulation, unilateral stimulation and bilateral stimulation. Unilateral stimulation results are the grand averages of the means of stimulating each side in each patient. (A) Turn task duration (s). (B) Turn task cadence (steps/min). Differences within the PD-FOG group are indicated by bridges: +P < 0.01, *P < 0.05. Compared to the off stimulation state, bilateral stimulation improved both parameters more than unilateral stimulation. Differences between groups were as follows: (i) turn task duration was longer for PD-FOG patients during all stimulation conditions than in either control group (P < 0.001); (ii) turn task cadence was smaller for PD-FOG patients when off stimulation and with unilateral stimulation than either control group (P < 0.05) but did not differ between PD-FOG patients when on bilateral stimulation and control groups (P = 0.882).
In PD-FOG, turn task duration differed between stimulation conditions [F(1,6) = 16.825, P < 0.001; Fig. 2A]. Post hoc tests revealed that compared with off stimulation, turn task duration reduced with bilateral stimulation [mean 31.1–11.0 s, t(6) = −5.053, P = 0.006] and unilateral stimulation [to 17.5 s, t(6) = 3.068, P = 0.022]. Bilateral stimulation reduced turn task duration more than unilateral stimulation [t(6) = −3.308, P = 0.032]. Percentage improvement of turn task duration with bilateral stimulation was also greater than unilateral stimulation [57.9 versus 35.5%, t(6) = 2.924, P = 0.026].The impact of unilateral stimulation was not influenced by the direction of turning that provoked freezing [19.2 s ipsilateral turning versus 14.4 s contralateral turning, t(6) = 0.729, P = 0.494].
Cadence
Cadence during turning differed between PD-FOG patients off stimulation, Parkinson’s disease controls and healthy subjects [F(2,21) = 9.885, P = 0.001]. Post hoc tests revealed a deficit in cadence during turning in PD-FOG patients off stimulation compared with Parkinson’s disease controls [mean cadence 77.6 steps/min PD-FOG versus 105.9 steps/min Parkinson’s disease controls, t(13) = −3.093, P = 0.032] and healthy controls [106.1 steps/min, t(14) = −3.132, P = 0.032]. With PD-FOG patients on bilateral stimulation, cadence during turning no longer differed between subject groups [F(2,21) = 0.126, P = 0.882].
In PD-FOG, cadence during turning differed between stimulation conditions [F(2,12) = 16.599, P < 0.001; Fig. 2B]. Post hoc tests revealed that compared with off stimulation, turning cadence increased with bilateral stimulation [77.6–110.1 steps/min, t(6) = −4.633, P = 0.012] and unilateral stimulation [to 91.9 steps/min, t(6) = −3.987, P = 0.014]. Bilateral stimulation increased cadence during turning more than unilateral stimulation [t(6) = 3.050, P = 0.023]. Percentage improvements of cadence were also greater with bilateral than unilateral stimulation [47.4 versus 19.7%, t(6) = 2.590, P = 0.041].The impact of unilateral stimulation on turning cadence was not influenced by the direction of turning that provoked freezing [89.4 steps/min ipsilateral turning versus 97.2 steps/min contralateral turning, t(6) = −1.215, P = 0.270].
Secondary outcomes
Unconstrained walking: straight task parameters
With PD-FOG patients off stimulation, there were significant differences between subject groups in step length [F(2,21) = 31.190, P < 0.001] and step length coefficient of variation [F(2,21) = 15.298, P < 0.001]. Cadence did not differ between subject groups [F(2,21) = 0.229, P = 0.797]. Post hoc tests revealed a deficit in step length in the PD-FOG group off stimulation compared with Parkinson’s disease controls [mean 34.9 cm PD-FOG versus 59.8 cm Parkinson’s disease controls, t(13) = 4.987, P = 0.002] and healthy controls [65.5 cm, t(14) = 5.874, P = 0.002]. Step length coefficient of variation was greater in patients in the PD-FOG group off stimulation than Parkinson’s disease controls [mean 0.09 cm PD-FOG versus 0.03 cm Parkinson’s disease controls, t(13) = −3.509, P = 0.004] and healthy controls [0.02 cm, t(14) = 5.947, P = 0.009]. These group differences remained with patients in the PD-FOG group on stimulation (Table 3).
Table 3.
Straight task outcomes for the three subject groups, including patients in the PD-FOG group in all conditions of stimulation, mean (SD)
| Step length (cm) | Step length CoV (cm) | Cadence (steps/min) | |
|---|---|---|---|
| Healthy controls | 65.5 (6.8) | 0.02 (0.01) | 114.2 (10.6) |
| Parkinson’s disease Controls | 59.8 (6.3) | 0.03 (0.01) | 117.0 (6.9) |
| PD FOG Off DBS | 34.9 (9.6)a | 0.09 (0.04)a | 116.9 (12.4) |
| PD FOG Unilateral DBS | 36.1 (8.6)a | 0.09 (0.06)a | 123.6 (9.9) |
| PD FOG Bilateral DBS | 38.7 (7.0)a | 0.09 (0.05)a | 121.5 (13.0) |
a PD FOG different to Parkinson’s disease controls and healthy controls, P < 0.01.
CoV = coefficient of variation; DBS = deep brain stimulation.
In the PD-FOG group, a multivariate ANOVA revealed no differences between stimulation conditions during the straight task in step length [F(2,12) = 1.074, P = 0.372], step length coefficient of variation [F(2,12) = 0.215, P = 0.810] or cadence [F(2,12) = 1.589, P = 0.244].
Falls during recordings
In Parkinson’s disease freezing of gait, falls were recorded in only one patient when turning; five times when off stimulation and a mean of 3.5 times for left and right unilateral stimulation (data from these trials with falls were discarded and trials repeated). No falls occurred during bilateral stimulation or in the straight task.
Pre- and postoperative clinical scores
In patients in the PD-FOG group, chronic pedunculopontine nucleus stimulation improved scores in the Gait and Falls Questionnaire [44.9 versus 26.7, t(6) = 4.422, P = 0.008] and Freezing of Gait Question [19.7 versus 13.0, t(5) = 2.988, P = 0.031] compared with preoperatively (Table 2).
Discussion
Our primary outcome measure was the severity of gait freezing triggered by turning in a tight space under objective, double-blinded experimental conditions. Pedunculopontine nucleus stimulation below the pontomesencephalic junction reduced gait freezing, with bilateral stimulation more effective than unilateral stimulation. During unconstrained walking, Parkinsonian patients who experienced gait freezing had reduced step length and increased step length variability compared to patients without gait freezing, but these deficits were unchanged by pedunculopontine nucleus stimulation.
Before further discussion, the validity of our measures to capture freezing related deficits and quantify freezing needs consideration. Freezing is notorious for disappearing during single-session assessments, which are therefore prone to underestimating the disorder (Giladi and Nieuwboer, 2008). For this reason, we assessed patients OFF medication and employed a strong trigger of freezing; turning in a tight space. Furthermore, we were careful to include only those PD-FOG patients in whom freezing was clinically evident in the baseline condition. A limitation is that freezing was then assessed only with objective spatiotemporal methods and not also with clinical methods (Giladi et al., 2000). Our turn task measures aimed to quantify rather than characterize gait freezing. For example, we could not assess high frequency attempts at stepping that did not substantially displace the feet (Spildooren et al., 2010). Rather, we sought to provide objective measures of functional impairment from gait freezing (turn task duration) and of stepping that could progress position (turn task cadence).
This study contributes objective, double-blinded evidence that pedunculopontine nucleus stimulation can be therapeutic for gait freezing in Parkinson’s disease. To limit ascertainment bias, we recruited all 11 patients receiving established bilateral pedunculopontine nucleus stimulation from the study centres living within a reasonable distance. Of these, two patients could not perform the tasks due to severe OFF medication akinesia, a deficit that appears unresponsive to pedunculopontine nucleus stimulation (Moro et al., 2010). Two patients, apparently successfully treated with pedunculopontine nucleus stimulation, had persistent remission of freezing despite having ceased stimulation for >12 h. This lack of freezing could have reflected the well described phenomenon of freezing improving during medical assessments, thought to result from attentional recruitment (Chee et al., 2009). Alternatively, the lack of freezing could have reflected failure of stimulation ‘washout’, as studies have suggested that therapeutic effects may sometimes persist beyond the period of pedunculopontine nucleus stimulation for up to several days (Ostrem et al., 2010; Thevathasan et al., 2011a). Exclusion of PD-FOG patients without baseline freezing was necessary for freezing related deficits to be accurately captured in the baseline condition and to avoid the introduction of floor effects, whereby the intervention (pedunculopontine nucleus stimulation) could not possibly yield any benefit. Note that this approach differs from ‘enrichment’, a strategy employed by some studies whereby only treatment responsive patients are selected for assessment (Leber and Davis, 1998). Although we excluded the two patients who did not exhibit freezing despite having ceased stimulation for over 12 h, washout effects could still have influenced our results, persisting from either chronic therapy or over the 30-min interval, which could reasonably be provided between conditions. This would tend to bias towards underestimating the impact of pedunculopontine nucleus stimulation. Furthermore, wash-in effects (e.g. delays to reach optimal treatment effects) may also have limited the measured impact of pedunculopontine nucleus stimulation. Even on bilateral stimulation, PD-FOG patients remained substantially impaired during turning relative to controls. It is not clear if continuous pedunculopontine nucleus stimulation for >30 min might yield further benefits or if such improvements found experimentally would enhance quality of life. Such questions call for a randomized clinical trial.
An important constraint is that the outcomes presented here reflect the specific selection criteria, target location and stimulation strategies employed for pedunculopontine nucleus stimulation in this study, which differ in some respects from previous reports (Stefani et al., 2007; Ferraye et al., 2009; Moro et al., 2010). Selected patients were an uncommon subgroup of Parkinson’s disease who experience extremely severe gait freezing, postural instability and falls, persisting even ON medication (as established preoperatively using clinical methods). Severe motor fluctuations were absent, although these later developed in one patient who was then implanted with subthalamic nucleus stimulators, which were switched off for experiments. Thus, our results reflect lone pedunculopontine nucleus stimulation, excluding any interference from stimulation elsewhere (Ferraye et al., 2011). The relative efficacy of differing pedunculopontine nucleus stimulation strategies remains to be objectively examined. Stimulation frequencies employed here (35–40 Hz) were intermediate between those reported as clinically optimal in previous studies, namely 15–25 Hz (Stefani et al., 2007; Ferraye et al., 2009) and 60 Hz (Moro et al., 2010). Stimulation was specifically applied more caudally in the pedunculopontine nucleus region than previous reports, beneath the pontomesencephalic junction. This target was chosen based on the experience of two authors (N.J and T.Z.A.) in applying pedunculopontine nucleus stimulation in the non-human primate model of Parkinson’s disease and on the distribution of cholinergic cells in humans identified by ChAT5 immunohistochemistry (Olszewski and Baxter, 1954; Mesulam et al., 1989; Manaye et al., 1999; Nandi et al., 2002; Jenkinson et al., 2004). Furthermore, at least in animal models, the pedunculopontine nucleus is argued to be topographically organized with the caudal pedunculopontine nucleus subregion being identified as most relevant to locomotor control (Martinez-Gonzalez et al., 2011). However, the limits of anatomical specificity from electrical stimulation must also be acknowledged, particularly in the brainstem. In typical subthalamic nucleus stimulation, electrical fields are estimated to activate axonal elements up to 4 mm from the active contact (McIntyre et al., 2004). On the one hand, such a broad field of influence may allow locomotor relevant pedunculopontine nucleus neurons to be activated despite some variability in electrode location. Equally, however, the effects of stimulation in this region could actually result from pedunculopontine nucleus projections or even surrounding nuclei, some of which are also implicated in locomotor control (Orlovskii et al., 1966; Zrinzo et al., 2007; Piallat et al., 2009).
The relative efficacy of unilateral versus bilateral pedunculopontine nucleus stimulation has been controversial. Given the state of equipoise, some have elected to implant unilaterally, given the greater risks inherent in bilateral implantation (Moro et al., 2010). However, in an experimental setting, we found that bilateral pedunculopontine nucleus stimulation improved OFF medication gait freezing approximately twice as much as unilateral stimulation (in terms of percentage improvements). We did not find that the effectiveness of unilateral pedunculopontine nucleus stimulation was influenced by the direction of turning that triggered freezing. Thus, we cannot explain the greater impact of bilateral stimulation by a unilateral effect of unilateral stimulation.
During unconstrained walking, patients with Parkinson’s disease freezing of gait had reduced step length and increased step length variability compared to well-matched Parkinson’s disease controls without gait freezing. Thus, these background deficits, unless due to the pedunculopontine nucleus electrodes or failed stimulation washout, appear associated with gait freezing and corroborate findings from previous studies (Hausdorff et al., 2003b; Chee et al., 2009; Snijders et al., 2011). Although we did not clinically observe gait freezing during straight task trials, the abnormalities of step length could still reflect covert freezing interrupting the smooth execution of gait. Against this, cadence was not abnormal in PD-FOG patients during straight walking and the step length deficits did not improve with pedunculopontine nucleus stimulation despite improvements in triggered freezing. The step length deficits could simply be epiphenomenal to gait freezing. However, previous studies have found that gait freezing episodes are commonly preceded by a sequential reduction in step length—a deficit that would account for the increased step length variability in our PD-FOG patients (Nieuwboer et al., 2001; Chee et al., 2009). Furthermore, small steps, deliberately taken, can trigger freezing (Chee et al., 2009). Step length along with other manifestations of akinesia, are potentially responsive to levodopa and subthalamic nucleus stimulation—suggesting a potential mechanism by which these therapies can improve ‘OFF medication freezing’ (Faist et al., 2001). However, we found that pedunculopontine nucleus stimulation did not improve step length or its variability, supporting the proposition that pedunculopontine nucleus stimulation may improve gait freezing through alternative, potentially complementary, pathways (Jenkinson et al., 2006; Thevathasan et al., 2011b).
Funding
NIHR Oxford Biomedical Research Centre; Medical Research Council (UK); Melbourne Brain Centre University of Melbourne Fellowship (to W.T.); Queensland University of Technology; Queensland Government International Fellowship (to G.K.) and Medtronic (honoraria to T.Z.A., P.B., P.A.S. and T.J.C.).
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- PD-FOG
patients with Parkinson’s disease with freezing of gait and implanted with bilateral pedunculopontine nucleus stimulators
- UPDRS
Unified Parkinson’s Disease Rating Scale
References
- Almeida QJ, Lebold CA. Freezing of gait in Parkinson’s disease: a perceptual cause for a motor impairment? J Neurol Neurosurg Psychiatry. 2010;81:513–8. doi: 10.1136/jnnp.2008.160580. [DOI] [PubMed] [Google Scholar]
- Bejamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture. 2003;17:68–74. doi: 10.1016/s0966-6362(02)00053-x. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–84. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- Chee R, Murphy A, Danoudis M, Georgiou-Karistianis N, Iansek R. Gait freezing in Parkinson’s disease and the stride length sequence effect interaction. Brain. 2009;132:2151–60. doi: 10.1093/brain/awp053. [DOI] [PubMed] [Google Scholar]
- Chien SL, Lin SZ, Liang CC, Soong YS, Lin SH, Hsin YL, et al. The efficacy of quantitative gait analysis by the GAITRite system in evaluation of Parkinsonian bradykinesia. Parkinsonism Relat Disord. 2006;12:438–42. doi: 10.1016/j.parkreldis.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Factor SA. The clinical spectrum of freezing of gait in atypical parkinsonism. Mov Disord. 2008;23(Suppl 2):S431–8. doi: 10.1002/mds.21849. [DOI] [PubMed] [Google Scholar]
- Faist M, Xie J, Kurz D, Berger W, Maurer C, Pollak P, et al. Effect of bilateral subthalamic nucleus stimulation on gait in Parkinson’s disease. Brain. 2001;124:1590–600. doi: 10.1093/brain/124.8.1590. [DOI] [PubMed] [Google Scholar]
- Ferraye MU, Debu B, Fraix V, Goetz L, Ardouin C, Yelnik J, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson’s disease. Brain. 2009;133:205–14. doi: 10.1093/brain/awp229. [DOI] [PubMed] [Google Scholar]
- Ferraye MU, Debu B, Fraix V, Krack P, Charbardes S, Seigneuret E, et al. Subthalamic nucleus versus pedunculopontine nucleus stimulation in Parkinson disease: synergy or antagonism? J Neural Transm. 2011;118:1469–75. doi: 10.1007/s00702-011-0673-y. [DOI] [PubMed] [Google Scholar]
- Ferraye MU, Debu B, Fraix V, Xie-Brustolin J, Chabardes S, Krack P, et al. Effects of subthalamic nucleus stimulation and levodopa on freezing of gait in Parkinson disease. Neurology. 2008;70:1431–7. doi: 10.1212/01.wnl.0000310416.90757.85. [DOI] [PubMed] [Google Scholar]
- Giladi N, McDermott MP, Fahn S, Przedborski S, Jankovic J, Stern M, et al. Freezing of gait in PD: prospective assessment in the DATATOP cohort. Neurology. 2001;56:1712–21. doi: 10.1212/wnl.56.12.1712. [DOI] [PubMed] [Google Scholar]
- Giladi N, Nieuwboer A. Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage. Mov Disord. 2008;23(Suppl 2):S423–5. doi: 10.1002/mds.21927. [DOI] [PubMed] [Google Scholar]
- Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism Relat Disord. 2000;6:165–70. doi: 10.1016/s1353-8020(99)00062-0. [DOI] [PubMed] [Google Scholar]
- Giladi N, Tal J, Azulay T, Rascol O, Brooks DJ, Melamed E, et al. Validation of the freezing of gait questionnaire in patients with Parkinson’s disease. Mov Disord. 2009;24:655–61. doi: 10.1002/mds.21745. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Balash Y, Giladi N. Statistical mechanics and its applications: time series analysis of leg movements during freezing of gait in Parkinson’s disease: akinesia, rhyme or reason? Physica A. 2003a;321:565–70. [Google Scholar]
- Hausdorff JM, Schaafsma JD, Balash Y, Bartels AL, Gurevich T, Giladi N. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp Brain Res. 2003b;149:187–94. doi: 10.1007/s00221-002-1354-8. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–76. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Jenkinson N, Nandi D, Miall RC, Stein JF, Aziz TZ. Pedunculopontine nucleus stimulation improves akinesia in a Parkinsonian monkey. Neuroreport. 2004;15:2621–4. doi: 10.1097/00001756-200412030-00012. [DOI] [PubMed] [Google Scholar]
- Jenkinson N, Nandi D, Oram R, Stein JF, Aziz TZ. Pedunculopontine nucleus electric stimulation alleviates akinesia independently of dopaminergic mechanisms. Neuroreport. 2006;17:639–41. doi: 10.1097/00001756-200604240-00016. [DOI] [PubMed] [Google Scholar]
- Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology. 2010;75:116–24. doi: 10.1212/WNL.0b013e3181e7b688. [DOI] [PubMed] [Google Scholar]
- Leber PD, Davis CS. Threats to the validity of clinical trials employing enrichment strategies for sample selection. Control Clin Trial. 1998;19:178–87. doi: 10.1016/s0197-2456(97)00118-9. [DOI] [PubMed] [Google Scholar]
- Macht M, Kaussner Y, Moller JC, Stiasny-Kolster K, Eggert KM, Kruger HP, et al. Predictors of freezing in Parkinson’s disease: a survey of 6,620 patients. Mov Disord. 2007;22:953–6. doi: 10.1002/mds.21458. [DOI] [PubMed] [Google Scholar]
- Manaye KF, Zweig R, Wu D, Hersh LB, De Lacalle S, Saper CB, et al. Quantification of cholinergic and select non-cholinergic mesopontine neuronal populations in the human brain. Neuroscience. 1999;89:759–70. doi: 10.1016/s0306-4522(98)00380-7. [DOI] [PubMed] [Google Scholar]
- Martinez-Gonzalez C, Bolam JP, Mena-Segovia J. Topographical organization of the pedunculopontine nucleus. Front Neuroanat. 2011;5:22. doi: 10.3389/fnana.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone P, Lozano A, Stanzione P, Galati S, Scarnati E, Peppe A, et al. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson’s disease. Neuroreport. 2005;16:1877–81. doi: 10.1097/01.wnr.0000187629.38010.12. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Mori S, Sherman DL, Thakor NV, Vitek JL. Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin Neurophysiol. 2004;115:589–95. doi: 10.1016/j.clinph.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Geula C, Bothwell MA, Hersh LB. Human reticular formation: cholinergic neurons of the pedunculopontine and laterodorsal tegmental nuclei and some cytochemical comparisons to forebrain cholinergic neurons. J Comp Neurol. 1989;283:611–33. doi: 10.1002/cne.902830414. [DOI] [PubMed] [Google Scholar]
- Moore O, Peretz C, Giladi N. Freezing of gait affects quality of life of peoples with Parkinson’s disease beyond its relationships with mobility and gait. Mov Disord. 2007;22:2192–5. doi: 10.1002/mds.21659. [DOI] [PubMed] [Google Scholar]
- Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, Hutchison WD, et al. Unilateral pedunculopontine stimulation improves falls in Parkinson’s disease. Brain. 2010;133:215–24. doi: 10.1093/brain/awp261. [DOI] [PubMed] [Google Scholar]
- Nandi D, Aziz TZ, Giladi N, Winter J, Stein JF. Reversal of akinesia in experimental parkinsonism by GABA antagonist microinjections in the pedunculopontine nucleus. Brain. 2002;125:2418–30. doi: 10.1093/brain/awf259. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, Dom R, De Weerdt W, Desloovere K, Fieuws S, Broens-Kaucsik E. Abnormalities of the spatiotemporal characteristics of gait at the onset of freezing in Parkinson’s disease. Mov Disord. 2001;16:1066–75. doi: 10.1002/mds.1206. [DOI] [PubMed] [Google Scholar]
- Okuma Y. Freezing of gait in Parkinson’s disease. J Neurol. 2006;253(Suppl 7):VII27–32. doi: 10.1007/s00415-006-7007-2. [DOI] [PubMed] [Google Scholar]
- Olszewski J, Baxter D. Cytoarchitecture of the human brain stem. Philadelphia: Lippencott; 1954. [Google Scholar]
- Orlovskii GN, Severin FV, Shik ML. [Locomotion induced by stimulation of the mesencephalon] Dokl Akad Nauk SSSR. 1966;169:1223–6. [PubMed] [Google Scholar]
- Ostrem JL, Christine CW, Glass GA, Schrock LE, Starr PA. Pedunculopontine nucleus deep brain stimulation in a patient with primary progressive freezing gait disorder. Stereotact Funct Neurosurg. 2010;88:51–5. doi: 10.1159/000268742. [DOI] [PubMed] [Google Scholar]
- Peppe A, Pierantozzi M, Chiavalon C, Marchetti F, Caltagirone C, Musicco M, et al. Deep brain stimulation of the pedunculopontine tegmentum and subthalamic nucleus: effects on gait in Parkinson’s disease. Gait Posture. 2010;32:512–8. doi: 10.1016/j.gaitpost.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Pereira EA, Muthusamy KA, De Pennington N, Joint CA, Aziz TZ. Deep brain stimulation of the pedunculopontine nucleus in Parkinson’s disease. Preliminary experience at Oxford. Br J Neurosurg. 2008;22(Suppl 1):S41–4. doi: 10.1080/02688690802448335. [DOI] [PubMed] [Google Scholar]
- Piallat B, Chabardes S, Torres N, Fraix V, Goetz L, Seigneuret E, et al. Gait is associated with an increase in tonic firing of the sub-cuneiform nucleus neurons. Neuroscience. 2009;158:1201–5. doi: 10.1016/j.neuroscience.2008.10.046. [DOI] [PubMed] [Google Scholar]
- Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson’s disease. Neuroreport. 2005;16:1883–7. doi: 10.1097/01.wnr.0000187637.20771.a0. [DOI] [PubMed] [Google Scholar]
- Selikhova M, Williams DR, Kempster PA, Holton JL, Revesz T, Lees AJ. A clinico-pathological study of subtypes in Parkinson’s disease. Brain. 2009;132:2947–57. doi: 10.1093/brain/awp234. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, et al. Gait-related cerebral alterations in patients with Parkinson’s disease with freezing of gait. Brain. 2011;134:59–72. doi: 10.1093/brain/awq324. [DOI] [PubMed] [Google Scholar]
- Spildooren J, Vercruysse S, Desloovere K, Vandenberghe W, Kerckhofs E, Nieuwboer A. Freezing of gait in Parkinson’s disease: the impact of dual-tasking and turning. Mov Disord. 2010;25:2563–70. doi: 10.1002/mds.23327. [DOI] [PubMed] [Google Scholar]
- Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007;130:1596–607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- Thevathasan W, Coyne TJ, Hyam JA, Kerr G, Jenkinson N, Aziz TZ, et al. Pedunculopontine nucleus stimulation improves gait freezing in Parkinson disease. Neurosurgery. 2011a;69:1248–53. doi: 10.1227/NEU.0b013e31822b6f71. [DOI] [PubMed] [Google Scholar]
- Thevathasan W, Pogosyan A, Hyam JA, Jenkinson N, Bogdanovic M, Coyne TJ, et al. A block to pre-prepared movement in gait freezing, relieved by pedunculopontine nucleus stimulation. Brain. 2011b;134:2085–95. doi: 10.1093/brain/awr131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrinzo L, Zrinzo LV, Hariz M. The peripeduncular nucleus: a novel target for deep brain stimulation? Neuroreport. 2007;18:1301–2. doi: 10.1097/WNR.0b013e3282638603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.