Abstract
A fundamental aspect of visual cognition is our disposition to see the ‘forest before the trees’. However, damage to the posterior parietal cortex, a critical brain region along the dorsal visual pathway, can produce a neurological disorder called simultanagnosia, characterized by a debilitating inability to perceive the ‘forest’ but not the ‘trees’ (i.e. impaired global processing despite intact local processing). This impairment in perceiving the global shape persists even though the ventral visual pathway, the primary recognition pathway, is intact in these patients. Here, we enabled global processing in patients with simultanagnosia using a psychophysical technique, which allowed us to bias stimuli such that they are processed predominantly by the intact ventral visual pathway. Our findings reveal that the impairment in global processing that characterizes simultanagnosia stems from a disruption in the processing of low-spatial frequencies through the dorsal pathway. These findings advance our understanding of the relationship between visuospatial attention and perception and reveal the neural mechanism mediating the disposition to see the ‘forest before the trees’.
Keywords: brain circuits, cerebral ischaemia, visual system, dorsal stream, neurological disorders
Introduction
A typical visual scene contains many individual objects. However, the human visual system normally resolves the global properties of a scene first (Fabre-Thorpe et al., 2001) and is able to categorize scenes rapidly, based solely on their gist (Torralba et al., 2006). This phenomenon is known as the ‘global precedence effect’ (Navon, 1977). Individuals who suffer from lesions in the posterior parietal cortex, a major way station of the dorsal visual pathway, present with a condition called simultanagnosia (Luria, 1959; Rafal, 2001). These patients exhibit a profound inability to integrate multiple visual elements within a scene and do not show the global precedence effect (Kinsbourne and Warrington, 1962). They are, nevertheless, able to recognize individual or local elements of a scene normally and show a local precedence effect. Since the parietal cortex plays a critical role in evaluating the spatial relationship between multiple objects (Ungerleider and Haxby, 1994), the deficit of simultanagnosic patients in global recognition has been proposed to emerge from an inability to modulate visuospatial attention (Rizzo and Hurtig, 1987; Rizzo and Robin, 1990; Jackson et al., 2006). However, the ventral visual pathway, which is considered the primary recognition pathway (Ungerleider and Haxby, 1994), is typically intact in simultanagnosic patients and yet does not appear to be sufficient for normal global processing.
In healthy individuals, numerous psychophysical studies have shown that the global precedence effect is mediated by low-spatial frequencies (Shulman et al., 1986; Hughes et al., 1996; Robertson, 1996). For example, when stimuli were presented without low-spatial frequencies, subjects did not show the global precedence effect (Badcock et al., 1990; LaGasse, 1993) and, interestingly, showed a local precedence effect (Hughes et al., 1990). Could the global processing impairment in simultanagnosia therefore be attributed to a disruption in processing low-spatial frequency information? Can global processing be restored in simultanagnosia by biasing the intact ventral pathway to process low-spatial frequencies? What is the mechanism underlying the local precedence effect? To address these questions, we used psychophysical techniques to bias visual processing through the dorsal or ventral visual pathways and examined the mechanism underlying the impairment in global processing in patients with simultanagnosia. We focus on the dorsal and ventral visual pathways because of the unique response properties of neurons in the magnocellular and parvocellular layers of the lateral geniculate nucleus of the thalamus, which contribute significantly to these two pathways.
The magnocellular and parvocellular cells are known to have complementary response properties. The magnocellular cells are less responsive to colour and have high contrast gain, particularly at low luminance contrast (Purpura et al., 1988). Most parvocellular cells, on the other hand, are highly responsive to opposing colours (red–green or blue–yellow) (Lee et al., 1988), have lower gain to luminance changes and cannot resolve luminance contrast when it is less than ∼8% (Tootell et al., 1988a). Although there is some overlap in the spatial frequency ranges to which magnocellular and parvocellular cells are sensitive (Merigan and Maunsell, 1993), the magnocellular cells are highly sensitive to low-spatial frequencies (Derrington and Lennie, 1984; Tootell et al., 1988b), which are transmitted faster (Breitmeyer, 1975; Lupp et al., 1976) and are critical for global processing (Hughes et al., 1990, 1996). In contrast, the parvocellular cells are more sensitive to high-spatial frequencies (Tootell et al., 1988b; Merigan et al., 1991) that are considered to be crucial for local processing (Shulman et al., 1986). However, parvocellular cells function like low-pass filters and convey only low-spatial frequencies if the luminance differences in stimuli with opposing colour spectra (e.g. red–green) are equated (i.e. isoluminant) (Gegenfurtner and Kiper, 2003). Therefore, stimuli that are achromatic with low luminance (<8%) contrast will activate the magnocellular cells predominantly (i.e. magnocellular-biased), but stimuli that are isoluminant and defined only by colour (i.e. parvocellular-biased) are likely to activate the parvocellular cells more and will contain mostly low-spatial frequency information. Importantly, a recent study showed that a sizeable number of cells in the primate V4 region, a major cortical hub along the ventral visual pathway, respond selectively to isoluminant coloured form (Bushnell et al., 2011). Thus, these distinct physiological response properties can be exploited to bias the activation of magnocellular or parvocellular cells and even to convey a particular range of spatial frequencies to the visual pathways that receive their input signals. Indeed, a recent study combined functional MRI with visual psychophysics and showed that recognition of everyday objects that were either magnocellular- or parvocellular-biased using the psychophysical biasing technique described above activated predominantly key regions either in the dorsal pathway (e.g. bilateral inferior parietal lobule, middle temporal gyrus) or in the ventral pathway (e.g. bilateral fusiform cortex and occipitotemporal sulcus), respectively (Kveraga et al., 2007).
Therefore, in two experiments, we used the same biasing technique to test the relative contributions of the dorsal and ventral pathways in mediating global and local visual processing in patients with simultanagnosia, a group of elderly healthy volunteers and a group of young healthy volunteers. For each participant, we first established the achromatic contrast threshold and chromatic isoluminance threshold (Kveraga et al., 2007). Next, to test global and local visual processing, we used these thresholds to dynamically generate magnocellular- and parvocellular-biased stimuli from a set of ‘unbiased’ hierarchical letter stimuli (Fig. 2). We used hierarchical letter stimuli for two reasons: (i) they are a well-established and controlled approximation of the global and local components in natural scenes (Navon, 2003); and (ii) previous studies have shown that patients with simultanagnosia fail to recognize the global shape and can only report the identity of the local shape (Karnath et al., 2000; Huberle and Karnath, 2006).
Figure 2.
The three kinds of stimuli used to bias processing through the visual pathways. This example illustrates the incongruent case where the global identity is ‘H’ and local identity is ‘S’. Note that the contrast and luminance properties of the magnocellular-biased (M-biased) and parvocellular-biased (P-biased) stimuli illustrated here have been altered to be more discernable to readers.
Subjects and methods
Participants
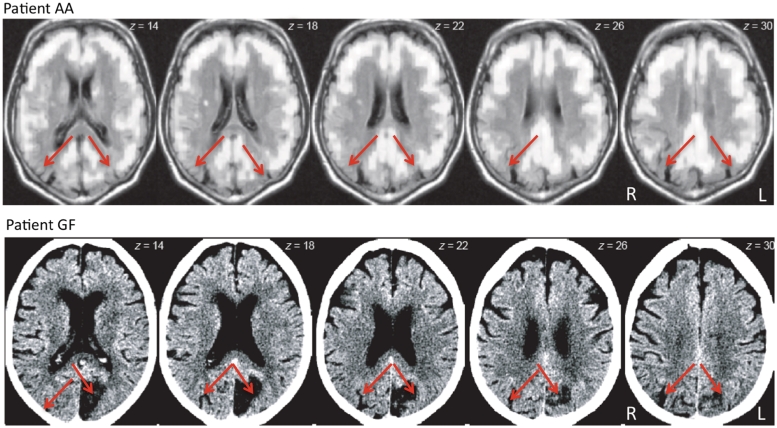
For Experiment 1, we recruited two patients (Patient AA: male, 64 years; Patient GF: female, 79 years) who presented with lesions in the bilateral posterior parietal cortex (Fig. 1) and showed classic signs of simultanagnosia (see Supplementary material for biographical details), as well as a group of five healthy elderly controls (mean age: 66 years, range: 65–69 years). For Experiment 2, we recruited a group of 10 young healthy controls (mean age: 25 years, range: 24–26 years). The control participants in both experiments did not present with any history of neurological or psychiatric disorders. All participants were right handed, had normal or corrected-to-normal vision and gave their informed consent before volunteering for the study. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki, and procedures approved by Massachusetts General Hospital (Human Studies Protocol 2001P-001754).
Figure 1.
Brain scans of the two patients with simultanagnosia. In Patient AA, 18-fluorodeoxyglucose PET revealed reduced metabolism (dark regions) in bilateral posterior parietal cortex (red arrows) consistent with posterior cortical atrophy. In Patient GF, computed tomography revealed damage to bilateral posterior parietal cortex (red arrows) due to cerebral ischaemia. L = left; R = right.
Stimuli
In both experiments, the stimuli for estimating the psychophysical thresholds (Supplementary material) were identical. However, for testing global–local visual processing the set of hierarchical stimuli used for the two experiments were slightly different and these are noted below.
In Experiment 1, the hierarchical stimuli (Fig. 2) consisted of global patterns of three different letters (E, H and S) formed from smaller local patterns of the same letters. All possible combinations of incongruent stimuli (identity of the global and local shapes were incongruent) were applied resulting in a total number of six different combinations. In Experiment 2, the hierarchical stimuli comprised global patterns of the letters H and S formed from smaller local patterns of the same letters that resulted in two categories. In the congruent case (e.g. a big H made up of small H's), the global and the local shapes shared identity, whereas in the incongruent case (e.g. a big H made up of small S's), the global and local levels had different identities.
In both experiments, the background of the hierarchical stimuli subtended 5.1° horizontally and 5.4° vertically, the global letters subtended a visual angle of 3.4° × 2.3° and the local letters subtended 0.5° × 0.3°, respectively. The distance between the local letters was 0.03° visual angle. For each participant, the unbiased stimuli were dynamically converted into achromatic magnocellular-biased stimuli and the red–green parvocellular-biased stimuli using the luminance contrast threshold and the red–green isoluminance threshold estimated in an independent experiment (Supplementary material). This resulted in three stimulus types: unbiased, magnocellular-biased and parvocellular-biased (Fig. 2).
Design and procedure
Estimation of the psychophysical thresholds and the subsequent experiments were conducted in a low-lit room. Stimulus presentation and data collection were controlled by a custom-made program using MatLab (MathWorks) and the Psychophysics Toolbox (Brainard, 1997).
In Experiment 1, the stimuli were presented on a laptop and the responses were coded by one of the experimenters (E.H.). The stimuli across the three conditions (unbiased, magnocellular- and parvocellular-biased) were presented in a pseudorandom order so that identical stimuli were not presented consecutively, and each letter combination was repeated five times yielding a total of 90 trials, with 30 trials for each condition, for a specific task (global report or local report). Each trial was initiated by the experimenter when the participant indicated readiness. The stimulus was presented at the centre of the monitor for a duration of 5000 ms for the patients and 2000 ms for the elderly control group. Only accuracy was collected as the dependent measure.
In Experiment 2, all stimuli were presented on a colour monitor placed 75 cm in front of the observers and responses were recorded using a USB keyboard. A chin rest was used to stabilize the viewing distance. All participants were required to focus at a specific level (global or local) across an entire block of trials. The order of the level (global/local) was randomized. Each block started with instructions to focus on one level (for example global) and report the identity of the hierarchical letters by key press. For a specific target level (global/local), the two congruency levels (congruent/incongruent), two letter levels (H/S) and three bias levels (unbiased, magnocellular- and parvocellular-biased) yielded 12 types of stimuli. These stimuli were pseudorandomly presented in three blocks with 60 trials in each block, yielding a total of 180 trials. Thus, for the global and local condition, there were a total of 360 trials. Each trial began with the appearance of a fixation cross at the centre of the screen for 1250 ms and was replaced by the stimulus, which remained on the monitor for an unlimited duration or until the subject pressed one of the marked keys. Participants were given 24 practice trials containing an equal number of the 12 stimulus types prior to the beginning of each experiment. Participants were offered an opportunity to rest at the end of each block. Accuracy and reaction time were collected as the dependent measures.
Results
Experiment 1
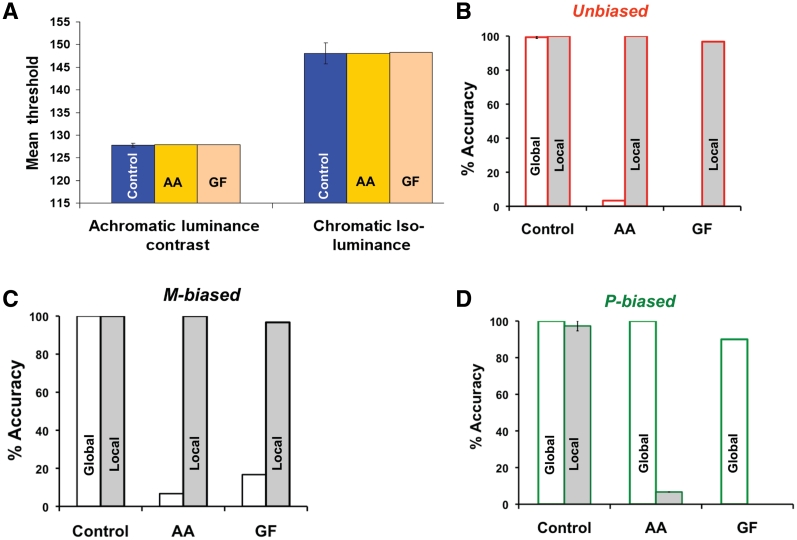
Assessment of the psychophysical thresholds in the Patients AA and GF revealed that they were well within the range observed in the control group for both achromatic luminance contrast threshold [control mean: 127.81, standard deviation (SD) = 0.24; Patient AA: 127.95, Patient GF: 127.85] and chromatic isoluminance threshold (control mean: 148.07, SD = 0.88; Patient AA = 148.03, Patient GF = 148.32) (Fig. 3A). This suggests that, in terms of low-level visual processing, the two groups were not different. With regard to global–local visual processing, we used the average percentage of accuracy across the three biasing conditions (unbiased/magnocellular-biased/parvocellular-biased) and tasks (global–local) as the dependent measure, rather than reaction time because the responses from the patients were coded by one of the experimenters. Performance above the chance level was defined by applying the 95% confidence interval for the binomial distribution.
Figure 3.
(A) The psychophysical thresholds (in arbitrary units) and accuracy for global–local processing across the three biasing conditions (B–D) in the patients (AA and GF) with simultanagnosia and the elderly control group. Error bars indicate the standard error of the mean. M-biased = magnocellular-biased; P-biased = parvocellular-biased.
Not surprisingly, the elderly control group showed a ceiling effect in accuracy for the local task, as well as the global task, across all three conditions (Fig. 3B–D). More importantly, in the unbiased condition, unlike the elderly control group, both patients consistently reported the local shape during global processing, resulting in very low response accuracy (control mean: 99.33%, SD = 1.5%; Patient AA = 3.33%, Patient GF = 0%). However, their accuracy for local processing (Fig. 3B) remained close to ceiling (control mean: 100%, SD = 0; Patient AA = 100%, Patient GF = 96.7%). Thus, we replicated the standard findings concerning global and local visual processing of unbiased hierarchical stimuli in simultanagnosia (Karnath et al., 2000; Huberle and Karnath, 2006). In the magnocellular-biased condition (Fig. 3C), both patients showed a behavioural profile similar to the unbiased condition for the global (control mean: 100%, SD = 0; Patient AA = 6.67% correct, Patient = GF 16.7% correct) and local tasks (control mean: 100%, SD = 0; Patient AA = 100%, Patient GF = 96.7%). This behavioural pattern is not surprising since the damage to the dorsal pathway, which receives the bulk of its input from magnocellular cells (Maunsell et al., 1990), would disrupt the processing of low-spatial frequencies necessary for global recognition. In the parvocellular-biased condition, however, (Fig. 3D) both patients showed almost complete recovery in global recognition (control mean: 100%, SD = 0; Patient AA = 100%, Patient GF = 90%), while local performance dropped precipitously (control mean: 97.33%, SD = 6%; AA 6.67%, GF 0%). This is because when the chromatic, isoluminant stimuli activate the parvocellular cells, they function as low-pass filters and convey only low-spatial frequencies to the intact ventral pathway, which facilitates normal global processing. However, because the colour channel of the parvocellular cells does not convey high-spatial frequencies, the patients, for the first time, were almost completely unable to perform local processing.
One could argue that the restoration of global processing in the parvocellular-biased condition is due to a disproportionate reduction in the relative salience of the global and local levels. Indeed, studies involving non-human primates have shown that at isoluminance, there is a significant reduction in the response profiles of both magnocellular and parvocellular cells (Schiller and Colby, 1983). This, in turn, could reduce the ability to resolve the identity of the local level at isoluminance and contribute to the recovery in normal global processing in the patients. Alternatively, given that high-spatial frequencies are important for normal local processing (Shulman et al., 1986), it is possible that the impairment in local processing is due to the lack of high-spatial frequencies conveyed by the parvocellular-biased stimuli. One way to clarify this issue is to test for reaction time differences between magnocellular- and parvocellular-biased conditions in implicit processing of the local or global levels. Specifically, given that the global letter is composed of local letters with an incongruent identity, if the local elements in the magnocellular-biased stimuli are more salient than the local elements in the parvocellular-biased stimuli, one would expect significant differences in interference from the local level when reporting the global identity in the magnocellular-biased condition, compared with the parvocellular-biased condition. Conversely, if processing of the global level dominates over the local level, one would expect to see greater global-to-local interference for the parvocellular-biased relative to the magnocellular-biased conditions. To test these predictions, we examined global and local visual processing in a group of young healthy volunteers (n = 10) using the same paradigm, with some minor modifications in the stimulus set that are noted above.
Experiment 2
A complete description of the results is provided in Supplementary Fig. 1. Of greater relevance is whether participants show reaction time differences in implicit processing of the global or local level due to differences in the saliency of the local elements of the magnocellular- and parvocellular-biased stimuli. We, therefore, computed for each subject, across the three conditions, a normalized measure of interference [(congruent – incongruent)/(congruent + incongruent)] from the local level during global report (local-to-global interference) and from the global level during local report (global-to-local interference). Pair-wise t-tests comparing local-to-global interference between unbiased, magnocellular and parvocellular-biased conditions did not reveal any significant differences (Supplementary Fig. 2A). A similar comparison of global-to-local interference between the three biasing conditions (Supplementary Fig. 2B) revealed that both magnocellular- and parvocellular-biased stimuli evoked significantly greater global-to-local interference than the unbiased stimuli [unbiased versus magnocellular-biased (t = −2.764, P < 0.02); unbiased versus parvocellular-biased (t = −3.952, P < 0.003)]. More importantly, however, a comparison of global-to-local interference between magnocellular- and parvocellular-biased conditions did not reveal any significant differences (t = −1.381, P < 0.20). In summary, these results suggest that differences in saliency of local elements between the magnocellular- and parvocellular-biased stimuli could not have contributed to the restoration of global processing in the patient group.
Discussion
Previous studies have shown that when the size, numerosity and spatial distance between local elements are manipulated, simultanagnosic patients can process the global shape and the local shape. For example, normal global processing was observed in the patients when the inter-element distance between local elements or the size of the global shape was decreased and when the number of local elements was increased (Huberle and Karnath, 2006; Dalrymple et al., 2007). However, this type of recovery in global processing was found to be mediated by the reduction in retinal and physical spacing between the local elements (Huberle et al., 2010). In contrast, we maintained a constant distance between the local elements and enabled normal global processing in the patients by biasing the stimuli using visual psychophysics such that it is processed by the intact ventral pathway. Thus, even though global processing can be enabled in simultanagnosia by different experimental manipulations, the underlying mechanisms may be very different.
With regard to facilitating global processing by reducing the space between local elements, one plausible mechanism is that collinear integration in early visual cortex mediates the representation of the densely arranged local elements into a unitary global shape. Indeed, studies have shown that in the early visual cortex, the number of neurons that facilitate collinear integration of coaxially arranged spatially independent lines increases as the spatial distance between two individual lines decreases (Kapadia et al., 1995). However, this mechanism cannot facilitate rapid processing of the gist of real-world scenes in which the local elements or objects are spatially distant and rarely collinear. In the current study, we conveyed low-spatial frequency information through the intact ventral pathway using chromatic, isoluminant, hierarchical stimuli and reversed the behavioural profile of simultanagnosic patients. Thus, the key component of the mechanism we propose here is the availability of low-spatial frequency information, which has been shown to be necessary for the global precedence effect and normal global processing (Hughes et al., 1996). By this account, if the stimuli are parvocellular-biased, the patients would be able to report the global shape even if the spatial distance between local elements in the hierarchical stimuli is increased and, in principle, normal global processing in simultanagnosia could be extended to processing the gist of scenes.
However, because chromatic, isoluminant stimuli are low-pass filtered by the P cells and thus lack high-spatial frequencies, the patients show a striking impairment in local processing. This reversal in the behavioural profile can be viewed as being mediated by either the availability of low-spatial frequencies or suppression of local processing due to the lack of high-spatial frequency information, which in turn reduces the saliency of the local elements. However, it is important to note that an alteration in saliency cannot fully account for the normal global processing observed in the patients for the following reasons.
First, even though the spatial distance between the local elements was small (i.e. 0.03°), the patients showed a clear deficit in identifying the global shape of the unbiased and magnocellular-biased stimuli, but showed complete recovery of global processing only when the same stimuli were parvocellular-biased. Second, qualitatively, the local elements of magnocellular-biased stimuli are also less salient than the unbiased stimuli, but no significant recovery of global processing is observed in that condition. Third, if there were differences in the saliency of the local elements between magnocellular- and parvocellular-biased stimuli, one would predict significant differences in local-to-global interference between the two types of stimuli. However, the data from Experiment 2 do not support this conjecture. Fourth, subjects tend to report the parvocellular-biased stimuli as appearing more visible than the magnocellular-biased stimuli (Kveraga et al., 2007). Finally, although luminance differences have been proposed to be critical for form perception (Lu and Fender, 1972; Gregory, 1977; Schiller and Colby, 1983) chromatic contrast also contributes to form perception (Cavanagh, 1991). Indeed, this is evident from the high accuracy rate demonstrated by the elderly controls (Fig. 3D), which suggests that local processing of the parvocellular-biased stimuli is certainly possible, although the slower reaction times in the young control group (Supplementary Fig. 1C) indicates that it is not trivial.
Based on these observations, we propose that the slow local processing of parvocellular-biased stimuli exhibited by the young control group, and the impairment in local processing observed in the patient group, can be attributed to the attenuation of high-spatial frequency information through the ventral pathway. Nevertheless, despite reduced availability of high-spatial frequency information, healthy volunteers may accomplish local processing by focusing on the borders of adjacent local elements to extract some diagnostic information pertaining to the identity of the local shape. Likewise, the patients might also be capable of extracting the identity of the local elements. But they may require longer exposure duration to the stimuli (i.e. >5000 ms) than what was employed in the present study (for example, see Karnath et al., 2000). While the lack of high-spatial frequency information disrupts local processing, the availability of low-spatial frequency information enables the patients to recognize the global shape. Taken together, the present findings suggest that the simultanagnosic's inability to see the ‘forest’ emerges from a disruption in processing low-spatial frequency information. In addition, these findings provide a deeper understanding of the relationship between the two major visual pathways and global–local visual processing.
Both patients in the current study present with bilateral damage to the posterior parietal cortex, a core region along the dorsal pathway. Given that the bulk of visual input to the dorsal pathway is considered to be from magnocellular channels (Maunsell et al., 1990) that are sensitive to low-spatial frequencies, this pathway may function as the dominant pathway for global processing. Indeed, a recent psychophysics study reported that magnocellular input to the dorsal pathway contributes to conscious vision by top–down modulation of the ventral (recognition) pathway (Tapia and Breitmeyer, 2011). This is consistent with evidence from other studies that suggests rapid, coarse, low-spatial frequency information, possibly conveyed through the dorsal pathway, serves as a ‘frame’ within which relatively fine grained but slower processing in the ventral pathway is modulated in the service of visual recognition (Schyns and Oliva, 1994; Bar et al., 2006; Chen et al., 2007; Peyrin et al., 2010). In the present study, the data from Experiment 2 indicate a significant difference in global processing only between unbiased (i.e. when both pathways are engaged) and parvocellular-biased (i.e. when the dorsal pathway is attenuated and ventral pathway is engaged) conditions (see Supplementary material). This suggests that magnocellular input to the dorsal pathway is critical for rapid global processing and supports the view that the dorsal stream is the dominant pathway for global visual processing. Therefore, damage along the dorsal pathway, as in the case of dorsal simultanagnosia, may be particularly devastating because it disrupts the processing of low-spatial frequency information that is necessary for rapid global processing. While channelling low-spatial frequencies via the intact ventral pathway can enable global processing in patients with dorsal simultanagnosia, the same strategy may not succeed in cases of simultanagnosia associated with lesion within the ventral pathway.
Indeed, it is important to bear in mind that the degree of the behavioural impairment and recovery in simultanagnosia may depend on the extent and location of the lesion. For example, some studies have reported evidence for implicit processing of the global shape in patients with simultanagnosia (Karnath et al., 2000; Jackson et al., 2004). This could be attributed to the spatial extent of the lesion because if some portion of the posterior parietal cortex (for example) in one of the hemispheres is relatively intact in the patient, the processing of low-spatial frequency information may only be partially disrupted. While the residual low-spatial frequency information may not be sufficient for normal global processing, it could contribute to implicit processing of the global form. With regard to the location of the lesion, it is noteworthy that simultanagnosia is predominantly attributed to bilateral lesions of the posterior parietal cortex and there are very few cases in which patients with unilateral lesions present with the simultanagnosia (Karpov et al., 1979; Clavagnier et al., 2006). This could be attributed to compensation by the intact hemisphere in order to overcome difficulties in global processing, especially if the hierarchical stimuli are presented foveally. However, in the context of the present findings, a simple prediction is that patients with a unilateral lesion in the posterior parietal cortex will present with difficulties in global processing if the stimuli are presented in the contralesional visual field. Although not explicitly tested so far, Posner et al. (1984) found that patients with unilateral lesions predominantly within the parietal lobe have difficulty detecting visual targets presented contralesional visual field in an attentional-cueing paradigm. One way to interpret this finding is that unilateral posterior parietal damage disrupts access to low-spatial frequency information in the contralesional visual field. While this manifests as slower reaction times in detecting a target away from fixation in patients with unilateral damage, in patients with bilateral posterior parietal cortex damage, as in the present study, the disruption in processing low-spatial frequencies from both visual fields results in simultanagnosia.
An important question that remains to be addressed concerns the simultanagnosic's disposition to focus on the ‘trees’ or the local level. Evidence from lesion studies (Merigan, 1989; Schiller et al., 1991) suggests that the ventral pathway may serve as the dominant pathway for local processing, much like the dorsal pathway functions as the dominant pathway for global processing. In this context, one plausible hypothesis is that damage to core regions along the dorsal pathway disrupts processing of the coarse, low-spatial frequency information that is critical for generating a ‘frame’ within which relatively fine-grained processing via the ventral pathway can take place. In the absence of the modulatory influence of the dorsal pathway, the ventral pathway is biased to process at the local level, giving rise to paradoxical facilitation of local processing that is characteristic of simultanagnosia (Karnath et al., 2000). Although speculative, this hypothesis can be tested directly by examining the effect of reversible lesions induced by transcranial magnetic stimulation of key cortical regions along the dorsal and ventral pathways, on global–local visual processing of magnocellular- and parvocellular-biased hierarchical stimuli. It is worth noting, however, that similar paradoxical facilitations between the dorsal and ventral pathways have been documented in a patient with sulcal abnormalities in the parietal cortex (Morland et al., 1996), and also in healthy volunteers by inducing reversible lesions in area MT with transcranial magnetic stimulation (Walsh et al., 1998).
We recognize that the mapping of magnocellular and parvocellular cell response profiles to the characteristics of the dorsal and ventral pathways may be an over-simplification. Indeed, even though some degree of functional segregation of signals from the magnocellular and parvocellular cells is maintained from the lateral geniculate nucleus to the primary visual cortex (V1), there is considerable mixing of signals from the different layers of the lateral geniculate nucleus, including the koniocellular layer, at V1 (Sincich and Horton, 2005). But a more striking functional segregation of visual information is observed beyond V1 and V2 and forms the basis for the characterization of the dorsal and ventral visual pathways. In particular, the behavioural profile that emerges following lesions of the magnocellular and parvocellular cells indicates a distinct relationship between the response properties of the magnocellular and parvocellular cells and the functions of the cortical regions along the two visual pathways. For example, parvocellular lesions cause a deficit in chromatic vision, texture perception, pattern perception (Schiller et al., 1991), acuity and a striking loss in contrast sensitivity at low temporal and high-spatial frequencies (Merigan, 1989). Magnocellular lesions, in comparison, result in deficits in the perception of flicker and motion (Schiller et al., 1991). These findings suggest that although both pathways receive inputs from different types of cells in the lateral geniculate nucleus, there are strong asymmetries in the functional properties of the two pathways that are consistent with the response profiles of the magnocellular and parvocellular cells. Furthermore, such functional asymmetries evoke more activity in cortical regions along the dorsal pathway or the ventral pathway during recognition of line drawings of objects that were magnocellular-biased or parvocellular-biased, respectively (Kveraga et al., 2007). Therefore, in the present study, we do not assume that magnocellular and parvocellular biasing of the stimuli activates only one of the pathways, but only that the manipulation biases processing in one pathway over the other.
In conclusion, we have demonstrated that despite the lesion along the dorsal pathway, normal global processing of a scene can be enabled in the patients with simultanagnosia as long as low-spatial frequency information is made available to the intact ventral pathway. These findings provide a deeper understanding of the relation between subcortical pathways, cortical pathways, spatial frequencies and visual processing.
Funding
Bundesministerium für Bildung und Forschung (BMBF-Verbund Grant Number 01GW0654 to H-O.K.) and by the National Institutes of Health (Grant Number R01EY019477 to M.B.).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors would like to thank Bernd Ritzinger for help with the data acquisition.
References
- Badcock JC, Whitworth FA, Badcock DR, Lovegrove WJ. Low-frequency filtering and the processing of local-global stimuli. Perception. 1990;19:617–29. doi: 10.1068/p190617. [DOI] [PubMed] [Google Scholar]
- Bar M, Kassam KS, Ghuman AS, Boshyan J, Schmidt AM, Dale AM, et al. Top-down facilitation of visual recognition. Proc Natl Acad Sci USA. 2006;103:449–54. doi: 10.1073/pnas.0507062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard D. The psychophysics toolbox. Spatial Vis. 1997;10:433–6. [PubMed] [Google Scholar]
- Breitmeyer BG. Simple reaction time as a measure of the temporal response properties of transient and sustained channels. Vis Res. 1975;15:1411–12. doi: 10.1016/0042-6989(75)90200-x. [DOI] [PubMed] [Google Scholar]
- Bushnell BN, Harding PJ, Kosai Y, Bair W, Pasupathy A. Equiluminance cells in visual cortical area V4. J Neurosci. 2011;31:12398–412. doi: 10.1523/JNEUROSCI.1890-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh P. Vision at equiluminance. In: Kulikowski JJ, Murray IJ, Walsh V, editors. Vision and visual dysfunction: limits of vision. Boca-Raton, FL: CRC; 1991. pp. 234–50. [Google Scholar]
- Chen CM, Lakatos P, Shah AS, Mehta AD, Givre SJ, Javitt DC, et al. Functional anatomy and interaction of fast and slow visual pathways in macaque monkeys. Cereb Cortex. 2007;17:1561–9. doi: 10.1093/cercor/bhl067. [DOI] [PubMed] [Google Scholar]
- Clavagnier S, Fruhmann Berger M, Klockgether T, Moskau S, Karnath HO. Restricted ocular exploration does not seem to explain simultanagnosia. Neuropsychologia. 2006;44:2330–6. doi: 10.1016/j.neuropsychologia.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Dalrymple K, Kingstone A, Barton J. Seeing trees OR seeing forests in simultanagnosia: attentional capture can be local or global. Neuropsychologia. 2007;45:871–5. doi: 10.1016/j.neuropsychologia.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Derrington AM, Lennie P. Spatial and temporal contrast sensitivities of neurons in lateral geniculate nucleus of macaque. J Physiol. 1984;357:219–40. doi: 10.1113/jphysiol.1984.sp015498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre-Thorpe M, Delorme A, Marlot C, Thorpe S. A limit to the speed of processing in ultra-rapid visual categorization of novel natural scenes. J Cogn Neurosci. 2001;13:171–80. doi: 10.1162/089892901564234. [DOI] [PubMed] [Google Scholar]
- Gegenfurtner KR, Kiper DC. Color vision. Ann Rev Neurosci. 2003;26:181–206. doi: 10.1146/annurev.neuro.26.041002.131116. [DOI] [PubMed] [Google Scholar]
- Gregory RL. Vision with isoluminant colour contrast: 1. A projection technique and observations. Perception. 1977;6:113–9. doi: 10.1068/p060113. [DOI] [PubMed] [Google Scholar]
- Huberle E, Driver J, Karnath HO. Retinal versus physical stimulus size as determinants of visual perception in simultanagnosia. Neuropsychologia. 2010;48:1677–82. doi: 10.1016/j.neuropsychologia.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberle E, Karnath H. Global shape recognition is modulated by the spatial distance of local elements—evidence from simultanagnosia. Neuropsychologia. 2006;44:905–11. doi: 10.1016/j.neuropsychologia.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Hughes HC, Fendrich R, Reuter-Lorenz PA. Global versus local processing in the absence of low spatial frequencies. J Cogn Neurosci. 1990;2:272–82. doi: 10.1162/jocn.1990.2.3.272. [DOI] [PubMed] [Google Scholar]
- Hughes HC, Nozawa G, Kitterle F. Global precedence, spatial frequency channels, and the statistics of natural images. J Cogn Neurosci. 1996;8:197–230. doi: 10.1162/jocn.1996.8.3.197. [DOI] [PubMed] [Google Scholar]
- Jackson G, Shepherd T, Mueller S, Husain M, Jackson S. Dorsal simultanagnosia: an impairment of visual processing or visual awareness? Cortex. 2006;42:740–9. doi: 10.1016/s0010-9452(08)70412-x. [DOI] [PubMed] [Google Scholar]
- Jackson GM, Swainson R, Mort D, Husain M, Jackson SR. Implicit processing of global information in Balint's syndrome. Cortex. 2004;40:179–80. doi: 10.1016/s0010-9452(08)70941-9. [DOI] [PubMed] [Google Scholar]
- Kapadia MK, Ito M, Gilbert CD, Westheimer G. Improvement in visual sensitivity by changes in local context: parallel studies in human observers and in V1 of alert monkeys. Neuron. 1995;15:843–56. doi: 10.1016/0896-6273(95)90175-2. [DOI] [PubMed] [Google Scholar]
- Karnath H, Ferber S, Rorden C, Driver J. The fate of global information in dorsal simultanagnosia. Neurocase. 2000;6:295–306. [Google Scholar]
- Karpov B, Meerson YA, Tonkonogii I. On some peculiarities of the visuomotor system in visual agnosia. Neuropsychologia. 1979;17:281–94. doi: 10.1016/0028-3932(79)90074-5. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M, Warrington EK. A disorder of simultaneous form perception. Brain. 1962;85:461–86. doi: 10.1093/brain/85.3.461. [DOI] [PubMed] [Google Scholar]
- Kveraga K, Boshyan J, Bar M. Magnocellular projections as the trigger of top-down facilitation in recognition. J Neurosci. 2007;27:13232–40. doi: 10.1523/JNEUROSCI.3481-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGasse LL. Effects of good form and spatial frequency on global precedence. Atten Percept Psychophys. 1993;53:89–105. doi: 10.3758/bf03211718. [DOI] [PubMed] [Google Scholar]
- Lee B, Martin P, Valberg A. The physiological basis of heterochromatic flicker photometry demonstrated in the ganglion cells of the macaque retina. J Physiol. 1988;404:323–47. doi: 10.1113/jphysiol.1988.sp017292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Fender DH. The interaction of color and luminance in stereoscopic vision. Invest Ophthalmol Visual Sci. 1972;11:482–90. [PubMed] [Google Scholar]
- Lupp U, Hauske G, Wolf W. Perceptual latencies to sinusoidal gratings. Vis Res. 1976;16:969–72. doi: 10.1016/0042-6989(76)90228-5. [DOI] [PubMed] [Google Scholar]
- Luria AR. Disorders of “simultaneous perception” in a case of bilateral occipito-parietal brain injury. Brain. 1959;82:437–49. doi: 10.1093/brain/82.3.437. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Nealey TA, DePriest DD. Magnocellular and parvocellular contributions to responses in the middle temporal visual area (MT) of the macaque monkey. J Neurosci. 1990;10:3323–34. doi: 10.1523/JNEUROSCI.10-10-03323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan WH. Chromatic and achromatic vision of macaques: role of the P pathway. J Neurosci. 1989;9:776–83. doi: 10.1523/JNEUROSCI.09-03-00776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan WH, Katz LM, Maunsell JH. The effects of parvocellular lateral geniculate lesions on the acuity and contrast sensitivity of macaque monkeys. J Neurosci. 1991;11:994–1001. doi: 10.1523/JNEUROSCI.11-04-00994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JHR. How parallel are the primate visual pathways? Ann Rev Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Morland A, Ogilvie J, Ruddock K, Wright J. A new abnormality of human vision provides evidence of interactions between cortical mechanisms sensitive to movement and those sensitive to colour. Proc Biol Sci. 1996;263:1087–94. doi: 10.1098/rspb.1996.0160. [DOI] [PubMed] [Google Scholar]
- Navon D. Forest before trees: the precedence of global features in visual perception. Cogn Psychol. 1977;9:353–83. [Google Scholar]
- Navon D. What does a compound letter tell the psychologist's mind? Acta Psychologica. 2003;114:273–309. doi: 10.1016/j.actpsy.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Peyrin C, Michel CM, Schwartz S, Thut G, Seghier M, Landis T, et al. The neural substrates and timing of top-down processes during coarse-to-fine categorization of visual scenes: a combined fMRI and ERP study. J Cogn Neurosci. 2010;22:2768–80. doi: 10.1162/jocn.2010.21424. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. J Neurosci. 1984;4:1863–74. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purpura K, Kaplan E, Shapley RM. Background light and the contrast gain of primate P and M retinal ganglion cells. Proc Natl Acad Sci. 1988;85:4534–7. doi: 10.1073/pnas.85.12.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafal RD. Balint's syndrome. In: Behrmann M, editor. Disorders of visual behavior. Amsterdam: Elsevier Science; 2001. [Google Scholar]
- Rizzo M, Hurtig R. Looking but not seeing: attention, perception, and eye movements in simultanagnosia. Neurology. 1987;37:1642–8. doi: 10.1212/wnl.37.10.1642. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Robin D. Simultanagnosia: a defect of sustained attention yields insights on visual information processing. Neurology. 1990;40:447–55. doi: 10.1212/wnl.40.3_part_1.447. [DOI] [PubMed] [Google Scholar]
- Robertson LC. Attentional persistence for features of hierarchical patterns. J Exp Psychol: General. 1996;125:227–49. doi: 10.1037//0096-3445.125.3.227. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Colby CL. The responses of single cells in the lateral geniculate nucleus of the rhesus monkey to color and luminance contrast. Vis Res. 1983;23:1631–41. doi: 10.1016/0042-6989(83)90177-3. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Logothetis NK, Charles ER. Parallel pathways in the visual system: their role in perception at isoluminance. Neuropsychologia. 1991;29:433–41. doi: 10.1016/0028-3932(91)90003-q. [DOI] [PubMed] [Google Scholar]
- Schyns PG, Oliva A. From blobs to boundary edges: evidence for time-and spatial-scale-dependent scene recognition. Psychol Sci. 1994;5:195–200. [Google Scholar]
- Shulman G, Sullivan MA, Gish K, Sakoda WJ. The role of spatial frequency channels in the perception of local and global structure. Perception. 1986;15:259–79. doi: 10.1068/p150259. [DOI] [PubMed] [Google Scholar]
- Sincich LC, Horton JC. The circuitry of V1 and V2: integration of color, form, and motion. Ann Rev Neurosci. 2005;28:303–26. doi: 10.1146/annurev.neuro.28.061604.135731. [DOI] [PubMed] [Google Scholar]
- Tapia E, Breitmeyer BG. Visual consciousness revisited: magnocellular and parvocellular contributions to conscious and nonconscious vision. Psychol Sci. 2011;22:934–42. doi: 10.1177/0956797611413471. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Hamilton SL, Switkes E. Functional anatomy of macaque striate cortex: IV. Contrast and magno-parvo streams. J Neurosci. 1988a;8:1594–609. doi: 10.1523/JNEUROSCI.08-05-01594.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, Silverman MS, Hamilton SL, Switkes E, De Valois RL. Functional anatomy of macaque striate cortex: V. Spatial frequency. J Neurosci. 1988b;8:1610–24. doi: 10.1523/JNEUROSCI.08-05-01610.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torralba A, Oliva A, Castelhano MS, Henderson JM. Contextual guidance of eye movements and attention in real-world scenes: the role of global features in object search. Psychol Rev. 2006;113:766–86. doi: 10.1037/0033-295X.113.4.766. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. 'What' and 'where' in the human brain. Curr Opin Neurobiol. 1994;4:157–65. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Walsh V, Ellison A, Battelli L, Cowey A. Task-specific impairments and enhancements induced by magnetic stimulation of human visual area V5. Proc R Soc B: Biol Sci. 1998;265:537–43. doi: 10.1098/rspb.1998.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.