Abstract
In this observer-blind study (NCT00423046), women (N = 1,106), stratified by age (18–26, 27–35, 36–45 y), were randomized (1:1) to receive the HPV-16/18 vaccine (Cervarix®, GlaxoSmithKline Biologicals, Months 0, 1, 6) or the HPV-6/11/16/18 vaccine (Gardasil® Merck and Co., Inc., Months 0, 2, 6). Month 7 results were previously reported; we now report Month 24 results. In the according-to-protocol cohort for immunogenicity (seronegative and DNA-negative at baseline for HPV type analyzed), seropositivity rates of neutralizing antibodies (nAbs) [pseudovirion-based neutralization assay] were, across all age strata, 100% (HPV-16/18 vaccine) and 97.5–100% (HPV-6/11/16/18 vaccine) for HPV-16, and 99.0–100% (HPV-16/18 vaccine) and 72.3–84.4% (HPV-6/11/16/18 vaccine) for HPV-18. Corresponding geometric mean titers (GMTs) were 2.4–5.8-fold higher for HPV-16 and 7.7–9.4-fold higher for HPV-18 with the HPV-16/18 vaccine vs. the HPV-6/11/16/18 vaccine; HPV-16 and HPV-18 GMTs were significantly higher with the HPV-16/18 vaccine than the HPV-6/11/16/18 vaccine (p < 0.0001) in the total vaccinated cohort (received ≥1 vaccine dose, irrespective of baseline sero/DNA-status). Similar results were obtained using enzyme-linked immunosorbent assay (ELISA ). Positivity rates and GMTs of antigen-specific IgG antibodies in cervicovaginal secretions (ELISA) were not significantly different between vaccines. At Month 24, CD4+ T-cell responses for HPV-16 and HPV-18 were higher with the HPV-16/18 vaccine; memory B-cell response was higher for HPV-18 with the HPV-16/18 vaccine and similar between vaccines for HPV-16. Both vaccines were generally well tolerated. Although an immunological correlate of protection has not been defined, differences in the magnitude of immune response between vaccines may represent determinants of duration of protection.
Key words: Cervarix®, Gardasil®, human papillomavirus, immunogenicity, safety
Introduction
Cervical cancer is the second most frequent cancer in women worldwide, leading to an estimated 270,000 deaths annually.1,2 Persistent infection with oncogenic human papillomavirus (HPV) is a necessary cause of cervical cancer.3,4 Together, HPV types 16 and 18 account for approximately 70% of invasive cervical cancers worldwide.1,5
Two prophylactic HPV vaccines, Cervarix® (HPV-16/18 vaccine): Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Recombinant, adjuvanted, adsorbed) (GlaxoSmithKline Biologicals) and Gardasil® (HPV-6/11/16/18 vaccine): Human Papillomavirus Quadrivalent (Types 6, 11, 16 and 18) Vaccine, Recombinant (Merck and Co., Inc.,), are currently licensed in over 100 countries. The HPV-16/18 vaccine contains virus-like particles (VLPs) assembled from the L1 major capsid proteins of HPV-16 and HPV-18, formulated with the proprietary immunostimulatory Adjuvant System 04 (AS04; 3-O-desacyl-4′-monophosphoryl lipid A [MPL] and aluminum hydroxide).6,7 The HPV-6/11/16/18 vaccine contains L1 VLPs for HPV types 6 and 11, which cause genital warts, and 16 and 18, and is formulated with a proprietary amorphous aluminum hydroxyphosphate sulfate (AAHS) adjuvant.8,9
In the short to medium term, clinical studies have shown both vaccines to be highly efficacious against persistent HPV-16/18 infection and their associated precancerous lesions.10–13 Protection has been demonstrated for up to 6.4 y post-vaccination for the HPV-16/18 vaccine10,13–15 and up to 5 y post-vaccination for the licensed formulation of the HPV-6/11/16/18 vaccine,12,16–18 although there has been a longer follow-up with a prototype HPV-16 monovalent vaccine.19 Any clinically significant difference in efficacy between the two HPV vaccines (e.g., waning protection) may not become apparent for many years and would not be evaluable through clinical trials, in part due to the extremely high efficacy of both of these vaccines. We believe that differences will become apparent during long-term assessments of vaccinated females in countries with organized registries and population-based tracking of breakthrough lesions and associated HPV types.
Genital HPV infections are rapidly acquired after initiation of sexual activity,20–22 hence prophylactic HPV vaccination programs generally target young adolescents prior to sexual debut. However, women are at risk for HPV infection for as long as they are sexually active.23 Therefore, induction of enduring protection is an important element in cervical cancer prevention.
Neutralizing antibodies are the hallmark of most known successful vaccines today. As HPV evades the host immune system, antibody levels induced after natural infection are low and may not be sufficient to protect against re-infection. Serum neutralizing antibodies, which transudate to the site of HPV infection (i.e., the cervical mucosa), are thought to be the major basis of protection afforded by L1 VLP-based vaccines.24–28 Induction of antigen-specific memory B-cells, a process in which CD4+ T-cells play an essential role, may also be important for long-term vaccine- induced antibody protection.6,25
Both the HPV-16/18 vaccine and the HPV-6/11/16/18 vaccine were licensed based on the efficacy of each vaccine observed in adolescents and young adults and the immunogenicity demonstrated across all licensed age groups. We conducted a randomized, observer-blind, study to compare the immunogenicity and safety of the HPV-16/18 vaccine and the HPV-6/11/16/18 vaccine in healthy women aged 18–45 y (Study HPV-010; NCT00423046). The primary objective was to compare vaccine-induced geometric mean titers (GMTs) of serum anti-HPV-16 and anti-HPV-18 neutralizing antibodies by a pseudovirionbased neutralization assay (PBNA). PBNA measures a range of neutralizing antibodies, whereas enzyme-linked immunosorbent assay (ELISA) measures neutralizing and non-neutralizing antibodies.29 Secondary objectives included the assessment of serum anti-HPV-16 and -18 IgG antibodies by ELISA, anti-HPV-16 and -18 antibodies in cervicovaginal secretions (CVS), HPV-16/18-specific memory B-cell and CD4+ T-cell responses and a safety analysis.
Findings at Month 7 (i.e., one month after completion of the full vaccination series) were previously reported: the HPV-16/18 vaccine induced superior anti-HPV-16/18 neutralizing antibody levels in serum, higher anti-HPV-16/18 antibody positivity rates in CVS, and significantly higher HPV-16/18-specific circulating memory B-cell frequencies, when compared with the HPV-6/11/16/18 vaccine.30 Both vaccines were generally well tolerated and compliance with the full vaccination course was similarly high in both groups.30
We now report follow-up of this study cohort through Month 24 (i.e., 18 mo after completion of the full vaccination series). The data presented extend the relevance and implications of our initial findings30 for clinicians and other stakeholders in cervical cancer prevention.
Results
Study population.
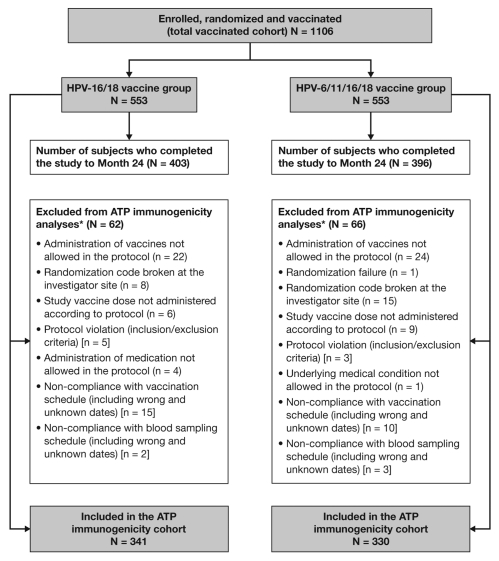
A total of 1,106 women (553 women in each group) were enrolled and vaccinated. The number of women excluded from the Month 24 immunogenicity analyses was similar in the HPV-16/18 vaccine (n = 62) and HPV-6/11/16/18 vaccine (n = 66) groups, as were the reasons for exclusion (Fig. 1). The according-to-protocol (ATP) cohort for immunogenicity (all evaluable subjects who received three vaccine doses and for whom data concerning immunogenicity endpoint measures were available), comprised 671 women at Month 24 (341 women in the HPV-16/18 vaccine group and 330 women in the HPV-6/11/16/18 vaccine group). The total vaccinated cohort (TVC; all subjects who received at least one vaccine dose) comprised 799 women at Month 24: 403 women in the HPV-16/18 vaccine group and 396 women in the HPV-6/11/16/18 vaccine group. In both the ATP cohort for immunogenicity and the TVC at Month 24, the demographic profiles of subjects were similar between the two vaccine groups (mean age 31 y, racial distribution 84–90% Caucasian).
Figure 1.
Subject disposition. ATP, according to protocol. *Women may have been excluded for more than one reason, but were only counted for the primary reason for exclusion. Primary and secondary between-group comparisons to assess non-inferiority were performed in the according-to-protocol (ATP) cohort for immunogenicity on women who were seronegative and DNA-negative (by PCR) prior to vaccination for the antigen under analysis. Exploratory analyses of superiority and analyses of reactogenicity/safety were performed in the total vaccinated cohort on all women irrespective of their serostatus and DNA status prior to vaccination.
Antibody responses in serum.
The seropositivity rates and GMTs of anti-HPV-16 and -18 neutralizing antibodies measured by PBNA in women in the ATP cohort for immunogenicity (irrespective of serostatus and DNA status prior to vaccination) are presented in Table 1. The seropositivity rates and GMTs of anti-HPV-16 and -18 neutralizing antibodies measured by PBNA in women in the ATP cohort for immunogenicity who were seronegative and DNA-negative for the antigen under analysis prior to vaccination, which is the target population for HPV vaccination programs, are shown in Figure 2. At Month 24, 100% of women in the HPV-16/18 vaccine group and 97.5–100% of women in the HPV-6/11/16/18 vaccine group remained seropositive for HPV-16, across all age strata; 99.0–100% of women in the HPV-16/18 vaccine group and 72.3–84.4% of women in the HPV-6/11/16/18 vaccine group remained seropositive for HPV-18.
Table 1.
Seropositivity rates and geometric mean titers (GMTs) for anti-HPV-16 and anti-HPV-18 serum neutralizing antibodies measured by pseudovirion-based neutralization assay at Months 0, 6, 7, 12, 18 and 24 (ATP cohort for immunogenicity, irrespective of serostatus and DNA status prior to vaccination)
| A. 18–26 years | |||||||
| HPV-16/18 vaccine | HPV-6/11/16/18 vaccine | ||||||
| Antigen | Month | N | % SP [95% CI] | GMT [95% CI] | N | % SP [95% CI] | GMT [95% CI] |
| HPV-16 | 0 | 132 | 17.4 [11.4, 25.0] | 30.1 [25.4, 35.7] | 137 | 19.7 [13.4, 27.4] | 29.4 [25.5, 34] |
| 6 7 12 18 24 |
132 132 127 125 122 |
100 [97.2, 100] 100 [97.2, 100] 100 [97.1, 100] 100 [97.1, 100] 100 [97.0, 100] |
1928 [1556, 2390] 34417 [28161, 42063] 14446 [11400, 18306] 5724 [4598, 7127] 5060 [4080, 6274] |
136 137 134 121 116 |
99.3 [96.0, 100] 100 [97.3, 100] 100 [97.3, 100] 100 [97.0, 100] 98.3 [93.9, 99.8] |
2096 [1623, 2705] 10402 [8634, 12533] 3932 [3145, 4916] 1561 [1203, 2026] 1135 [870, 1481] |
|
| HPV-18 | 0 | 132 | 6.8 [3.2, 12.5] | 22.5 [20.6, 24.5] | 137 | 3.6 [1.2, 8.3] | 21.9 [20.1, 23.8] |
| 6 7 12 18 24 |
132 132 127 125 122 |
99.2 [95.9, 100] 100 [97.2, 100] 100 [97.1, 100] 100 [97.1, 100] 100 [97.0, 100] |
770 [616, 961] 16204 [13336, 19689] 4393 [3536, 5458] 2308 [1840, 2895] 1710 [1368, 2137] |
136 137 134 121 116 |
93.4 [87.8, 96.9] 100 [97.3, 100] 96.3 [91.5, 98.8] 93.4 [87.4, 97.1] 85.3 [77.6, 91.2] |
260 [206, 329] 2301 [1859, 2849] 635 [503, 803] 280 [218, 360] 196 [149, 259] |
|
| B. 27–35 years | |||||||
| HPV-16/18 vaccine | HPV-6/11/16/18 vaccine | ||||||
| Antigen | Month | N | % SP [95% CI] | GMT [95% CI] | N | % SP [95% CI] | GMT [95% CI] |
| HPV-16 | 0 | 117 | 16.2 [10.1, 24.2] | 31.2 [25.5, 38.2] | 116 | 23.3 [15.9, 32.0] | 32.4 [26.7, 39.4] |
| 6 7 12 18 24 |
117 117 118 113 109 |
100 [96.9, 100] 100 [96.9, 100] 100 [96.9, 100] 100 [96.8, 100] 100 [96.7, 100] |
1839 [1300, 2600] 25389 [20559, 31354] 8574 [6602, 11134] 3468 [2668, 4508] 2852 [2243, 3626] |
115 116 116 113 105 |
99.1 [95.3, 100] 100 [96.9, 100] 99.1 [95.3, 100] 99.1 [95.2, 100] 98.1 [93.3, 99.8] |
2181 [1530, 3111] 7667 [5930, 9913] 2852 [2118, 3839] 1217 [874, 1696] 968 [705, 1327] |
|
| HPV-18 | 0 | 117 | 10.3 [5.4, 17.2] | 23.5 [21.2, 26.1] | 116 | 12.1 [6.8, 19.4] | 26.2 [22.3, 30.7] |
| 6 7 12 18 24 |
117 117 118 113 109 |
97.4 [92.7, 99.5] 100 [96.9, 100] 99.2 [95.4, 100] 100 [96.8, 100] 100 [96.7, 100] |
580 [427, 788] 10009 [8026, 12482] 2616 [2049, 3340] 1464 [1138, 1883] 1128 [878, 1449] |
115 116 116 113 105 |
86.1 [78.4, 91.8] 98.3 [93.9, 99.8] 91.4 [84.7, 95.8] 77.9 [69.1, 85.1] 75.2 [65.9, 83.1] |
234 [173, 315] 1217 [927, 1598] 367 [271, 498] 179 [133, 240] 158 [115, 217] |
|
| C. 36–45 years | |||||||
| HPV-16/18 vaccine | HPV-6/11/16/18 vaccine | ||||||
| Antigen | Month | N | % SP [95% CI] | GMT [95% CI] | N | % SP [95% CI] | GMT [95% CI] |
| HPV-16 | 0 | 121 | 19.0 [12.4, 27.1] | 29.2 [24.7, 34.6] | 111 | 24.3 [16.7, 33.4] | 34.6 [28.0, 42.8] |
| 6 7 12 18 24 |
121 121 116 113 109 |
99.2 [95.5, 100] 100 [97.0, 100] 100 [96.9, 100] 100 [96.8, 100] 100 [96.7, 100] |
3027 [2109, 4344] 20286 [16324, 25211] 9323 [7262, 11969] 3177 [2461, 4101] 2865 [2207, 3719] |
109 111 111 110 108 |
100 [96.7, 100] 100 [96.7, 100] 100 [96.7, 100] 100 [96.7, 100] 100 [96.6, 100] |
3134 [2244, 4378] 9506 [7397, 12216] 3898 [2920, 5202] 1562 [1146, 2128] 1347 [990, 1833] |
|
| HPV-18 | 0 | 121 | 7.4 [3.5, 13.7] | 24.0 [21.0, 27.5] | 111 | 18.0 [11.4, 26.4] | 28.1 [24.0, 32.8] |
| 6 7 12 18 24 |
121 121 116 113 109 |
97.5 [92.9, 99.5] 100 [97.0, 100] 100 [96.9, 100] 99.1 [95.2, 100] 99.1 [95.0, 100] |
717 [524, 980] 9675 [7826, 11960] 3198 [2492, 4104] 1534 [1183, 1990] 1116 [856, 1455] |
109 111 111 110 108 |
89.9 [82.7, 94.9] 100 [96.7, 100] 99.1 [95.1, 100] 89.1 [81.7, 94.2] 81.5 [72.9, 88.3] |
292 [207, 411] 1856 [1429, 2411] 639 [474, 861] 276 [203, 376] 222 [158, 311] |
|
GMT, geometric mean titer; N, number of subjects with available results; SP, seropositivity [defined as neutralizing antibody titer ≥40 ED50 (effective dose producing 50% response)]. The ATP cohort for immunogenicity included all evaluable subjects who received three vaccine doses (i.e., those meeting all eligibility criteria and complying with the procedures defined in the protocol) for whom data concerning immunogenicity endpoint measures were available. This included subjects for whom assay results were available for antibodies against at least one study vaccine antigen (HPV-16 or HPV-18) at the timepoint under analysis.
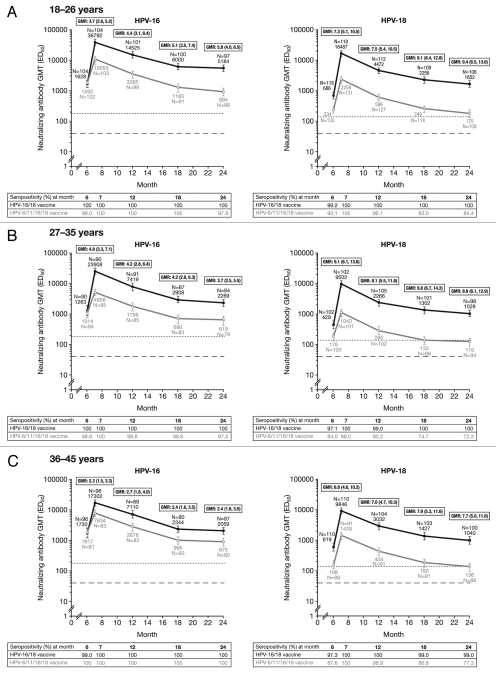
Figure 2.
Geometric mean titers (GMTs), GMT ratios (GMR) and seropositivity rates for anti-HPV-16 and anti-HPV-18 serum neutralizing antibodies measured by pseudovirion-based neutralization assay at Months 6, 7, 12, 18 and 24 (ATP cohort for immunogenicity, seronegative and DNA-negative prior to vaccination). Solid black lines, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Recombinant, adjuvanted, adsorbed) (Cervarix®); solid gray lines, Human Papillomavirus Quadrivalent (Types 6, 11, 16 and 18) Vaccine, Recombinant (Gardasil®). Error bars denote 95% confidence intervals (CIs) of GMTs. Dotted line, neutralizing antibody GMTs measured by PBNA in women in the total vaccinated cohort of the HPV-010 study who had cleared natural infection (prior to vaccination) [i.e., those who were seropositive and DNA-negative at Month 0]: 180.1 ED50 for HPV-16 and 137.3 ED50 for HPV-18.30 Dashed line, PBNA limit of detection (40 ED50). ED50, effective dose producing 50% response; GMR, geometric mean titer ratio; GMT, geometric mean titer. GMR = HPV-16/18 vaccine GMT divided by HPV-6/11/16/18 vaccine GMT at Month 7, 12, 18 or 24 computed using an ANOVA model on the log10 transformation of the titers in each age cohort. 95% CIs for GMRs are presented in parentheses. Seropositivity defined as neutralizing antibody titer ≥40 ED50. The ATP cohort for immunogenicity included all evaluable subjects who received three vaccine doses (i.e., those meeting all eligibility criteria and complying with the procedures defined in the protocol) for whom data concerning immunogenicity endpoint measures were available. This included subjects for whom assay results were available for antibodies against at least one study vaccine antigen (HPV-16 or HPV-18) at the timepoint under analysis.
At Month 24, in women aged 18–26 y, anti-HPV-16 and -18 neutralizing antibody GMTs were 5.8- and 9.4-fold higher, respectively, in the HPV-16/18 vaccine group than in the HPV-6/11/16/18 vaccine group. Compared with the HPV-6/11/16/18 vaccine at the same timepoint, anti-HPV-16 and -18 GMTs with the HPV-16/18 vaccine were 3.7- and 8.9-fold higher in women aged 27–35 y and 2.4- and 7.7-fold higher in women aged 36–45 y, respectively. Non-inferiority of the HPV-16/18 vaccine vs. the HPV-6/11/16/18 vaccine was demonstrated in the ATP cohort for immunogenicity (seronegative and DNA-negative prior to vaccination) for both anti-HPV-16 and -18 at all timepoints through to Month 24 and in all age groups (Fig. 2). Exploratory superiority testing performed in the TVC (irrespective of serostatus and DNA status prior to vaccination) showed anti-HPV-16 and −18 neutralizing antibody levels induced by the HPV-16/18 vaccine to be superior to those induced by the HPV-6/11/16/18 vaccine in all age groups and at all timepoints up to Month 24 (p < 0.0001) (Table 2).
Table 2.
Exploratory superiority assessments of geometric mean titers (GMTs) of anti-HPV-16 and anti-HPV-18 serum neutralizing antibodies measured by pseudovirion-based neutralization assay at Months 7, 12, 18 and 24 (total vaccinated cohort, irrespective of serostatus and DNA status prior to vaccination)
| A. 18–26 years | ||||||
| Antigen | Month | HPV-16/18 vaccine | HPV-6/11/16/18 vaccine | ANOVA p-value* | ||
| N | GMT | N | GMT | |||
| HPV-16 | 7 12 18 24 |
167 153 147 145 |
31715 13671 5442 4855 |
168 162 147 135 |
8682 3506 1396 1055 |
<0.0001 <0.0001 <0.0001 <0.0001 |
| HPV-18 | 7 12 18 24 |
167 153 147 145 |
13732 4234 2252 1660 |
168 162 147 135 |
1886 574 246 191 |
<0.0001 <0.0001 <0.0001 <0.0001 |
| B. 27–35 years | ||||||
| Antigen | Month | HPV-16/18 vaccine | HPV-6/11/16/18 vaccine | ANOVA p-value* | ||
| N | GMT | N | GMT | |||
| HPV-16 | 7 12 18 24 |
146 140 133 129 |
25134 8713 3584 2870 |
148 141 136 124 |
7322 2883 1206 1006 |
<0.0001 <0.0001 <0.0001 <0.0001 |
| HPV-18 | 7 12 18 24 |
146 140 133 129 |
9390 2765 1548 1193 |
148 141 136 124 |
1178 397 200 171 |
<0.0001 <0.0001 <0.0001 <0.0001 |
| C. 36–45 years | ||||||
| Antigen | Month | HPV-16/18 vaccine | HPV-6/11/16/18 vaccine | ANOVA p-value* | ||
| N | GMT | N | GMT | |||
| HPV-16 | 7 12 18 24 |
143 137 131 128 |
21874 9731 3750 3069 |
143 141 137 136 |
9828 4046 1553 1313 |
<0.0001 <0.0001 <0.0001 <0.0001 |
| HPV-18 | 7 12 18 24 |
143 137 131 128 |
9760 3385 1672 1186 |
143 141 137 136 |
1709 607 268 226 |
<0.0001 <0.0001 <0.0001 <0.0001 |
GMT, geometric mean titer; N, number of subjects with available results.
p-values refer to superiority testing; superiority of the HPV-16/18 vaccine demonstrated if p ≤ 0.05 (p-values are exploratory for Months 12, 18 and 24).
Across all age groups in the ATP cohort for immunogenicity (seronegative and DNA-negative prior to vaccination) and at all timepoints, the HPV-16/18 vaccine induced anti-HPV-16 and -18 neutralizing antibody levels which were above levels observed in women with evidence of previous exposure to natural infection (i.e., seropositive and DNA-negative at Month 0).30 For the HPV-6/11/16/18 vaccine, anti-HPV-16 neutralizing antibody GMTs were also above those associated with previous exposure in all age groups and at all timepoints up to Month 24; however, anti-HPV-18 neutralizing antibody GMTs at Month 24 were above those associated with previous exposure only in women aged 18–26 y (Fig. 2).
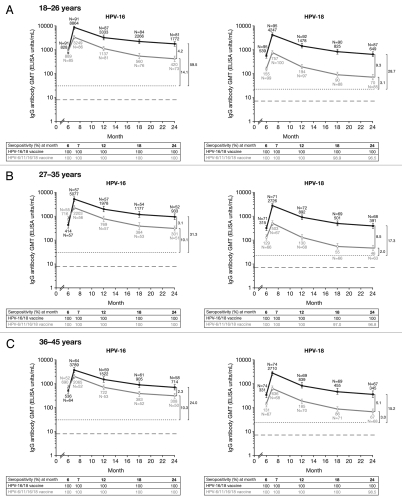
Vaccine-induced HPV type-specific serum antibody responses assessed by ELISA were consistent with PBNA results (Fig. 3). Across all age groups in the ATP cohort for immunogenicity (seronegative and DNA-negative prior to vaccination), GMTs of anti-HPV-16 and -18 IgG antibodies at Month 24 were 2.3- to 4.2-fold and 5.1- to 9.3-fold higher, respectively, in the HPV-16/18 vaccine group than in the HPV-6/11/16/18 vaccine group.
Figure 3.
Geometric mean titers (GMTs), Month 24 GMT ratios (GMR) and seropositivity rates for anti-HPV-16 and anti-HPV-18 IgG antibodies measured by enzyme-linked immunosorbent assay at Months 6, 7, 12, 18 and 24 (ATP cohort for immunogenicity, seronegative and DNA negative prior to vaccination). Solid black lines, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Recombinant adjuvanted, adsorbed) [Cervarix®]; solid gray lines, Human Papillomavirus Quadrivalent (Types 6, 11, 16 and 18) Vaccine, Recombinant [Gardasil®]. Error bars denote 95% confidence intervals (CIs) of GMTs. Dotted line, GMTs for natural infection antibody levels (measured by ELISA) in the HPV-008 study: 29.8 ELISA units/mL for HPV-16 and 22.6 ELISA units/mL for HPV-18.10 GMTs for natural infection antibody levels (measured by ELISA) in the HPV-001 14 were 50 EU/mL for anti HPV-16 antibodies and 41 EU/mL anti HPV-18 antibodies. Vaccine induced antibody levels remained above the levels associated with natural infection in women in the ATP cohort for immunogenicity who received all three doses of HPV-16/18 vaccine through to 7.3 y follow-up of Study HPV-001 (HPV-007, HPV-023) 14,15,44 and through to Month 36 follow-up of Study HPV-008.10,13 Dashed line, ELISA limit of detection (8 ELISA units/mL for HPV-16 and 7 ELISA units/mL for HPV-18). Black square brackets, GMRs = HPV-16/18 vaccine GMT divided by HPV-6/11/16/18 vaccine GMT at Month 24 computed using an ANOVA model on the log10 transformation of the titers in each age cohort. Seropositivity defined as neutralizing antibody titer ≥8 ELISA units/mL for HPV-16 and ≥7 ELISA units for HPV-18. The ATP cohort for immunogenicity included all evaluable subjects who received three vaccine doses (i.e., those meeting all eligibility criteria and complying with the procedures defined in the protocol) for whom data concerning immunogenicity endpoint measures were available. This included subjects for whom assay results were available for antibodies against at least one study vaccine antigen (HPV-16 or HPV-18) at the timepoint under analysis.
Antibody responses in cervicovaginal secretions.
For all age groups combined, anti-HPV-16 positivity rates in CVS at Month 24 were 77.8% (95% CI: 62.9, 88.8) in the HPV-16/18 vaccine group and 55.8% (39.9, 70.9) in the HPV-6/11/16/18 vaccine group, respectively (Table 3). Anti-HPV-18 IgG antibody positivity rates in CVS were 68.9% (53.4, 81.8) in the HPV-16/18 vaccine group compared with 39.5% (25.0, 55.6) in the HPV-6/11/16/18 vaccine group at Month 24. GMTs of antigen-specific IgG antibodies in CVS calculated on positive subjects in the subset at Month 24 were similar between groups for both HPV-16 and HPV-18.
Table 3.
Positivity rates and geometric mean titers (GMTs) of anti-HPV-16 and anti-HPV-18 IgG antibodies measured in cervicovaginal secretions by enzyme-linked immunosorbent assay at Months 7, 12, 18 and 24 (ATP cohort for immunogenicity, irrespective of serostatus and DNA status prior to vaccination)
| HPV-16/18 vaccine | HPV-6/11/16/18 vaccine | ||||||||
| Antigen | Month | N | n | % P [95% CI] | GMT* [95% CI] | N | n | % P [95% CI] | GMT* [95% CI] |
| HPV-16 | Baseline 7 12 18 24 |
24 48 50 42 45 |
0 46 43 33 35 |
0.0 [0.0, 14.2] 95.8 [85.7, 99.5] 86.0 [73.3, 94.2] 78.3 [63.2, 89.7] 77.8 [62.9, 88.8] |
- [-, -] 168.9 [114.4, 249.4] 135.6 [93.8, 195.9] 96.0 [64.8, 142.3] 85.0 [57.9, 124.8] |
36 57 46 43 43 |
0 51 35 26 24 |
0.0 [0.0, 9.7] 89.5 [78.5, 96.0] 76.1 [61.2, 87.4] 60.5 [44.4, 75.0] 55.8 [39.9, 70.9] |
- [-, -] 90.7 [62.6, 131.6] 46.8 [33.0, 66.5] 52.8 [35.6, 78.3] 45.1 [27.0, 75.2] |
| HPV-18 | Baseline 7 12 18 24 |
24 48 50 42 45 |
1 43 40 28 31 |
4.2 [0.1, 21.1] 89.6 [77.3, 96.5] 80.0 [66.3, 90.0] 66.7 [50.5, 80.4] 68.9 [53.4, 81.8] |
15.4 [-, -] 88.6 [65.0, 120.8] 59.0 [42.5, 81.9] 33.2 [21.3, 51.8] 43.9 [28.1, 68.8] |
36 57 47 43 43 |
3 40 21 13 17 |
8.3 [1.8, 22.5] 70.2 [56.6, 81.6] 44.7 [30.2, 59.9] 30.2 [17.2, 46.1] 39.5 [25.0, 55.6] |
13.5 [0.3, 701.8] 43.4 [30.1, 62.6] 29.1 [18.6, 45.3] 16.4 [10.4, 25.9] 21.5 [10.5, 44.3] |
GMT, geometric mean titer; N, number of subjects with available results; n, number of subjects with an antibody titer ≥ the limit of quantification; P, positivity (defined as antibody titer ≥0.58 EU/mL for HPV-16 and ≥0.35 EU/mL for HPV-18).
GMTs were calculated on positive subjects (n values) because data for all subjects in the subset did not follow a normal distribution. Dashes (-) indicate where there were insufficient values (i.e., n ≤ 1) to calculate GMTs.
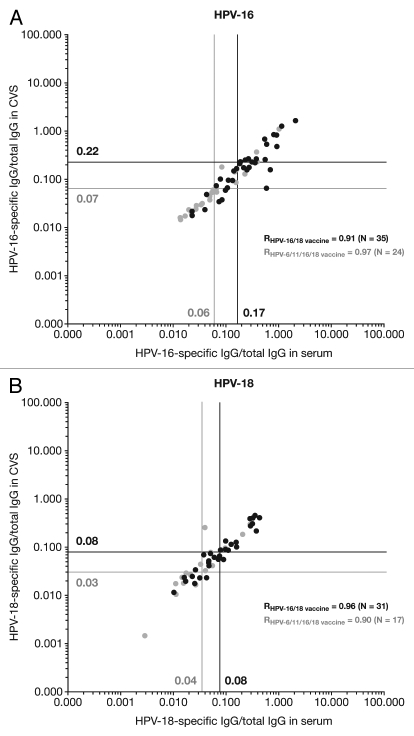
A linear relationship (high Pearson correlation coefficient; r) between HPV-specific antibody titers in serum and CVS was observed in both the HPV-16/18 vaccine and HPV-6/11/16/18 vaccine groups; for HPV-16, r = 0.91 and 0.97 and HPV-18, r = 0.96 and 0.90 (Fig. 4). For each vaccine, geometric means (GM) of the ratios between HPV-specific IgG and total IgG antibodies were generally similar for serum and CVS samples, for both HPV-16 and HPV-18. This suggests that for both vaccines a similar proportion of HPV-specific IgG antibodies transudate from serum to CVS. GM ratios in serum and CVS were approximately 2–3-fold higher with the HPV-16/18 vaccine than with the HPV-6/11/16/18 vaccine; for HPV-16, GMT ratios were 4.45, 4.23 and 2.65 for the three age ranges and for HPV-18 were 7.51, 8.09 and 6.99.
Figure 4.
Correlation between serum and cervicovaginal secretions for (A) HPV-16 and (B) HPV-18 IgG antibodies (standardized for total IgG antibodies) measured by enzyme-linked immunosorbent assay at Month 24 (ATP cohort for immunogenicity). CVS, cervicovaginal secretions; IgG, immunoglobulin G; R, Pearson correlation coefficient. Solid black lines and filled black circles, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Recombinant, adjuvanted, adsorbed) [Cervarix®]; solid gray lines and filled gray circles, Human Papillomavirus Quadrivalent (Types 6, 11, 16 and 18) Vaccine, Recombinant [Gardasil®]. Only samples that tested positive for the antigen under analysis in both serum and CVS (i.e., double-positive samples) were analyzed. Vertical and horizontal lines represent the geometric means of the ratios between HPV-specific antibody titers and total IgG antibody content in serum and CVS samples, respectively. HPV-16/18-specific IgG levels were measured by VLP-specific ELISA. The total IgG antibody concentration of each sample was measured using an ELISA developed and validated in-house by GSK.
Memory B-cell responses.
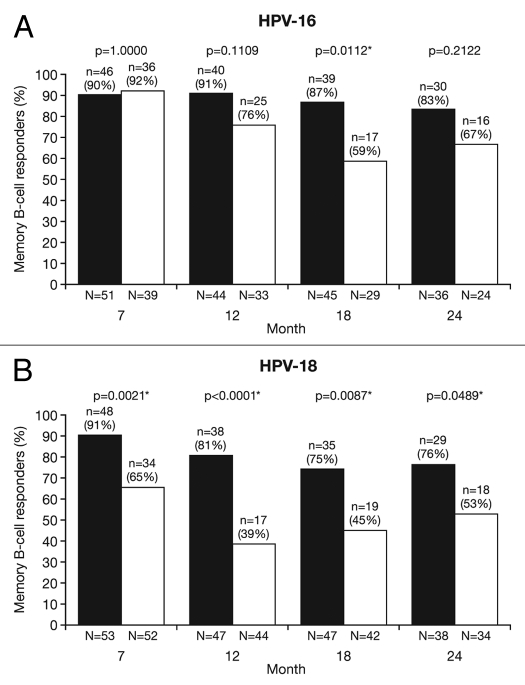
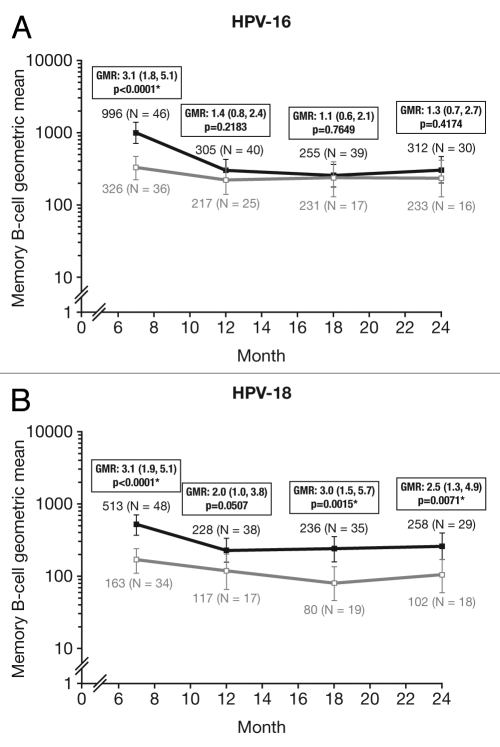
At Month 24, for all age groups combined, the proportion of responders (subjects with detectable HPV type-specific memory B-cells, i.e., >1 cell/million cells) was not statistically different between groups for HPV-16 (HPV-16/18 vaccine, 83.3%; HPV-6/11/16/18 vaccine, 66.7%: p = 0.2122) and was significantly higher for HPV-18 with the HPV-16/18 vaccine compared with the HPV-6/11/16/18 vaccine (76.3% vs. 52.9%; p = 0.0489) (Fig. 5). Because memory B-cell response data for all subjects in the subset did not follow a normal distribution, the mean frequency of circulating antigen-specific memory B-cells was assessed in responders only (i.e., positive subjects); at Month 24 this was not significantly different between the vaccine groups for HPV-16 [GM ratio, 1.3 (95% CI: 0.7, 2.7); p = 0.4174] and was significantly higher in the HPV-16/18 vaccine group for HPV-18 [GM ratio, 2.5 (1.3, 4.9); p = 0.0071] (Fig. 6).
Figure 5.
Proportion of responders for (A) HPV-16- and (B) HPV-18-specific B-cell responses at Months 7, 12, 18 and 24 (ATP cohort for immunogenicity; seronegative, DNA-negative and with no detectable HPV type-specific B-cells prior to vaccination). *p < 0.05. N, number of subjects with available results. Black bars, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Recombinant, adjuvanted, adsorbed) (Cervarix®); white bars, Human Papillomavirus Quadrivalent (Types 6, 11, 16 and 18) Vaccine, Recombinant (Gardasil®). Responders defined as subjects with detectable HPV type-specific memory B-cells [>1 cell/million cells]. p-values were calculated using Fisher's exact test to compare proportion of responders.
Figure 6.
Geometric means (GM) and GM ratios in responders only for (A) HPV-16- and (B) HPV-18-specific B-cells at Months 7, 12, 18 and 24 (ATP cohort for immunogenicity; seronegative, DNA negative and with no detectable HPV type-specific B-cells prior to vaccination). *p < 0.05. GMR, geometric mean ratio; N, number of responders [i.e., subjects with detectable HPV type-specific memory B-cells (>1 cell/million cells)]. Solid black lines, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Recombinant, adjuvanted, adsorbed) (Cervarix®); solid gray lines, Human Papillomavirus Quadrivalent (Types 6, 11, 16 and 18) Vaccine, Recombinant (Gardasil®). Error bars denote 95% confidence intervals of geometric means. Statistical comparison (GMR ANOVA p-value) was performed on B-cell responders only because data for all subjects in the subset did not follow a normal distribution.
CD4+ T-cell responses.
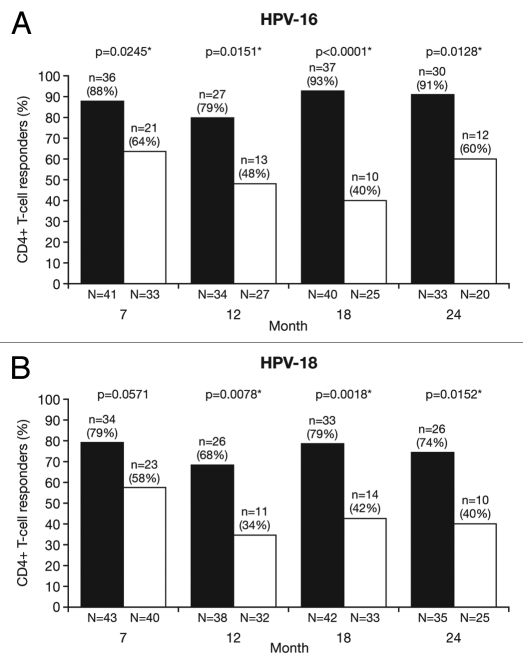
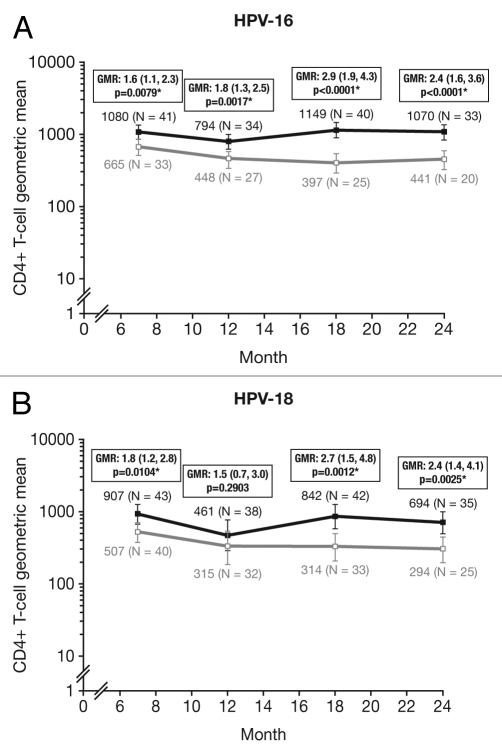
At Month 24, for all age groups combined, the proportion of responders (subjects with ≥500 HPV type-specific memory CD4+ T cells) was significantly higher in the HPV-16/18 vaccine group than in the HPV-6/11/16/18 vaccine group for both HPV-16 (90.9% vs. 60.0%; p = 0.0128) and HPV-18 (74.3% vs. 40.0%; p = 0.0152) (Fig. 7). The geometric mean frequency of circulating antigen-specific CD4+ T cells in all subjects (i.e., responders and non-responders) at Month 24 was significantly higher in the HPV-16/18 vaccine group than in the HPV-6/11/16/18 vaccine group for both HPV-16 [GM ratio, 2.4 (1.6, 3.6); p < 0.0001] and HPV-18 [GM ratio, 2.4 (1.4, 4.1); p = 0.0025] (Fig. 8).
Figure 7.
Proportion of responders for (A) HPV-16- and (B) HPV-18-specific CD4+ T-cell response at Months 7, 12, 18 and 24 (ATP cohort for immunogenicity; seronegative, DNA-negative and with a HPV type-specific CD4+ T-cell response below 500 cells per million cells prior to vaccination). *p < 0.05. N, number of subjects with available results. Black bars, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Recombinant, adjuvanted, adsorbed) (Cervarix®); white bars, Human Papillomavirus Quadrivalent (Types 6, 11, 16 and 18) Vaccine, Recombinant (Gardasil®). Responders defined as subjects with ≥500 HPV type-specific memory CD4+ T-cells expressing at least two of four immune markers [(CD40L, IL-2, TNFα, IFNγ)/million cells]. p-values were calculated using Fisher's exact test to compare proportion of responders.
Figure 8.
Geometric means (GM) and GM ratios for (A) HPV-16- and (B) HPV-18-specific CD4+ T-cell response at Months 7, 12, 18 and 24 (ATP cohort for immunogenicity; seronegative, DNA-negative and with a HPV type-specific CD4+ T-cell response below 500 cells per million cells prior to vaccination). *p < 0.05. GMR, geometric mean ratio; N, number of subjects with available results. Solid black lines, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Recombinant, adjuvanted, adsorbed) (Cervarix®); solid gray lines, Human Papillomavirus Quadrivalent (Types 6, 11, 16 and 18) Vaccine, Recombinant (Gardasil®). Error bars denote 95% confidence intervals of geometric means. Statistical comparison (GMR ANOVA p-value) was performed on all subjects.
Safety.
The proportion of subjects reporting medically significant conditions (MSCs; conditions prompting physician visits), new onset of chronic diseases (NOCDs) and new onset of autoimmune diseases (NOADs) in the TVC were similar between the vaccine groups (Table 4). The proportion of subjects reporting MSCs was 40.0% (95% CI: 35.9, 44.2) in the HPV-16/18 vaccine group and 34.7% (30.8, 38.9) in the HPV-6/11/16/18 vaccine group. The most commonly reported MSC in the HPV-16/18 vaccine group was bronchitis [HPV-16/18 vaccine, 2.7% (95% CI: 1.5, 4.4); HPV-6/11/16/18 vaccine, 1.6% (0.7, 3.1)] and in the HPV-6/11/16/18 vaccine group was depression [HPV-16/18 vaccine, 2.0% (1.0, 3.5); HPV-6/11/16/18 vaccine, 2.0% (1.0, 3.5)]. A total of 45 non-fatal SAEs were reported during this period (Month 0 to Month 24), 32 of which were reported from Month 7 to Month 24; two SAEs (one in each vaccine group) were considered possibly related to vaccination and were described in the Month 7 paper.30 During the subsequent follow-up period, one woman died from metastatic renal cell carcinoma before the Month 18 visit; her death was considered not related to vaccination by the investigator at the study site (this case remains blinded since the study is ongoing).
Table 4.
Safety outcomes at Month 24; number and proportion of subjects reporting at least one event (total vaccinated cohort, irrespective of serostatus and DNA status prior to vaccination)
| Proportion of responders, % (95% CI) [n] | ||
| HPV-16/18 vaccine N = 553 | HPV-6/11/16/18 vaccine N = 553 | |
| Medically significant conditions | 40.0 (35.9, 44.2) [221] | 34.7 (30.8, 38.9) [192] |
| New onset of chronic diseases* | 3.6 (2.2, 5.5) [20] | 3.8 (2.4, 5.7) [21] |
| New onset of autoimmune diseases‡ | 1.1 (0.4, 2.3) [6] | 1.8 (0.9, 3.3) [10] |
| Serious adverse events | 4.2 (2.7, 6.2) [23] | 4.0 (2.5, 6.0) [22] |
CI, confidence interval; N, number of subjects who received at least one vaccine dose; n, number of subjects reporting at least one event; Medically significant conditions, conditions prompting physician visits. Decisions relating adverse events to vaccination were based on the judgment of the investigator at the study site reporting the event.
All adverse events reported were compared with a pre-defined list of potential chronic diseases derived from the Medical Dictionary for Regulatory Activities; determination of whether a chronic disease was of new onset was based on blinded review of the reported symptoms and the subject's pre-vaccination medical history by a GSK physician.
New onset autoimmune diseases were identified from events categorized as new onset chronic diseases using a list detailing potential autoimmune events, which excluded allergy-related events or isolated signs and symptoms, plus events not considered to be autoimmune in origin.
A total of 77 pregnancies were reported up to Month 24, with similar outcomes between groups (Table 5).
Table 5.
Pregnancy outcomes up to Month 24 (total vaccinated cohort, irrespective of serostatus and DNA status prior to vaccination)
| Number (%) | ||
| HPV-16/18 vaccine N = 38 | HPV-6/11/16/18 vaccine N = 39 | |
| Normal infant | 20 (52.6) | 24 (61.5) |
| Premature birth | 2 (5.3) | 2 (5.1) |
| Abnormal infant | 0 (0.0) | 2 (5.1) |
| Elective termination | 5 (13.2) | 1 (2.6) |
| Therapeutic abortion | 0 (0.0) | 0 (0.0) |
| Ectopic pregnancy | 1 (2.6) | 0 (0.0) |
| Spontaneous abortion | 6 (15.8) | 6 (15.4) |
| Still birth | 0 (0.0) | 0 (0.0) |
| Lost to follow-up | 1 (2.6) | 3 (7.7) |
| Missed abortion | 0 (0.0) | 0 (0.0) |
N, number of cases identified; n, number of cases in a given category. At the time of this analysis, pregnancy was ongoing in three women (7.9%) in the HPV-16/18 vaccine group and one woman (2.6%) in the HPV-6/11/16/18 vaccine group.
Discussion
In general, the higher anti-HPV-16 and -18 immune responses induced by the HPV-16/18 vaccine when compared with the HPV-6/11/16/18 vaccine at Month 7 in Study HPV-010 30 were maintained up to Month 24 after first vaccination. GMTs of anti-HPV-16 and -18 serum neutralizing antibodies measured by PBNA remained significantly higher up to Month 24 in women aged 18–26 y who received the HPV-16/18 vaccine than in women who received the HPV-6/11/16/18 vaccine. The HPV-16/18 vaccine also induced significantly higher anti-HPV-16 and -18 neutralizing antibody titers in women aged 27–35 and 36–45 y at all timepoints through to Month 24. For both neutralizing and IgG anti-HPV-16 and -18 ELISA antibody titers, the magnitudes of the differences between the two vaccines (GMRs) at Month 24 were similar to those observed at previous timepoints. In the HPV-naive subset of the ATP cohort for immunogenicity (i.e., seronegative and DNA-negative for the antigen under analysis prior to vaccination), which approximates the target population for HPV vaccination programs, GMRs of anti-HPV-16 and -18 neutralizing antibody titers between the vaccine groups were greater in the younger age groups studied (18–26 y and 27–35 y) compared with the older age group (36–45 y) at all timepoints from Month 7 to 24. As expected from observations in other studies,10,13–15,17,18 for both vaccines, levels of vaccine-induced anti-HPV-16 and -18 antibodies peaked at Month 7 30 and subsequently declined toward plateau by Month 18.31,32 Through to Month 24, both vaccines induced higher anti-HPV-16 neutralizing antibody titers than those observed in women who had cleared natural infection (as measured by PBNA in the TVC);30 vaccine-induced anti-HPV-18 neutralizing antibody titers remained higher than those observed after natural infection in women who received the HPV-16/18 vaccine and reached levels similar to those associated with natural infection with the HPV-6/11/16/18 vaccine. In a previous study, for HPV-16, analysis by antibody titer quartile showed a significantly reduced risk of new HPV-16 infection with an increasing naturally-acquired antibody titer (measured by ELISA);33 an epidemiological study reported similar findings with women in the highest HPV-18 antibody tertile having a significantly reduced risk of subsequent infection with HPV-18.34 In our current study, across all age groups, at Month 24 the ELISA antibody titers are higher in the ATP cohort for immunogenicity with the HPV-16/18 vaccine compared with the HPV-6/11/16/18 vaccine for HPV-16 (2.3–4.2-fold) and HPV-18 (5.2–9.7-fold). Although an immunological correlate of protection has not been defined, these observed differences in the magnitude of immune response between both vaccines might therefore represent determinants of duration of protection.
In the ATP cohort for immunogenicity (seronegative and DNA-negative prior to vaccination), seropositivity rates for anti-HPV-16 neutralizing antibodies remained high (≥97.5%) in both vaccine groups at all timepoints up to Month 24. In the HPV-16/18 vaccine group, seropositivity rates for anti-HPV-18 neutralizing antibodies were ≥97.1% at all timepoints. In the HPV-6/11/16/18 vaccine group, seropositivity rates for anti-HPV-16 and −18 IgG antibodies remained high (≥96.5%) through Month 24 in both vaccine groups.
High serum antibody titers have been shown to induce enhanced concentrations of antibodies in CVS, thereby providing the first line of defense against HPV infection and subsequent disease.35,36 At Month 7, a greater proportion of women who received the HPV-16/18 vaccine compared with those who received the HPV-6/11/16/18 vaccine had detectable HPV type-specific neutralizing antibodies in CVS, correlating with the higher serological immune response observed with the HPV-16/18 vaccine.30 CVS antibody levels in women with detectable antibodies were similar between groups at most timepoints through Month 24. For both HPV-16 and HPV-18, the HPV-16/18 vaccine induced higher GM ratios of HPV-specific IgG antibodies to total IgG antibody content in CVS at Month 24, as previously observed at Month 7.30 This suggests that, up to 18 mo after the third dose, vaccination with the HPV-16/18 vaccine resulted in the transudation of more HPV-specific antibodies from serum to the site of potential infection, when compared with the HPV-6/11/16/18 vaccine. Interpretation of CVS data are, however, limited by the small number of samples analyzed, the technical difficulty and the limited sensitivity of the analyses, which we believe may be due to the presence of inhibitors such as blood and sample dilution resulting from heterogeneity of the sampling and from the IgG extraction process.
The proportion of HPV-18-specific circulating memory B-cell responses was statistically higher at all timepoints up to Month 24 in the HPV-16/18 vaccine group compared with the HPV-6/11/16/18 vaccine group, as was the mean frequency of circulating antigen-specific cells in responders at all timepoints except Month 12. In terms of HPV-16-specific circulating memory B-cell responses, at Month 7, although the proportion of responders was similar in both groups, the geometric mean of HPV-16-specific circulating memory B cells was significantly higher in the HPV-16/18 vaccine group compared with the HPV-6/11/16/18 vaccine group.30 After Month 7, no significant differences were observed between the vaccine groups in the proportion of responders and geometric means of HPV-16-specific circulating memory B-cells, except for at Month 18 when a significant difference in the proportion of responders was observed for HPV-16-specific circulating memory B-cells. Within each vaccine group, HPV-16- and HPV-18-specific circulating memory B-cell frequencies generally remained stable between Months 12–24. It should be noted that B-cells may migrate to central lymphatic organs (e.g., spleen) at later timepoints.
For CD4+ T-cells, the proportion of responders and mean frequency of circulating antigen-specific cells calculated on all subjects (i.e., responders and non-responders) were significantly higher with the HPV-16/18 vaccine compared with the HPV-6/11/16/18 vaccine at Month 24, for both HPV-16 and HPV-18. For the HPV-16/18 vaccine, higher values observed at later timepoints might in part be due to variability in the batch testing utilized, as samples from different timepoints were evaluated in different testing runs. For instance, assay variability may be a result of the batch of reagents used at that timepoint or the assay set up, which, even if bridged, may have evolved. The most consistent comparisons are with the group responses at each timepoint. In the HPV-6/11/16/18 vaccine group, HPV-16- and HPV-18-specific circulating CD4+ T-cell frequencies plateaued between Months 12–24.
Both vaccines were generally well tolerated and exhibited similar safety profiles up to Month 24; incidences of MSCs, NOCDs, SAEs and pregnancy outcomes were similar between the groups and consistent with previous studies of each vaccine. The incidence of autoimmune diseases was similar in women vaccinated with the HPV-16/18 vaccine [1.1% (95% CI: 0.4, 2.3)] and women vaccinated with the HPV-6/11/16/18 vaccine [1.8% (0.9, 3.3)]; these values are comparable with those from a large integrated safety database of participants in studies of the HPV-16/18 vaccine [N = 19,723; 0.49% (0.39, 0.59)] vs. control [N = 19,437; 0.54% (0.44, 0.65)].37 Additionally, incidence rates of autoimmune disease in vaccinated women were comparable with rates of other neuroinflammatory events within the general population.
The differences in anti-HPV-16/18 immunogenicity observed between the HPV-16/18 vaccine and the HPV-6/11/16/18 vaccine may reflect the different adjuvant systems used in each. The HPV-16/18 vaccine employs AS04, which contains aluminum hydroxide and MPL, a detoxified derivative of the immunomodulatory cell wall lipopolysaccharide (LPS) molecule of the Gram-negative Salmonella minnesota R595 strain.38 MPL is an agonist of Toll-like receptor 4 (TLR4) 39 and TLR4 stimulation can contribute to activation of the innate immune response.40 In vitro and in vivo data suggest that the addition of MPL to aluminum hydroxide enhances vaccine-induced immune response by rapidly triggering a local cytokine response leading to optimal activation of antigen-presenting cells.39 A separate study demonstrated enhanced antibody and memory B-cell responses when HPV-16/18 L1 VLP vaccine was formulated with AS04 compared with aluminum hydroxide alone.6 The HPV-6/11/16/18 vaccine contains a proprietary AAHS adjuvant. When formulated with HPV-16 vaccine, AAHS has demonstrated greater inherent capacity for adsorption of HPV-16 L1 VLPs and greater anti-HPV-16 L1 VLP antibody responses compared with aluminum hydroxide.8 However, no study has directly compared the relative contributions of AS04 and AAHS (using identically expressed antigens) to vaccine-induced anti-HPV-16/18 immune responses.
Strengths of our study include the use of a PBNA, which measures a range of neutralizing antibodies, and identical methodology for assessment of both vaccine groups. The cell line used in the PBNA is not used in the production of either vaccine and the pseudovirions closely resemble the natural viral particles, making the PBNA unbiased to either vaccine. As discussed previously, our results are also unlikely to be biased by the ELISA and B-cell ELISPOT assays used to measure HPV type-specific immune responses.30 Although these assays are based on the HPV-16/18 vaccine constructs, data are not expected to be significantly impacted by the use of these truncated proteins, given their overall similarity of 93% with the full-length L1 protein sequences. In a sub-analysis of sera from women included in Study HPV-010, a good correlation was observed between GMTs (HPV-16/18 vaccine over HPV-6/11/16/18 vaccine) generated using GSK's ELISA and Merck's competitive Luminex immunoassay (cLIA); the HPV-16/18 vaccine induced higher GMTs of anti-HPV-16 and -18 serum antibodies compared with the HPV-6/11/16/18 vaccine irrespective of the assay used.41 The assay used to evaluate CD4+ T-cell responses is also unlikely to favor either vaccine, as the HPV peptide pools used for in vitro stimulation were designed from the HPV-16 and HPV-18 L1 VLP sequences used in the HPV-16/18 vaccine but included the portions truncated from the HPV-16/18 vaccine but present in the HPV-6/11/16/18 vaccine.
Study limitations include the exclusion of pre-teen/young adolescent girls (as discussed previously in ref. 30) and reliance on subjects for accurate reporting of history (e.g., sexual activity, number of partners, previous abnormal Pap results and HPV positivity). This study primarily evaluated vaccine-induced immune responses by measuring GMTs of serum neutralizing antibodies (by PBNA) and, in the absence of a defined correlate of vaccine-induced protection, our data may or may not reflect clinical outcomes. Given the very high efficacy observed with both vaccines, conducting a trial capable of detecting a difference between the two would require a very large study population and a prolonged follow-up period. Due to the methodological challenges of assessing CVS samples by PBNA (e.g., presence of inhibitors such as blood, timing relative to menstrual cycle, sample dilution), antibody titers in CVS were measured by ELISA. A previous study of women vaccinated with the HPV-16/18 vaccine observed a high correlation when anti-HPV-16 and -18 antibody responses were measured by direct ELISA (which is based on multiple epitopes) and PBNA, suggesting that the direct ELISA is a surrogate for neutralizing activity.42
Both the HPV-16/18 vaccine and the HPV-6/11/16/18 vaccine have demonstrated high efficacy against disease and virological endpoints in clinical trials and are expected to substantially reduce HPV-related disease burden.43 Both vaccines were licensed based on their excellent efficacy profiles in adolescents and young adults, with high immunogenicity demonstrated across all licensed age groups. Currently, the longest published immunogenicity follow-up for either vaccine is 7.3 y.44 Higher immunogenicity can reasonably be expected to contribute to a longer duration of vaccine-induced protection.
In the absence of a surrogate marker for a correlate of protection against HPV, the number of breakthrough cases in vaccinees (fortunately rare) for each type will become particularly important to establish normalized serocurves. If a correlate of protection against HPV is established, a standardized assay would be important to ensure that this is appropriately and universally defined. PBNA, developed independently without using antigens from specific vaccines, could be used for such comparison.
In this follow-up analysis of Study HPV-010, the HPV-16/18 vaccine demonstrated generally higher immunogenicity and a similar safety profile to the HPV-6/11/16/18 vaccine through 24 mo after first vaccination in healthy women aged 18–45 y. This observation may be of relevance for healthcare providers and other stakeholders in cervical cancer prevention. Any clinical efficacy differences between the two vaccines in terms of prevention of HPV-16/18-associated cervical disease, should they exist, are not currently evident and will only become apparent through long-term assessment.
Materials and Methods
Study design, immunogenicity and safety assessments.
This follow-up study was conducted in 40 centers in the US; we previously reported findings at Month 7.30 Study participants, ethics, design and vaccine composition were as described previously in reference 30. Women were stratified by age (18–26, 27–35 and 36–45 y) and randomized (1:1 ratio in each age group) to receive 0.5 mL of either the HPV-16/18 vaccine or the HPV-6/11/16/18 vaccine according to their recommended three-dose schedules (Months 0, 1, 6 or Months 0, 2, 6, respectively). The study was conducted in an observer-blind manner and to maintain the blind, women received one dose of placebo (aluminum hydroxide) at either Month 1 or 2, as appropriate. Analyses were performed at baseline and Months 7, 12, 18 and 24 with all analysts being blind to sample identity. Long-term follow-up through Month 48 is ongoing.
Blood, cervicovaginal secretion and cervical sampling were performed as described previously in reference 30. CVS samples were collected in a subset of subjects from preselected sites capable of handling collection according to the predefined conditions. HPV type-specific IgG antibody positivity rates were assessed in CVS by ELISA in the ATP cohort for immunogenicity (irrespective of serostatus and DNA status prior to vaccination) (Table 3).
Blood samples for assessment of immune response were also collected from all women at Months 12, 18 and 24. As prespecified, additional samples were collected from a subset of women in all age groups in both vaccine groups at Months 12, 18 and 24 for further immunological assessment; this included evaluation of (1) HPV type-specific antibody levels in CVS (2) HPV type-specific memory B cell and (3) CD4+ T-cell responses. Antibody extraction from CVS samples, PBNA, ELISA, B-cell ELISPOT assay and safety assessments were performed as described previously in reference 30. In the absence of a serological correlate of protection afforded by HPV vaccines, GMTs of anti-HPV-16 and -18 neutralizing antibodies (measured by PBNA) induced by natural infection were used to evaluate vaccine-induced antibody responses. These were defined as GMTs in women in the TVC who were DNA negative but seropositive at Month 0 for the antigen under analysis, indicating clearance of natural infection. For each antigen, positivity was defined as a serum dilution greater than or equal to the assay threshold of 40 ED50 (effective dose producing 50% response).
CD4+ T-cell responses. CD4+ T-cell responses were evaluated in a subset of subjects (women in the ATP cohort for immunogenicity who were seronegative, DNA-negative and had no detectable CD4+ T-cell response prior to vaccination). Prior to analyses, peripheral blood mononuclear cells were separated from heparinized whole blood using a Ficoll-Hypaque gradient and were cultured in vitro in RPMI 1640 medium supplemented with 100 U/mL penicillin, 0.1 mg/mL streptomycin, 0.3 mg/mL glutamine, 50 µM β-mercaptoethanol and 10% heat-inactivated human AB serum.45
Vaccine-induced CD4+ T-cell responses were evaluated in vitro by using a pool of HPV peptides to stimulate peripheral blood antigen-specific CD4+ T-cells to produce cytokines. The HPV-16 and HPV-18 L1 patented sequences for the HPV-16/18 vaccine and the HPV-6/11/16/18 vaccine are 99.6% and 99.4% identical, respectively, by protein level comparison. The main differences between the sequences are the 33 and 35 amino acid C-terminal truncations of the L1 sequences used in the HPV-16/18 vaccine. The HPV peptide pool used for in vitro stimulation included the HPV-16 and HPV-18 L1 VLP sequences used in the HPV-16/18 vaccine and the portions truncated from the HPV-16/18 vaccine but present in the HPV-6/11/16/18 vaccine. Overall, the peptide pool comprised 55 and 53 peptides (including 20-mer peptides overlapping by 10 amino acids) covering the L1 VLP sequences for HPV-16 and HPV-18, respectively. Following intracellular cytokine staining, flow cytometry was used to quantify the number of cells producing at least two of four different immune markers (CD40L, IL-2, TNFα and IFNγ) in response to the HPV peptide pool. Expressing specific T-cell response in this way was based on studies in the literature.45–47 Evaluations performed in-house by GSK consistently found that this approach reduced background without significantly compromising sensitivity, and constituted a robust method for analyzing a large number of samples. A positive CD4+ T-cell response was set as a frequency ≥500 HPV-specific CD4+ T-cells per million CD4+ T-cells (0.05%), the cut-off value corresponding to the 95th percentile of the specific response in a HPV-negative population from the study HPV-010 (NCT00423046). Cell culture medium alone (i.e., without the HPV peptide pool) was used as a negative control and a mitogen (staphylococcal enterotoxin B [SEB]) was used as a positive control in each testing run to ensure consistency.
Statistical analyses.
The primary exploratory objective of this follow-up analysis was to compare GMTs of anti-HPV-16 and -18 serum neutralizing antibodies measured by PBNA at Month 24 after first vaccination with either the HPV-16/18 vaccine or the HPV-6/11/16/18 vaccine in women aged 18–26 y. Secondary objectives were to compare the immune response to HPV-16 and -18 induced by the two vaccines measured at Month 24: (1) in serum by PBNA in women aged 27–35 and 36–45 y; (2) in serum by ELISA in all women; (3) in CVS by PBNA/ELISA in a subset of women; (4) in terms of cell-mediated immunity (CMI; HPV-16/18-specific B-cell and CD4+ T-cell responses) in a subset of women; and (5) to compare the safety of the two vaccines. The prespecified target for CVS and CMI analyses was 30% of subjects per age cohort per vaccine group. Statistical analyses (withingroup and between-group comparisons) of immunogenicity (ATP cohort for immunogenicity) and safety (TVC) were performed at Months 7, 12, 18 and 24, as described previously in reference 30.
For statistical comparison of serum PBNA data, non-inferiority of the HPV-16/18 vaccine to the HPV-6/11/16/18 vaccine was concluded if the lower limit of the two-sided 95% CI for the GMT ratio for a given antigen was greater than 0.5 in the ATP cohort for immunogenicity (seronegative and DNA-negative prior to vaccination). If the lower limit of the two-sided 95% CI for the GMT ratio was greater than 1, the p-value associated with a test of superiority (ANOVA model) was calculated for that antigen in the TVC (irrespective of serostatus and DNA status prior to vaccination).
To enable comparison of vaccine-specific antibody titers between serum and CVS, anti-HPV-16 and anti-HPV-18 antibody titers were measured relative to the total IgG antibody concentration of each sample. Ratios for anti-HPV-16 or anti-HPV-18 antibody titers divided by total IgG antibody concentration were expressed as EU/µg of total IgG, and the corresponding Pearson correlation coefficient (r) was calculated.
For CD4+ T-cell responses, the results for each stimulant at each timepoint were summarized for each group and age stratum by the geometric mean (GM). In an exploratory inferential analysis, the proportion of responders in each vaccine group was compared using a Fisher's exact test. The GM ratio between vaccine groups was obtained using an ANOVA model on the log10-transformed frequencies. The ANOVA model included the vaccine group as fixed effect. The GM ratio and its 95% CI were derived as exponential transformation. For the comparison of CD4+ T-cell GM ratios, p-values were computed using ANOVA model. The same statistical methods were used to compare B-cell responses between the vaccine groups, i.e., the proportion of responders and the GM ratio in responders.
Acknowledgments
We thank all study participants and their families. This study was sponsored by GlaxoSmithKline Biologicals, Belgium. The team from GlaxoSmithKline Biologicals, US, included Kevin Carrick (Senior Study Manager), J. Ann Jones (Field Monitor), and Joan Adler (Local Medical Monitor). Jesse C. Lepage (Cincinnati Children's Hospital, Cincinnati, Ohio, United States) provided technical expertise in the preparation of samples for the evaluation of B-cell responses. B-cell assays were performed by the Human Cellular Immunity Team at GlaxoSmithKline Biologicals, Belgium (Olivier Jauniaux, Sarah Charpentier, Valerie Mohy, Dinis Fernandez-Ferreira, Samira Hadiy, Michael P. Mestre, Murielle Carton, Pierre Libert and Luc Franssen). Philippe Moris (GlaxoSmithKline Biologicals, Belgium) led the memory B-cell analysis. Pseudovirion-based neutralization assays and enzyme-linked immunosorbent assays were performed by the Global Vaccine Clinical Laboratories Development Unit (GVCL DU) team at GlaxoSmithKline Biologicals, Belgium (Rudy Crudenaire, Stephanie Abderhamane, Rita Dereymaeker, France Dufranne, Benjamin Mathieu, Marie Gangarossa, Lieve Lauwers, Jeremy Leurquin, Mailys Pringels, Laurence Torset, Jessica Vanderhaegen, Laurence Luyten, Vinciane Lelivre). Sylviane Poncelet (GlaxoSmithKline Biologicals, Belgium) led the enzyme-linked immunosorbent assay analysis of cervico-vaginal secretions. Testing of HPV-16 and HPV-18 antibodies in serum was performed by the Global Vaccine Clinical Laboratories Clinical Immunology and Applied Microbiology (GVCL CI&AM) team at GlaxoSmithKline Biologicals, Belgium (Laetitia Gérard, Valérie Smuga, Natacha Casaert, Cathy Hosselet, Aurore Bernis, Gaby Eckstein, Françoise Stevens and Bénédicte Brasseur). Valérie Xhenseval (GlaxoSmithKline Biologicals, Belgium) led the enzyme-linked immunosorbent assay analysis of sera.
Jean-Louis Maroye (Modern Solutions for Business SPRI, Belgium) received the randomization list and performed the statistical analyses. Marie Lebacq (GlaxoSmithKline Biologicals, Belgium) supervised the data analyses (without access to the randomization list), and contributed to the statistical QC and writing of the statistical analysis report. The study report was prepared by Kim Nijs (Emtex, C/O GlaxoSmithKline Biologicals, Belgium) and Stéphanie Genevrois (GlaxoSmithKline Biologicals, Belgium). Medical writing assistance was provided by Meridian HealthComms Ltd., (Middlewich, UK) on behalf of GlaxoSmithKline Biologicals. Jenny M.E. Andersson and Dirk Saerens (GlaxoSmithKline Biologicals, Belgium) provided manuscript coordination on behalf of GlaxoSmithKline.
Abbreviations
- AAHS
amorphous aluminum hydroxyphosphate sulfate
- ANOVA
analysis of variance
- AS04
adjuvant system 04
- ATP
according-to-protocol
- CI
confidence interval
- CMI
cell-mediated immunity
- CVS
cervicovaginal secretion
- DNA
deoxyribonucleic acid
- ED50
effective dose producing 50% response
- ELISA
enzyme-linked immunosorbent assay
- EU
ELISA units
- GM
geometric mean
- GMR
geometric mean (titer) ratio
- GMT
geometric mean titer
- GSK
GlaxoSmithKline
- HPV
human papillomavirus
- IgG
immunoglobulin G
- LPS
lipopolysaccharide
- MPL
monophosphoryl lipid A
- MSC
medically significant condition
- NOAD
new onset autoimmune disease
- NOCD
new onset chronic disease
- PBNA
pseudovirion-based neutralization assay
- PCR
polymerase chain reaction
- SAE
serious adverse event
- TLR4
toll-like receptor 4
- TVC
total vaccinated cohort
- VLP
virus-like particle
Notes
Cervarix is a registered trade mark of the GlaxoSmithKline group of companies. Gardasil is a registered trade mark of Merck and Co., Inc.
Conflict of Interest Statement
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf and declare: The submitted work got financial support from GlaxoSmithKline Biologicals.
ME has advised or participated in educational speaking activities, but does not receive payments from any companies. In specific cases, Montefiore Medical Center has received payment for ME time spent for these activities from GSK and Merck. Also, Montefiore has received grant funding for research related costs of clinical trials that ME has been the Montefiore PI from GSK and Merck.
A.C., J.R., G.D. received support for travel to meetings for the study. A.C., J.R., M.J.L., N.C. received grants for their institutions. A.C. received financial support for board membership. A.C., M.J.L. received financial support for consultancy. A.C., B.F., N.C. received payment for lectures including service on speaker bureaus. D.D., D.M., F.D., G.D., S.D. are GSK employees. D.D., G.D., S.D. have GSK stock options. G.D. received royalties from Pfizer. S.S., M.B. declare no conflict of interest.
†HPV-010 Study Group
Principal and Co-Investigators: Keith Aqua (Visions Clinic Research, Boynton Beach, FL), Mira Baron (Rapid Medical Research, Cleveland, OH), Mark Blatter (Primary Physicians Research, Inc., Pittsburgh, PA), Archana Chatterjee (Creighton University, Omaha, NE), Christopher V. Chambers (Thomas Jefferson University, Philadelphia, PA), Nahida Chakhtoura (University of Miami, Miami, FL), Louis A. Civitarese (Preferred Primary Care Physicians, Inc., Carnegie, PA), Donna DeSantis (East Valley Family Physicians, Chandler, AZ), Rovena Reagan (Women's Health Care at Frost St., San Diego, CA), Mark H. Einstein (Montefiore Medical Center, Bronx, NY), Douglas K. Fenton (North Coast Women's Care, Vista, CA), Bradley Fox (Liberty Family Practice, Erie, PA), David L. Fried (Omega Medical Research, Warwick RI), Sidney A. Funk (Radiant Research, Atlanta, GA), Cheryl A. Hansen (Ridgeview Research, Chaska, MN), James A. Hedrick (Kentucky Pediatric and Adult Research, Bardstown, KY), Dan C. Henry (Foothill Family Clinic, Salt Lake City, UT), Bethany Hoffman (Aspen Medical Group, St. Paul, MN), Alan Johns (Texas Healthcare, Fort Worth, TX), Terry D. Klein (Heartland Research Assoc., Wichita, KS), Jacob Lalezari (Quest Clinical Research, San Francisco, CA), Myron J. Levin (University of Colorado Denver and Health Sciences Center, Aurora, CO), William Nebel (Chapel Hill OB/GYN, Chapel Hill, NC), Michael J. Noss (Radiant Research, Cincinnati, OH), Kevin Pitts (Wenatchee Valley Medical Center, Wenatchee, WA), Alfred N. Poindexter III (Advances in Health, Inc., Houston, TX), Anthony Puopolo (Milford Emergency Associates, Inc., Milford, MA), Jeffrey Rosen (Clinical Research of South Florida, Coral Gables, FL), L. Sofia Scholar (Walla Walla Clinic, Walla Walla, WA), Michael A. Scutella (OB/GYN Associates of Erie, Erie, PA), James H. Silverblatt (Lake Medical Research, LLC, Willoughby Hills, OH), Dirk Smith (Heartland Research Associates, Arkansas City, KS), Rhoda S. Sperling (Mount Sinai School of Medicine, New York, NY), Karen G. Swenson (Professional Quality Research, Austin, TX), Mark Turner (Advanced Clinical Research, Boise, ID), Michael W. Warren (Research Across America, Lancaster, PA).
Study sponsor contributors: Francis Dessy (GlaxoSmithKline Biologicals, Belgium) led the pseudovirion-based neutralization assay (PBNA); Sanjoy Datta and Dominique Descamps (GlaxoSmithKline Biologicals, Belgium), Gary Dubin and Anne Schuind (GlaxoSmithKline Biologicals, US) supervised study design and conduct, data collection, interpretation and reporting.
References
- 1.Castellsagué X, de Sanjosé S, Aguado T, Louie KS, Bruni L, Muñoz J, et al. HPV and cervical cancer in the world. Vaccine. 2007;25:1–230. [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de SS, Bruni L, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26:1–16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 6.Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24:5937–5949. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Keam SJ, Harper DM. Human papillomavirus types 16 and 18 vaccine (recombinant, AS04 adjuvanted, adsorbed) [Cervarix] Drugs. 2008;68:359–372. doi: 10.2165/00003495-200868030-00007. [DOI] [PubMed] [Google Scholar]
- 8.Caulfield MJ, Shi L, Wang S, Wang B, Tobery TW, Mach H, et al. Effect of alternative aluminum adjuvants on the absorption and immunogenicity of HPV16 L1 VLPs in mice. Hum Vaccin. 2007;3:139–145. doi: 10.4161/hv.3.4.4309. [DOI] [PubMed] [Google Scholar]
- 9.Tovar JM, Bazaldua OV. New quadrivalent HPV vaccine developments. Postgrad Med. 2008;120:14–16. doi: 10.3810/pgm.2008.11.1929. [DOI] [PubMed] [Google Scholar]
- 10.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 11.FUTURE II Study Group, author. Prophylactic efficacy of a quadrivalent human papillomavirus (HPV) vaccine in women with virological evidence of HPV infection. J Infect Dis. 2007;196:1438–1446. doi: 10.1086/522864. [DOI] [PubMed] [Google Scholar]
- 12.FUTURE II Study Group, author. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 13.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 14.Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 15.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised controlled trial. Lancet. 2006;367:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 16.Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007;25:4931–4939. doi: 10.1016/j.vaccine.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 17.Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16 and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 18.Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95:1459–1466. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowhani-Rahbar A, Mao C, Hughes JP, Alvarez FB, Bryan JT, Hawes SE, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine. 2009;27:5612–5619. doi: 10.1016/j.vaccine.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 21.Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102:3–8. doi: 10.1016/S0002-9343(97)00177-0. [DOI] [PubMed] [Google Scholar]
- 22.Woodman CB, Collins S, Winter H, Bailey A, Ellis J, Prior P, et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;357:1831–1836. doi: 10.1016/S0140-6736(00)04956-4. [DOI] [PubMed] [Google Scholar]
- 23.Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol. 2005;32:16–24. doi: 10.1016/j.jcv.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Alphs HH, Gambhira R, Karanam B, Roberts JN, Jagu S, Schiller JT, et al. Protection against heterologous human papillomavirus challenge by a synthetic lipopeptide vaccine containing a broadly cross-neutralizing epitope of L2. Proc Natl Acad Sci USA. 2008;105:5850–5855. doi: 10.1073/pnas.0800868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarz TF, Leo O. Immune response to human papillomavirus after prophylactic vaccination with AS04-adjuvanted HPV-16/18 vaccine: improving upon nature. Gynecol Oncol. 2008;110:1–10. doi: 10.1016/j.ygyno.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 26.Stanley MA. Immune responses to human papilloma viruses. Indian J Med Res. 2009;130:266–276. [PubMed] [Google Scholar]
- 27.Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci USA. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization, author. Human papillomavirus and HPV vaccines: technical information for policy-makers and health professionals. World Health Organization. 2007. Available from: URL: http://whqlibdoc.who.int/hq/2007/WHO_IVB_07.05_eng.pdf.
- 29.Schiller JT, Lowy DR. Immunogenicity testing in human papillomavirus virus-like-particle vaccine trials. J Infect Dis. 2009;200:166–171. doi: 10.1086/599988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, et al. Comparison of the immunogenicity and safety of Cervarix™ and Gardasil® human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin. 2009;5:705–719. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 31.Joura EA, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, Hernandez-Avila M, et al. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine. 2008;26:6844–6851. doi: 10.1016/j.vaccine.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 32.Moscicki AB, Wheeler C, Romanowski B, Hedrick J, Gall S, Ferris D, et al. Anamnestic response elicited by a fourth dose of the HPV-16/18 adjuvanted vaccine in young women; EUROGIN; February 17–20, 2010; Monte Carlo, Monaco. 2010. [Google Scholar]
- 33.Castellsagué X on behalf of the HPV PATRICIA study group, author. Risk of new infection with the same HPV-type in women with naturally-acquired HPV16/18 antibodies; Presented at International Gynecologic Cancer Society (IGCS)—13th Biennial Meeting; October 23–26, 2010; Prague, Czech Republic. 2010. [Google Scholar]
- 34.Safaeian M, Porras C, Schiffman M, Rodriguez AC, Wacholder S, Gonzalez P, et al. Epidemiologic study of anti HPV16/18 seropositivity and subsequent risk of HPV16 and 18 infections; 26th International Papillomavirus Conference; July 3–8, 2010; Montreal, Canada. 2010. [Google Scholar]
- 35.Stanley M, Lowy DR, Frazer I. Chapter 12: Prophylactic HPV vaccines: Underlying mechanisms. Vaccine. 2006;24(Suppl 3):S3/106–S3/113. doi: 10.1016/j.vaccine.2006.05.110. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Perona P, et al. Immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15–55 years. Vaccine. 2009;27:581–587. doi: 10.1016/j.vaccine.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 37.Verstraeten T, Descamps D, David MP, Zahaf T, Hardt K, Izurieta P, et al. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine. 2008;26:6630–6638. doi: 10.1016/j.vaccine.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 38.Garçon N, Chomez P, Van MM. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines. 2007;6:723–739. doi: 10.1586/14760584.6.5.723. [DOI] [PubMed] [Google Scholar]
- 39.Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;183:6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- 40.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 41.Dessy F, Poncelet S, Xhenseval V, Méric S, Datta S on behalf of the HPV-010 study group, author. Comparative evaluation of the immunogenicity of two prophylactic human papillomavirus cervical cancer vaccines by Merck's competitive Luminex immunoassay (cLIA) and GSK's binding ELISA; EUROGIN; February 17–20, 2010; Monte Carlo, Monaco. 2010. [Google Scholar]
- 42.Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008;4:425–434. doi: 10.4161/hv.4.6.6912. [DOI] [PubMed] [Google Scholar]
- 43.Schiller JT, Castellsague X, Villa LL, Hildesheim A. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine. 2008;26:53–61. doi: 10.1016/j.vaccine.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Carvalho N, Teixeira J, Roteli-Martins CM, Naud P, De Borba P, Zahaf T, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine. 2010;28:6247–6255. doi: 10.1016/j.vaccine.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, et al. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11:1118–1124. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 46.Chattopadhyay PK, Yu J, Roederer M. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat Med. 2005;11:1113–1117. doi: 10.1038/nm1293. [DOI] [PubMed] [Google Scholar]
- 47.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]