Abstract
Early rotavirus vaccine adopter countries in the Americas, Europe, and in Australia have documented substantial declines in rotavirus disease burden following the introduction of vaccination. However, the full public health impact of rotavirus vaccines has not been realized as they have not been introduced into routine immunization programs in countries of Africa and Asia with the highest rotavirus disease morbidity and mortality burden. In this article, we review the epidemiology of rotavirus disease, the development and current status of rotavirus vaccines including newly available vaccine impact data from early-introducer countries, and future priorities for implementation and monitoring of rotavirus vaccination programs in developing countries.
Key words: rotavirus, gastroenteritis, vaccines, rotavirus vaccines, vaccine impact
Rotaviruses are the most common cause of severe infant and childhood gastroenteritis worldwide, responsible for an estimated 23 million outpatient visits, 2.3 million hospitalizations, and over half a million deaths annually among children under 5 y of age.1,2 To mitigate this substantial burden of disease, two live, oral rotavirus vaccines—a monovalent rotavirus vaccine (RV1), Rotarix® (GSK Biologicals) and a pentavalent rotavirus vaccine (RV5), RotaTeq® (Merck and Company, Inc.,)—are now available for use in over 100 countries. Beginning in 2006, many countries in the Americas and Europe adopted rotavirus vaccines into their national immunization programs following availability of clinical trial data from these regions.3,4 In 2009, after data on efficacy of rotavirus vaccines became available from Africa and Asia,5–7 the Strategic Advisory Group of Experts (SAGE) of the World Health Organization (WHO) recommended inclusion of rotavirus vaccines in all national immunization programs worldwide.8 In this paper, we review the epidemiology of rotavirus disease, the development and current status of rotavirus vaccines including newly available vaccine impact data from early-introducer countries and future priorities for implementation and monitoring of rotavirus vaccination programs in developing countries.
Epidemiology of Rotavirus Disease
Rotaviruses are 100 nm, non-enveloped RNA viruses that are made up of a triple-layered protein capsid surrounding a viral genome of 11 segments of double-stranded RNA.9,10 These RNA segments code for six structural proteins and six nonstructural proteins. Two outer layer structural proteins, the VP7 glycoprotein (i.e., G-type glycoprotein) and the VP4 protein (i.e., P-type protease activated protein) determine the G and P serotypes. These structural proteins are the principal targets of neutralizing antibodies that are believed to be important in protection against disease and hence are used in vaccine development. Globally, five G types (G1–G4, and G9) and three P types (P[4], P[6] and P[8]) predominate,11–18 with G1P[8], G2P[4], G3P[8], G4P[8] and G9P[8] combinations accounting for more than 90% of circulating viruses (Fig. 1).14
Figure 1.
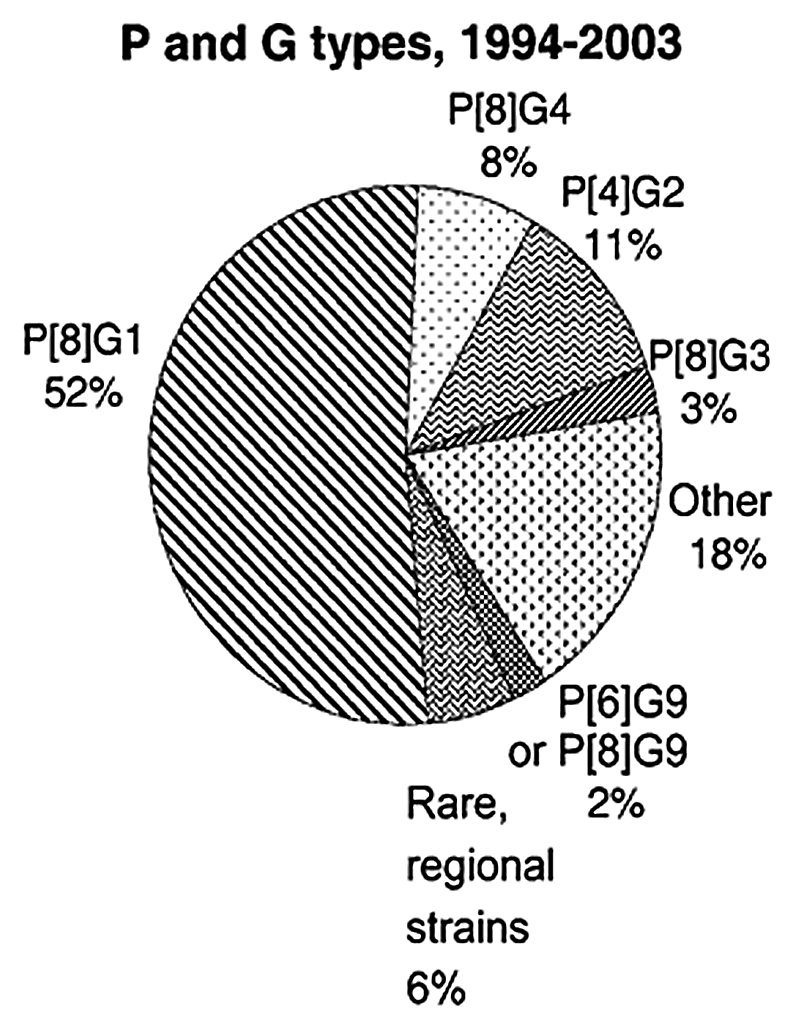
Global distribution of rotavirus strains, 1994–2003. Adapted from Gentsch J, et al.11
Spread of the virus occurs primarily through the fecal-oral route, by close person-to-person contact,10 and also likely through contaminated fomites. The latter may be especially important in out-of-home care settings and hospitals.19–21 Very few infectious virions are needed to cause disease in susceptible hosts.22 Clinical manifestations of illness range from mild, watery diarrhea to severe diarrhea with vomiting and fever that may result in severe dehydration.23–27 Complications and fatalities are related almost exclusively to severe dehydration,28 although rare cases of meningoencephalitis have been reported.29 Other reported complications include acute myositis, hepatitis, hemophagocytic lymphohistiocytosis and polio-like paralysis, but their relationship to rotavirus infection remains unclear.30 Rotavirus has also been detected in some surveys of children with intussusception, a form of bowel obstruction in which a portion of intestine invaginates into another, potentially resulting in bowel edema and ischemia. However, results have been equivocal and further study is needed to evaluate if rotavirus is associated with intussusception among children.31–33 Additionally, although rotavirus infection was originally thought to be confined to the gut, rotavirus antigenemia and viremia have been identified in children with rotavirus disease, but the clinical significance of these findings is unclear.34–36 Symptoms and asymptomatic viral shedding can be prolonged in patients with severely compromised immune systems, such as those with certain primary immunodeficiencies and those who have undergone bone marrow or solid organ transplantation;37–40 infection does not appear to cause more severe disease in children with human immunodeficiency virus (HIV).41–43
Almost all children are infected with rotavirus at least once by the age of 5 y,44,45 regardless of whether they live in industrialized or developing countries. The ubiquitous nature of rotavirus infection indicates that improvements in sanitation and hygiene will not adequately prevent transmission, and thus, vaccines remain the cornerstone of rotavirus disease prevention. The epidemiology of rotavirus disease, however, can differ among countries. Rotavirus infection has demonstrated winter seasonality in countries located in more temperate climates, but has demonstrated less distinct seasonality in countries located in more tropical climates.46 In these countries, disease typically tends to occur year-round and there is often greater diversity in the number of circulating rotavirus strains. Also, children in developing countries tend to acquire their first rotavirus infection at an earlier age (approximately 75% are infected by their first birthday) vs. children in developed countries.47,48 The rates of severe outcomes including mortality are greater in developing country settings possibly because children living in these settings tend to have more co-morbidity, such as co-infections and malnutrition, and limited access to medical care.1
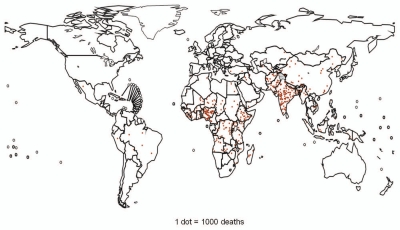
Worldwide, an estimated 527,000 children under 5 y of age die each year from rotavirus disease, translating to approximately 1,440 deaths due to rotavirus per day, with more than 85% of these deaths occurring in low-income countries (Fig. 2).1 Rotavirus is the most common cause of diarrhea requiring hospital care in young children. Data from a global rotavirus surveillance network of 43 countries demonstrated that, in 2009, approximately 25–47% of children under 5 y of age hospitalized with diarrhea tested positive for rotavirus. The lowest proportions occurred in countries in the Americas where rotavirus vaccines had already been introduced and reduced the overall rotavirus disease burden.49
Figure 2.
Estimated distribution of rotavirus deaths among children <5 y of age. Each dot represents 1,000 deaths. Dots are placed at random within each country according to the estimated number of deaths in that country. Adapted from Parashar UD, et al.1
Evolution of Rotavirus Vaccines
The development of rotavirus vaccines has been based on the premise that a live, attenuated rotavirus vaccine can mimic the immunologic response to natural infection without causing significant symptoms and therefore provide protection against clinical disease following subsequent wild-type rotavirus exposure.50–52 First generation rotavirus vaccines used naturally attenuated animal strains, but efficacy of these monovalent vaccines against severe rotavirus disease was variable, and none were licensed for use after completion of clinical trials, with the exception of a lamb-derived vaccine that is currently used in China.53–58 Second generation vaccines, including the currently licensed vaccines, use either naturally attenuated human-animal reassortant strains or an attenuated human strain to induce protection against disease.59
In 1998, a rhesus-human rotavirus vaccine (RotaShield®, Wyeth Lederle Vaccines) was licensed in the US after clinical trials in Finland, the US, and Venezuela demonstrated vaccine efficacy of 82–91% against severe rotavirus gastroenteritis.60–63 However, the vaccine was withdrawn from the US market less than one year after its licensure due to an association with intussusception at a rate of 1 case per 10,000 vaccinated infants that occurred primarily after the first dose.33,64–66 Fortunately, despite this setback, vaccine development continued due to recognition of the significant public health burden of rotavirus disease, and both currently licensed vaccines, RV1 and RV5, underwent large prelicensure safety trials with approximately 60,000 to 70,000 infants each.3,4 No increased risk of intussusception was observed with either vaccine in these clinical trials.
Monovalent Rotavirus Vaccine (RV1)
RV1 is a live, monovalent vaccine that contains an attenuated, human G1P[8] rotavirus strain. Two doses are orally administered early in infancy (first dose may be given as early as six weeks of age). RV1 vaccine trials performed mainly in Latin America and in Europe demonstrated a 2-dose vaccine efficacy of 83% [95% Confidence Interval (CI): 67–92] and 96% (95% CI: 90–99), respectively, against severe rotavirus gastroenteritis through the first year of life. Efficacy against rotavirus disease of any severity was measured in Europe and was 87% (95% CI: 80–92) (Table 1).3,67 In Latin America, efficacy against serotype-specific severe rotavirus gastroenteritis was approximately 92% (95% CI: 74–98) against G1P[8] strains and 87% (95% CI: 64–97) against pooled non-G1 strains (G3P[8], G4P[8] and G9P[8]).3,70 Efficacy was 41% (95% CI: −79–82) against G2P[4] strains which do not share a G- or P-type with the vaccine. However, confidence intervals crossed zero and limited the interpretation of the lower efficacy of RV1 against disease from G2P[4] strains. In Europe, RV1 efficacy against severe rotavirus gastroenteritis through the second year of life was approximately 86% (95% CI: 80–92) against G2P[4] strains and >90% against non-G2 strains, demonstrating significant heterotypic cross-protection.67 Clinical trials conducted in high income Asian countries demonstrated similar efficacy against severe rotavirus gastroenteritis of approximately 96% (95% CI: 85–100) in Hong Kong, Singapore and Taiwan, and 92% (95% CI: 62–99) in Japan through the second year of life (Table 1).68,69 In Hong Kong, Singapore and Taiwan, efficacy against serotype-specific severe rotavirus gastroenteritis was approximately 100% (95% CI: 81–100) against G1P[8] strains and 94% (95% CI: 75–99) against pooled non-G1 strains. In Japan, efficacy against serotype-specific severe rotavirus gastroenteritis was approximately 92% (95% CI: 31–99) against G1 strains, 100% (95% CI: 24–100) against G3 strains, and non-significantly, 75% (95% CI: −382–100) against G9 strains. However, clinical trials conducted in Africa demonstrated lower vaccine efficacy through the first year of life against severe rotavirus gastroenteritis of approximately 72% (95% CI: 40–88) in South Africa and 49% (95% CI: 11–72) in Malawi (Table 1).7 No difference in vaccine efficacy against severe gastroenteritis due to G1 strains and non-G1 strains was observed in either South Africa or Malawi. In Malawi, where greater strain diversity was encountered, almost 87% of strains circulating during the trial were non-G1.7 Despite lower vaccine efficacy in these African countries, the number of rotavirus cases prevented per 100 person-years was substantial (6.7 cases per 100 person-years in Malawi; 4.2 cases per 100 person-years in South Africa) and was greater than estimates of cases prevented in Latin America and Europe, largely because of the substantially greater baseline rates of severe rotavirus disease.
Table 1.
Summary of vaccine efficacy findings from clinical trials of rotavirus vaccines (RV1 and RV5)
| Location | Vaccine | Vaccine Efficacy (95% Cl) | Rotavirus Gastroenteritis Severity |
| High and middle income | |||
| Latin America and Finland3 | RV1 | 85% (72–92) | Severe (Vesikari score ≥11) |
| Europe67 | RV1 | 87% (80–92) | Any severity |
| Asia (Hong Kong, Singapore, Taiwan)68 | RV1 | 96% (85–100) | Severe (Vesikari score ≥11) |
| Japan69 | RV1 | 92% (62–99) | Severe (Vesikari score ≥11) |
| United States and Finland4 | RV5 | 98% (88–100) | Severe G1–G4 (Clark score >16) |
| 74% (67–80) | Any severity (G1–G4) | ||
| Middle-low and low income | |||
| Africa (South Africa and Malawi)7 | RV1 | 59% (36–74) | Severe (Vesikari score ≥11) |
| Africa (Kenya, Ghana, Mali)5 | RV5 | 64% (40–79) | Severe (Vesikari score ≥11) |
| Asia (Vietnam and Bangladesh)6 | RV5 | 51% (13–73) | Severe (Vesikari score ≥11) |
Pentavalent Rotavirus Vaccine (RV5)
RV5 is a live, pentavalent human-bovine reassortant vaccine that contains 5 reassortant viruses, 4 expressing a unique human G-type protein (G1–G4) and one expressing a human P-type protein (P[8]).4 Three doses are orally administered early in infancy (first dose may be given as early as 6 weeks of age). Pre-licensure clinical trials performed predominantly in the US and Finland demonstrated a vaccine efficacy of 98% (95% CI: 88–100) against severe G1–G4 rotavirus gastroenteritis and 74% (95% CI: 67–80) against G1–G4 rotavirus gastroenteritis of any severity through the first full rotavirus season following vaccination (Table 1); efficacy against G2 rotavirus gastroenteritis of any severity was approximately 63% (95% CI: 3–88).4 Similar to the RV1 trials, vaccine trials performed in Africa and Asia demonstrated lower efficacy; vaccine efficacy against severe rotavirus gastroenteritis through the first year of life was 64% (95% CI: 40–79) in Africa (Ghana, Kenya and Mali) and 51% (95% CI: 13–73) in Asia (Bangladesh and Vietnam) (Table 1).5,6 In these African and Asian countries, the majority (89–100%) of rotavirus strains circulating during the trials were of G and P genotypes contained in RV5. Similar to the RV1 results, despite lower vaccine efficacy, the number of rotavirus cases prevented per 100 person-years in Africa and Asia was substantial (2.7 cases per 100 person-years in Africa, 3.3 cases per 100 person-years in Asia).
Post-marketing Rotavirus Vaccine Impact and Effectiveness Studies
Many studies have demonstrated the real-world impact of rotavirus vaccines in early-introducer countries in the Americas, Europe and in Australia (Table 2). In the Americas, where rotavirus vaccines have been integrated into the national immunization programs of many countries since 2006, vaccine effectiveness in routine use has been similar to the vaccine efficacy seen in pre-licensure trials.71,79,84 Sustained reductions in diarrhea-related mortality and/or morbidity have been observed in Brazil, El Salvador, Mexico, Panama and the US,72,74–76,78,80,85 thus demonstrating the benefits of vaccination in countries of diverse socioeconomic status (Figs. 3 and 4). Similar findings have been observed in Europe, where Austria and Belgium were the earliest countries to introduce both rotavirus vaccines into their national immunization programs, and also in Australia.81–83 Evidence of indirect benefits (i.e., herd immunity) for older children has been noted after vaccine introduction in several countries, including the US, Austria, Australia and El Salvador.74,80,81,83
Table 2.
Summary of findings from vaccine impact studies from early-introduction countries
| Location | Vaccine | Key Findings from Major Vaccine Impact Studies |
| The Americas | ||
| Brazil | RV1 | Vaccine effectiveness of 85% (95% CI: 54–95) against G2P[4] rotavirus gastroenteritis among children 6–11 mo of age;71 ∼22% reduction in diarrhea-associated mortality rates and ∼17% reduction in diarrhea-associated hospitalization rates among children <5 y during postvaccine years 2007–200972 |
| El Salvador | RV1 | Vaccine effectiveness of 74% against severe rotavirus gastroenteritis (Vesikari score ≥11) and 88% against very severe rotavirus gastroenteritis (Vesikari score ≥15);73 ∼69–81% decline in rotavirus hospitalizations rates and ∼35–48% decline in all-cause diarrhea events (outpatient and inpatient) among children <5 y in 2008 and 200974 |
| Mexico | RV1 | ∼35% reduction in diarrhea-related mortality rate among children <5 y in 2008; 75 ∼11–40% reduction in all-cause diarrhea hospitalizations among children <5 y in 2008 and 200976 |
| Nicaragua | RV5 | Vaccine effectiveness of 52–63% against severe rotavirus gastroenteritis (Vesikari score ≥11) and 73–86% against very severe rotavirus gastroenteritis (Vesikari score ≥15) in the first year post vaccine introduction77 |
| Panama | RV1 | ∼22–37% reduction in all-cause diarrhea hospitalizations among children <5 y in 2007 and 200878 |
| US | RV1 and RV5 | RV5 vaccine effectiveness of 83–86% against rotavirus gastroenteritis ED visits or hospitalizations over 2 rotavirus seasons, 2008–2009;79 ∼46% decline in all-cause diarrhea hospitalization rates among children <5 y in 18 states in 2008 resulting in ∼40,000 to 60,000 fewer gastroenteritis-related hospitalizations;80 delayed, shorter rotavirus seasons and a sustained reduction in the number of rotavirus antigen-positive tests through the 2009–2010 rotavirus season72 |
| Europe | ||
| Austria | RV1 and RV5 | Vaccine effectiveness of 60–97% against rotavirus hospitalization; ∼79–87% reduction in rotavirus hospitalizations among children age-eligible to receive vaccine in 2008 and 200981 |
| Belgium | RV1 and RV5 | ∼65–83% reduction in rotavirus hospitalizations during postvaccine years 2007–201082 |
| Australia | RV1 and RV5 | Vaccine effectiveness of 89–94% against rotavirus hospitalizations; ∼53–93% reduction in rotavirus hospitalizations among children ≤3 y83 |
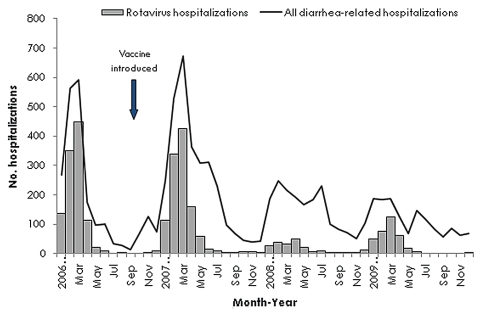
Figure 3.
Number of diarrhea-related and rotavirus hospitalizations among children <5 y of age at 7 sentinel surveillance hospitals, El Salvador, January 2006—December 2009. Adapted from Yen C, et al.74
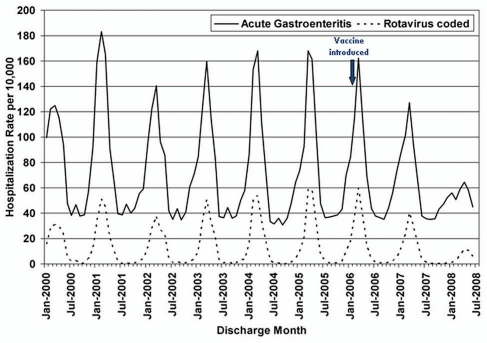
Figure 4.
Monthly acute gastroenteritis and rotavirus—coded hospitalization rates among children aged <5 y from January 2000 through June 2008, in 18 states—US. Adapted from Curns AT, et al.80
Of specific interest to the global community are findings of significant reductions in under-5 mortality related to diarrhea in Mexico and Brazil after the introduction of rotavirus vaccine.72,75 In both settings, the reduction has been large, amounting to declines of over 1,300 childhood deaths annually, and sustained for more than 3 y since the introduction of vaccine. The effect of the vaccines on diarrhea mortality was not assessed in clinical trials. Thus, these findings provide welcome news for developing countries of Africa and Asia where over 85% of the global rotavirus mortality occurs.
Post-Marketing Rotavirus Vaccine Safety Surveillance
As mentioned previously, no increased risk of intussusception was observed during large pre-licensure safety trials for either of both currently licensed vaccines, RV1 and RV5.3,4 However, some post-marketing safety studies have detected a low risk of intussusception, primarily in the first week after the first vaccine dose. Studies conducted in Mexico (RV1) and Australia (RV1 and RV5) found a low-level, increased risk of intussusception of approximately 1 per 50,000 to 100,000 vaccinated infants following the first dose of vaccine, risks much lower than the 1 per 10,000 seen with Rotashield®.86–88 A risk with the first dose was not observed in a similar study conducted in Brazil (RV1),87 although a low-level risk with the second dose of vaccine was noted. Post-marketing data on RV5 available from the US have not demonstrated an increased risk of intussusception, although currently available US data cannot reliably exclude the level of risk seen in Mexico and Australia.89,90
The WHO Global Advisory Committee for Vaccine Safety reviewed available data in late 2010, and concluded that the substantial benefits of vaccination outweigh the small risk of intussusception seen in some post-licensure studies.8 For example, in Mexico, rotavirus vaccination has prevented some 330 deaths and 280 hospitalizations related to rotavirus diarrhea for every death and hospitalization related to vaccine-associated intussusception. WHO continues to recommend universal rotavirus vaccination of infants.
Considerations and Future Priorities
Despite the WHO recommendation for inclusion of rotavirus vaccination in all national immunization programs and the welldocumented benefits of vaccination, only 27 of 193 WHO member states have introduced rotavirus vaccines nationally to date.49 As many countries weigh the introduction of rotavirus vaccines into their national immunization programs and as other countries decide whether to continue to fund their existing rotavirus vaccination programs, several areas of consideration and future priorities should be addressed. These include monitoring of the impact of rotavirus vaccination programs in other countries to support the introduction of rotavirus vaccines, monitoring of existing rotavirus vaccination programs to support sustained use, understanding vaccine effectiveness (including indirect effects and the economic benefits across different settings), understanding the impact on circulating strains, monitoring the safety of rotavirus vaccines, improving the performance of rotavirus vaccines in developing country settings, and improving financial access to rotavirus vaccines.
Evidence of the benefits of vaccination can be useful to help countries decide whether to introduce rotavirus vaccine into their national immunization programs and, once introduced, whether to provide continued support for the rotavirus vaccination program. In countries considering introduction of rotavirus vaccines, documentation of the burden of rotavirus disease and the cost-effectiveness of a vaccination program may provide decision makers the evidence required for prioritizing limited health care funding on a new vaccine. This will be particularly important for developing countries in Africa and Asia, where the impact of vaccination will likely be substantial even after taking into account the lower vaccine efficacies seen in the clinical trials, given high disease burdens.5–7 In countries that have existing rotavirus vaccination programs, documenting the impact of the vaccination program can be achieved through studies assessing the field effectiveness of vaccination and the direct and indirect benefits of vaccination on diarrhea-related disease trends and healthcare costs. Targeted vaccine introduction projects may help demonstrate the health benefits of vaccination and allow appraisal of potential logistical or programmatic issues related to rotavirus vaccine introduction. Additionally, rotavirus strain surveillance can provide a better understanding of the impact of vaccination on strain diversity and evolution. These studies are important since it is not known whether vaccination will provide protection against all circulating strains and there is a question of whether some of these strains may be selected over the longterm in highly vaccinated populations, either through reassortment events between different rotavirus strains, antigenic drift in viral antigens, or both. Documenting changes occurring in strains through whole genome sequencing may help elucidate potential rotaviral resistance mechanisms to vaccine immunity if they occur.
Understanding the overall balance between the benefits and possible risks of vaccination, especially with regards to intussusception, is essential. The reasons for the low-level risk of intussusception seen in recent studies in Mexico, Brazil and Australia are currently unclear.86–88 Post-licensure assessments will help better characterize the potential risk of intussusception and may elucidate the potential mechanism of vaccine-associated intussusception. To do this, strong surveillance systems are necessary to help establish baseline rates of intussusception and to monitor for adverse events following the introduction of a new vaccine. However, given the limitations of passive reporting systems, relying on active surveillance at sentinel hospitals may be a more efficient and economical approach to assess the risk of intussusception following vaccination.
Optimizing the performance of rotavirus vaccines in developing country settings is a priority as data from studies in Africa, Asia, and the Americas indicate lower vaccine efficacy/effectiveness and suggest waning immunity against rotavirus disease in the second year of life that has not been seen in higher income countries.5–7,77 In these settings, the immune response to oral vaccines in early infancy may be reduced because of higher levels of transplacental maternal antibody, interference by immune and nonimmune components of breast milk, micronutrient malnutrition (e.g., vitamin A and zinc), interfering gut flora, and the disease state of the infant (e.g., HIV infection or other concomitant infections).91–93 More research in the area of immune response to oral rotavirus vaccines in early infancy is needed, and future studies with the aim of improving the immune response to vaccination, including the use of micronutrient supplementation (i.e., zinc and/or probiotics), withholding breastfeeding at the time of vaccination, and determining the optimal dosing schedule, including providing neonatal or booster doses of vaccine, will be important to determine if protection in low-resource settings during the first year of life can be improved and extended through the second year of life. Protection against severe rotavirus disease through the second year of life is necessary for maximal public health impact. Additionally, continued investment in the research and development of new rotavirus vaccines may lead to vaccines with greater effectiveness in developing countries, as well as lower cost vaccines. Currently, emerging vaccine manufacturers in Brazil, China, Germany, India, Indonesia and Vietnam are working on the development of new rotavirus vaccines. The original rhesus-based reassortant vaccine is also undergoing renewed development with a manufacturer in Germany.94 Finally, there is renewed interest in exploring non-replicating, parenteral rotavirus vaccine candidates.95 All of this work may lead to the development of better oral vaccines and/or oral delivery systems or of vaccines with alternative delivery systems, such as inactivated vaccines, that may potentially have better efficacy and safety profiles.
One of the most significant barriers to the introduction of rotavirus vaccines is financial. Current rotavirus vaccines are more expensive than most other vaccines supplied by national immunization programs. Therefore, for those countries that are not eligible for new vaccine support through the GAVI Alliance (formerly known as the Global Alliance for Vaccines and Immunizations), the costs and benefits of a national rotavirus vaccination program must be weighed carefully. For rotavirus vaccines to have maximal public health impact globally, financial access to these vaccines must be improved, either through reduced prices or additional funding mechanisms. Recent successful fundraising efforts by the GAVI Alliance and the reduction in the price of RV1 to US$2.50 per dose by GlaxoSmithKline for GAVI-eligible countries are great steps toward improving access to these vaccines for populations in countries with the highest risk for the disease and in the greatest need of protection,96,97 but these steps alone are not enough, particularly for populations not supported by the GAVI Alliance.
Conclusion
Substantial declines in rotavirus disease burden have been documented in early rotavirus vaccine adopter countries in the Americas, Europe, and in Australia. However, the full public health impact of these vaccines has not been completely realized as they have been introduced in few countries in Africa and Asia (i.e., those with the highest rotavirus disease morbidity and mortality). As rotavirus vaccines are adopted and used more widely, it is imperative that evidence of the benefits of vaccination be documented, the safety of these vaccines continue to be monitored, efforts to maximize the impact of current and future vaccines be made, and financial barriers to introduction and maintenance of vaccination programs be reduced to allow countries to make informed decisions that are in the best interest of public health. To monitor these parameters, sustainable surveillance systems will need to be established and maintained in more countries adopting rotavirus vaccines.
Acknowledgements
The authors have indicated that they have no conflicts of interest relevant to this article to disclose.
Abbreviations
- SAGE
strategic advisory group of experts
- WHO
World Health Organization
- HIV
human immunodeficiency virus
- GAVI Alliance
formerly known as the Global Alliance for Vaccines and Immunizations
- RV1
monovalent rotavirus vaccine
- RV5
pentavalent rotavirus vaccine
Financial Support
The authors have indicated that they have no financial relationships relevant to this article to disclose.
Disclaimer
The findings and conclusions of this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, et al. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis. 2009;200:9–15. doi: 10.1086/605025. [DOI] [PubMed] [Google Scholar]
- 2.Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 4.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 5.Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:606–614. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 6.Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–623. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 7.Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization, author. Meeting of the Global Advisory Committee on Vaccine Safety, December 2010. Wkly Epidemiol Rec. 2011;86:38–43. [PubMed] [Google Scholar]
- 9.Greenberg HB, Estes MK. Rotaviruses: from pathogenesis to vaccination. Gastroenterology. 2009;136:1939–1951. doi: 10.1053/j.gastro.2009.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estes MK, Kapikian AZ. Rotaviruses. In: Knipe DM, Howley PM, editors. Field's Virology. Philadelphia, Pennsylvania: Lippincott, Willliams & Wilkins; 2007. [Google Scholar]
- 11.Gentsch JR, Laird AR, Bielfelt B, Griffin DD, Banyai K, Ramachandran M, et al. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. J Infect Dis. 2005;192:146–159. doi: 10.1086/431499. [DOI] [PubMed] [Google Scholar]
- 12.Gentsch JR, Woods PA, Ramachandran M, Das BK, Leite JP, Alfieri A, et al. Review of G and P typing results from a global collection of rotavirus strains: implications for vaccine development. J Infect Dis. 1996;174:30–36. doi: 10.1093/infdis/174.Supplement_1.S30. [DOI] [PubMed] [Google Scholar]
- 13.Matthijnssens J, Bilcke J, Ciarlet M, Martella V, Banyai K, Rahman M, et al. Rotavirus disease and vaccination: impact on genotype diversity. Future Microbiol. 2009;4:1303–1316. doi: 10.2217/fmb.09.96. [DOI] [PubMed] [Google Scholar]
- 14.Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol. 2005;15:29–56. doi: 10.1002/rmv.448. [DOI] [PubMed] [Google Scholar]
- 15.Kang G, Arora R, Chitambar SD, Deshpande J, Gupte MD, Kulkarni M, et al. Multicenter, hospital-based surveillance of rotavirus disease and strains among indian children aged <5 years. J Infect Dis. 2009;200:147–153. doi: 10.1086/605031. [DOI] [PubMed] [Google Scholar]
- 16.Linhares AC, Stupka JA, Ciapponi A, Bardach AE, Glujovsky D, Aruj PK, et al. Burden and typing of rotavirus group A in Latin America and the Caribbean: systematic review and meta-analysis. Rev Med Virol. 2011;21:89–109. doi: 10.1002/rmv.682. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Padilla E, Grais RF, Guerin PJ, Steele AD, Burny ME, Luquero FJ. Burden of disease and circulating serotypes of rotavirus infection in sub-Saharan Africa: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:567–576. doi: 10.1016/S1473-3099(09)70179-3. [DOI] [PubMed] [Google Scholar]
- 18.Todd S, Page NA, Duncan Steele A, Peenze I, Cunliffe NA. Rotavirus strain types circulating in Africa: Review of studies published during 1997–2006. J Infect Dis. 2010;202:34–42. doi: 10.1086/653555. [DOI] [PubMed] [Google Scholar]
- 19.Ansari SA, Springthorpe VS, Sattar SA. Survival and vehicular spread of human rotaviruses: possible relation to seasonality of outbreaks. Rev Infect Dis. 1991;13:448–461. doi: 10.1093/clinids/13.3.448. [DOI] [PubMed] [Google Scholar]
- 20.Butz AM, Fosarelli P, Dick J, Cusack T, Yolken R. Prevalence of rotavirus on high-risk fomites in day-care facilities. Pediatrics. 1993;92:202–205. [PubMed] [Google Scholar]
- 21.Pickering LK, Bartlett AV, Woodward WE. Acute infectious diarrhea among children in day care: epidemiology and control. Rev Infect Dis. 1986;8:539–547. doi: 10.1093/clinids/8.4.539. [DOI] [PubMed] [Google Scholar]
- 22.Bishop RF. Natural history of human rotavirus infection. Arch Virol Suppl. 1996;12:119–128. doi: 10.1007/978-3-7091-6553-9_14. [DOI] [PubMed] [Google Scholar]
- 23.Black RE, Lopez de Romana G, Brown KH, Bravo N, Bazalar OG, Kanashiro HC. Incidence and etiology of infantile diarrhea and major routes of transmission in Huascar, Peru. Am J Epidemiol. 1989;129:785–799. doi: 10.1093/oxfordjournals.aje.a115193. [DOI] [PubMed] [Google Scholar]
- 24.Kovacs A, Chan L, Hotrakitya C, Overturf G, Portnoy B. Serum transaminase elevations in infants with rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr. 1986;5:873–877. doi: 10.1097/00005176-198611000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Mata L, Simhon A, Padilla R, del Mar Gamboa M, Vargas G, Hernandez F, et al. Diarrhea associated with rotaviruses, enterotoxigenic Escherichia coli, Campylobacter, and other agents in Costa Rican children 1976–1981. Am J Trop Med Hyg. 1983;32:146–153. doi: 10.4269/ajtmh.1983.32.146. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez WJ, Kim HW, Arrobio JO, Brandt CD, Chanock RM, Kapikian AZ, et al. Clinical features of acute gastroenteritis associated with human reovirus-like agent in infants and young children. J Pediatr. 1977;91:188–193. doi: 10.1016/S0022-3476(77)80810-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staat MA, Azimi PH, Berke T, Roberts N, Bernstein DI, Ward RL, et al. Clinical presentations of rotavirus infection among hospitalized children. Pediatr Infect Dis J. 2002;21:221–227. doi: 10.1097/00006454-200203000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Matson DO. Rotaviruses. In: Long SS, Pickering LK, Prober CG, editors. Principles and Practice of Pediatric Infectious Diseases. New York, NY: Churchill Livingstone; 2003. [Google Scholar]
- 29.Dickey M, Jamison L, Michaud L, Care M, Bernstein DI, Staat MA. Rotavirus meningoencephalitis in a previously healthy child and a review of the literature. Pediatr Infect Dis J. 2009;28:318–321. doi: 10.1097/INF.0b013e31818ddbe9. [DOI] [PubMed] [Google Scholar]
- 30.Gilger MA, Matson DO, Conner ME, Rosenblatt HM, Finegold MJ, Estes MK. Extraintestinal rotavirus infections in children with immunodeficiency. J Pediatr. 1992;120:912–917. doi: 10.1016/S0022-3476(05)81959-6. [DOI] [PubMed] [Google Scholar]
- 31.Bines JE, Liem NT, Justice FA, Son TN, Kirkwood CD, de Campo M, et al. Risk factors for intussusception in infants in Vietnam and Australia: adenovirus implicated, but not rotavirus. J Pediatr. 2006;149:452–460. doi: 10.1016/j.jpeds.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Konno T, Suzuki H, Kutsuzawa T, Imai A, Katsushima N, Sakamoto M, et al. Human rotavirus infection in infants and young children with intussusception. J Med Virol. 1978;2:265–269. doi: 10.1002/jmv.1890020310. [DOI] [PubMed] [Google Scholar]
- 33.Peter G, Myers MG. Intussusception, rotavirus and oral vaccines: summary of a workshop. Pediatrics. 2002;110:67. doi: 10.1542/peds.110.6.e67. [DOI] [PubMed] [Google Scholar]
- 34.Blutt SE, Conner ME. Rotavirus: to the gut and beyond! Curr Opin Gastroenterol. 2007;23:39–43. doi: 10.1097/MOG.0b013e328011829d. [DOI] [PubMed] [Google Scholar]
- 35.Blutt SE, Kirkwood CD, Parreno V, Warfield KL, Ciarlet M, Estes MK, et al. Rotavirus antigenaemia and viraemia: a common event? Lancet. 2003;362:1445–1449. doi: 10.1016/S0140-6736(03)14687-9. [DOI] [PubMed] [Google Scholar]
- 36.Fischer TK, Ashley D, Kerin T, Reynolds-Hedmann E, Gentsch J, Widdowson MA, et al. Rotavirus anti-genemia in patients with acute gastroenteritis. J Infect Dis. 2005;192:913–919. doi: 10.1086/432549. [DOI] [PubMed] [Google Scholar]
- 37.Liakopoulou E, Mutton K, Carrington D, Robinson S, Steward CG, Goulden NJ, et al. Rotavirus as a significant cause of prolonged diarrhoeal illness and morbidity following allogeneic bone marrow transplantation. Bone Marrow Transplant. 2005;36:691–694. doi: 10.1038/sj.bmt.1705127. [DOI] [PubMed] [Google Scholar]
- 38.Rayani A, Bode U, Habas E, Fleischhack G, Engelhart S, Exner M, et al. Rotavirus infections in paediatric oncology patients: a matched-pairs analysis. Scand J Gastroenterol. 2007;42:81–87. doi: 10.1080/00365520600842179. [DOI] [PubMed] [Google Scholar]
- 39.Saulsbury FT, Winkelstein JA, Yolken RH. Chronic rotavirus infection in immunodeficiency. J Pediatr. 1980;97:61–65. doi: 10.1016/S0022-3476(80)80131-4. [DOI] [PubMed] [Google Scholar]
- 40.Stelzmueller I, Dunst KM, Hengster P, Wykypiel H, Steurer W, Wiesmayr S, et al. A cluster of rotavirus enteritis in adult transplant recipients. Transpl Int. 2005;18:470–474. doi: 10.1111/j.1432-2277.2004.00073.x. [DOI] [PubMed] [Google Scholar]
- 41.Steele AD, Cunliffe N, Tumbo J, Madhi SA, De Vos B, Bouckenooghe A. A review of rotavirus infection in and vaccination of human immunodeficiency virus-infected children. J Infect Dis. 2009;200:57–62. doi: 10.1086/605027. [DOI] [PubMed] [Google Scholar]
- 42.Cunliffe NA, Gondwe JS, Kirkwood CD, Graham SM, Nhlane NM, Thindwa BD, et al. Effect of concomitant HIV infection on presentation and outcome of rotavirus gastroenteritis in Malawian children. Lancet. 2001;358:550–555. doi: 10.1016/S0140-6736(01)05706-3. [DOI] [PubMed] [Google Scholar]
- 43.Jere C, Cunliffe NA, Hoffman IF, Stewart PW, Kilaru R, Broadhead RL, et al. Plasma HIV burden in Malawian children co-infected with rotavirus. AIDS. 2001;15:1439–1442. doi: 10.1097/00002030-200107270-00016. [DOI] [PubMed] [Google Scholar]
- 44.Gurwith M, Wenman W, Hinde D, Feltham S, Greenberg H. A prospective study of rotavirus infection in infants and young children. J Infect Dis. 1981;144:218–224. doi: 10.1093/infdis/144.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Velázquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, et al. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022–1028. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 46.Levy K, Hubbard AE, Eisenberg JN. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol. 2009;38:1487–1496. doi: 10.1093/ije/dyn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bahl R, Ray P, Subodh S, Shambharkar P, Saxena M, Parashar U, et al. Incidence of severe rotavirus diarrhea in New Delhi, India, and G and P types of the infecting rotavirus strains. J Infect Dis. 2005;192:114–119. doi: 10.1086/431497. [DOI] [PubMed] [Google Scholar]
- 48.Cunliffe NA, Kilgore PE, Bresee JS, Steele AD, Luo N, Hart CA, et al. Epidemiology of rotavirus diarrhoea in Africa: a review to assess the need for rotavirus immunization. Bull World Health Organ. 1998;76:525–537. [PMC free article] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention, author. Rotavirus surveillance—worldwide, 2009. MMWR Morb Mortal Wkly Rep. 2011;60:514–516. [PubMed] [Google Scholar]
- 50.Fischer TK, Valentiner-Branth P, Steinsland H, Perch M, Santos G, Aaby P, et al. Protective immunity after natural rotavirus infection: a community cohort study of newborn children in Guinea-Bissau, west Africa. J Infect Dis. 2002;186:593–597. doi: 10.1086/342294. [DOI] [PubMed] [Google Scholar]
- 51.Moulton LH, Staat MA, Santosham M, Ward RL. The protective effectiveness of natural rotavirus infection in an American Indian population. J Infect Dis. 1998;178:1562–1566. doi: 10.1086/314504. [DOI] [PubMed] [Google Scholar]
- 52.Ward RL, Bernstein DI. Protection against rotavirus disease after natural rotavirus infection. US Rotavirus Vaccine Efficacy Group. J Infect Dis. 1994;169:900–904. doi: 10.1093/infdis/169.4.900. [DOI] [PubMed] [Google Scholar]
- 53.De Mol P, Zissis G, Butzler JP, Mutwewingabo A, Andre FE. Failure of live, attenuated oral rotavirus vaccine. Lancet. 1986;2:108. doi: 10.1016/S0140-6736(86)91643-0. [DOI] [PubMed] [Google Scholar]
- 54.Hanlon P, Hanlon L, Marsh V, Byass P, Shenton F, Hassan-King M, et al. Trial of an attenuated bovine rotavirus vaccine (RIT 4237) in Gambian infants. Lancet. 1987;1:1342–1345. doi: 10.1016/S0140-6736(87)90649-0. [DOI] [PubMed] [Google Scholar]
- 55.Lanata CF, Black RE, del Aguila R, Gil A, Verastegui H, Gerna G, et al. Protection of Peruvian children against rotavirus diarrhea of specific serotypes by one, two, or three doses of the RIT 4237 attenuated bovine rotavirus vaccine. J Infect Dis. 1989;159:452–459. doi: 10.1093/infdis/159.3.452. [DOI] [PubMed] [Google Scholar]
- 56.Vesikari T. Clinical trials of live oral rotavirus vaccines: the Finnish experience. Vaccine. 1993;11:255–261. doi: 10.1016/0264-410X(93)90026-T. [DOI] [PubMed] [Google Scholar]
- 57.Vesikari T, Isolauri E, Delem A, d'Hondt E, Andre FE, Beards GM, et al. Clinical efficacy of the RIT 4237 live attenuated bovine rotavirus vaccine in infants vaccinated before a rotavirus epidemic. J Pediatr. 1985;107:189–194. doi: 10.1016/S0022-3476(85)80123-2. [DOI] [PubMed] [Google Scholar]
- 58.Vesikari T, Isolauri E, D'Hondt E, Delem A, Andre FE, Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984;1:977–981. doi: 10.1016/S0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]
- 59.Tate JE, Patel MM, Steele AD, Gentsch JR, Payne DC, Cortese MM, et al. Global impact of rotavirus vaccines. Expert Rev Vaccines. 2010;9:395–407. doi: 10.1586/erv.10.17. [DOI] [PubMed] [Google Scholar]
- 60.Bernstein DI, Glass RI, Rodgers G, Davidson BL, Sack DA. Evaluation of rhesus rotavirus monovalent and tetravalent reassortant vaccines in US children. US Rotavirus Vaccine Efficacy Group. JAMA. 1995;273:1191–1196. doi: 10.1001/jama.273.15.1191. [DOI] [PubMed] [Google Scholar]
- 61.Joensuu J, Koskenniemi E, Pang XL, Vesikari T. Randomised placebo-controlled trial of rhesus-human reassortant rotavirus vaccine for prevention of severe rotavirus gastroenteritis. Lancet. 1997;350:1205–1209. doi: 10.1016/S0140-6736(97)05118-0. [DOI] [PubMed] [Google Scholar]
- 62.Perez-Schael I, Garcia D, Gonzalez M, Gonzalez R, Daoud N, Perez M, et al. Prospective study of diarrheal diseases in Venezuelan children to evaluate the efficacy of rhesus rotavirus vaccine. J Med Virol. 1990;30:219–229. doi: 10.1002/jmv.1890300315. [DOI] [PubMed] [Google Scholar]
- 63.Rennels MB, Glass RI, Dennehy PH, Bernstein DI, Pichichero ME, Zito ET, et al. Safety and efficacy of high-dose rhesus-human reassortant rotavirus vaccines—report of the National Multicenter Trial. United States Rotavirus Vaccine Efficacy Group. Pediatrics. 1996;97:7–13. [PubMed] [Google Scholar]
- 64.Centers for Disease Control and Prevention, author. Intussusception among recipients of rotavirus vaccine—United States 1998–1999. MMWR Morb Mortal Wkly Rep. 1999;48:577–581. [PubMed] [Google Scholar]
- 65.Kramarz P, France EK, Destefano F, Black SB, Shinefield H, Ward JI, et al. Population-based study of rotavirus vaccination and intussusception. Pediatr Infect Dis J. 2001;20:410–416. doi: 10.1097/00006454-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 66.Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344:564–572. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- 67.Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370:1757–1763. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- 68.Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak SH, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double-blind, controlled study. Vaccine. 2009;27:5936–5941. doi: 10.1016/j.vaccine.2009.07.098. [DOI] [PubMed] [Google Scholar]
- 69.Kawamura N, Tokoeda Y, Oshima M, Okahata H, Tsutsumi H, Van Doorn LJ, et al. Efficacy, safety and immunogenicity of RIX4414 in Japanese infants during the first two years of life. Vaccine. 2011;29 doi: 10.1016/j.vaccine.2011.05.017;21640780. [DOI] [PubMed] [Google Scholar]
- 70.Linhares AC, Velazquez FR, Perez-Schael I, Saez-Llorens X, Abate H, Espinoza F, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1181–1189. doi: 10.1016/S0140-6736(08)60524-3. [DOI] [PubMed] [Google Scholar]
- 71.Correia JB, Patel MM, Nakagomi O, Montenegro FM, Germano EM, Correia NB, et al. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. J Infect Dis. 2010;201:363–369. doi: 10.1086/649843. [DOI] [PubMed] [Google Scholar]
- 72.do Carmo GM, Yen C, Cortes J, Siqueira AA, de Oliveira WK, Cortez-Escalante JJ, et al. Decline in diarrhea mortality and admissions after routine childhood rotavirus immunization in Brazil: a time-series analysis. PLoS Med. 2011;8:1001024. doi: 10.1371/journal.pmed.1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Palma O, Cruz L, Ramos H, de Baires A, Villatoro N, Pastor D, et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case-control study. BMJ. 2010;340:2825. doi: 10.1136/bmj.c2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yen C, Armero Guardado JA, Alberto P, Rodriguez Araujo DS, Mena C, Cuellar E, et al. Decline in rotavirus hospitalizations and health care visits for childhood diarrhea following rotavirus vaccination in El Salvador. Pediatr Infect Dis J. 2011;30:6–10. doi: 10.1097/INF.0b013e3181fefa05. [DOI] [PubMed] [Google Scholar]
- 75.Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, Esparza-Aguilar M, Johnson B, Gomez-Altamirano CM, et al. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med. 2010;362:299–305. doi: 10.1056/NEJMoa0905211. [DOI] [PubMed] [Google Scholar]
- 76.Quintanar-Solares M, Yen C, Richardson V, Esparza-Aguilar M, Parashar UD, Patel MM. Impact of rotavirus vaccination on diarrhea-related hospitalizations among children <5 years of age in Mexico. Pediatr Infect Dis J. 2011;30:11–15. doi: 10.1097/INF.0b013e3181fefb32. [DOI] [PubMed] [Google Scholar]
- 77.Patel M, Pedreira C, De Oliveira LH, Tate J, Orozco M, Mercado J, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA. 2009;301:2243–2251. doi: 10.1001/jama.2009.756. [DOI] [PubMed] [Google Scholar]
- 78.Molto Y, Cortes JE, De Oliveira LH, Mike A, Solis I, Suman O, et al. Reduction of diarrhea-associated hospitalizations among children aged <5 Years in Panama following the introduction of rotavirus vaccine. Pediatr Infect Dis J. 2011;30:16–20. doi: 10.1097/INF.0b013e3181fefc68. [DOI] [PubMed] [Google Scholar]
- 79.Boom JA, Tate JE, Sahni LC, Rench MA, Quaye O, Mijatovic-Rustempasic S, et al. Sustained protection from pentavalent rotavirus vaccination during the second year of life at a large, urban United States pediatric hospital. Pediatr Infect Dis J. 2010;29:1133–1135. doi: 10.1097/INF.0b013e3181ed18ab. [DOI] [PubMed] [Google Scholar]
- 80.Curns AT, Steiner CA, Barrett M, Hunter K, Wilson E, Parashar UD. Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J Infect Dis. 2010;201:1617–1624. doi: 10.1086/652403. [DOI] [PubMed] [Google Scholar]
- 81.Paulke-Korinek M, Rendi-Wagner P, Kundi M, Kronik R, Kollaritsch H. Universal mass vaccination against rotavirus gastroenteritis: impact on hospitalization rates in austrian children. Pediatr Infect Dis J. 2010;29:319–323. doi: 10.1097/INF.0b013e3181c18434. [DOI] [PubMed] [Google Scholar]
- 82.Braeckman T, Van Herck K, Raes M, Vergison A, Sabbe M, Van Damme P. Rotavirus vaccines in Belgium: policy and impact. Pediatr Infect Dis J. 2011;30:21–24. doi: 10.1097/INF.0b013e3181fefc51. [DOI] [PubMed] [Google Scholar]
- 83.Buttery JP, Lambert SB, Grimwood K, Nissen MD, Field EJ, Macartney KK, et al. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia's National Childhood vaccine schedule. Pediatr Infect Dis J. 2011;30:25–29. doi: 10.1097/INF.0b013e3181fefdee. [DOI] [PubMed] [Google Scholar]
- 84.Boom JA, Tate JE, Sahni LC, Rench MA, Hull JJ, Gentsch JR, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics. 2010;125:199–207. doi: 10.1542/peds.2009-1021. [DOI] [PubMed] [Google Scholar]
- 85.Tate JE, Mutuc JD, Panozzo CA, Payne DC, Cortese MM, Cortes JE, et al. Sustained decline in rotavirus detections in the United States following the introduction of rotavirus vaccine in 2006. Pediatr Infect Dis J. 2011;30:30–34. doi: 10.1097/INF.0b013e3181ffe3eb. [DOI] [PubMed] [Google Scholar]
- 86.Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, et al. Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine. 2011;29:3061–3066. doi: 10.1016/j.vaccine.2011.01.088. [DOI] [PubMed] [Google Scholar]
- 87.Patel MM, Lopez-Collada VR, Bulhoes MM, De Oliveira LH, Bautista Marquez A, Flannery B, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011;364:2283–2292. doi: 10.1056/NEJMoa1012952. [DOI] [PubMed] [Google Scholar]
- 88.Australian Government Therapeutic Goods Administration, author. Rotavirus vaccination and risk of intussusception. [August 24, 2011]. http://www.tga.gov.au/safety/alertsmedicine-rotavirus-110225.htm.
- 89.Belongia EA, Irving SA, Shui IM, Kulldorff M, Lewis E, Yin R, et al. Real-time surveillance to assess risk of intussusception and other adverse events after pentavalent, bovine-derived rotavirus vaccine. Pediatr Infect Dis J. 2010;29:1–5. doi: 10.1097/INF.0b013e3181af8605. [DOI] [PubMed] [Google Scholar]
- 90.Haber P, Patel M, Izurieta HS, Baggs J, Gargiullo P, Weintraub E, et al. Postlicensure monitoring of intussusception after RotaTeq vaccination in the United States, February 1, 2006, to September 25, 2007. Pediatrics. 2008;121:1206–1212. doi: 10.1542/peds.2007-3793. [DOI] [PubMed] [Google Scholar]
- 91.Levine MM. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol. 2010;8:129. doi: 10.1186/1741-7007-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patel M, Shane AL, Parashar UD, Jiang B, Gentsch JR, Glass RI. Oral rotavirus vaccines: how well will they work where they are needed most? J Infect Dis. 2009;200:39–48. doi: 10.1086/605035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moon SS, Wang Y, Shane AL, Nguyen T, Ray P, Dennehy P, et al. Inhibitory effect of breast milk on infectivity of live oral rotavirus vaccines. Pediatr Infect Dis J. 2010;29:919–923. doi: 10.1097/INF.0b013e3181e232ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.BusinessWire website B. Rotavirus Vaccine Manufacturing Agreement Between BIOVIRx and IDT 2006. [July 22, 2011]. http://www.businesswire.com/news/home/20060327005013/en/Rotavirus-Vaccine-Manufacturing-Agreement-BIOVIRx-IDT.
- 95.Wang Y, Azevedo M, Saif LJ, Gentsch JR, Glass RI, Jiang B. Inactivated rotavirus vaccine induces protective immunity in gnotobiotic piglets. Vaccine. 2010;28:5432–5436. doi: 10.1016/j.vaccine.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 96.GlaxoSmithKline website. Millions of children in the world's poorest countries could receive vaccination against rotavirus diarrhoeal disease under new offer made by GSK to the GAVI Alliance. [July 22, 2011]. http://www.gsk.com/media/pressreleases/2011/2011-pressrelease-462284.htm.
- 97.Donors commit vaccine funding to achieve historic milestone. [July 22, 2011]. GAVI Alliance website. http://www.gavialliance.org/library/news/press-releases/2011/donors-commit-vaccine-funding-to-achieve-historicmilestone-in-global-health/ [Google Scholar]