Abstract
Primary progressive aphasia is a neurodegenerative syndrome characterized by gradual dissolution of language but relative sparing of other cognitive domains, especially memory. It is associated with asymmetric atrophy in the language-dominant hemisphere (usually left), and differs from typical Alzheimer-type dementia where amnesia is the primary deficit. Various pathologies have been reported, including the tangles and plaques of Alzheimer’s disease. Identification of Alzheimer pathology in these aphasic patients is puzzling since tangles and related neuronal loss in Alzheimer’s disease typically emerge in memory-related structures such as entorhinal cortex and spread to language-related neocortex later in the disease. Furthermore, Alzheimer pathology is typically symmetric. How can a predominantly limbic and symmetric pathology cause the primary progressive aphasia phenotype, characterized by relative preservation of memory and asymmetric predilection for the language-dominant hemisphere? Initial investigations into the possibility that Alzheimer pathology displays an atypical distribution in primary progressive aphasia yielded inconclusive results. The current study was based on larger groups of patients with either primary progressive aphasia or a typical amnestic dementia. Alzheimer pathology was the principal diagnosis in all cases. The goal was to determine whether Alzheimer pathology had clinically-concordant, and hence different distributions in these two phenotypes. Stereological counts of tangles and plaques revealed greater leftward asymmetry for tangles in primary progressive aphasia but not in the amnestic Alzheimer-type dementia (P < 0.05). Five of seven aphasics had more leftward tangle asymmetry in all four neocortical regions analysed, whereas this pattern was not seen in any of the predominantly amnestic cases. One aphasic case displayed higher right-hemisphere tangle density despite greater left-hemisphere hypoperfusion and atrophy during life. Although there were more tangles in the memory-related entorhinal cortex than in language-related neocortical areas in both phenotypes (P < 0.0001), the ratio of neocortical-to-entorhinal tangles was significantly higher in the aphasic cases (P = 0.034). Additionally, overall numbers of tangles and plaques were greater in the aphasic than amnestic cases (P < 0.05), especially in neocortical areas. No significant hemispheric asymmetry was found in plaque distribution, reinforcing the conclusion that tangles have greater clinical concordance than plaques in the spectrum of Alzheimer pathologies. The presence of left-sided tangle predominance and higher neocortical-to-entorhinal tangle ratio in primary progressive aphasia establishes clinical concordance of Alzheimer pathology with the aphasic phenotype. The one case with reversed asymmetry, however, suggests that these concordant clinicopathological relationships are not universal and that individual primary progressive aphasia cases with Alzheimer pathology exist where distributions of plaques and tangles do not account for the observed phenotype.
Keywords: neurodegenerative disorders, primary progressive aphasia, AD pathology, hemispheric differences, stereology
Introduction
The neuropathology of Alzheimer’s disease most commonly leads to a predominantly amnestic dementia, also known as ‘dementia of the Alzheimer’s type’. The relationship between this clinical syndrome and the anatomical distribution of the two major histopathological markers of Alzheimer’s disease, amyloid plaques and neurofibrillary tangles, has been investigated in great detail. Most of these investigations show that the distribution and density of neurofibrillary tangles are more closely associated with the neuronal loss, atrophy and nature of the cognitive deficits than the distribution and density of amyloid plaques (Arriagada et al., 1992; Bierer et al., 1995; Bobinski et al., 1996; Mesulam, 1999; Guillozet et al., 2003; Nelissen et al., 2007). In the course of the continuum that leads to dementia of the Alzheimer’s type, the initial emergence and gradual accumulation of neurofibrillary tangles in hippocampo-entorhinal areas are associated with the focal atrophy of medial temporal cortex and the relatively isolated amnesia of mild cognitive impairment, while the subsequent spread of neurofibrillary tangles to neocortical areas is associated with the additional cognitive impairments that fulfil the diagnostic criteria for amnestic dementia of the Alzheimer’s type (Hyman et al., 1984; Braak and Braak, 1996; Mesulam, 1999; Mesulam et al., 2004).
Alzheimer neuropathology, however, has also been reported in distinctly different dementia syndromes. For example, some patients with the clinical syndrome of progressive visuospatial dysfunction (Weintraub and Mesulam, 1993) also known as posterior cortical atrophy (Benson et al., 1988) have been shown to have Alzheimer pathology. These patients, who display generalized visuospatial disorientation and a biparietal syndrome, show neurofibrillary tangle densities in visual areas such as Brodmann area (BA) 17, BA 18 and superior colliculus, which are up to 25-fold higher than in patients with amnestic dementia of the Alzheimer’s type and the neuropathology of Alzheimer’s disease (DAT/AD) (Hof et al., 1997; Tang-Wai and Mapstone, 2006). The subicular component of the hippocampo-entorhinal complex in these patients was also shown to have fewer tangles than in BA 18, a reversal of the pattern seen in typical DAT/AD (Tang-Wai and Mapstone, 2006).
Another atypical presentation of Alzheimer neuropathology has been described in patients with early and disproportionately severe impairment of frontal lobe function. In such cases, neurofibrillary tangle densities in the frontal cortex were 10-times greater than in DAT/AD (Johnson et al., 1999). Additionally, neurofibrillary tangle densities were higher in the frontal neocortex than in the hippocampo-entorhinal complex. In what is probably the single most atypical clinical picture associated with Alzheimer neuropathology, progressive left hemiparesis was associated with an asymmetric neurofibrillary tangle density that was 10 times greater in the contralateral right somatosensory cortex than in the left (Jagust et al., 1990).
Primary progressive aphasia (PPA) is another non-amnestic neurodegenerative syndrome associated with Alzheimer pathology. PPA is diagnosed when a language impairment (aphasia), caused by a neurodegenerative disease (progressive), constitutes the most salient aspect of the clinical picture (primary) (Mesulam et al., 2009). In the initial stages, episodic memory is generally preserved though scores on verbally mediated memory assessments may be reduced. The most common finding in autopsies of patients with PPA is frontotemporal lobar degeneration with either tauopathy or TDP-43 proteinopathy. However, in ∼30–40% of PPA cases, the post-mortem examination reveals the characteristic plaques and tangles of Alzheimer’s disease (Mesulam, 1982, 2003; Greene et al., 1996; Knibb et al., 2006; Rogalski and Mesulam, 2007; Rohrer et al., 2010).
As in the case of PPA with frontotemporal lobar degeneration, patients with PPA and the neuropathology of Alzheimer’s disease (PPA/AD) also show asymmetric atrophy of the language dominant hemisphere (Mesulam et al., 2008). However, the relationship of the histopathological Alzheimer’s disease markers to the asymmetric atrophy and aphasic phenotype remains unsettled. Is the typical hippocampo-entorhinal predominance of neurofibrillary tangles altered in PPA/AD, and is there hemispheric asymmetry of plaques and tangles to mirror the asymmetry of the atrophy? Most of the reports that address these questions have been based on qualitative evaluations and have led to partial and inconsistent answers. For example, the patient of Engel and Fleming (1997) had a progressive non-fluent aphasia in life, asymmetric left perisylvian atrophy in imaging studies and the neuropathology of Alzheimer’s disease. Despite the aphasic phenotype, neurofibrillary tangle density was higher in the entorhinal cortex than in language-related neocortical areas and asymmetry was not mentioned. Green et al. (1990) reported two autopsied patients with features of PPA, one of whom had the neuropathological findings of Alzheimer’s disease. As in the Engel and Fleming (1997) case, neurofibrillary tangles were maximal in the hippocampo-entorhinal complex and did not seem to have displayed hemispheric asymmetry in language-related neocortical areas. One of the three cases with PPA reported by Kempler et al. (1990) was found to have Alzheimer’s disease at autopsy but there was no mention of atypical neurofibrillary tangle distribution. Li et al. (2000) reviewed the literature on Alzheimer pathology in PPA and presented a case of their own without quantitation but with apparently ‘typical’ neurofibrillary tangle distribution favouring the hippocampo-entorhinal complex over language areas. The eight ‘possible’ PPA cases with Alzheimer pathology reported by Munoz et al. (2007) are also said to have a ‘typical’ distribution of Alzheimer-specific lesions although no quantitation was reported.
The six PPA/AD cases reported by Galton et al. (2000) were also evaluated qualitatively. Sections from both hemispheres were available but left–right comparisons are mentioned for only one case where it was reported to be symmetric (Case OM). In Case OM and two others (Cases CT and CM), the entorhinal cortex had as many or more neurofibrillary tangles as temporal, parietal and frontal areas. In one of the cases (Case PG) ‘well-defined plaques were not a feature’ so that this case would more appropriately be classified as displaying the ‘tangle only’ pathology that probably belongs to the frontotemporal lobar degeneration group (McKhann et al., 2001). The remaining two of the six cases with PPA/AD in the Galton et al. (2000) series (Cases AS and PB) had atypical neurofibrillary tangle distributions with some language-related neocortical areas showing a higher neurofibrillary tangle density than the entorhinal cortex. However, information on asymmetry of neurofibrillary tangle density was not provided.
The question of clinicopathological concordance was further addressed in an investigation of 23 autopsies of patients with PPA, 11 of whom had Alzheimer pathology. Only four of these cases had sufficient tissue for quantitation of neurofibrillary tangles and amyloid plaques in both hemispheres. The results were compared with a group of four cases with typical DAT/AD. Although the PPA/AD cases did have significantly more left hemisphere neocortical neurofibrillary tangles than the DAT/AD group, the magnitude of the asymmetry was low and inconsistent (Mesulam et al., 2008). This lack of clear-cut concordance led some investigators to question whether there is sufficient correspondence between the anatomical distribution of disease markers and clinical features in PPA/AD (Munoz et al., 2007), and consequently, whether there may be some other unidentified concomitant process that is responsible for the emergence of the PPA phenotype (Mesulam et al., 2008). The current study was undertaken to address further this question with a larger sample of 12 autopsied brains, seven PPA/AD and five DAT/AD, each with systematically sampled, identically stained and quantitatively analysed tissue from five representative cortical areas in both hemispheres. In the majority of these PPA/AD cases, the unbiased stereological analysis of the tissue showed that neurofibrillary tangles did display regional variations that deviated from those of DAT/AD and that closely mirrored the salience of aphasic rather than amnestic impairments.
Materials and methods
Cases and tissue processing
The holdings of the Northwestern Alzheimer's Disease Centre Brain Bank were surveyed first to identify right-handed cases with the clinical diagnosis of PPA and pathological diagnosis of Alzheimer’s disease for which fixed tissue representing both hemispheres in at least three of the five target areas was available. The four cases in the previously reported quantitative analysis (Mesulam et al., 2008) were excluded. Seven PPA/AD cases fit these requirements and were included in this study. We then selected the five most recent right-handed DAT/AD cases that had the same tissue availability. The PPA root diagnosis was based on the criteria of Mesulam (2001, 2003). Further classification into agrammatic and logopenic subtypes was based on retrospective chart review guided by the criteria of Gorno-Tempini et al. (2011) and Mesulam et al. (2009). The clinical amnestic dementia of the Alzheimer’s type diagnosis was based on CERAD criteria (McKhann et al. 1984; Morris et al., 1989).
Post-mortem intervals ranged from 5 to 24 h with one outlier, which had a 71 h post-mortem interval (Table 1). The difference in post-mortem interval in the two groups was not statistically significant (P = 0.41). Following autopsy, the right and left hemispheres from each brain were cut coronally into 2- to 3-cm slabs and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, at 4°C for 30 h, cryoprotected in ascending sucrose gradients and stored in 40% sucrose. Bilateral samples were taken from middle frontal gyrus (BA 8–9), inferior frontal gyrus (BA 44), superior temporal gyrus (BA 22), inferior parietal lobule (BA 39–40) and entorhinal cortex (BA 28)/anterior hippocampus and embedded in paraffin. Sections were cut at 5 µm on a rotary microtome, mounted on charged slides and stored at room temperature until used.
Table 1.
Demographic information
| Case | Dementia profile | Gender | Education (years) | Imaging | ApoE | Age at symptom onset | Age at death | PMI (h) | Brain weight (g) | Braak staging |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | PPA-G | M | 20 | MRI: non-specific signal abnormalities in white matter; EEG: L slight slowing | 3,3 | 68 | 81 | 6 | 1100 | V |
| P2 | PPA-L | F | 19 | No consistent asymmetry | 3,3 | 51 | 66 | 19 | 890 | VI |
| P3 | PPA-L | M | 19 | SPECT: mild decreased perfusion in the L temporo-parietal region; EEG: L slowing | 3,4 | 62 | 75 | 8 | 1030 | VI |
| P4 | PPA-L | M | 16 | MRI: atrophy L temporal pole and bilateral frontal | 3,4 | 80 | 87 | 6 | 1100 | VI |
| P5 | PPA-L | F | 16 | MRI: L perisylvian atrophy | 3,3 | 71 | 77 | 18 | 1250 | VI |
| P6 | PPA-Mixed | M | 15 | MRI: unremarkable | 3,4 | 59 | 71 | 24 | 1250 | VI |
| P7 | PPA-L | M | 16 | MRI: atrophy L temporoparietal cortex | 3,3 | 56 | 61 | 6 | 1250 | VI |
| D1 | DAT | F | 18 | No asymmetry | 3,4 | 76 | 89 | 71 | 980 | VI |
| D2 | DAT | M | 14 | No asymmetry | 3,4 | 74 | 80 | 20 | 1390 | V |
| D3 | DAT | F | 12 | No asymmetry | 3,4 | 74 | 85 | 5 | 1140 | VI |
| D4 | DAT | F | 16 | No asymmetry | 3,3 | 73 | 82 | 7 | 1170 | V |
| D5 | DAT | M | 12 | No asymmetry | 4,4 | 54 | 71 | 6 | 1210 | VI |
F = Female; L = left hemisphere; M = Male; PMI = post-mortem interval; PPA-G = agrammatic subtype of PPA; PPA-L = logopenic subtype of PPA; PPA-Mixed = mixed subtype of PPA; R = right hemisphere; SPECT = single-photon emission computed tomography.
Histopathology
Three adjacent sections from each cortical area were stained with 1.0% thioflavin-S (Sigma-Aldrich), which recognizes β-pleated sheet protein conformations, to visualize neurofibrillary tangles and mature/compact amyloid plaques.
Modified stereological quantitative analysis of Alzheimer pathology
Modified stereological methods were used to estimate the density of thioflavin-S-stained plaques and tangles using fluorescence (380–420 nm) microscopy. For each case, the five selected bilateral regions (middle frontal gyrus, inferior frontal gyrus, superior temporal gyrus, inferior parietal lobule and entorhinal cortex) were analysed at ×60 magnification by a viewer blind to the clinical diagnosis, brain region and brain hemisphere. Modified stereological analysis was carried out on three adjacent sections according to procedures previously described in detail (Gliebus et al., 2010), employing the fractionator method and the StereoInvestigator software (MicroBrightField). An area of interest was selected at random from each cortical region under study per slide, and traced from the cortical surface to the white matter. The top and bottom 1 μm of each section were set as guard height. The dimensions of the counting frame were 100 × 100 μm. Stereological counts obtained were combined for the three sections per brain area and expressed as mean neurofibrillary tangles or plaques per cubic millimetre, based on planimetric calculation of volume by the fractionator software. Mean neurofibrillary tangle and plaque counts were compared between groups to evaluate quantitative differences in Alzheimer pathology in amnestic versus aphasic phenotypes. Additionally, since PPA is an asymmetric syndrome, mean counts were compared by hemisphere in both groups.
Imaging acquisition
MRI of Case P7 and a comparison cohort of 27 healthy individuals of a similar age was performed at the Northwestern University Department of Radiology Centre for Advanced MRI using a T1-weighted 3D magnetization prepared-rapid acquisition gradient echo (MP-RAGE) sequence (repetition time, 2300 ms; echo time, 2.86 ms; flip angle, 9°; field of view, 256 mm) recording 160 slices at a slice thickness of 1.0 mm acquired on a 3 T Siemens TRIO system using a 12-channel birdcage head coil. The MRI scans were processed using the image analysis suite FreeSurfer (version 4.5.0). Cortical thickness estimates were calculated by measuring the distance between representations of the white-grey and pial-CSF boundaries across each point of the cortical surface.
Statistical analysis
Data were analysed using independent sample t-tests, Wilcoxon rank-sum tests, and a mixed-model repeated measures ANOVA. A two-tailed Fisher’s exact test was used to analyse differences in apolipoprotein ε4 (ApoE-ε4) allele frequency across samples. Statistical analysis was carried out using the SAS software (version 8; SAS Institute).
For the MRI analysis, statistical surface maps were generated with the FreeSurfer software using a general linear model, which displayed differences in cortical thickness between the PPA and healthy groups for each vertex along surface representations of the entire neocortex. False discovery rate was applied at 0.05 to adjust for multiple comparisons (Genovese et al., 2002).
Results
Clinical findings, demographics and imaging
Of the seven cases with PPA/AD, five were clinically diagnosed with the logopenic variant, one with the mixed variant and one with the agrammatic variant (Table 1). The PPA/AD and DAT/AD groups did not differ significantly in education, age at death or disease duration (P > 0.05). Although mean age at onset was not significantly different between groups, symptom onset occurred at 70 years or younger in ∼71% of patients with PPA/AD, but only in 20% of patients with DAT/AD. Neuroimaging in the PPA/AD group revealed a general pattern of greater atrophy, hypoperfusion and hypometabolism in the left hemisphere (Table 1).
Apolipoprotein genotyping
The most extensively documented genetic risk factor for DAT/AD is the ApoE-ε4 allele. In our clinical core database of a larger sample of 330 non-demented age-matched control subjects, 14.4% had the ApoE-ε4 allele. In the current sample of five subjects with DAT/AD, the 50% ApoE-ε4 frequency was significantly higher than in our larger sample of non-demented control subjects (P = 0.009). The 21.4% ApoE-ε4 frequency in subjects with PPA/AD, however, did not differ significantly from the ApoE-ε4 frequency in controls (P = 0.44).
Pathological staging and stereological quantitation of overall neurofibrillary tangles and amyloid plaques
Alzheimer pathology was at Braak stage VI in nine cases and Braak stage V in three (Cases P1, D2, and D4) (Braak and Braak, 1991). The thioflavin-S stain provided the material for stereological analyses (Fig. 1). The average number of neurofibrillary tangles in the two hemispheres and in all regions including the entorhinal cortex was greater in PPA/AD [mean = 9845.34, standard deviation (SD) = 10023.88] compared with DAT/AD (mean = 5387.02, SD = 8730.96) (P = 0.017) (Table 2). This difference was more pronounced in neocortical areas. Similarly, the average number of thioflavin-S-positive plaques in all regions was greater in PPA/AD (mean = 4846.17, SD = 4632.09) than in DAT/AD (mean = 3145.84, SD = 2615.74) (P = 0.022) (Table 2).
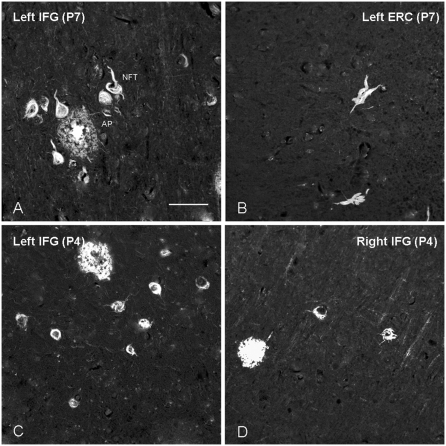
Figure 1.
Thioflavin-S staining of neurofibrillary tangles and amyloid plaques. Photomicrograph (×20) of thioflavin-S-stained sections showing examples of neurofibrillary tangles and amyloid plaques in the left inferior frontal gyrus versus the left entorhinal cortex in Case P7 (A and B), and in left versus right inferior frontal gyrus in Case P4 (C and D). Magnification bar in A is 20 μm, and applies to B–D. AP = amyloid plaque; ERC = entorhinal cortex; IFG = inferior frontal gyrus; NFT = neurofibrillary tangle.
Table 2.
Stereological counts of neurofibrillary tangles and amyloid plaques in PPA/AD (Cases P1–P7) and DAT/AD (Cases D1–D5)
| Cases | Middle frontal gyrus |
Inferior frontal gyrus |
Superior temporal gyrus |
Inferior parietal lobule |
Entorhinal cortex |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | |
| PPA NFTs | ||||||||||
| P1 | 780 | 207 | 1450 | 935 | 4140 | 2115 | 6587 | 1045 | 17418 | – |
| P2 | 23696 | 8393 | 13499 | 7049 | 11738 | 11621 | 40393 | 15513 | 31308 | 38112 |
| P3 | 6799 | 18108 | 9046 | 11552 | 8870 | 10728 | 6221 | 17408 | 21698 | – |
| P4 | 2356 | 761 | 4808 | 736 | 3856 | 269 | 6887 | 1649 | 20601 | 5453 |
| P5 | 1042 | 1262 | 3245 | 1303 | – | 3774 | – | 1137 | 20638 | 20155 |
| P6 | 6600 | 3467 | 7618 | 6760 | 5780 | 2809 | 16638 | 5074 | 30742 | 25297 |
| P7 | 8431 | 4754 | 11512 | 3056 | 25072 | 7718 | 24032 | 9057 | 14716 | 6108 |
| Mean (SD) | 7101 (7923) | 5279 (6326) | 7311 (4401) | 4484 (4093) | 9909 (8016) | 5576 (4448) | 16793 (13590) | 7269 (6913) | 22446 (6320) | 19025 (13748) |
| PPA Plaques | ||||||||||
| P1 | 1561 | 414 | 2486 | 936 | 2277 | 2350 | 2864 | 1881 | 281 | – |
| P2 | 5294 | 3659 | 4219 | 1828 | 7043 | 3021 | 13895 | 7256 | 1327 | 1401 |
| P3 | 9179 | 9613 | 11915 | 6470 | 11709 | 12200 | 17025 | 10108 | 1825 | – |
| P4 | 1885 | 2539 | 5267 | 2210 | 8139 | 3499 | 4879 | 5223 | 2168 | 2077 |
| P5 | 869 | 1578 | 2434 | 2281 | – | 1332 | – | 284 | 279 | 265 |
| P6 | 776 | 612 | 2143 | 2080 | 1530 | 4359 | 2638 | 1614 | 1708 | 1150 |
| P7 | 16060 | 14859 | 13392 | 10698 | 13866 | 9049 | 10372 | 10092 | 965 | 643 |
| Mean (SD) | 5089 (5719) | 4753 (5445) | 5979 (4713) | 3786 (3526) | 7427 (4935) | 5116 (3983) | 8612 (6074) | 5208 (4088) | 1222 (747) | 1107 (699) |
| DAT NFTs | ||||||||||
| D1 | 1442 | 861 | 845 | 774 | 1948 | 351 | 771 | 1278 | 40920 | 28568 |
| D2 | 178 | 524 | 303 | 308 | 224 | 268 | 748 | 518 | 11509 | 11391 |
| D3 | 1827 | 1835 | 3786 | 3462 | 1676 | 1359 | 1123 | 2121 | 17225 | – |
| D4 | 1185 | 1651 | 680 | 1110 | 963 | 741 | 790 | 4280 | 10226 | 16811 |
| D5 | 5647 | 8794 | 3459 | 2685 | 2699 | 2221 | 5084 | 4349 | 27344 | 25097 |
| Mean (SD) | 2056 (2098) | 2733 (3431) | 1815 (1666) | 1668 (1343) | 1502 (947) | 988 (813) | 1703 (1896) | 2509 (1743) | 21445 (12811) | 20467 (7806) |
| DAT Plaques | ||||||||||
| D1 | 6129 | 8614 | 5984 | 3613 | 6233 | 3156 | 1280 | 3514 | 0 | 0 |
| D2 | 712 | 1049 | 303 | 308 | 896 | 1341 | 4863 | 2591 | 548 | 422 |
| D3 | 997 | 612 | 757 | 1237 | 2095 | 1942 | 1572 | 1515 | 2768 | – |
| D4 | 3556 | 1888 | 681 | 2777 | 5058 | 4197 | 6435 | 6513 | 2556 | 1789 |
| D5 | 7187 | 8040 | 7783 | 5670 | 4661 | 4038 | 7430 | 8185 | 326 | 326 |
| Mean (SD) | 3716 (2929) | 4041 (3945) | 3102 (3515) | 2721 (2093) | 3789 (2213) | 2935 (1263) | 4316 (2794) | 4464 (2791) | 1240 (1315) | 634 (791) |
Counts were quantitatively estimated as numbers per cubic millimetre.
NFTs = neurofibrillary tangles.
Hemispheric differences in neocortical distribution of neurofibrillary tangles and amyloid plaques
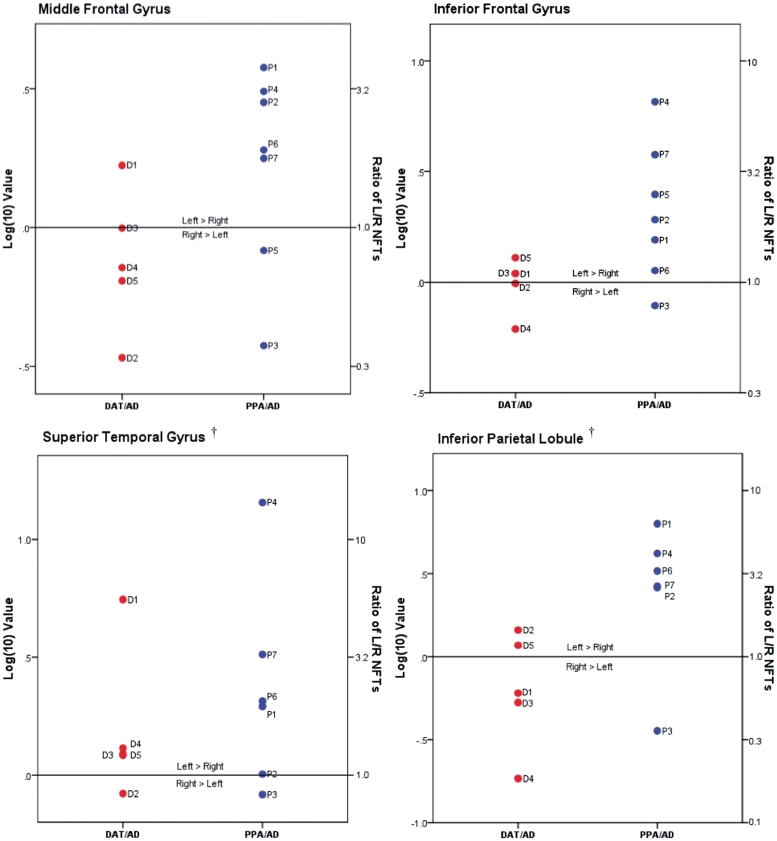
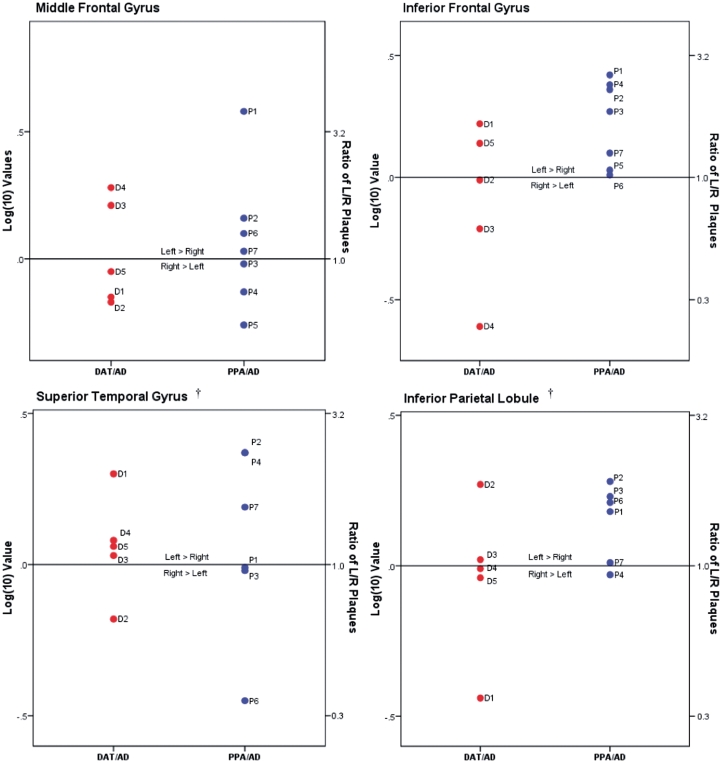
As indicated in Table 2, there were large interindividual variations of neurofibrillary tangle and plaque counts and equally large standard deviations of group means. Relying on group statistics alone would therefore tend to overlook the broad spectrum of individual patterns. Figures 4 and 5 provide logarithmic transformations of left-to-right ratios and are included to illustrate patterns of regional variations in hemispheric asymmetry at the single case level.
Figure 4.
Neurofibrillary tangles (NFTs) in left/right neocortical regions. Ratios of neurofibrillary tangle density in individual left/right neocortical regions, as shown in Table 2, were transformed logarithmically (base 10) to illustrate relative distributional patterns of left-to-right neurofibrillary tangle pathology in DAT/AD and PPA/AD cases; the 0.0 mark represents no left/right asymmetry. Actual ratio values are indicated on the y-axis on right side. Dagger indicates left superior temporal gyrus and left inferior parietal lobule not available from Case P5. L = left; NFTs = neurofibrillary tangles; R = right.
Figure 5.
Amyloid plaques in left/right neocortical regions. Ratios of amyloid plaque density in individual left/right neocortical regions, as shown in Table 2, were transformed logarithmically (base 10) to illustrate relative distributional patterns of left-to-right plaque pathology in DAT/AD and PPA/AD cases; the 0.0 mark represents no left/right asymmetry. Actual ratio values are indicated on the y-axis on right side. Dagger indicates left superior temporal gyrus and left inferior parietal lobule not available from Case P5.
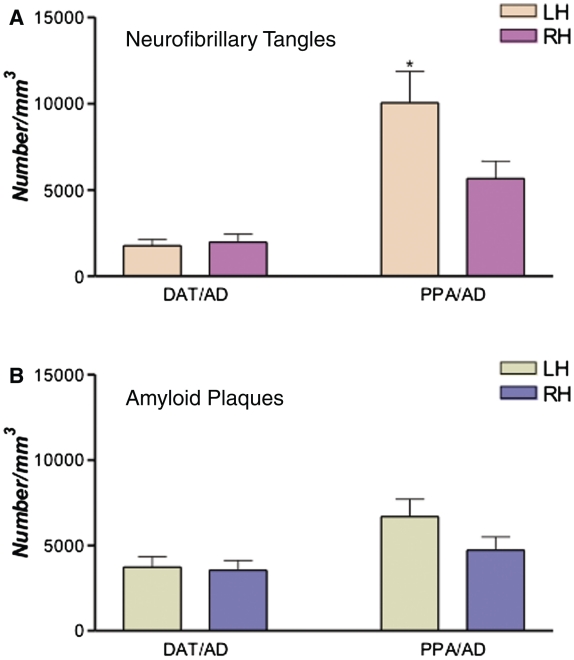
Of the seven cases with PPA/AD, five (Cases P1, P2, P4, P6 and P7) showed greater left than right neurofibrillary tangles in all four neocortical areas where counts were obtained (Table 2, Figs 3B and 4). None of the five subjects with DAT/AD showed such a pattern. In one case with PPA/AD where counts were available in only two neocortical areas (Case P5), the neurofibrillary tangles in the left inferior frontal gyrus (Broca’s area) were ∼2.5 times more numerous as those on the right, while the counts in the middle frontal gyrus were higher on the right by <20% (Fig. 4 and Table 2). In two of the PPA/AD cases (Cases P2 and P7), amyloid plaques were also more numerous in all left neocortical areas (Figs 5 and 3C). Case P3, however, showed ‘reverse’ right-sided asymmetry of neurofibrillary tangles in all neocortical areas (Fig. 4 and Table 2). Although the patient was right handed, the aphasia was associated with left hemispheric dysfunction as evidenced by single-photon emission computed tomography and EEG, and the left hemisphere was more atrophic than the right at autopsy. Statistical analysis at the group level revealed greater leftward asymmetry of neurofibrillary tangles in PPA/AD versus DAT/AD (P < 0.05), but not of amyloid plaques (P = 0.09) (Figs 2A and B, 4 and 5). Only two cases with amnestic dementia of the Alzheimer’s type had asymmetry, one favoured the right side and the other favoured the left side for neurofibrillary tangles, but not amyloid plaques. In general, asymmetry (both left and right) was significantly more apparent in neurofibrillary tangle density than amyloid plaque density within the PPA/AD group (P = 0.016) (Figs 4 and 5). Leftward neurofibrillary tangle asymmetry in cases with PPA/AD was particularly conspicuous in the inferior frontal gyrus and inferior parietal lobule where the majority of cases, with the exception of Case P3, displayed left-to-right ratios well beyond the range seen in any case with DAT/AD (Fig. 4). This type of pronounced asymmetry was not seen in counts of plaques in either group (Fig. 5).
Figure 3.
Concordance of atrophy with plaques and tangles in Case P7. (A) Cortical thickness map shows regional distribution of cortical thinning (i.e. atrophy) in Case P7 compared with 27 healthy controls. For each hemisphere, the top panels are lateral views, the bottom panels are medial views. Significance is displayed as a log(10) P-value; red and yellow areas designate peak atrophy sites. (B and C) Stereological counts of neurofibrillary tangles and amyloid plaques in Case P7. Heights of the bars represent quantitative estimated counts of Alzheimer’s disease pathological markers per cubic millimetre. CG = cingulate gyrus; ERC = entorhinal cortex; IFG = inferior frontal gyrus; IPL = inferior parietal lobule; LH = left hemisphere; NFTs = neurofibrillary tangles; MFG = middle frontal gyrus; PHG = parahippocampal gyrus; RH = right hemisphere; STG = superior temporal gyrus; TPJ = temporoparietal junction.
Figure 2.
Mean stereological counts of neurofibrillary tangles (NFTs) and amyloid plaques in neocortical areas in DAT/AD versus PPA/AD. Heights of the bars represent mean estimated counts of neurofibrillary tangles and amyloid plaques per cubic millimetre in left versus right hemisphere in DAT/AD and PPA/AD. (A) The asterisk represents significantly greater leftward asymmetry of neurofibrillary tangles in PPA/AD (*P < 0.05); no significant difference was detected in DAT/AD. (B) No significant differences were found between neocortical plaque density in the left versus right hemisphere in either PPA/AD or DAT/AD. LH = left hemisphere; RH = right hemisphere.
Differences in entorhinal versus neocortical distributions of neurofibrillary tangles and amyloid plaques
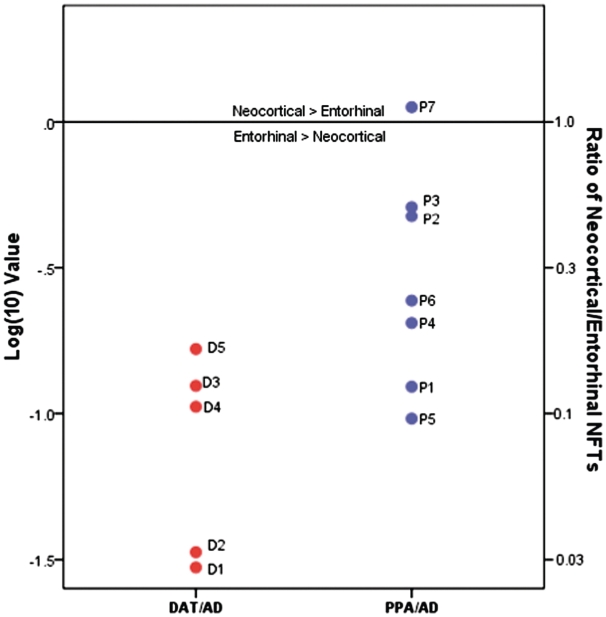
Greater neurofibrillary tangle mean density was observed in the entorhinal cortex than in neocortical regions in both DAT/AD (P < 0.0001) and PPA/AD (P < 0.0001). Greater mean density of thioflavin-S-positive plaques was observed in neocortical regions than in the entorhinal cortex in both DAT/AD (P = 0.0005) and PPA/AD (P = 0.0088) (Table 2). Overall, PPA/AD displayed greater neocortical-to-entorhinal cortex ratios than DAT/AD, and differences were statistically significant (P = 0.034) (Fig. 6). Figure 6 demonstrates the log ratio of neocortical-to-entorhinal cortex tangles stained with thioflavin-S. Of the PPA/AD cases, only one case (Case P7) demonstrated greater neurofibrillary tangles in neocortical areas when compared with the entorhinal cortex; interestingly, this individual had the earliest age at death and the shortest disease duration (Table 1).
Figure 6.
Mean neurofibrillary tangles in neocortical/entorhinal regions. Ratios of average neurofibrillary tangle (NFT) density in neocortical regions versus entorhinal cortex, as shown in Table 2, were transformed logarithmically (base 10) to illustrate relative distributional patterns of neocortical-to-entorhinal neurofibrillary tangle pathology in DAT/AD and PPA/AD cases; the 0.0 mark represents unity of the neocortical/entorhinal ratio. Actual ratio values are indicated on the y-axis on right side. Case P7 is the only case to demonstrate a higher density of neurofibrillary tangles in each neocortical region compared with the entorhinal cortex. Note: right entorhinal cortex was not available in Cases D3, P1 and P3.
Comparison of histopathology with atrophy in Case P7
In one of the PPA/AD cases (Case P7), quantitative MRI scanning was obtained 7 months before death, as part of a prospective longitudinal study. At the time that the MRI was obtained, the patient had logopenic and mildly agrammatic speech but intact comprehension. His non-verbal delayed memory score on the Wechsler Memory Scale-III Faces II sub-test (Wechsler, 1945) was within one standard deviation of normal, whereas his aphasia quotient on the Western Aphasia Battery (Shewan and Kertesz, 1980) yielded a particularly abnormal score of 68 (control values are 99 ± 1). The FreeSurfer software was used to examine cortical atrophy by comparison with a previously described control group of healthy adults (n = 27) (Rogalski et al., 2011). Areas of peak atrophy were much more pronounced in the left hemisphere and appeared to spare the parahippocampal-entorhinal region (Fig. 3A). In keeping with the anatomy of atrophy, neocortical neurofibrillary tangles and plaques were more numerous in the left hemisphere, and there were more neurofibrillary tangles in neocortex than the entorhinal cortex (Fig. 3B and C). The areas of peak atrophy, the left inferior parietal lobule and superior temporal gyrus, had the most neurofibrillary tangles but not the most plaques. While the left inferior frontal gyrus, considered critical for grammatical competence and fluency, did not display peak atrophy, it contained neurofibrillary tangles and one of the highest plaque concentrations.
Additional neuropathology in Case P3
Since Case P3 showed neurofibrillary tangle distributions that were inconsistent with the phenotype, additional investigations were carried out to look for alternative disease processes. Pathological diagnostic findings revealed gross atrophy, which was greater in the left versus right hemisphere in frontal, temporal and parietal lobes. In general, there was a substantial difference in brain weight between cerebral hemispheres stripped of pia, vessels and brainstem (left = 390 g; right = 450 g). Superficial microvacuolation was greater in the left hemisphere in the frontal lobe, particularly in the inferior frontal region, and microscopic neuronal loss and gliosis was also greater in the left versus right parietal lobe. Interestingly, higher densities of argyrophilic thorny astrocyte clusters, in numbers not usually encountered in typical Alzheimer’s disease, were found in the left versus right frontal lobe.
Discussion
Amnestic dementia of the Alzheimer’s type and PPA reflect two very different dementia phenotypes. In amnestic dementia of the Alzheimer’s type, memory impairment is the most conspicuous component of the dementia and is accompanied by a progressive and usually symmetric atrophy of entorhinal and hippocampal areas in the medial temporal lobe. In PPA, an impairment of word usage and comprehension is the most conspicuous clinical component and is accompanied by asymmetric temporal and perisylvian atrophy of the left hemisphere language network. While the neuropathology of Alzheimer’s disease is most commonly seen in conjunction with the amnestic dementia of the Alzheimer’s type phenotype, it has also been reported in brains of cases with PPA. In amnestic dementia of the Alzheimer’s type, the neurofibrillary tangles of Alzheimer’s disease show a predilection for memory-related parts of the brain such as the entorhinal and hippocampal areas. The assumption is that neurofibrillary tangles interfere with crucial cytoskeletal functions and eventually lead to neuronal death and the characteristic amnesia. Although the presence of Alzheimer pathology in PPA autopsies had been known for a long time (Mesulam and Weintraub, 1992), whether Alzheimer’s disease markers in PPA also display clinically concordant anatomical distribution patterns had remained unresolved. This is the question addressed by the current study. With the notable exception of Case P3, we found that the cases with PPA in this study displayed lesion distributions that differed from those of amnestic dementia of the Alzheimer’s type. The majority of the PPA/AD cases had pronounced leftward asymmetry of neurofibrillary tangles (Figs 2A and 4), with a median of left to right ratio (across all cases and regions) of 2.06 (interquartile range 1.02–3.28) versus a median ratio of 1.09 (0.61–1.29) in DAT/AD, P = 0.001. PPA/AD cases also had a significantly higher neocortical-to-entorhinal ratio of neurofibrillary tangles than DAT/AD cases (Fig. 6); with a median (interquartile range) of 0.23 (0.12 to 0.52) for PPA/AD and 0.11 (0.03 to 0.12) for DAT/AD, P = 0.034. These distinctive features of lesion distribution fit the salience of aphasia and relative preservation of memory that distinguish PPA from the amnestic dementia of the Alzheimer’s type phenotype.
The clinically concordant distribution of Alzheimer pathology in PPA was significant for neurofibrillary tangles (Fig. 2A) but not for amyloid plaques (Fig. 2B). This conclusion is consistent with a vast literature on amnestic dementia of the Alzheimer’s type, which has repeatedly demonstrated a closer correlation of clinical deficits with neurofibrillary tangles than plaques (Arriagada et al., 1992; Mesulam, 1999; Guillozet et al., 2003). Amyloid imaging of patients with amnestic dementia of the Alzheimer’s type also supports this as it has been shown to be a marker of presence of disease rather than a correlate of cognitive decline (Jagust et al., 2009). Amyloid imaging has recently been explored in patients with atypical dementias such as PPA. Ng et al. (2007) used Pittsburgh compound B as a positron-emitting amyloid ligand in one patient with PPA and reported labelling patterns that were mostly similar to those of amnestic dementia of the Alzheimer’s type with the exception of greater left-sided labelling. In a larger study with Pittsburgh compound B, Rabinovici et al. (2008) found that the anatomical distribution of Pittsburgh compound B uptake in PPA was indistinguishable from that of patients with typical amnestic dementias, that it did not display consistent asymmetry, and that the distribution patterns did not distinguish among PPA subtypes despite the marked differences in subtype-specific patterns of atrophy and dysfunction.
Despite our finding of clinical concordance of neurofibrillary tangle distribution in the majority of the PPA/AD cases, there was a notable exception. Case P3 had more numerous neurofibrillary tangles in the right hemisphere. The PPA syndrome has been described in association with right hemisphere atrophy in patients with right hemisphere language dominance (Mesulam et al., 2005). However, this is an unlikely explanation for the rightward neurofibrillary tangle predominance in Case P3 because the patient was right handed, showed left hemisphere hypoperfusion on single-photon emission computed tomography and had distinctly greater left hemisphere atrophy at autopsy as shown by the hemispheric weights of 390 g on the left as opposed to 450 g on the right. It seems, therefore, that neurofibrillary tangle pathology in this case cannot account for the left hemisphere atrophy or aphasic phenotype. Although the number of amyloid plaques in Case P3 were higher in the left versus right inferior frontal gyrus and inferior parietal lobule (Fig. 5), it would be difficult to suggest that this is the one case where plaques rather than tangles were responsible for the neuronal death and dysfunction. The search for an alternative neuropathological process in Case P3 did not reveal concomitant vascular lesions or other currently known pathological inclusions. However, the frontal lobes did contain more superficial microvacuolation in the left hemisphere and there were argyrophilic thorny astrocyte clusters in larger numbers than usual in the left inferior frontal gyrus. It is interesting that Munoz et al. (2007) found that seven of eight ‘possible’ PPA cases with Alzheimer pathology also showed abnormally high argyrophilic thorny astrocyte clusters in the fronto-temporo-parietal cortex. Given that their sample of PPA cases revealed no topographic correlations between Alzheimer’s disease markers and language-related areas, the authors suggested that argyrophilic thorny astrocyte clusters may in fact represent a marker of a concomitant process that may be responsible for the aphasia (Munoz et al., 2007). In the absence of a convincing explanation for the discrepancy between the location of atrophy and neurofibrillary tangles in Case P3, the possibility of an unknown pathological process that might have initiated the left atrophy and aphasic phenotype, but which subsequently became overshadowed by Alzheimer pathology that arose during the 13-year disease course, remains open.
In Case P7, the availability of quantitative imaging and clinical data obtained 7 months prior to death allowed a particularly close correlation of Alzheimer pathology with the PPA phenotype. In all four neocortical areas, the neurofibrillary tangles in Case P7 were more numerous on the left. Case P7 was the only case in which mean neurofibrillary tangles were higher in neocortical areas than in the entorhinal cortex (Fig. 6). This rarely described pattern of absolute neocortical neurofibrillary tangle predominance was in keeping with the sparing of memory at the last clinical examination of Case P7 and the absence of medial temporal atrophy on imaging at that time. The inferior parietal lobule and superior temporal gyrus in Case P7 had largest neurofibrillary tangle numbers and also greater atrophy compared with other areas of interest in that case (Fig. 3A and B). The one potential inconsistency was the absence of inferior frontal gyrus atrophy despite low fluency and mild agrammatism. However, the inferior frontal gyrus contained neurofibrillary tangles as well as amyloid plaques, which undoubtedly interfered with normal function. In neurodegenerative disease, therefore, clinicopathological correlations based on atrophy need to be interpreted with caution since atrophy is preceded by cytotoxic events that also impair functionality.
Despite the small number of cases, the current study shows that PPA can arise as one of the atypical but clinicopathologically concordant phenotypes of Alzheimer pathology. Greater awareness of this relationship may encourage a more widespread use of biomarkers such as amyloid imaging so that patients with PPA/AD can be differentiated from PPA with frontotemporal lobar degeneration pathology as early as possible, at a time when disease-specific treatment is likely to have optimal impact. As in the case of all neurodegenerative diseases, the molecular determinants of selective vulnerability remain puzzling and raise numerous questions. For example, would it be possible to identify molecular factors that differentially lead Alzheimer pathology to yield amnestic versus aphasic phenotypes? The ApoE-ε4 results are potentially relevant to this question. In sporadic DAT/AD the ε4 allele is the single most important genetic risk factor. Its incidence in this population is at least twice the control values (Corder et al., 1993). Table 1 and previous results from our laboratory show that the incidence of ε4 is not elevated in PPA/AD (Rogalski et al., 2011). It appears, therefore, that DAT/AD and PPA/AD arise from two different genetic pools and that ε4 is a risk factor specifically for the type of Alzheimer pathology that leads to a predominantly amnestic phenotype. Circumstantial support for this contention comes from imaging studies that show reduced hippocampal volume in non-demented carriers of the ε4 allele, as if the ε4 allele makes medial limbic structures more susceptible to neuronal dysfunction and pathology (Lind et al., 2006). The preferential vulnerability of the language network in PPA may be determined by a different set of developmental and genetic risk factors. Those that have been suggested include a family history of dyslexia, the H1/H1 haplotype of the tau gene and the methionine/valine (M/V) polymorphism of codon 129 of the prion protein gene (Sobrido et al., 2003; Li et al., 2005; Rogalski et al., 2008). The identification of factors that determine the atypical distribution of neurofibrillary tangles in PPA/AD may eventually shed light on the mechanisms of Alzheimer’s disease as well as on the molecular peculiarities of the language network.
Funding
NS047987 T-32 Neuroscience of Human Cognition Training Program from the National Institute of Neurological Disorders and Stroke, DC008552 from the National Institute on Deafness and Communication Disorders, AG13854 (Alzheimer Disease Centre) from the National Institute on Aging, 5KL2RR025740 from the National Centre for Research Resources, The Louis Family Foundation, and The Davee Foundation Neurobiology Research Initiative Fund.
Glossary
Abbreviations
- ApoE-ε4
apolipoprotein E4 allele
- DAT/AD
amnestic dementia of the Alzheimer’s type and the neuropathology of Alzheimer’s disease
- PPA
primary progressive aphasia
- PPA/AD
primary progressive aphasia and the neuropathology of Alzheimer’s disease
References
- Arriagada PV, Growdon JH, Hedley-Whyte T, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42(Pt 1):631–9. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Arch Neurol. 1988;45:789–93. doi: 10.1001/archneur.1988.00520310107024. [DOI] [PubMed] [Google Scholar]
- Bierer LM, Hof PR, Purohit DP, Carlin L, Schmeidler J, Davis KL, et al. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer's disease. Arch Neurol. 1995;52:81–8. doi: 10.1001/archneur.1995.00540250089017. [DOI] [PubMed] [Google Scholar]
- Bobinski M, Wegiel J, Wisniewski HM, Tarnawski M. Neurofibrillary pathology—correlation with hippocampal formation atrophy in Alzheimer disease. Neurobiol Aging. 1996;17:909–19. doi: 10.1016/s0197-4580(97)85095-6. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Evolution of the neuropathology of Alzheimer's disease. Acta Neurol Scand Suppl. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Engel PA, Fleming PD. Primary progressive aphasia, left anterior atrophy, and neurofibrillary hippocampal pathology: observations in an unusual case. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10:213–8. [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Xuereb JH, Hodges JR. Atypical and typical presentations of Alzheimer's disease: a clinical neuropsychological, neuroimaging and pathological study of 13 cases. Brain. 2000;123:484–98. doi: 10.1093/brain/123.3.484. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gliebus G, Bigio EH, Gasho K, Mishra M, Caplan D, Mesulam MM, et al. Asymmetric TDP-43 distribution in primary progressive aphasia with progranulin mutation. Neurology. 2010;74:1607–10. doi: 10.1212/WNL.0b013e3181df0a1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Morris JC, Sandson J, McKeel DW, Jr, Miller JW. Progressive aphasia: a precursor of global dementia? Neurology. 1990;40(Pt 1):423–9. doi: 10.1212/wnl.40.3_part_1.423. [DOI] [PubMed] [Google Scholar]
- Greene JDW, Patterson K, Xuereb J, Hodges JR. Alzheimer disease and nonfluent progressive aphasia. Arch Neurol. 1996;53:1072–8. doi: 10.1001/archneur.1996.00550100158027. [DOI] [PubMed] [Google Scholar]
- Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol. 2003;60:729–36. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- Hof PR, Vogt BA, Bouras C, Morrison JH. Atypical form of Alzheimer's disease with prominent posterior cortical atrophy: a review of lesion distribution and circuit disconnection in cortical visual pathways. Vision Res. 1997;37:3609–25. doi: 10.1016/S0042-6989(96)00240-4. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–70. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Jagust WJ, Davies P, Tiller-Borcich JK, Reed BR. Focal Alzheimer's disease. Neurology. 1990;40:14–9. doi: 10.1212/wnl.40.1.14. [DOI] [PubMed] [Google Scholar]
- Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–9. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56:1233–9. doi: 10.1001/archneur.56.10.1233. [DOI] [PubMed] [Google Scholar]
- Kempler D, Metter EJ, Riege WH, Jackson CA. Slowly progressive aphasia: three cases with language, memory, CT and PET data. J Neurol Neurosurg Psych. 1990;53:987–93. doi: 10.1136/jnnp.53.11.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006;59:156–65. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- Li F, Iseki E, Kato M, Adachi Y, Akagi M, Kosaka K. An autopsy case of Alzheimer's disease presenting with primary progressive aphasia: a clinicopathological and immunohistochemical study. Neuropathology. 2000;20:239–45. doi: 10.1046/j.1440-1789.2000.00343.x. [DOI] [PubMed] [Google Scholar]
- Li X, Rowland LP, Mitsumoto H, Przedborski S, Bird TD, Schellenberg GD, et al. Prion protein codon 129 genotype prevalence is altered in primary progressive aphasia. Ann Neurol. 2005;58:858–64. doi: 10.1002/ana.20646. [DOI] [PubMed] [Google Scholar]
- Lind J, Larsson A, Persson J, Ingvar M, Nilsson LG, Backman L, et al. Reduced hippocampal volume in non-demented carriers of the apolipoprotein E epsilon4: relation to chronological age and recognition memory. Neurosci Lett. 2006;20:23–7. doi: 10.1016/j.neulet.2005.11.070. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia. Arch Neurol. 2001;58:1803–9. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11:592–8. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Neuroplasticity failure in Alzheimer's disease: bridging the gap between plaques and tangles. Neuron. 1999;24:521–9. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49:425–32. [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia: a language-based dementia. N Engl J Med. 2003;349:1535–42. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Shaw P, Mash D, Weintraub S. Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann Neurol. 2004;55:815–28. doi: 10.1002/ana.20100. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Weintraub S, Parrish T, Gitelman D. Primary progressive aphasia: reversed asymmetry of atrophy and right hemisphere language dominance. Neurology. 2005;64:556–7. doi: 10.1212/01.WNL.0000150545.46351.DE. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63:709–19. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wieneke C, Rogalski E, Cobia D, Thompson C, Weintraub S. Quantitative template for subtyping primary progressive aphasia. Arch Neurol. 2009;66:1545–51. doi: 10.1001/archneurol.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Weintraub S. Spectrum of primary progressive aphasia. In: Rossor MN, editor. Unusual Dementias Balliere's clinical neurology. London: Baillière Tindall; 1992. pp. 583–609. [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP. The consortium to establish a registry for Alzheimer's disease (CERAD): I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–65. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Munoz DG, Woulfe J, Kertesz A. Argyrophilic thorny astrocyte clusters in association with Alzheimer's disease pathology in possible primary progressive aphasia. Acta Neuropathol. 2007;114:347–57. doi: 10.1007/s00401-007-0266-x. [DOI] [PubMed] [Google Scholar]
- Nelissen N, Vandenbulcke M, Fannes K, Verbruggen A, Peeters R, Dupont P, et al. A beta amyloid deposition in the language system and how the brain responds. Brain. 2007;130:2055–69. doi: 10.1093/brain/awm133. [DOI] [PubMed] [Google Scholar]
- Ng SY, Villemagne VL, Masters CL, Rowe CC. Evaluating atypical dementia syndromes using positron emission tomography with carbon 11-labeled Pittsburgh Compound B. Arch Neurol. 2007;64:1140–4. doi: 10.1001/archneur.64.8.1140. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, et al. A beta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64:388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, et al. Anatomy of language impairments in primary progressive aphasia. J Neurosci. 2011;31:3344–50. doi: 10.1523/JNEUROSCI.5544-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Johnson N, Weintraub S, Mesulam M. Increased frequency of learning disability in patients with primary progressive aphasia and their first-degree relatives. Arch Neurol. 2008;65:244–8. doi: 10.1001/archneurol.2007.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Mesulam M. An update on primary progressive aphasia. Curr Neurol Neurosci Rep. 2007;7:388–92. doi: 10.1007/s11910-007-0060-0. [DOI] [PubMed] [Google Scholar]
- Rogalski EJ, Rademaker A, Harrison TM, Helenowski IH, Johnson N, Bigio E, et al. ApoE E4 is a susceptibility factor in amnestic but not aphasic dementias. Alzheimer Dis Associat Dis. 2011;25:159–63. doi: 10.1097/WAD.0b013e318201f249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Rossor MN, Warren JD. Alzheimer's pathology in primary progressive aphasia. Neurobiol Aging. 2010;33:744–52. doi: 10.1016/j.neurobiolaging.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewan CM, Kertesz A. Reliability and validity characteristics of the western aphasia battery (WAB) J Speech Hear Disord. 1980;45:308–24. doi: 10.1044/jshd.4503.308. [DOI] [PubMed] [Google Scholar]
- Sobrido MJ, Abu-Khalil A, Weintraub S, Johnson N, Quinn B, Cummings JL, et al. Possible association of the tau H1/H1 genotype with primary progressive aphasia. Neurology. 2003;60:86–4. doi: 10.1212/01.wnl.0000049473.36612.f2. [DOI] [PubMed] [Google Scholar]
- Tang-Wai D, Mapstone M. What are we seeing? Is posterior cortical atrophy just Alzheimer disease? Neurology. 2006;66:300–1. doi: 10.1212/01.wnl.0000202093.81603.d8. [DOI] [PubMed] [Google Scholar]
- Wechsler D. A standardized memory scale for clinical use. J Psychol. 1945;19:87–95. [Google Scholar]
- Weintraub S, Mesulam M-M. Four neuropsychological profiles in dementia. In: Boller F, Grafman J, editors. Handbook of neuropsychology. Vol. 8. Amsterdam: Elsevier; 1993. pp. 253–82. [Google Scholar]